Abstract
Coriander leaves are widely valued in cooking due to their rich nutrition and unique volatiles, and the flowers are also beneficial for oil extraction from seeds. With the growing interest in LED lights for controlled environments, research on coriander leaves has been reported, but studies on flowers are limited. We investigated the effects of various LED lights, including white (W), red (R), blue (B), and green (G) lights, on flowering. Coriander plants under B light were twice as tall and flowered approximately 4 weeks earlier than those under other lights. However, plants under B light exhibited overgrowth, resulting in fewer flowers at a PAR of 100 µmol·m−2·s−1. To reduce this shade avoidance effect, we tested various combinations of W and B light at a PAR of 120 µmol·m−2·s−1. The 50:50 ratio of W and B light enhanced growth and accelerated flowering, resulting in twice as many flowers as plants under W light. Total volatile compound levels were higher under W light and highest under 50% W and 50% B lights. Key volatiles specific to coriander leaves, such as (E)-2-decenal, 2-undecenal, and 2-dodecenal, were high under G light at 100 µmol·m−2·s−1 of PAR. These findings provide valuable insights into the effects of light on flower development.
1. Introduction
Light-emitting diodes (LEDs) have become the primary light source for growing crops in controlled environments. One of the most significant benefits is the easy adjustment of light quality according to different plant developmental stages [1]. Light quality refers to spectral distribution and significantly affects plant growth and development [2]. Different wavelengths of blue (B, 445–500 nm), green (G, 500–580 nm), red (R, 620–700 nm), and far-red (FR, 700–775 nm) have been used on various crops to find optimal light conditions [1,3,4]. Phytochromes absorb R and FR light and are involved in photomorphogenesis and efficient photosynthesis [5]. The main photoreceptors for B light in plants are cryptochromes and phototropins. Cryptochromes participate in de-etiolation, the inhibition of stem and petiole elongation, promote leaf expansion, regulate circadian rhythms, flowering, flavonoid biosynthesis, and shade response [1]. In contrast, phototropins are associated with leaf flattening, phototropism, chloroplast relocation, and the promotion of stomatal opening [1,6]. G light is also involved in the absorption of cryptochromes and has been identified in synergistic action with phytochromes [1,7]. Since both B and R lights are essential for plant growth and photosynthetic rates [1], many studies have been reported on these lights to promote plant growth. Coriander grown under R and B light (ratios of 10:1 and 19:1) increased fresh and dry mass [8], while plants grown under R and B light (ratio of 3:1) produced higher total yield compared to other light treatments [2]. Similarly, coriander grown under R and B light (ratio of 87:13) showed improved biomass and chlorophyll levels [9].
While R and B light are crucial for plant growth, B light is more effective than R light, especially for flowering [7]. For example, lettuce grown under R light exhibited etiolation of the hypocotyl, but the addition of B light compensated for this effect through improved chlorophyll synthesis [1]. Flowering is known to be regulated by cryptochromes [10], which play a crucial role in activating the floral induction that promotes flower bud development [11]. Much research has shown that B light can enhance flowering in various plants, including Arabidopsis, calibrachoa, geranium, marigold, and petunia [3,10,12]. Blueberry plants grown under B light flowered 15 days earlier than those under R light, producing approximately three times more flowers than those under R light, and four times more than those grown under fluorescent light [13]. Saffron plants grown under B light also exhibited a higher number of flowers derived from corms than combinations of B and R lights [14]. Chrysanthemum flower buds emerged earlier, and the number increased as supplemental B light was extended to W light [15].
Coriandrum sativum, commonly known as coriander, is an annual herb that belongs to the Apiaceae family. All parts of the plant are edible, with fresh leaves and dried seeds commonly used in cooking. The leaves are rich in vitamins and contain essential minerals, antioxidants, and carotenoids [16,17]. Flowers and seeds are also highly beneficial, including microorganism inhibition and oil extraction. Due to the high demand for quality coriander, there is increasing interest in cultivating it in controlled environments, where light quality is one of the most important factors for optimal growth. Although extensive research has been conducted on LED lights, relatively few studies have been reported on coriander. Additionally, most existing research has focused on the early stages, leaving a significant gap in understanding how different LED lighting conditions affect the flowering of coriander plants. Coriander is an ideal model plant for studying flowering because it grows rapidly, typically flowering within 6–8 weeks from seed, and produces a substantial number of flowers. Flowers are vital not only for plant reproduction, such as leaves and seeds, but also for secondary metabolite production, such as phenyl propanoids. For example, a study found that a hectare of coriander can produce about 500 kg of honey for honeybees [17].
