Abstract
Excessive nitrogen (N) fertilizer usage in agriculture has prompted the exploration of sustainable strategies to enhance nitrogen use efficiency (NUE) while maintaining crop yield and quality. Processed tomatoes (Solanum lycopersicum L.) were grown for two years (2023 and 2024) following a two-way factorial randomized complete block (RCBD) design, considering three biostimulants and three N regimes as two factors, to assess their morphophysiological, biochemical, anatomical and yield performances. Nitrogen application significantly influenced biomass accumulation, the leaf area index (LAI), nitrogen uptake and yield with notable comparable values between reduced and optimal nitrogen dose, indicating improved nitrogen use efficiency. Biostimulants showed limited effects alone but enhanced plant performance under reduced nitrogen conditions, particularly improving chlorophyll content, crop growth, N uptake, yield and anatomical adaptations. Moreover, compared to 2024, biostimulant application enhanced tomato growth more evidently in 2023 due to environmental variations, likely due to the occurrence of stress conditions. Importantly, biostimulants, together with N regimes, i.e., optimal and reduced doses, showed improved anatomical traits, especially regarding leaf thickness and thickness between the two epidermises, indicating adaptive responses that may support sustained productivity under N-limited conditions. Among the biostimulants used, the processed tomatoes responded better to protein hydrolysate and endophytic N-fixing bacteria than to seaweed extract. These findings suggest that although biostimulants alone were not affected, integrating them with reduced N fertilization provides a viable strategy for optimizing tomato production, conserving resources and minimizing the environmental impact without compromising yield or quality.
1. Introduction
The agricultural sector faces significant challenges due to climate change, which affects crop productivity through direct and indirect mechanisms. A comprehensive, multi-model analysis conducted by the Intergovernmental Panel on Climate Change (IPCC) projected a mean reduction of 17% in the yield of four staple crops (coarse grains, oilseeds, wheat, and rice) by the year 2050 under a baseline scenario of static climatic conditions [1]. These crops, which constitute approximately 70% of the global harvested area, were assessed using a suite of climate–crop simulation models [2]. This projection underscores the potential for significant reductions in global agricultural productivity, even in the absence of further climate perturbations, emphasizing the vulnerability of major food systems to current environmental trends. This reduction, coupled with a growing global population, underscores the urgency of developing sustainable agricultural practices. As agricultural challenges evolve, traditional fertilization methods are no longer effective, making innovative strategies essential for ensuring both crop productivity and environmental sustainability.
Nitrogen (N) is one of the most essential macronutrients required for plant growth and development, playing a crucial role in various physiological and biochemical processes. To ensure high crop yields, nitrogen fertilizers have been extensively applied in large quantities. However, excessive N fertilization has led to severe environmental consequences, including soil and water pollution [3]. In response to these challenges, contemporary fertilization practices are being revised to optimize nitrogen use efficiency (NUE) while minimizing environmental impacts and reducing fertilizer costs [4].
Tomato is a globally cultivated vegetable crop produced in both open-field and greenhouse systems. According to the Food and Agriculture Organization (FAO), global tomato production reached approximately 186.8 million tonnes in 2022 and cultivated over nearly 5 million hectares, with an average yield of about 37 tonnes per hectare [5]. The main producers include China, India, Turkey, the United States, Egypt and Italy, collectively accounting for most of the global output. The continuous expansion of tomato cultivation highlights the need for sustainable strategies to enhance yield, quality and resource use efficiency. According to the World Processing Tomato Council (WPTC) [6], Italy ranked third in tomato production after China and the USA. Several studies conducted in Central Italy have extensively investigated fertilization strategies for processed tomatoes, focusing on optimizing N inputs while minimizing environmental impacts [7,8,9]. Extensive research has shown that fruit yield generally increases with nitrogen supplementation, but only up to a specific limit—after which additional nitrogen offers no further benefit to productivity [10]. Furthermore, marketable fruit yield, rather than total fruit yield, should be the primary consideration in optimizing N fertilization strategies. Previous research has demonstrated that increasing N supply from 50 to 250 kg/ha affected the total fruit yield but not necessarily the marketable yield [11]. The impact of N supply on fruit quality parameters remains a topic of ongoing investigation. While fruit color has been reported to be largely unaffected by N levels, fruit firmness has shown variability [12]. Some studies suggest that reducing N supply could lead to higher sugar content in tomatoes, while titratable acidity may decrease with increasing N supply [10]. However, other findings indicate that increased N can enhance sugar and acid content, potentially improving overall fruit quality [13].
Previous studies have shown that the application of biostimulants such as seaweed extracts, protein hydrolysates and beneficial microorganisms can significantly enhance tomato growth, yield, NUE, and fruit quality under both optimal and reduced N conditions [14,15,16,17]. Seaweed extracts and protein hydrolysates have been particularly effective in improving marketable yield, dry matter accumulation, and fruit firmness while also increasing NUE by enhancing N uptake and utilization [17]. Arbuscular mycorrhizal fungi and Trichoderma, used as microbial inoculants, can work synergistically to promote plant growth and boost the production of bioactive compounds, especially under low N availability [16]. Furthermore, studies combining biostimulants with environmental modifications, such as light-diffusing films, revealed substantial gains in yield and fruit quality under sub-optimal N inputs [14]. However, there is still limited comparative research evaluating multiple categories of biostimulants, such as seaweed extracts, protein hydrolysates, and nitrogen-fixing bacteria, under varying N regimes, particularly in terms of their integrated effects on tomato growth, yield, NUE and fruit quality.
