Abstract
Tuta absoluta (Meyrick), a new invasive pest in China, is a major threat to global tomato production. Trichogramma egg parasitoids are an effective approach to controlling this pest. In this study, we examined the potential of seven strains from four Trichogramma species, encompassing three native and commercially available representatives in China—namely, Trichogramma chilonis Ishii (strains TC-HN and TC-JL), T. dendrolimi Matsumura (TD-JL), and T. ostriniae Pang and Chen (TO-JL and TO-MY)—and one of South America origin—T. pretiosum Riley (TP-GS and TP-HN), a species commercially available for T. absoluta control but not evaluated in any previous studies in China. The host acceptance of the seven Trichogramma strains by T. absoluta was examined by placing parasitoid females with T. absoluta eggs on cardboard in tubes. The performance (life history traits and lifetable parameters) of four prospective strains, TC-HN, TC-JL, TO-JL, and TP-HN, was tested by using cardboard with T. absoluta eggs. The most promising strains, TC-HN, TC-JL, and TP-HN, were evaluated on a larger scale using cages in the laboratory to assess their parasitism capacity. The most promising strain, TC-JL (and TP-HN), was tested in field cages to assess its control efficiency under cropping conditions. The TC-JL and TC-HN strains of T. chilonis, the TO-JL strain of T. ostriniae, and the TP-HN strain of T. pretiosum showed greater host acceptance; the TP-HN strain of T. pretiosum showed a greater egg-card parasitism rate. Strain TC-JL outperformed other species/strains under laboratory conditions. In field cage tests, the larval population size and percentages of damaged plants and leaves in cages with TC-JL released were significantly reduced by 75.10%, 55.56%, and 64.69%, respectively, compared with those of the non-Trichogramma-release control. Our results indicate that the Asian native T. chilonis (particularly strain TC-JL), a dominant commercial biocontrol agent, should be included in IPM programs targeting T. absoluta in China. T. pretiosum (particularly strain TP-HN) could be a potential candidate for biocontrol of T. absoluta.
1. Introduction
The South American tomato leafminer Tuta absoluta (Meyrick) (synonymy Phthorimaea absoluta Meyrick []) (Lepidoptera: Gelechiidae) is a devastating tomato pest that has invaded over 110 countries and regions outside of South America []. T. absoluta affects tomato plants across all developmental stages. Besides mining plant leaves, larval feeding can damage fruits, stems, apical buds, and flower buds []. This pest also impacts other economically important solanaceous crops, including potato, eggplant, pepino fruit, pepper, and tobacco [,,]. If inadequately controlled, this pest may cause up to 80–100% production losses in tomato crops [,]. Given the cryptic nature of its larvae, its high fecundity with overlapping generations, and the rapid emergence of resistant populations to commonly used chemical insecticides [,,], T. absoluta has become a challenging pest to manage worldwide [,], particularly for organic vegetable producers.
China is the largest producer of tomatoes globally []. T. absoluta is a new invasive insect pest in China. However, since the first detection of T. absoluta in Xinjiang, northwestern China [,], and Yunnan, southwestern China [], in 2017 and 2018, respectively, this species has invaded more than 20 provincial-level regions and become a serious threat to greenhouse and open-field tomato production [,]. Because of the lack of other crop protection tools, chemical insecticides are usually the main measures used to control T. absoluta in newly invaded areas (and even in their native region). However, this approach does not consistently achieve the desired results, leading to resistance [,], toxicity to natural enemies [], insecticide residues in crop products [], and environmental contamination [,]. Thus, alternative approaches that are effective and environmentally friendly, i.e., “green” management methods [], are required.
Biological control, namely, the use of living organisms (i.e., nature enemies, including predators, parasitoids, and pathogens) to reduce/suppress pest populations [], is a crucial component of integrated pest management (IPM). Trichogramma egg parasitoids (Hymenoptera: Trichogrammatidae), the most widely used biological control measure globally, have been used to control T. absoluta. For example, Trichogramma dendrolimi Matsumura, T. pretiosum Riley, T. exiguum (Girault) [,], T. chilonis Ishii, and T. ostriniae Pang and Chen [,,] have been reported in association with T. absoluta in native and recently invaded areas, although with different results [].
Trichogramma species have been successfully used to control economically important lepidopterous insect pests on crops, particularly in China []. In an extensive field survey conducted beginning in the late 1990s in China, 24 Trichogramma species were identified, of which 10, including 4 well-studied ones (namely, T. dendrolimi, T. japonicum Ashmead, T. chilonis, and T. ostriniae) [], were further developed and mass-reared for field application []. Mass-rearing is achieved via factitious hosts, such as the small eggs of Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) or Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) or the large eggs of Samia cynthia ricini Boisduval or Antheraea pernyi Guerin-Meneville (Lepidoptera: Saturniidae) (only successful for two species: T. dendrolimi and T. chilonis) [].
Trichogramma chilonis is native to Asia [,] and naturally distributed across many provinces of both southern and northern China [] and other regions across Asia (13 countries or regions), Africa (3), Europe (1), North America (4), and Oceania (3) [,]. This Trichogramma species could be released in the field, as it has been used to control various species of lepidopterous cereal stem borers, such as Chilo infuscatellus Snellen, C. sacchariphagus Bojer (Lepidoptera: Pyralidae), and Argyroploce schistaceana Snellen (Lepidoptera: Tortricidae) in Guangxi, China [,]. Additionally, they have been deployed against C. partellus Swinhoe in South Africa [] and used in sugarcane IPM in Pakistan [], as well as against Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) in cotton fields in Xinjiang, China, for over 10 yrs [].
Trichogramma pretiosum (Riley), a generalist [] and the most frequently used egg parasitoid for controlling T. absoluta in South and Latin America and Europe, is commercially available in various countries [,], including Peru, where T. absoluta was first documented []. This Trichogramma species was first introduced to China in the 1970s [] and was later re-introduced in 1992 and 2016 to control Plutella xylostella (L.) (Lepidoptera: Plutellidae) [,]. Currently, it is commercialized for the biocontrol of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Hainan, Guizhou, and Guangzhou [,] and S. litura (Fabricius) in northern and northwestern China [], although it is not yet widely applied in China. Moreover, the control efficacy of T. pretiosum against T. absoluta in China is still unknown.
Using Trichogramma species as a biocontrol measure could prevent T. absoluta from damaging crops [,]. T. absoluta has invaded northern and southern China [], so seven strains from four commercially available Trichogramma species candidates from different geographic areas were used in this study. These included five strains from three native commercialized species—T. chilonis (previously proven effective for T. absoluta control [,]) (arrhenotokous: two strains, TC-HN (from Hainan, southern China) and TC-JL (from Jilin, northern China)), T. dendrolimi (arrhenotokous: one strain, TD-JL (from northern China)), and T. ostriniae (arrhenotokous: two strains, TO-JL (from northern China) and TO-MY (from eastern Myanmar))—and two strains from the South American commercialized T. pretiosum (thelytokous: TP-GS (from northwestern China) and TP-HN (from southern China)) (Table 1). The control effects of these species/strains on T. absoluta were assessed at three different spatial scales. We first compared (1) host acceptance—represented by the percentage of parasitized egg cards and number of parasitized host eggs—of the seven strains from these four Trichogramma species and assessed (2) the life history traits (longevity and fecundity in the F0 generation and the emergence rate in the F1 generation) and lifetable parameters (intrinsic rate of increase, r; net reproductive rate, R0; finite rate of increase, λ; and mean generation time, T) of four selected prospective strains. Then, after choosing three promising strains (including TP-HN), we examined (3) the parasitism capacity. Finally, we evaluated (4) the pest control efficiency of the selected strains based on the larval population size and the proportion of damaged plants and leaves in a protected tomato crop. In particular, we aimed to determine the control effect of T. pretiosum, a species that has not been previously evaluated in China, on T. absoluta. The experiments were conducted from May 2022 to September 2024.

Table 1.
Year of collection or introduction, initial host insect and host plant, country of origin, and thelytoky status (females produced from unfertilized eggs) of the seven Trichogramma strains in this study.