This study aims to investigate the impact of various light conditions on coriander plants, specifically examining flowering and changes in volatile compounds. The findings of this research could enhance understanding of how LED lighting influences flowering.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Coriander (Coriandrum sativum) seeds were soaked in water overnight and then germinated in PRO-MIX BX potting soil (Premier Tech Horticulture, Quakertown, PA, USA). The seeds were placed under cool white, fluorescent lamps (T12, 40 W, 4100 K; Philips Co., Amsterdam, The Netherlands) for 16 h a day at 18 °C in a plant growth chamber (Percival Scientific, Perry, IA, USA). After 6 weeks, the individual seedlings were transplanted into 4-inch pots, and three plants per treatment were grown under various light conditions.
The light experiments were conducted in a walk-in growth room maintained at a temperature of 18 °C for 8 weeks. Four individual boxes (86.36 cm × 82.55 cm × 60 cm) were equipped with LED lighting systems (Heliospectra AB, Gothenburg, Sweden). The boxes were set for a 16 h photo period. Light spectra were measured using a StellarNet spectroradiometer and visualized with SpectraWiz software, version 5.33 (Stellar Net Inc., Tampa, FL, USA). In the first experiment, four different types of light were used: white (W, mixed bandwidth range of 380–700 nm), R (660 nm at peak), B (420 nm at peak), and G (530 nm at peak) with photosynthetic active radiation (PAR) set to 100 µmol·m−2·s−1 (Figure 1A). In the second experiment, the effect of B light was further examined through various combinations of white and B light (ratios of white to blue: 100:0, 90:10, 80:20, or 50:50), while maintaining a PAR of 120 µmol·m−2·s−1 (Figure 1B).
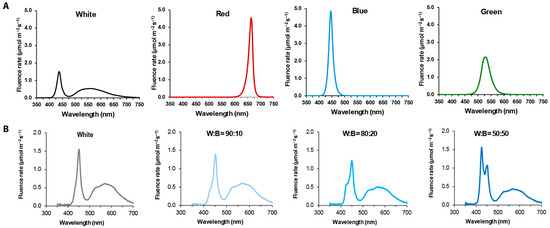
Figure 1.
Spectroradiometer reading of the light qualities used in this study. All treatments represent the narrow-bandwidth or combined lights at 100 µmol·m−2·s−1 (A) and 120 µmol·m−2·s−1 (B) of PAR.
2.2. Analyses of Growth and Volatiles
The growth biomass of each plant was measured around 8 weeks after light treatments, assessing factors such as height, leaf length, leaf width, leaf number, branch number, and flower number.
Flowers were harvested around 8 weeks after growing under LED lights, and their volatiles were collected for one hour in glass tubes using a push-pull dynamic collection system, as described by Johnson et al. [18]. Briefly, volatiles were collected from 0.5 g flowers with three biological replications in glass columns filled with approximately 50 mg HaySep Q 80–100 porous polymer adsorbent (Hayes Separations Inc., Bandera, TX, USA) and eluted with methylene chloride. Quantification of the volatiles in the elution matrix was conducted using an Agilent 7890A Series gas chromatograph (GC) equipped with an Agilent 5977A single quadrupole mass spectrum detector (MSD), utilizing a DB-5 column (Agilent Technologies, Santa Clara, CA, USA). The volatile mass emission rates (ng·gFW−1·h−1) were calculated as previously described [18].
Chlorophyll was extracted from leaves according to the method of Hiscox and Israelstam [19]. Briefly, three fresh leaf segments (~20 mg total) were collected using a hole punch, and 1 mL of 100% dimethyl sulfoxide was added. The mixture was then incubated at 65 °C for 30 min. Absorbance was measured at 645 nm and 663 nm with three biological replications. The concentrations of chlorophyll (mg/mL) were calculated.