2. Materials and Methods
2.1. Insect
2.1.1. Tuta absoluta Colonies
The T. absoluta colonies were obtained from tomato (Solanum lycopersicon L.) fields at the organic vegetable production base (GPS coordinates: 24°20’28.68″ N latitude, 102°34’43.08″ E longitude, and 1677–1738 m ASL) at Yuxi, Yunnan, southwestern China, in December 2018. Individuals from the T. absoluta colonies were reared for over 50 generations under insectary conditions (25–30 °C and 40–60% RH, with a 14:10 light/dark photoperiod) in the National Agricultural Biosafety Science Center, Institute of Plant Protection, at the Chinese Academy of Agricultural Sciences (IPP, CAAS), Beijing, China. The laboratory colonies of T. absoluta were maintained on tomato plants contained in mesh cages (rectangular solid, 40 × 40 × 60 cm; mesh size, 150 × 150 µm; Xunon Instrument (Beijing) Co., Ltd., Beijing, China). The colonies were periodically replenished using populations collected from organic crops in Yunnan or Beijing, China. The tomato plants (cv. Maofen; Vegetable and Flower Research Institute, CAAS, Beijing, China) used to rear T. absoluta colonies were grown in pots (14.5 cm in diameter; Hebei Yujie Plastic Industry Co., Ltd., Tangshan, China) using black turfy soil in greenhouses and offered to insects when 6 to 8 true leaves were well developed.
2.1.2. Trichogramma Species and Strains
The seven Trichogramma strains were grown on inactivated (UV-irradiated) C. cephalonica eggs (Shandong Lubao Technology Development Co., Ltd., Jinan, China) in climate chambers (Ningbo Saifu Experimental Instrument Co., Ltd., Ningbo, China) at 26 ± 1 °C with a 14:10 (light/dark) photoperiod. C. cephalonica has been used as a factitious host for the commercial production of Trichogramma in China. Trichogramma species cultures were established in transparent polystyrene tubes (95 mm length × 25 mm diameter; Nantong Fengyuxin Biotechnology Co., Ltd, Nantong, China), sealed with a matching high-density sponge plug, and parasitoid wasps were fed honey water (15% v/v). The identities of the laboratory cultures of Trichogramma species and strains were confirmed through DNA barcoding [] every two months. The Trichogramma species were maintained for at least 20 generations on C. cephalonica eggs prior to experimentation. The distinction between female and male Trichogramma adults was made primarily based on antennal morphology []. The female Trichogramma adults used in the laboratory assessment were <12 h old. All seven Trichogramma strains had no oviposition experience on T. absoluta eggs or a factitious host in the presence of tomato leaves or odor.
2.2. Test of Host Acceptance of Trichogramma
2.2.1. T. absoluta Egg Cards
To obtain a stable population of T. absoluta eggs of approximately the same age, three clean tomato seedlings (with 4–6 well-developed true leaves) were put in a mass-rearing cage of moths from 5:00 to 10:00 pm. After that period, all of the adults were removed. The tomato plants with host eggs were grown in a climate-controlled chamber (26 ± 1 °C and 65 ± 5% RH, with a 14L:10D photoperiod) (Ningbo Saifu Experimental Instrument Co., Ltd., Ningbo, China). T. absoluta eggs were gently removed with a fine-hair brush from tomato leaves and used to make egg cards the following morning. Eggs <12 h old were used to evaluate host suitability. Graph paper (cardboard) with 1 mm grids was cut into 2 × 6 cm sized cardboard pieces. In the middle of the cardboard, five sticky circles (2 mm diameter, equidistant, arranged with 8 mm intervals) were made using double-sided tape (Chuan 1Z, Shenzhen Changdasheng Electronics Co., Ltd., Shenzhen, China), and then 45 T. absoluta eggs (determined via preliminary experiment) were gently glued in each sticky circle. Fine quartz sand was used to fill gaps between eggs to avoid harming Trichogramma adults. The large 5-row egg cards were cut into five small egg cards, and the small egg cards with 45 T. absoluta eggs (<12 h old) attached were used for further experiments.
2.2.2. Host Acceptance Test
The host acceptance of the seven tested Trichogramma strains was assessed based on the percentage of parasitized egg cards (%) and number of parasitized eggs (identified via melanized eggs) in 24 h. Prepared egg cards, each containing 45 T. absoluta eggs (<12 h), were transferred into transparent polystyrene tubes. Then, one male and one female (<12 h old) of a specific Trichogramma strain were placed onto egg-card-equipped polystyrene tubes. For the thelytokous strain T. pretiosum, only one female was introduced. The tubes containing a host egg card and a Trichogramma female, with or without a male adult, were incubated at 26 ± 1 °C and 65 ± 5% RH, with a 14:10 (light/dark) photoperiod for 24 h. Subsequently, Trichogramma adults were removed, and the parasitized egg cards were transferred individually into polystyrene tubes for further incubation under the same conditions. Six days later, parasitized melanized (black in color) eggs (and egg cards with at least one black egg) were counted. The proportion of parasitoid females that had parasitized at least one host egg (defined as the egg-card parasitism rate, %) was also documented []. In total, 5 replicates, each containing 10 tubes (each tube contained one T. absoluta egg card and one female adult, with or without a male adult), were performed, amounting to 50 females evaluated for each of the seven Trichogramma strains. Percentage of parasitized egg cards (%) = (number of egg cards with at least one black egg/total number of egg cards provided) × 100%.
2.3. Life History Traits and Lifetable Analysis of Trichogramma
2.3.1. Life History Traits
The life history traits were evaluated based on the longevity and fecundity (identified via melanized host eggs) of the F0 generation, and the emergence rate (%) and female proportion (%) of the F1 offspring. Four strains were assessed based on the findings of the host acceptance test: strains TC-HN and TC-JL of T. chilonis; strain TO-JL of T. ostriniae (with higher host acceptance than TO-MY); and TP-HN (showing higher host acceptance than TP-GS) of T. pretiosum. To investigate the longevity and fecundity of the F0 generation, as well as the emergence rate and female proportion of the F1 offspring, a new egg card with 45 fresh T. absoluta eggs was provided daily until the female adults died. Honey water (15% v/v) was used to supply parasitoid nutrition. The egg cards were replaced daily and set under the conditions described above to analyze parasitism until Trichogramma adult emergence of F1 offspring. The number of parasitized eggs (black eggs) and the longevity of female adults of the F0 generation, as well as the number of host eggs with emergence holes and the number of female offspring in the emerged parasitoids in the F1 generation, were recorded for each F0 female. Subsequently, the average total number of parasitized eggs during the adult lifetime (defined as the fecundity) and the longevity (calculated as the day of death minus the day of emergence) of the F0 parasitoid females were calculated; the emergence rate and female proportion of the F1 offspring were determined [,]. Fifteen replications were assessed for strains TC-HN and TP-HN, and twelve replications for strains TO-JL and TC-JL. Emergence rate (%) = (number of host eggs with emergence hole/number of black host eggs) × 100%. Proportion of female offspring (%) = (number of emerged female wasps/total number of emerged wasps) × 100%.
2.3.2. Lifetable Analysis
The raw data on the survivorship, longevity, and daily fecundity of individual females were collected and analyzed based on the age-stage, two-sex life table method using the computer program TWOSEX-MSChart V1 [,,]. Based on Chi and Liu [], the age-stage-specific survival rate (Sxj, where x is age and j is the stage), age-specific survival rate (lx), age-stage-specific fecundity (fxj), age-specific fecundity (mx), net reproductive rate (R0), intrinsic rate of increase (r), finite rate of increase (λ), and mean generation time (T) were calculated. The net reproductive rate (R0) was calculated as follows:
The intrinsic rate of increase (r) was estimated using the iterative bisection method and the Euler–Lotka equation with the age indexed from 0 []:
The finite rate of increase (λ) and the mean generation time (T) were calculated as follows:
2.4. Parasitism of Selected Trichogramma Strains on Tomato Plants
The parasitism tests, namely, the capacity for host location and acceptance, were performed on potted tomato plants in cages placed in an insectary. Three selected strains were assessed based on the findings of the above laboratory assessment: strains TC-HN and TC-JL of T. chilonis and TP-HN of T. pretiosum. Clean tomato plants (with 6 to 8 well-developed true leaves and not infected by any pests) were placed in a mass reproduction cage to allow T. absolua adults to lay eggs freely overnight. After 12 h (from 18:00 to 06:00), all adults were removed; between 90 and 105 eggs were retained on each plant, and extra eggs were removed using a fine-hair brush. The tomato plants were placed individually in cages (25 × 25 × 30 cm; mesh size, 150 × 150 µm; Xunon Instrument (Beijing) Co., Ltd., Beijing, China). Honey water (15% v/v) was used to provide nutrition to parasitoids. Trichogramma adults, aged less than 12 h old and fully mated (reared on the factitious hosts, inactivated C. cephalonica eggs), were released in defined parasitoid–host ratios of 2:1, 4:1, and 6:1, namely, inundative release [,], onto the middle parts of the tomato plants. After 24 h, parasitoids were removed, potted tomato plants were placed in another clean cage, and eggs were incubated on potted tomato plants in the insectary at 25–30 °C and 40–60% RH with a 14:10 (light/dark) photoperiod. After 6 d, the number of parasitized eggs (black eggs) was recorded. In total, the trials were performed 5 or 6 times to obtain 5 or 6 replications (TC-HN 4:1, 6 replications; all other strains and ratios, 5 replications) for each Trichogramma strain and each ratio. The parasitism capacity of the three selected Trichogramma strains on the caged potted tomato plants was identified via the proportion of parasitized host eggs (%). Parasitism rate (%) = (number of black host eggs/total number of provided host eggs) × 100%.