Chlorophyll A = (0.0127 × A663 nm) − (0.00269 × A645 nm)
Chlorophyll B = (0.0229 × A645 nm) − (0.00468 × A663 nm)
Total Chlorophyll = (0.0202 × A645 nm) + (0.00802 × A663 nm)
Phenolic compounds were extracted using the method described by Stadnik and Buchenauer [20]. 0.5 g of ground dried leaves were soaked in 4 mL of 50% methanol and incubated at 80 °C for 1.5 h. After centrifugation at 3000× g for 5 min, the supernatant (free phenolic compounds) was transferred to new tubes. The remaining tissue was treated with 2 mL of 0.5 M NaOH at room temperature for 24 h, and then 0.5 mL of 2 M HCl was added. Following another centrifugation, the supernatant (wall-bound phenolics) was transferred to new tubes. For the Folin-Ciocalteau assay, 950 µL of water was added to 50 µL of the supernatant, followed by 50 µL of 2 M Folin-Ciocalteau reagent (Millipore Sigma, St. Louis, MO, USA) and 100 µL of 20% Na2CO3. After allowing the mixture to sit for 20 min, the absorbance of the samples was measured at 725 nm with three biological replications. The phenolic content was calculated using a standard curve of p-coumaric acid (Millipore Sigma, St. Louis, MO, USA).
2.3. Statistical Data Analysis
Each treatment included three replicated plants, and data from each treatment were collected and compared to those of plants grown under W light using Tukey’s multiple range test (one-way ANOVA, p < 0.05) in JASP (Version 0.19.3; JASP Team, 2025).
3. Results
3.1. Analysis of the Growth of Coriander Plants Grown Under W, R, B, or G Light
Light quality significantly influenced the growth of coriander plants. Plants grown under B light were taller (Figure 2A). In contrast, plants under R light were shorter but exhibited denser foliage, more branches, and longer and wider leaves (Figure 2B). The B light accelerated the reproductive development of coriander, resulting in early flowering at 36 days after light treatments (Figure 2 and Table 1). This occurred 26–30 days earlier than those under other lighting conditions, resulting in a higher number of flowers. Plants grown under W, R, or G light continued vegetative growth until 8 weeks, and produced their first flower at 9 weeks (at 62–69 days) after light treatment. After flowering, the coriander developed thinner and shorter leaves, a process known as bolting. They exhibited significantly fewer leaves and branches. Overall, the coriander plants grown under relatively lower intensity of light (100 µmol·m−2·s−1 of PAR) did not thrive, as even those grown under W light remained small and exhibited delayed flower development.
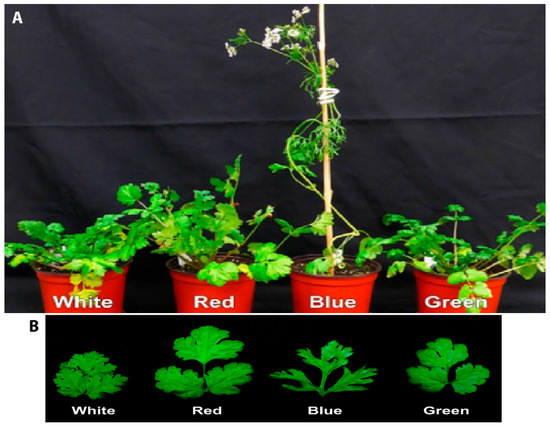
Figure 2.
Effects of light quality on the growth and flowering (A) and the leaf patterns (B) of coriander plants. The plants were grown under different LEDs with a PAR of 100 µmol·m−2·s−1 for 7 weeks in a growth chamber maintained at 18 °C.

Table 1.
Morphological characteristics and flowering of coriander plants grown under various LEDs, providing 100 µmol·m−2·s−1 of PAR for 7 weeks.
Chlorophyll levels were measured because light quality was reported to affect the levels of chlorophyll and phenolic compounds [6,9]. The chlorophyll a content was significantly higher in coriander grown under R light and lower in plants under B light (Table 2). However, there was no significant difference in chlorophyll b and total chlorophyll levels, although plants under R light showed slightly higher amounts than those under B light.

Table 2.
Effects of various LED qualities on chlorophyll contents in coriander leaves grown with a PAR of 100 µmol·m−2·s−1 for 7 weeks in a growth chamber at 18 °C.
Volatiles were collected and analyzed from the leaves of coriander plants. The total amount of volatile compounds was highest in plants grown under W light and lowest in plants under B light compared to other plants (Figure 3 and Table 3). The primary volatile compounds identified in coriander, such as (E)-2-decenal, 2-undecenal, and 2-dodecenal, were higher in plants grown under G light.
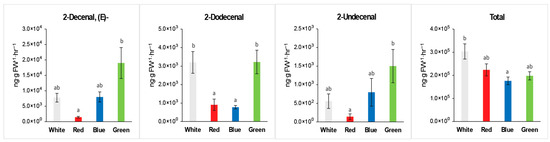
Figure 3.
Representative volatile compounds from coriander leaves grown under different LEDs providing 100 µmol·m−2·s−1 of PAR for 7 weeks. The bars indicate standard errors; the letters denote statistically significant differences between the plants (p < 0.001, Tukey’s test).

Table 3.
Volatile compounds in coriander leaves detected using GC-MS. The plants were grown under various LEDs with a PAR of 100 µmol·m−2·s−1 for 7 weeks in a growth chamber maintained at 18 °C.
3.2. Analysis of the Growth in Coriander Plant Under Various Intensities of B Light
In the second experiment, we investigated the effects of varying intensities of B light (0%, 10%, 20%, or 50%) when combined with W light. Since coriander plants did not grow well at a PAR of 100 µmol·m−2·s−1, even under W light, we increased PAR to 120 µmol·m−2·s−1. Under the conditions of 10% and 20% B light (W:B, 90:10 and 80:20), coriander growth and flowering were inhibited, causing the leaves to turn yellow (Figure 4). However, when the B light intensity was increased to 50% (W:B, 50:50), coriander plants showed significantly improved growth without overgrowth. The plants began to grow taller after 6 weeks of light treatment and bloomed earlier, producing many flowers, more flower clusters, and longer flower nodes (Figure 4 and Table 4). The first flower appeared 38 days after the light treatments. In contrast, plants grown under the other B light intensities produced their first flowers after 52 days of treatment. There were no significant differences in leaf length, leaf width, leaf number, or branch number across the different light conditions.

Figure 4.
Effects of light quality and intensity on the growth and flowering of coriander plants. The plants were grown under different combinations of white and blue LEDs with a PAR of 120 µmol·m−2·s−1 for 8 weeks in a growth chamber maintained at 18 °C.

Table 4.
Morphological characteristics and flowering of coriander plants grown under various combinations of white and blue LEDs, providing 120 µmol·m−2·s−1 of PAR for 8 weeks.
Chlorophyll and phenolic compound contents were measured to analyze the effect of B light on photosynthesis. Chlorophyll levels were not measured in coriander plants grown under 50% B light (W:B, 50:50), because most leaves were old and dead (Table 5). The levels of chlorophyll a were lower in plants under 20% B light (W:B, 80:20), but there was no distinct difference in chlorophyll b and total contents, although the amounts were slightly lower. Phenolic compound levels were significantly lower in plants grown under 10% B light (W:B, 90:10) but increased in plants grown under 20% (W:B, 80:20). Plants grown under 50% B light (W:B, 50:50) showed relatively low phenolic compound levels because the leaves were senesced due to early flowering.

Table 5.
Effects of various qualities and intensities of light on chlorophyll and phenolic contents in coriander leaves. The plants were grown under different combinations of white and blue LEDs with 120 µmol·m−2·s−1 of PAR for 8 weeks in a growth chamber at 18 °C.
Volatiles were collected from flowers grown under various intensities of B light when all plants had flowers on Day 54 after light treatments. As the intensity of B light increased, the total amount of floral volatiles also increased, with significant elevations observed in key compounds of coriander plants (Figure 5 and Table 6).
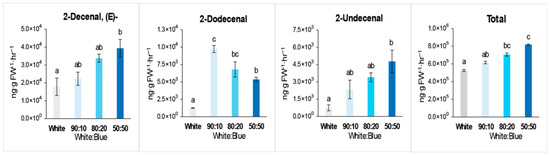
Figure 5.
Representative volatile compounds from coriander flowers grown under various combinations of white and blue LEDs, providing 120 µmol·m−2·s−1 of PAR for 8 weeks. The bars indicate standard errors; the letters denote statistically significant differences between the plants (p < 0.001, Tukey’s test).

Table 6.