2.5. Pest Control Efficiency Test of Trichogramma in Field Cage Trial
Based on the findings of the above experiments, the most promising strains, TC-JL of T. chilonis and TP-HN (thelytoky) of T. pretiosum, were evaluated for pest control efficiency in field cages in a tomato production greenhouse (i.e., these cages were installed in a greenhouse used for tomato production) (temperature: minimum 21.5 °C, maximum 28.1 °C, mean 24.8 °C; RH: minimum 41.0%, maximum 86.5%, mean 60.5%; natural ambient light: August–September). The field cage (walk-in cage, 2.2 × 1.8 × 2.0 m; covered with insect-proof mesh) trial was performed in an organic vegetable production base in Hongta, Yuxi, Yunnan, southwestern China (GPS coordinates: 24°20’28.68″ N latitude, 102°34’43.08″ E longitude, and 1677–1738 m ASL). Twelve clean tomato plants (6 rows × 2 columns, each with 6–8 well-developed true leaves) were planted (spacing between plants and rows was 0.3 m and 0.4 m, respectively) in each walk-in cage in a greenhouse. When the tomato plants had developed 10–12 true leaves, 10 T. absoluta adults (sex ratio, 1:1) were released simultaneously alongside about 500 Trichogramma individuals (the recommended commercial release of Trichogramma for T. absoluta management in a tomato greenhouse experiencing a high level of infestation []) for each field cage. Parasitoids were released on a parasitized egg card of C. cephalonica placed in an open plastic tube on the middle part of the central tomato plant. On the 14th day following Trichogramma release, the numbers of T. absoluta larvae per leaf, damaged plants per cage, and damaged leaves per plant were recorded for each cage. The average number of larvae per leaf was estimated, and the percentages of damaged plants and leaves were calculated. In total, the field cage trials were performed five times to obtain five replications for each of the two Trichogramma strains (in total, 10 walk-in field cages used) and three replications (in total, three walk-in field cages used) for the no-Trichogramma-release control. Percentage of damaged plants = (number of damaged plants/total number of plants) × 100%. Percentage of damaged leaves = (number of damaged leaves/total number of leaves) × 100%.
2.6. Statistical Analysis
All data were analyzed using DPS software (DPS DP_V9.01) []. A Kolmogorov–Smirnov test was performed to assess the normality of the data. The percentage and longevity data were arcsine- and square-root-transformed, respectively, prior to analysis. Statistical significance was defined as p < 0.05 (unadjusted).
For the host acceptance tests, differences in the percentage of parasitized cardboards with T. absoluta eggs and the number of successfully parasitized host eggs among the seven Trichogramma strains (TC-HN, TC-JL, TD-JL, TO-JL, TO-MY, TP-GS, and TP-HN) were analyzed using nonparametric one-way (for the same parameter among different Trichogramma strains) ANOVA (Kruskal–Wallis H test for K independent samples).
For the life history traits, differences in longevity and fecundity in the F0 generation and the emergence rate and female proportion in the F1 generation among the four Trichogramma strains (TC-HN, TC-JL, TO-JL, and TP-HN) were analyzed using nonparametric one-way (for the same parameter among different Trichogramma strains) ANOVA (Kruskal–Wallis H test for K independent samples). The paired-sample t-test was applied to evaluate differences in daily egg production among the four tested Trichogramma strains (TC-HN, TC-JL, TO-JL, and TP-HN) during the first six days of female emergence. The log-rank (Mantel–Cox) test was used to test the survival differences among the four Trichogramma strains (TC-HN, TC-JL, TO-JL, and TP-HN). For lifetable parameters, differences in the intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), and mean generation time (T) among the four Trichogramma strains (TC-HN, TC-JL, TO-JL, and TP-HN) were analyzed using Twosex-MSChart software V1 [,].
For the parasitism tests conducted on potted tomato plants in the laboratory, differences in the parasitism rate among the three Trichogramma strains (TC-HN, TC-JL, and TP-HN) at the same ratio of parasitoids to host eggs and that among the three ratios (2:1, 4:1, and 6:1) of parasitoids to host eggs in the same Trichogramma strain were analyzed. Two-way (Trichogramma strains and initial ratios of Trichogramma to T. absoluta eggs) factorial ANOVA with Tukey’s post hoc test was performed to assess differences in the parasitism rate among the Trichogramma strains across the three ratios (2:1, 4:1, and 6:1) of parasitoids to host eggs, and no significant interaction was found between these two main effects.
For the pest control efficiency assessed in walk-in field cages, differences in the number of larvae per leaf and the percentages of damaged plants and leaves among the three treatments (two treatments with Trichogramma release and one with no-Trichogramma-release as a control) were subjected to nonparametric one-way ANOVA (Kruskal–Wallis H test for K independent samples). Two-way ANOVA (three treatments and three damage-related variables) with Tukey’s post hoc test was performed to assess differences in control efficacy between Trichogramma release and no-release treatments.
3. Results
3.1. Host Acceptance
3.1.1. Percentage of Parasitized Egg Cards
All seven tested Trichogramma strains could parasitize the eggs of T. absoluta on egg cards (Figure 1A). The percentage of parasitized egg cards in 24 h varied significantly among the seven tested Trichogramma strains (H6,28 = 23.2553, p = 0.0007); those of strains TC-HN, TC-JL, TP-GS, and TP-HN in 24 h were significantly higher than those of strains TD-JL, TO-JL, and TO-MY (TC-HN vs. TD-JL: p = 0.0360; TC-HN vs. TO-JL: p = 0.0430; TC-HN vs. TO-MY: p = 0.0360; TC-JL vs. TD-JL: p = 0.0030; TC-JL vs. TO-JL: p = 0.0040; TC-JL vs. TO-MY: p = 0.0030; TP-GS vs. TD-JL: p = 0.0070; TP-GS vs. TO-JL: p = 0.0090; TP-GS vs. TO-MY: p = 0.0070; TP-HN vs. TD-JL: p = 0.0100; TP-HN vs. TO-JL: p = 0.0130; TP-HN vs. TO-MY: p = 0.0100) (Figure 1A).
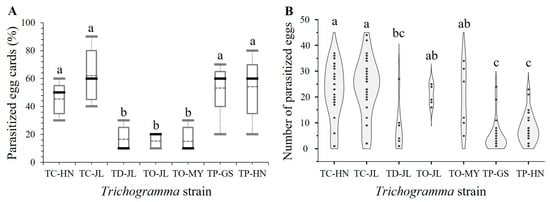
Figure 1.
Percentage of parasitized egg cards (A) and number of parasitized T. absoluta eggs (B) per parasitoid female in 24 h (n = 50 parasitoid females per strain). Box plots show the medians (horizontal solid lines) and means (horizontal dotted lines) (the mean percentage of parasitized egg cards was calculated from 5 replicates of 10 egg cards each: n = 10 egg cards × 5 replications = 50 egg cards per strain; the mean number of parasitized eggs was calculated across 132 egg cards distributed as follows: TC-HN (n = 23), TC-JL (n = 31), TD-JL (n = 8), TO-JL (n = 8), TO-MY (n = 8), TP-GS (n = 27), and TP-HN (n = 27), with each card containing 45 host eggs); whiskers (A) and black dots (B) show the range of data. Different lowercase letters denote significant differences between Trichogramma strains at the p < 0.05 level (Kruskal–Wallis H test for K independent samples). TC-HN, T. chilonis Hainan, southern China; TC-JL, T. chilonis Jilin, northern China; TD-JL, T. dendrolimi Jilin, northern China; TO-JL, T. ostriniae Jilin, northern China; TO-MY, T. ostriniae Shan, eastern Myanmar; TP-GS, T. pretiosum Gansu, northwestern China; TP-HN, T. pretiosum Hainan, southern China.
3.1.2. Number of Parasitized Host Eggs
The number of parasitized T. absoluta eggs per parasitoid female in 24 h varied significantly among the seven tested Trichogramma strains (H6,125 = 69.0230, p < 0.0001) (Figure 1B). In 24 h, the two strains TC-HN and TC-JL parasitized more than 22 host eggs on average, significantly more than the three strains TD-JL, TP-GS, and TP-HN (TC-HN vs. TD-JL: p = 0.0060; TC-HN vs. TP-GS: p < 0.0001; TC-HN vs. TP-TH: p < 0.0001; TC-JL vs. TD-JL: p = 0.0010; TC-JL vs. TP-GS: p < 0.0001; TC-JL vs. TP-TH: p < 0.0001); the two strains TO-JL and TO-MY parasitized about 20 host eggs on average, significantly more than the two strains TP-GS and TP-HN (TO-JL vs. TP-GS: p < 0.0001; TO-JL vs. TP-TH: p = 0.0090; TO-MY vs. TP-GS: p = 0.0010; TO-MY vs. TP-TH: p = 0.0130) (Figure 1B).