Volatile compounds in coriander flowers detected using GC-MS. The plants were grown under various combinations of white and blue LEDs, with a PAR of 120 µmol·m−2·s−1 for 8 weeks in a growth chamber maintained at 18 °C.
4. Discussion
Light quality influences plant growth and development, with B light significantly affecting flowering. Coriander plants grown under B light were taller and flowered approximately four weeks earlier than those under R, G, or W lights (Figure 2). B light has been shown to suppress shoot elongation by inhibiting the synthesis of auxins and gibberellins [21,22], though responses can vary depending on plant species. It has been reported to promote stem elongation in petunia plants [23], as well as in marigold, geranium, and salvia plants [3,24]. This phenomenon may be related to the shading avoidance response triggered by B light, which is associated with low phytochrome activity [25]. Fukuda et al. [23] also suggested that B light influences plant growth through cryptochromes, and the observed stem elongation may partly result from the absence of R light, which is known to inhibit gibberellin synthesis through phytochromes. Although coriander plants flowered quickly under B light, they produced relatively few flowers (Figure 2). Yang et al. [15] also observed an inverse relationship between the number of flowers and branches or leaves under B light, suggesting a possible competitive interaction between flower induction and leaf or branch development. B light plays a key role in flowering, but other lights such as R and FR are also involved in this process [23,26]. The regulation of floral bud formation and stem elongation is reported to be controlled by B and R light via cryptochromes and phytochromes [23]. The deficit of R light seems to be compensated for by adding W light, because W light includes all visible wavelengths. When W light was added to B light, the overgrowth of coriander plants was reduced, and they flowered earlier with more abundant flowers (Figure 4). The supporting evidence can be found in petunia plants. B light promoted stem elongation in petunia plants under low natural light conditions, but its effect decreased as natural light levels increased [26,27].
Light intensity is also vital for plant growth. Coriander plants grown under W light showed stunted growth at a relatively low PAR of 100 µmol·m−2·s−1 but exhibited normal growth at a PAR of 120 µmol·m−2·s−1. They also displayed overgrowth with fewer leaves and branches under B light at 100 µmol·m−2·s−1 of PAR, while this overgrowth decreased at 120 µmol·m−2·s−1 of PAR. The overgrowth may be due to the absence of the R spectrum, as we explained above, but light intensity also probably influences growth and development. The benefits of higher light intensity on plant growth seem to be limited to a certain point. We tested the effects of supplemental B light (25%, 50%, and 75%) to 100% W light, resulting in 130, 150, and 165 µmol·m−2·s−1 of PAR, respectively. Coriander plants under supplemental B light at 130 and 150 µmol·m−2·s−1 of PAR flowered earlier than those grown under W light (Supplementary Table S1). However, there was no difference in plants grown under higher intensity compared to those under W light. Although these results suggest that a combination of W and B light with 120–150 µmol·m−2·s−1 of PAR promotes rapid flower development in coriander plants, further research on various light conditions would be necessary to find optimal flowering conditions.
Research on the volatile changes in coriander under LED light is limited, with no studies focusing primarily on the flowers. The dominant volatile compound found in coriander leaves is (E)-2-decenal, which makes up 46.1% of the total, followed by (E)-2-dodecenal at 10.3% [28]. The major volatile compounds identified in coriander flowers were octanol, linalool, (E)-2-decenal, (E)-2-nonenol, and dodecanal [29]. The intensity and quality of light affected the composition of volatile compounds in coriander. Leaves grown under W light produced higher amounts of volatile compounds, while the primary volatile compounds were more prevalent when grown under G light (Figure 3). The influence of G light on plant development may be particularly significant when light intensity is lower. Ahmed et al. [30] found that G light increased the photosynthetic rate of lettuce leaves in the lower canopy. Moreover, the absence of B light may induce the conversion of B light to G light based on the research of Ooms et al. [31]. They reported that this conversion can enhance light penetration into high-density canopies by increasing the proportion of highly penetrating wavelengths. However, we cannot explain the exact effect of G light on floral volatiles, and coriander plants grown under G light did not exhibit the highest levels of total volatile compounds, suggesting a need for further studies to understand how G light specifically influences the production of volatile compounds. The addition of B light to W light had a significant impact on the volatiles of coriander (Figure 5). As B light intensity increased, both coriander-specific volatile compounds and total volatile compounds levels were enhanced. While it is well-established that B light promotes flower development through the activation of cryptochromes and the regulation of gibberellin [12], the response can vary depending on the crop and its variety [3]. Additionally, since R light is also involved in regulating gibberellin synthesis, further investigations into the effects of R light on flowering would be beneficial in understanding the influence of light quality on plant volatile production.