Consequently, four strains, namely, strains TC-HN and TC-JL of T. chilonis, strain TO-JL of T. ostriniae, and strain TP-HN of T. pretiosum (a species not evaluated in previous studies in China), were selected for life history trait and lifetable parameter tests. As strain TD-JL of T. dendrolimi showed poor performance (lower percentage of parasitized egg cards and lower number of parasitized eggs) (Figure 1), strain TO-MY of T. ostriniae native to Myanmar had fewer parasitized eggs, and strain TP-GS of T. pretiosum had fewer parasitized eggs than TP-HN (Figure 1B), they were not included in the following tests.
3.2. Life History Traits and Lifetable Parameters
3.2.1. Life History Traits: Longevity and Fecundity in F0 Generation
Longevity. All four Trichogramma strains survived longer than 11 d, but with significant differences between Trichogramma species/strains (F3,50 = 12.117, p = 0.0001) (Table 2). Specifically, longevity was greater in strain TP-HN (TP-HN vs. TC-HN: p = 0.0003; TP-HN vs. TC-JL: p = 0.0293; TP-HN vs. TO-JL: p = 0.0001), followed by strain TC-JL (TC-JL vs. TO-JL: p = 0.0010), with the shortest survival observed in strain TO-JL. Marginal significant differences were detected between strains TC-HN and TO-JL (p = 0.0628) (Table 2). Additionally, the survival curves were significantly or marginally significantly different between the four Trichogramma strains (TP-HN vs. TC-HN: x2 = 9.06, df = 1, p = 0.0026; TP-HN vs. TC-JL: x2 = 7.124, df = 1, p = 0.0076; TP-HN vs. TO-JL: x2 = 18.91, df = 1, p < 0.0001; TC-JL vs. TO-JL: x2 = 9.464, df = 1, p = 0.0021; TC-HN vs. TO-JL: x2 = 2.895, df = 1, p = 0.0888) (Figure 2).

Table 2.
Longevity and lifetime fecundity in F0 generation and emergence rate and proportion of female offspring in F1 generation (mean ± SE) of strains TC-HN and TC-JL of T. chilonis, strain TO-JL of T. ostriniae, and strain TP-HN of T. pretiosum on T. absoluta egg cards.
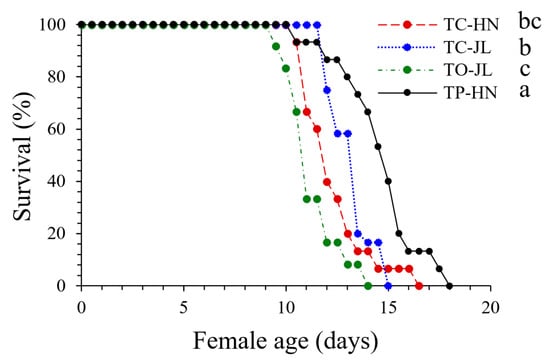
Figure 2.
Survival of strains TC-HN and TC-JL of T. chilonis, strain TO-JL of T. ostriniae, and strain TP-HN of T. pretiosum on T. absoluta egg cards. Different lowercase letters indicate significant differences among survival curves at the p < 0.05 significance level (p-values are from log-rank (Mantel–Cox) test).
Fecundity. All four Trichogramma strains parasitized more than one hundred T. absoluta eggs during their lifetime, but with significant differences between Trichogramma species/strains (F3,50 = 19.173, p < 0.0001) (Table 2). The lifetime fecundity was highest in strain TC-JL (TC-JL vs. TO-JL, p < 0.0001; TC-JL vs. TP-HN, p < 0.0001), followed by strains TC-HN (TC-HN vs. TO-JL, p = 0.0120) and TP-HN (TP-HN vs. TO-JL, p = 0.0010), with the lowest value observed in strain TO-JL; significant differences were observed between the two strains (TC-JL vs. TC-HN, p < 0.0001) of T. chilonis (Table 2). Moreover, investigation of daily egg production showed that fecundity was highest on the first day of adult emergence (Figure 3). During the first six days of egg oviposition, during which strains TC-HN, TC-JL, TO-JL, and TP-HN laid 80.36%, 77.56%, 85.57%, and 74.33% of their total number of eggs, respectively, the fecundity of strain TC-JL was significantly higher than that of the other three strains, TC-HN, TO-JL, and TP-HN (TC-JL vs. TC-HN, t = 2.8355, p = 0.0364; TC-JL vs. TO-JL, t = 3.4052, p = 0.0191; TC-JL vs. TP-HN, t = 3.9249, p = 0.0111) (Figure 3).
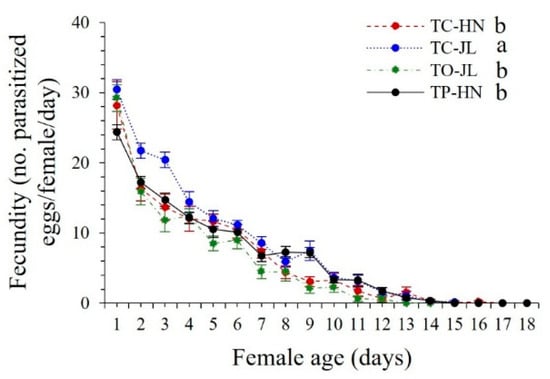
Figure 3.
Daily fecundity (mean ± SE) (the mean daily fecundity was calculated for each strain using 15 (TC-HN), 12 (TC-JL), 12 (TO-JL), and 15 (TP-HN) female parasitoids; total n = 54 females) of strains TC-JL and TC-HN of T. chilonis, strain TO-JL of T. ostriniae, and strain TP-HN of T. pretiosum parasitized on T. absoluta egg cards. Different lowercase letters indicate significant differences among different Trichogramma strains during the first six days of female emergence at the p < 0.05 significance level (paired-sample t-test).
3.2.2. Life History Traits: Emergence Rate and Proportion of Females in F1 Generation
Emergence rate. The emergence rate in the F1 generation of the four tested Trichogramma strains, developed in T. absoluta eggs on cardboard, was equal to or exceeded 80% (79.87–88.36%) and was over 85% in two strains, TC-JL and TO-JL, with significant differences (F3,50 = 6.132, p = 0.0010) (Table 2). The emergence rate in the F1 generation of strains TC-HN, TC-JL, and TO-JL was significantly higher than that of strain TP-HN (TC-HN vs. TP-HN, p = 0.0350; TC-JL vs. TP-HN, p = 0.0010; TO-JL vs. TP-HN, p < 0.0001) (Table 2).
Proportion of females. The proportion of female offspring in the F1 generation varied significantly among the four tested Trichogramma strains (F3,50 = 103.111, p < 0.0001) (Table 2). All three arrhenotokous Trichogramma strains exhibited female-biased sex ratios in F1 progeny (Table 2). The F1 generation of the thelytokous strain TP-HN was exclusively female. The female proportion in the F1 generation of strain TC-JL was significantly higher than those of both strains TC-HN (p < 0.0001) and TO-JL (p = 0.0150); moreover, significant differences were observed between two strains (TC-JL vs. TC-HN, p < 0.0001) of T. chilonis (Table 2).
3.2.3. Lifetable Parameters
The lifetable parameters, including the intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), and mean generation time (T), varied significant among the four Trichogramma strains (Table 3). The intrinsic rate of increase (r) and finite rate of increase (λ) were significantly greater in strains TC-JL and TC-HN than in TO-JL and TP-HN (r: TC-HN vs. TO-JL, p = 0.0155; TC-HN vs. TP-HN, p = 0.0481; TC-JL vs. TO-JL, p = 0.0002; TC-JL vs. TP-HN, p = 0.0011; λ: TC-HN vs. TO-JL, p = 0.0154; TC-HN vs. TP-HN, p = 0.0479; TC-JL vs. TO-JL, p = 0.0002; TC-JL vs. TP-HN, p = 0.0010) (Table 3). The net reproductive rate (R0) was significantly greater in strain TC-JL than in TO-JL (p = 0.0401); marginal significant differences were observed between the two strains of T. chilonis (TC-JL vs. TC-HN, p = 0.0769). The mean generation time (T) was significantly longer in strains TO-JL of T. ostriniae and TP-HN of T. pretiosum than in the other two strains, TC-HN and TC-JL of T. chilonis (TO-JL vs. TC-HN, p = 0.0001; TO-JL vs. TC-JL, p < 0.0001; TP-HN vs. TC-HN, p = 0.0002; TP-HN vs. TC-JL, p < 0.0001) (Table 3).

Table 3.
Lifetable parameters (r, intrinsic rate of increase; λ, finite rate of increase; R0, net reproductive rate; T, mean generation time) of strains TC-JL and TC-HN of T. chilonis, strain TO-JL of T. ostriniae, and strain TP-HN of T. pretiosum parasitized on T. absoluta egg cards.
Consequently, based on the lifetable parameters, strains TC-HN and TC-JL of T. chilonis and strain TP-HN of T. pretiosum (a species that has not been evaluated in previous studies in China) were selected for parasitism capacity tests.
3.3. Parasitism Capacity
The three examined strains (TC-HN, TC-JL, and TP-HN) exhibited similar parasitic efficiency against T. absoluta, regardless of the ratios of released parasitoids to host eggs (F2,41 = 0.687, p = 0.5093) (Figure 4). However, significant differences were detected among different ratios of parasitoids to host eggs (F2,41 = 4.144, p = 0.0238). As the ratio of parasitoids increased, the parasitism capacity increased significantly (p = 0.0378) or marginally (p = 0.0559) significantly under respective the ratios 6:1 and 4:1 compared with 2:1 (Figure 4). Moreover, a marginal significant difference (p = 0.063) was identified in TP-HN under the ratio 4:1 compared with 2:1. These findings demonstrate that the three examined Trichogramma strains can locate and parasitize T. absoluta eggs on tomato plants.
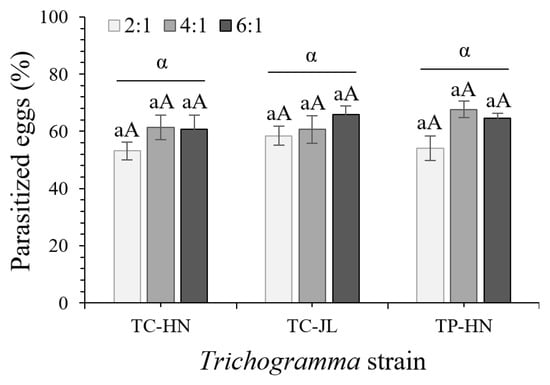
Figure 4.
Parasitism of the three examined Trichogramma strains (TC-HN, TC-JL, and TP-HN) on caged potted tomato plants (with 90–105 T. absoluta eggs/plant) at three ratios of parasitoids to host eggs. The percentage of parasitized (mean ± SE) T. absoluta eggs at three parasitoid–host ratios (2:1, 4:1, and 6:1) was determined with five or six replications (TC-HN 4:1, six replications; all other strains and ratios, five replications). The same lowercase letters indicate no significant differences between ratios of parasitoids to host eggs for the same Trichogramma strain at the p < 0.05 significance level; the same uppercase letters represent no significant differences between Trichogramma strains for the same ratios of parasitoids to host eggs at the p < 0.05 significance level; the same lowercase Greek letters above the horizontal lines indicate no significant differences among Trichogramma strains at the p < 0.05 significance level (two-way ANOVA and Tukey’s post hoc tests).
Based on these findings, together with lifetable parameters, the TC-JL strain and the thelytokous TP-HN strain were used in the field cage trial.
3.4. Pest Control Efficiency in Field Cage Trial
Releasing the TC-JL strain of T. chilonis successfully reduced the population of T. absoluta larvae (F2,10 = 12.4693, p = 0.0019) and the percentages of damaged plants (F2,10 = 4.9505, p = 0.0320) and leaves (F2,10 = 7.4055, p = 0.0106) (Table 4). There were significant differences in the number of larvae per leaf between strain TC-JL of T. chilonis and strain TP-HN of T. pretiosum (p = 0.0033). The larval population of T. absoluta was significantly reduced in the TC-JL strain treatment, while the no-Trichogramma-release control supported a higher population; strain TC-JL effectively reduced the T. absoluta population by 75.10% compared with the no-Trichogramma-release control treatment (p = 0.0011). Furthermore, the proportions of damaged plants (p = 0.0138) and leaves (p = 0.0078) were significantly decreased by 55.56% and 64.69%, respectively, in the TC-JL treatment compared with the no-Trichogramma-release control. Significant (leaf damage: p = 0.0095) or marginally significant (plant damage: p = 0.0523) differences were identified between the TC-JL and TP-HN treatments (Table 4).

Table 4.
Influence of the two tested Trichogramma strains, TC-JL of T. chilonis and TP-HN of T. pretiosum, on the T. absoluta population as well as plant and leaf damage of tomatoes in a walk-in field cage trial in a tomato production greenhouse.
Results based on the evaluation of the three damage-related variables (i.e., larvae number per leaf and percentages of damaged plants and leaves) indicated that release of strain TC-JL of T. chilonis and TP-HN of T. pretiosum could exert control effects (F2,36 = 41.973, p < 0.0001) (Table 4). The TC-JL strain of T. chilonis could effectively control T. absoluta on tomatoes (TC-JL vs. control, p = 0.0346). There were significant differences (p < 0.0001) between the strain TP-HN treatment and the no-release control, although the larval population and damaged plant and leaf percentages were only reduced by 20.55%, 20.00%, and 10.69%, respectively. The control effect of strain TC-JL was significantly greater than that of strain TP-HN (p < 0.0001) (Table 4).
4. Discussion
This study quantified the control potential of different strains of four commercially available Trichogramma species (initially seven strains included) on T. absoluta eggs at three different spatial scales: in a tube (host acceptance and performance (i.e., life history traits and lifetable parameters): seven strains from four species and four strains from three species), in a laboratory cage (parasitism capacity: three strains from two species), and in a walk-in field cage (pest control efficacy: two strains from two species). Based on host acceptance, four strains (TC-HN, TC-JL, TO-JL, and TP-HN) were deemed promising. The findings from tests using caged potted tomato plants did not align with those from tests on egg cards in tubes under laboratory conditions. T. chilonis strains TC-JL and TC-HN and T. pretiosum strain TP-HN on T. absoluta showed similar efficacy. However, the results obtained in the walk-in field cage trials under greenhouse conditions (used for tomato production) demonstrated the high efficiency of the T. chilonis strain TC-JL in controlling T. absoluta, as observed in host acceptance and performance (namely, life history traits and lifetable parameters) tests. These findings indicate that host acceptance and performance, i.e., the percentage of parasitized egg cards and number of parasitized eggs, as well as life history traits (fecundity in F0 generation and emergence rate and female proportion in F1 generation) and lifetable parameters (intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate (R0)), might be feasible parameters for predicting the efficacy of these strains as potential candidates for T. absoluta control. Nevertheless, the similar efficacy of the three examined Trichogramma strains on T. absoluta observed in caged potted tomato plants could be attributed to parasitoid aggregation, which induces mutual interference among individuals, subsequently diminishing host parasitic efficacy [].
Determining host acceptance is generally assumed to be the first significant step in assessing appropriate biological control agents [,]. In this study, four strains from three species—T. chilonis TC-HN and TC-JL, T. ostriniae TO-JL, and T. pretiosum TH-HN—yielded promising results. Host acceptance (or host suitability) significantly varied between species, as demonstrated in this study and previous research [,,,]. Moreover, a high percentage of parasitized egg cards does not always correspond with a large number of parasitized eggs, which could be related to the host’s immune system [], the thickness and structure of the chorion [], or the readiness of females to attack the host [], as well as the host choice behavior [] or aggregation effect [] of female parasitoids. In this study, the opposite phenomenon was observed in the two strains of T. ostriniae, TO-JL and TO-MY, which exhibited a low percentage of parasitized egg cards followed by a high number of parasitized eggs. However, the two strains of T. chilonis, TC-HN and TC-JL, exhibited consistency between these two parameters.
In the four evaluated Trichogramma strains, the percentage of emergence in the F1 generation developed in T. absoluta eggs was ≥79%. Three strains from two different species (TC-HN and TC-JL of T. chilonis and TO-JL of T. ostriniae) had an emergence rate of ≥84%, exhibiting higher host suitability than strain TP-HN of T. pretiosum. However, this trait might be of minor importance in an inundative release of Trichogramma species [,]. The proportion of females in the F1 generation in the three tested arrhenotokous strains from two species was 50.74–71.25%, which is beneficial for biological control, as only females kill the target pest via parasitization and direct killing []. The thelytokous strains, producing only female offspring, should be more favorable [], including strains TP-GS and TP-HN of T. pretiosum in this study. Most important, the total lifetime fecundity of the F0 generation of the four tested strains was over 100 eggs per female, with that of strain TC-JL being much higher than that of strains TC-HN, TP-HN, and TO-JL; the TC-JL strain also exhibited the highest intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate (R0) among the four tested strains. Despite adult survival capacities being beyond 10 d (11.25–14.67 d) across the four tested Trichogramma strains in this study, oviposition dynamics revealed a distinct temporal pattern, with ≥74% (74.33–85.57%) of egg deposition concentrated in the first 6 days after eclosion (also see Jiang et al.) [], suggesting early-life reproductive investment strategies []. These results, i.e., that T. chilonis strain TC-JL is a promising biocontrol agent against T. absoluta, are in line with those obtained in previous studies [,].