Chlorophyll is the green pigment found in plant cells that absorbs light energy, mainly from B and R spectra [30]. Chlorophyll a is essential in the light reaction of photosynthesis, and higher levels of chlorophyll a are associated with faster growth [32]. Saffron plants grown under B light exhibited higher levels of chlorophyll a and b compared to those under R light [14]. However, coriander plants grown under R light displayed higher chlorophyll contents than those under B light (Table 2). This difference may be attributed to the relatively lower intensity of light and the unique growth pattern of coriander. While the leaves developed well initially, additional leaf development halted after flowering due to a process known as bolting. Consequently, chlorophyll levels in plants grown under B light were lower, despite these plants exhibiting rapid growth and flowering. Since coriander plants under R light showed higher levels of chlorophyll than B light [2], we expected enhanced chlorophyll levels with the addition of W light. However, no significant increase was observed compared to the levels under W and B light. This may be attributed to the development stage or light intensity. Gao [2] measured chlorophyll contents after 20 days of light treatment at a PAR of 200 µmol·m−2·s−1, while the leaves in this experiment were collected on Day 54 at a PAR of 120 µmol·m−2·s−1. To investigate changes in metabolites in coriander under different intensities of B light, we measured phenolic compounds. The plants that produced many flowers showed reduced levels of phenolic compounds. For optimal growth and flower development, coriander plants appear to require a relatively high intensity.
5. Conclusions
B light promoted elongated heights and early flowering in coriander plants. It plays a crucial role in flowering, but its intensity significantly affects growth and flower development. Coriander plants grown under B light at a PAR of 100 µmol·m−2·s−1 exhibited overgrowth and fewer flowers. In contrast, plants grown under a balanced combination of W and B lights showed early flowering and abundant flowers at a PAR of 120 µmol·m−2·s−1. These suggest that a combination of 50% W and 50% B light is effective for accelerating flowering and flower development in coriander plants. Additionally, a relatively high intensity of light would be beneficial in coriander growth. While further studies are necessary, this research will help to understand flower development under LED light.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11091093/s1: Table S1: The growth of coriander plants grown under various intensities of supplementary blue lights after 7 weeks of treatment. Different levels of blue light were added to 100% white light.
Author Contributions
Conceptualization, T.A.C. and J.Y.K.; methodology, J.Y.K.; validation, J.Y.K. and K.H.C.; formal analysis, J.Y.K.; investigation, M.D.G.; resources, J.Y.K. and M.D.G.; data curation, K.H.C.; writing—original draft preparation, J.Y.K. and M.D.G.; writing—review and editing, T.A.C., J.M.P. and K.H.C.; visualization, J.Y.K.; supervision, J.Y.K.; project administration, T.A.C. and J.Y.K.; funding acquisition, T.A.C. and J.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the United States Department of Agriculture (USDA): Agricultural Research Service-Floriculture and Nursery Research Initiative (ARS-FNRI).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Reg. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED red and Blue light component on growth and photosynthetic characteristics of coriander in plant factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Diverse Flowering Response to Blue light Manipulation: Application of Electric Lighting in Controlled-Environment Plant Production. Horticulturae 2024, 10, 578. [Google Scholar] [CrossRef]