It has been observed that different Trichogramma species exhibit varying control potential against the same pest species [,,,,,], and that these differences are also apparent between strains of the same species [,]. These include greater fecundity (namely, total lifetime fecundity and fecundity within the first six days after emergence) in the F0 generation, a higher proportion of females in the F1 progeny, and marginally higher R0 detected in strain TC-JL of T. chilonis compared with TC-HN of the same species in this study. Consequently, the two T. chilonis strains, TC-JL and TC-HN, were differentiated. However, this does not necessarily mean they performed well in the field/on the tomato plants [].
The parasitism capacity test was evaluated on potted tomato plants in laboratory cages using naturally laid eggs of T. absoluta. The three tested Trichogramma strains, TC-HN, TC-JL, and TP-HN, achieved over 53% parasitism in 24 h, showing their ability to find and parasitize T. absoluta eggs on the tomato plant. No significant differences were observed among these three strains during the parasitism capacity tests that could be related to the assay method, i.e., parasitoid behavior might differ depending on whether the assay is conducted using egg cards or plants naturally infested with eggs []. Based on the results of our laboratory assessment, a high affinity between the target pest and T. chilonis and T. pretiosum was established even under field conditions, but further field validation is required. The performance of the tested T. pretiosum strain TP-HN was not as good as that of the T. chilonis strain TC-JL in our laboratory assessment (using a T. absoluta egg card). However, the examined strain possessed desirable traits, such as thelytoky (100% female offspring), high fecundity (120.00 eggs/female) in the F0 generation, and a high emergence rate (79.87%) in the F1 generation. Given these results, together with the commercial availability of Trichogramma species in areas where T. absoluta is native (Brazil, Chile, Colombia, Ecuador, and Peru) [,,] and invasive (Europe) [,], T. pretiosum remains attractive to us for future studies. As the most effective biological control agent against T. absoluta [], both strain TC-JL of T. chilonis and strain TP-HN of South American origin were used for further field cage tests.
In our field cage study, the TC-JL strain of T. chilonis was much more efficient than strain TP-HN of T. pretiosum at reducing the T. absoluta larval population and the proportion of damaged tomato plants and leaves. Our findings align with a previous study conducted by Li et al. [] on T. chilonis. In their greenhouse experiment, 76% control efficacy on T. absoluta was achieved at a release density of 20 parasitoids per tomato plant (i.e., 600,000 parasitoids/hectare) []. However, different results were obtained in Jiang et al.’s [,] studies conducted under controlled laboratory conditions: they found T. ostrinea to be more effective than T. chilonis against T. absoluta. In the future, the most promising strain, TC-JL of T. chilonis, should be examined on a large scale to assess its effectiveness under tomato production conditions. Moreover, other promising species or strains, such as TC-HN of T. chilonis and TO-JL of T. ostriniae, as demonstrated recently by Jiang et al. [,], should be tested, given that rearing conditions such as temperature match the abiotic conditions of the tomato [].
The effect of TP-HN of T. pretiosum when seeking to control T. absoluta in our field cage test was weaker than that of TC-JL of T. chilonis; this difference could be linked to the different strains tested and the commercial strains tested in South American countries []. In this study, we used the TP-HN strain, which was introduced to Guangdong, China, in 2016 and from Guangdong to Hainan, China, in 2017, where it was mainly used for biocontrol of P. xylostella and S. frugiperda. Therefore, introducing more promising strains of T. pretiosum, including those from South America, should be prioritized. Moreover, T. achaeae Nagaraja and Nagarkatti, as an effective agent of T. absoluta biological control in South American, North African, and European countries [,], should also be introduced.
To ensure more sustainable and effective pest management of T. absoluta, diverse tool combinations are critical []. The implementation of the Plantwise program, a global program led by the Centre for Agriculture and Bioscience International (CABI), has resulted in a reduction in pesticide use via incorporation of less toxic active ingredients and sustainable pest management strategies, such as biological control []. Trichogramma releases integrated with complementary strategies, such as predatory insects, pheromone traps, and microbial insecticides, have achieved significant T. absoluta suppression in Africa, Europe, and Asia, resulting in a significant reduction in T. absoluta populations and tomato crop damage [,]. The compatibility of T. chilonis (as well as T. ostriniae []) with other management measures in IPM packages against T. absoluta, including predators (e.g., Nesidiocoris tenuis (Reuter)) or parasitoids (e.g., larval parasitoid Neochrysocharis formosa (Westwood)) [,], biopesticides (e.g., Bacillus thuringiensis (Ernst Berliner), Bt) [], and sex-pheromone-based mating disruption [], should be evaluated in the near future [,].
5. Conclusions
Trichogramma egg parasitoids are a valuable tool for controlling pests of various crops, as they are easily mass-reared and attack the pest before target crops are damaged or otherwise affected. Identification of high-efficiency Trichogramma species or strains could allow for the establishment of an optimized, economically cost-effective biocontrol program against T. absoluta. Only the most promising strains under laboratory studies were tested on a large scale to evaluate their control efficacy in the field. Based on our laboratory assessment of T. absoluta egg cards, four Trichogramma strains were identified as promising candidates for the biocontrol of T. absoluta in China: the TC-JL and TC-HN strains of T. chilonis, the TO-JL strain of T. ostriniae, and the TP-HN strain of T. pretiosum showed greater host acceptance; strain TC-JL outperformed other species/strains; strains TC-JL and TC-HN of T. chilonis showed similar parasitism capacity on caged potted tomato plants; and strain TP-HN of T. pretiosum achieved a higher level of host acceptance and has not been evaluated in previous studies in China. Furthermore, our walk-in field cage findings indicate that TC-JL of T. chilonis could reduce the larval population and the percentages of damaged plants and leaves by 75.10%, 55.56%, and 64.69%, respectively. T. chilonis (particularly strain TC-JL), a commercially available species of Asian origin, demonstrates strong potential for integration into IPM programs against T. absoluta in China and even all of Asia. T. pretiosum (particularly strain TP-HN) is a potential candidate for biocontrol of T. absoluta in China.
Author Contributions
Conceptualization, G.-F.Z.; methodology, G.-F.Z., C.-M.Z., Y.-B.Z., D.-F.M., P.L., Y.-W.G., W.-C.L., Y.-S.W. and C.H.; formal analysis, G.-F.Z., C.-M.Z., Y.-B.Z., D.-F.M., P.L., Y.-W.G., W.-C.L., Y.-S.W., C.H., X.-Q.X. and F.-H.W.; data calculation, G.-F.Z., C.-M.Z. and Y.-B.Z.; statistical analysis, G.-F.Z., C.-M.Z. and X.-Q.X.; writing—original draft preparation, G.-F.Z., C.-M.Z., Y.-B.Z. and D.-F.M.; writing—review and editing, P.L., Y.-W.G., W.-C.L., Y.-S.W., C.H., X.-Q.X. and F.-H.W.; visualization, G.-F.Z., C.-M.Z., Y.-B.Z. and D.-F.M.; supervision, G.-F.Z. G.-F.Z., C.-M.Z., Y.-B.Z. and D.-F.M. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2021YFD1400200) and the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ZDRW202505 and CAASCX-2022-2027-IAS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All authors have given consent to publish this article.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are grateful to the Ministry of Science and Technology of the People’s Republic of China and the Chinese Academy of Agricultural Sciences for providing financial support; the State Key Laboratory for Biology of Plant Diseases and Insect Pests, Key Laboratory for Prevention and Control of Invasive Alien Species of Ministry of Agriculture and Rural Affairs; and the Yuxi Yangrui Organic Vegetable Production Base of IPP, CAAS for the research facilities. The authors are grateful to Hong-Yan Li and Yu-Xin Jiang from the College of Tropical Crops of Yunnan Agricultural University for their assistance in collecting data on host acceptance; Long Wang from the College of Tropical Crops of Yunnan Agricultural University and Sheng-Feng Li from the College of Plant Protection of Hunan Agricultural University for their assistance in collecting data on parasitism; He-Sen Yang from IPP, CAAS for his assistance in lifetable parameters’ analysis; Xiao-Wen Chen and Dan Gong from the College of Plant Protection of Hunan Agricultural University for their assistance in collecting data on host control ability; and Tao Jin from the Institute of Environment and Plant Protection, Chinese Academy of Tropical Agricultural Sciences, and Zhao-Xu Zhou from the Institute of Plant Protection, Gansu Academy of Agricultural Sciences for providing strains TP-HN and TP-GS of T. pretiosum, respectively.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chang, P.E.C.; Metz, M.A. Classification of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae: Gelechiinae: Gnorimoschemini) based on cladistic analysis of morphology. Proc. Entomol. Soc. Wash. 2021, 123, 41–54. [Google Scholar]
- Soares, M.A.; Campos, M.R. Phthorimaea absoluta (tomato leafminer). CABI Compendium. Datasheet: 30 November 2020. Data Last Updated on 6 February 2025. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49260 (accessed on 16 June 2025).
- Miranda, M.M.M.; PicancËo, M.; Zanuncio, J.C.; Guedes, R.N.C. Ecological life table of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Biocontrol Sci. Technol. 1998, 8, 597–606. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Desneux, N.; Han, P.; Mansour, R.; Arno, J.; Brévault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.C.; Karimi, J.; Konan, K.A.J.; et al. Integrated pest management of Tuta absoluta: Practical implementations across different world regions. J. Pest Sci. 2022, 95, 17–39. [Google Scholar] [CrossRef]
- Zhang, G.F.; Xian, X.Q.; Zhang, Y.B.; Liu, W.X.; Liu, H.; Feng, X.D.; Ma, D.Y.; Wang, Y.S.; Gao, Y.H.; Zhang, R.; et al. Outbreak of the South American tomato leafminer, Tuta absoluta, in the Chinese mainland: Geographic and potential host range expansion. Pest Manag. Sci. 2021, 77, 5475–5488. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, N.R.C.; Wan, F.H.; Desneux, N. Ecology, worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Cherif, A.; Attia-Barhoumi, S.; Mansour, R.; Zappalà, L.; Grissa-Lebdi, K. Elucidating key biological parameters of Tuta absoluta on different host plants and under various temperature and relative humidity regimes. Entomol. Gen. 2019, 39, 1. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Lietti, M.M.M.; Botto, E.; Alzogaray, R.A. Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2005, 34, 113–119. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). Online Statistical Database: Food and Agriculture Data. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 31 July 2025).
- Zhang, G.F.; Ma, D.Y.; Liu, W.X.; Wang, Y.S.; Fu, W.J.; Wang, J.; Gao, Y.H.; Wan, F.H. The arrival of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Biosaf. 2019, 28, 200–203. [Google Scholar]
- Zhang, G.F.; Ma, D.Y.; Wang, Y.S.; Gao, Y.H.; Liu, W.X.; Zhang, R.; Fu, W.J.; Xian, X.Q.; Wang, J.; Kuang, M.; et al. First report of the South American tomato leafminer, Tuta absoluta (Meyrick), in China. J. Integr. Agric. 2020, 19, 1912–1917. [Google Scholar] [CrossRef]
- Zhang, G.F.; Xian, X.Q.; Zhang, Y.B.; Zhang, R.; Ma, D.Y.; Liu, W.X.; Gao, Y.H.; Wang, J.; Yang, Z.L.; Li, Q.H.; et al. Warning of the dispersal of a newly invaded alien species, tomato leaf miner Tuta absoluta (Meyrick). Plant Prot. 2020, 46, 281–286. [Google Scholar]
- Li, P.; Ma, D.Y.; Ge, Z.Y.; Wang, S.Y.; Sun, Z.W.; Zhang, D.X.; Huang, J.X.; Zhang, G.F.; Guo, Y.W. Occurrence and damage of tomato leaf miner Tuta absoluta in China and application of prevention and control techniques. China Plant Prot. 2025, 45, 48–53. [Google Scholar]
- Cônsoli, F.L.; Parra, J.R.; Zucchi, R.A. Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Springer: Dordrecht, The Netherlands, 2010; Volume 9, 482p. [Google Scholar]
- Walgenbach, J.F.; Leidy, R.B.; Sheets, T.J. Persistence of insecticides on tomato foliage and implication for control of tomato fruit worm (Lepidoptera: Noctuidae). J. Econ. Entomol. 1991, 84, 978–986. [Google Scholar] [CrossRef]
- DeBach, P. Biological Control of Insect Pests and Weeds; Cambridge University Press: Cambridge, UK, 1964. [Google Scholar]
- Ferracini, C.; Bueno, V.H.P.; Dindo, M.L.; Ingegno, B.L.; Luna, M.G.; Gervassio, N.G.S.; Sánchez, N.E.; Siscaro, G.; van Lenteren, J.C.; Zappalà, L.; et al. Natural enemies of Tuta absoluta in the Mediterranean basin, Europe and South America. Biocontrol Sci. Technol. 2019, 29, 578–609. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Zhou, S.W.; Sun, Y.; Zou, K.; Li, T.A.; Zhang, J.L.; Chen, G.H.; Zhang, X.M. Assessment of the suitability of three native Trichogramma species for biological control of Tuta absoluta in China. Entomol. Gen. 2024, 44, 367–375. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Sun, Y.; Zhou, S.W.; Xiong, P.W.; Zhang, J.L.; Wu, D.H.; Chen, G.H.; Zhang, X.M. An evaluation of the growth, development, reproductive characteristics and pest control potential of three Trichogramma species on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Pest Manag. Sci. 2024, 80, 6107–6116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Chen, T.T.; Chen, L.M.; Ren, J.; Ullsh, F.; Yi, S.W.; Pan, Y.H.; Zhou, S.X.; Guo, W.C.; Fu, K.Y.; et al. Trichogramma chilonis is a promising biocontrol agent against Tuta absoluta in China: Results from laboratory and greenhouse experiments. Entomol. Gen. 2024, 44, 357–365. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Y.B.; Arnó, J.; Mansour, R. Research toward enhancing integrated management of Tuta absoluta, an ongoing invasive threat in Afro-Eurasia. Entomol. Gen. 2024, 44, 263–267. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–684. [Google Scholar] [CrossRef]
- Pang, X.F.; Chen, T.L. Trichogramma of China (Hymenoptera: Trichogrammatidae). Acta Entomol. Sin. 1974, 17, 441–454. [Google Scholar]
- CABI (Centre for Agriculture and Biosciences International). Trichogramma chilonis. CABI Compendium. Datasheet Type: Natural Enemy. Datasheet: 22 November 2019. Data Updated on 10 February 2022. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.54697 (accessed on 16 June 2025).