- Livadariu, O.; Maximilian, C.; Rahmanifar, B.; Cornea, C.P. LED Technology applied to plant development for promoting the accumulation of bioactive compounds: A review. Plants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Cammarisano, L.; Taylor, G.; Naznin, M.T.; Verdonk, J.C.; Ahamed, M.S. Impact of light spectral combinations on morphology, yield, and quality of indoor-grown cilantro. Front. Sustain. Food Syst. 2024, 8, 1499954. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Hao, X. Different ratios of red and blue LED light effects on coriander productivity and antioxidant properties. Acta Hortic. 2016, 1134, 223–230. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kitayama, M.; Lu, N.; Takagaki, M. Improving secondary metabolite accumulation, mineral content, and growth of coriander (Coriandrum sativum L.) by regulating light quality in a plant factory. J. Hortic. Sci. Biotechnol. 2020, 95, 356–363. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Mockler, T.C.; Lin, C. Regulation of flowering time by Arabidopsis photoreceptors. Science 1998, 279, 1360–1363. [Google Scholar] [CrossRef]
- Fukuda, N.; Ishii, Y.; Ezura, H.; Olsen, J.E. Effects of light quality under red and B light emitting diodes on growth and expression of FBP28 in petunia. Acta Hortic. 2011, 907, 361–366. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue-light-promoted elongation and flowering are not artifacts from 24-h lighting: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 659–666. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kadowaki, M.; Che, J.; Takahashi, S.; Horiuchi, N.; Ogiwara, I. Influence of light quality on flowering characteristics, potential for year-round fruit production and fruit quality of blueberry in a plant factory. Fruits 2019, 74, 3–10. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Blue light supplemented at intervals in long-day conditions intervenes in photoperiodic flowering, photosynthesis, and antioxidant properties in chrysanthemums. Antioxidants 2022, 11, 2310. [Google Scholar] [CrossRef]
- Diederichsen, A. Coriander: Coriandrum sativum L.; Bioversity International: Rome, Italy, 1996; Volume 3, pp. 22–26. [Google Scholar]
- Diederichsen, A.; Hammer, K. The infraspecific taxa of coriander (Coriandrum sativum L.). Genet. Resour. Crop Evol. 2003, 50, 33–63. [Google Scholar] [CrossRef]
- Johnson, T.S.; Schwieterman, M.L.; Kim, J.Y.; Cho, K.H.; Clark, D.G.; Colquhoun, T.A. Lilium floral fragrance: A biochemical and genetic resource for aroma and flavor. Phytochemistry 2016, 122, 103–112. [Google Scholar] [CrossRef]
- Hiscox, J.T.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Buchenauer, H. Inhibition of phenylalanine ammonia-lyase suppresses the resistance induced by benzothiadiazole in wheat to Blumeria graminis f. sp. tritici. Physiol. Mol. Plant Pathol. 2000, 57, 25–34. [Google Scholar] [CrossRef]
- Folta, K.M.; Pontin, M.A.; Karlin-Neumann, G.; Bottini, R.; Spalding, E.P. Genomic and physiological studies of early cryptochrome 1 action demonstrate roles for auxin and gibberellin in the control of hypocotyl growth by blue light. Plant J. 2003, 36, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Laux, V.Y.; Wallace-Springer, N.; Clark, D.G.; Folta, K.M.; Colquhoun, T.A. Effects of light quality on vegetative cutting and in vitro propagation of coleus (Plectranthus scutellarioides). HortScience 2019, 54, 926–935. [Google Scholar] [CrossRef]
- Fukuda, N.; Ajima, C.; Yukawa, T.; Olsen, J.E. Antagonistic action of Band red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 2016, 121, 102–111. [Google Scholar] [CrossRef]
- Heo, J.; Lee, C.; Chakrabarty, D.; Paek, K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Fukuda, N.; Yoshida, T.; Olsen, J.E.; Senaha, C.; Jikumaru, Y.; Kamiya, Y. Short main shoot length and inhibition of floral bud development under red light can be recovered by application of gibberellin and cytokinin. Acta Hortic. 2012, 956, 215–222. [Google Scholar] [CrossRef]
- Gautam, P.; Terfa, M.T.; Olsen, J.E.; Torre, S. Red and blue light effects on morphology and flowering of Petunia × hybrida. Sci. Hortic. 2015, 184, 171–178. [Google Scholar] [CrossRef]
- Potter, T.L.; Fagerson, I.S. Composition of coriander leaf volatiles. J. Agric. Food Chem. 1990, 38, 2054–2056. [Google Scholar] [CrossRef]
- Carrubba, A.; Ascolillo, V.; Pagan Domenech, A.T.; Saiano, F.; Aiello, P. Modifications over time of volatile compounds in coriander (Coriandrum sativum L.). Acta Hortic. 2007, 826, 43–50. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Tong, Y.-X.; Yang, Q.-C. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Ooms, M.D.; Dinh, C.T.; Sargent, E.H.; Sinton, D. Photon management for augmented photosynthesis. Nat. Commun. 2016, 7, 12699. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).