- Zhang, L.H.; Wang, H.S.; Xu, B.F.; Liu, W. Classification and natural distribution of Trichogramma spp. in China. J. Jilin For. Sci. Technol. 2014, 43, 33–35, 38. [Google Scholar]
- Miura, K.; Matsuda, S.; Murai, T. Esterase zymograms of Trichogramma chilonis Ishii and Trichogramma ostriniae Pang et Chen (Hymenoptera: Trichogrammatidae). Jpn. J. Entomol. 1990, 58, 689–692. [Google Scholar]
- Yuan, X.; Li, D.S.; Zhao, Y.L.; Feng, X.X.; Zuo, C.; Li, S.L. Investigation report on effect of large-area release Trichogramma to control sugarcane borers in Guangxi in 2013. Chin. J. Biol. Control 2014, 30, 630–634. [Google Scholar]
- Shi, Z.; Chen, H.; Qin, Z.; Guo, Q.; Bi, D.J.; Jiang, Q.; Huang, Z.; Tang, L.; Peng, C.; Ma, W.Q. Population dynamics of borers and its control effect evaluation by using Trichogramma chilonis Ishii in Chongzuo cane area. Chin. J. Biol. Control 2018, 34, 656–662. [Google Scholar]
- Kfir, R. Attempts at biological control of the stem borer Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) in South Africa. Afr. Entomol. 1994, 2, 67–68. [Google Scholar]
- Xu, J.J.; Guo, W.C.; Yao, J.; Turxun; Wang, Z.P.; Hamiti, A. Study on using mass-release Trichogramma chilonis to control Helicoverpa armigera in cotton field in Xinjiang. Xinjiang Agric. Sci. 2004, 41, 378–380. [Google Scholar]
- Gervassio, N.G.S.; Aquino, D.; Vallina, C.; Biondi, A.; Luna, M.G. A re-examination of Tuta absoluta parasitoids in South America for optimized biological control. J. Pest Sci. 2019, 92, 1343–1357. [Google Scholar] [CrossRef]
- Sarhan, A.A.; Osman, M.A.M.; Mandour, N.S.; Abd El-Hady, M.A. Parasitization capability of four Trichogrammatid species against the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under different releasing regimes. Egypt. J. Biol. Pest Control 2015, 25, 107–112. [Google Scholar]
- Editorial Department of Natural Enemies of Insects. Introducing three Trichogramma species from the United States of America to China. Nat. Enem. Insects 1979, 1, Back cover. [Google Scholar]
- He, Y.R.; Lü, L.H.; Chen, K.W. Parasitizing ability and interspecific competition of Trichogramma confusum Viggiani and T. pretiosum Riley on the eggs of Plutella xylostella (L.) in the laboratory. Acta Ecol. Sin. 2005, 25, 837–841. [Google Scholar]
- Deng, J.H.; Gu, J.R.; Dong, M.H.; Yang, D.F.; Liu, T.F. The parasitic differences of several Trichogramma species on Plutella xylostella eggs. Jiangsu Agric. Sci. 2012, 40, 77–78. [Google Scholar]
- Zhu, K.H.; Zhou, J.C.; Zhang, Z.T.; Zhang, S.; Che, W.N.; Zhang, L.S.; Dong, H. Parasitic efficacy and offspring fitness of Trichogramma pretiosum against Spodoptera fruguperda and Spodoptera litura at different egg ages. Plant Prot. 2019, 45, 54–59. [Google Scholar]
- Zhu, K.H.; Zhou, J.C.; Zhang, Z.T.; He, K.L.; Zhang, L.S.; Dong, H. Preliminary evaluation of field competition between Trichogramma pretiosum and Telenomus remus in the control of Spodoptera frugiperda. Plant Prot. 2020, 46, 267–271. [Google Scholar]
- Smith, S.M. Biological control with Trichogramma: Advances, success, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef] [PubMed]
- El-Arnaouty, S.A.; Pizzol, J.; Galal, H.H.; Kortam, M.N.; Afifi, A.I.; Beyssat, V.; Desneux, N.; Biondi, A.; Heikal, I.H. Assessment of two Trichogramma species for the control of Tuta absoluta in North African tomato greenhouses. Afr. Entomol. 2014, 22, 801–809. [Google Scholar] [CrossRef]
- Myint, Y.Y.; Bai, S.X.; Zhang, T.T.; Babendreier, D.; He, K.L.; Wang, Z.Y. Selection of the most effective Trichogramma strains (Hymenoptera: Trichogrammatidae) from Myanmar to control Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). J. Econ. Entomol. 2021, 115, 81–92. [Google Scholar] [CrossRef]
- Yue, J.; Dong, J.; Zhang, G.F.; Wang, P.S.; Qiao, Y.; Zhang, N.; Zhang, J.L.; Yuan, Z.Q. Identification of familiar Trichogramma species based on DNA barconding techniques. China Plant Prot. 2014, 34, 11–14. [Google Scholar]
- Romani, R.; Isidoro, N.; Bin, F. Antennal structures used in communication by egg parasitoids. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Cônsoli, F.L., Parra, J.R.P., Zucchi, R.A., Eds.; Progress in Biological Control; Springer: Dordrecht, The Netherlands, 2009; Volume 9, pp. 57–96. [Google Scholar]
- Schäfer, L.; Herz, A. Suitability of European Trichogramma species as biocontrol agents against the tomato leaf miner Tuta absoluta. Insects 2020, 11, 357. [Google Scholar] [CrossRef]
- Huang, J.; Hua, H.; Zhang, F.; Li, Y.X. Suitability assessment of three Trichogramma species in the control of Mythimna separata (Lepidoptera: Noctuidae). J. Appl. Entomol. 2018, 142, 131–140. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; You, M.S.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on the Age-Stage, Two-Sex Life Table. V1. 2022, Zenodo. Available online: https://zenodo.org/records/7482191 (accessed on 1 August 2025).
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chailleux, A.; Desneux, N.; Seguret, J.; Khanh, H.D.T.; Maignet, P.; Tabone, E. Assessing European egg parasitoids as a mean of controlling the invasive South American tomato pinworm Tuta absoluta. PLoS ONE 2012, 7, e48068. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Okuyama, T. Parasitoid aggregation and interference in host–parasitoid dynamics. Ecol. Entomol. 2016, 41, 473–479. [Google Scholar] [CrossRef]
- Khanh, D.T.H.; Chailleux, A.; Tiradon, M.; Desneux, N.; Colombel, E.; Tabone, E. Using new egg parasitoids (Trichogramma spp.) to improve integrated management against Tuta absoluta. EPPO Bull. 2012, 42, 249–254. [Google Scholar] [CrossRef]
- Reed, D.A.; Luhring, K.A.; Stafford, C.A.; Hansen, A.K.; Millar, J.G.; Hanks, L.M.; Paine, T.D. Host defensive response against an egg parasitoid involves cellular encapsulation and melanization. Biol. Control 2007, 4, 214–222. [Google Scholar] [CrossRef]
- Pak, G.; van Dalen, A.; Kaashoek, N.; Dijkman, H. Host egg chorion structure influencing host suitability for the egg parasitoid Trichogramma Westwood. J. Insect Physiol. 1990, 36, 869–875. [Google Scholar] [CrossRef]
- Taylor, T.A.; Stern, V.M. Host-preference studies with the egg parasite Trichogramma semifumatum (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. Am. 1971, 64, 1381–1390. [Google Scholar] [CrossRef]
- Ellers, J.; Jervis, M. Body size and the timing of egg production in parasitoid wasps. Oikos 2003, 102, 164–172. [Google Scholar] [CrossRef]
- Tabone, E.; Bardon, C.; Desneux, N.; Wajnberg, E. Parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. J. Pest Sci. 2010, 83, 251–256. [Google Scholar] [CrossRef]
- Pratissoli, D.; Thuler, R.T.; Andrade, G.S.; Zanotti, L.C.M.; Dasilva, A.F. Estimate of Trichogramma pretiosum to control Tuta absoluta in stalked tomato. Pesqui. Agropecu. Bras. 2005, 40, 715–718. [Google Scholar] [CrossRef]
- Cascone, P.; Carpenito, S.; Slotsbo, S.; Iodice, L.; Sørensen, J.G.; Holmstrup, M.; Guerrieri, E. Improving the efficiency of Trichogramma achaeae to control Tuta absoluta. BioControl 2015, 60, 761–771. [Google Scholar] [CrossRef]
- Cabello, T.; Gallego, J.R.; Vila, E.; Soler, A.; del Pino, M.; Carnero, A.; Hernandez-Suarez, E.; Polaszek, A. Biological control of the South American tomato pinworm, Tuta absoluta (Lep. Gelechiidae), with releases of Trichogramma achaeae (Hym. Trichogrammatidae) in tomato greenhouses of Spain. IOBC/WPRS Bull. 2009, 49, 225–230. [Google Scholar]
- Colmenárez, Y.C.; Vásquez, C.; Bde Freitas Bueno, A.; Cantor, F.; Hidalgo, E.; Corniani, N.; Lagrava, J.J. Sustainable management of the invasive Tuta absoluta (Lepidoptera: Gelechiidae): An overview of case studies from Latin American countries participating in Plantwise. J. Integr. Pest Manag. 2022, 13, 15. [Google Scholar] [CrossRef]
- Mansour, R.; Biondi, A. Releasing natural enemies and applying microbial and botanical pesticides for managing Tuta absoluta in the MENA region. Phytoparasitica 2021, 49, 179–194. [Google Scholar] [CrossRef]
- Guleria, P.; Sharma, P.L.; Verma, S.C.; Chandel, R.S. Life history traits and host-killing rate of Neochrysocharis formosa on Tuta absoluta. BioControl 2020, 65, 401–411. [Google Scholar] [CrossRef]
- Wang, H.; Gu, Y.J.; Song, R.R.; Zhang, C.; Liu, W.X.; Wan, F.H.; Desneux, N.; Zhang, G.F.; Zhang, Y.B. Thelytokous strains have better biocontrol potential than arrhenotokous strains: The parasitoid Neochrysocharis formosa on the invasive tomato leafminer Tuta absoluta as a case study. Entomol. Gen. 2024, 44, 377–384. [Google Scholar] [CrossRef]
- Zhang, G.F.; Zhang, Y.B.; Zhang, J.; Liu, W.X.; Wang, Y.S.; Wan, F.H.; Shu, C.L.; Liu, H.; Wang, F.L.; Zhao, L.; et al. Laboratory toxicity and field control efficacy of biopesticide Bacillus thuringiensis G033A on the South American tomato leafminer Tuta absoluta (Meyrick), a new invasive alien species in China. Chin. J. Biol. Control 2020, 36, 175–183. [Google Scholar]
- Huang, J.X.; Wu, E.; Yang, C.X.; Han, X.Y.; Zhang, J.H.; Cao, M.Y.; Deng, F.Z.; Guo, Q.S.; Du, Y.J. Mating disruption of the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae) on greenhouse tomatoes. Crop Health 2024, 2, 15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).