In this context, biostimulants have emerged as a promising sustainable strategy to enhance plant growth, improve nutrient uptake, and mitigate the adverse effects of reduced N application [18]. Biostimulant employment could represent a promising way to increase crop performance in an environmentally sustainable manner. Biostimulants, including protein hydrolysates [19], seaweed extracts [20], and beneficial microorganisms [21], have been reported to promote plant resilience to abiotic stresses, enhance soil microbial activity and improve overall crop performance [22]. Their use in tomato cultivation may serve as a viable alternative to excessive N fertilization, supporting both high productivity and environmental sustainability. Therefore, the objective of this study is to assess the efficacy of three types of biostimulants, i.e., (i) seaweed extract, (ii) protein hydrolysates and (iii) an endophytic nitrogen-fixing bacteria, in enhancing tomato growth, yield, NUE and fruit quality under different N fertilization regimes.
2. Materials and Methods
2.1. Experimental Site, Treatments and Crop Management
Two open-field experimental trials were conducted on tomato over the period May–September 2023 (the 1st growing season) and 2024 (the 2nd growing season) at the Experimental Station of the Department of Agricultural, Food and Environmental Sciences, the University of Perugia, Perugia, Italy, located in the middle of the Tiber plain (Central Italy, Papiano, 42.96° N, 12.37° E, 165 m a.s.l.). The soil was clay loam (Fluventic Haplustept, Soil Taxonomy) and samples were collected prior to the experiment in both seasons to determine the soil characteristics, which are presented in Table 1. The values reported in Table 1 reflect the average values in both years as the soil characteristics were almost similar.

Table 1.
Properties of soil used in the experiment.
The weather conditions, including the temperature and rainfall of both years, are described in Figure 1.
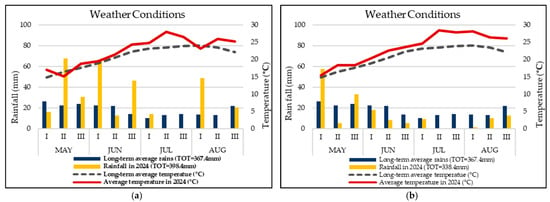
Figure 1.
Daily rainfall (vertical bars) and mean daily air temperature (line) during the periods May–August 2023 (a) and 2024 (b) and average values over the last 40 years.
The two-factorial experiments were designed followed by factorial randomized complete block design (RCBD) by considering three biostimulant (B) treatments together with the control, i.e., (i) BIOS0: no biostimulant, (ii) BIOS1: seaweed extract Macrocystis Integrifolia (Macys BC28, Cifo srl, Bologna, Italy), (iii) BIOS2: protein hydrolysates obtained as a by-product from the agri-food industry (not a commercial product, developed by Fomet Spa, Verona, Italy) and (iv) BIOS3: endophytic N-fixing bacteria Methylobacterium symbioticum (Utrisha™ N, Corteva AgriScience srl, Cremona, Italy), as the first factor. Three nitrogen (N) fertilization doses, i.e., N0: unfertilized control, STD: fertigation at the optimal N rate (200 kg N ha−1) and RED: reduced N fertilization at −30% of the optimal N rate, (140 kg N ha−1) represented the second factor. The experiment consisted of 4 Biostimulants (including the control) × 3 Nitrogen rates × 3 Replications = 36 experimental units with a plot (experimental unit) size of 40 m2 in both seasons. The biostimulants were applied as a foliar application following the recommended doses from the company providing the material. These were applied at three time points as follows: soon before flowering (30–40 DAT), at the end of flowering (50–60 DAT) and at the beginning of the fruit maturity phase (70–80 DAT). The application rates increased with the increase in plant growth and those were ranged as follows: BIOS1: 2–4 L ha−1, BIOS2: 2–3 L ha−1 and BIOS3: 300–500 g ha−1. Detailed biostimulant properties are described in Table 2.

Table 2.
Characteristics of the biostimulants used in the experiment.
The tomato crop (Solanum lycopersicum L., variety Heinz 5108, H.J. Heinz Company Brands LLC., Latina (LT), Italy) was transplanted on 22 May 2023 and 27 May 2024 for the first and second growing season, respectively, at 3 plants m−2. Plants were spaced 0.3 m apart within rows, with rows spaced 1 m apart. Each plot had five rows: three central rows for measurements and two border rows on the perimeter of each plot to reduce potential border effects.
Fertigation was applied with a mineral fertilizer (N.S.Z. 26, Cifo s.r.l., San Giorgio di Piano, BO, Italy) with the following composition: 6% N-NO3, 8% N-NH4+, 12% urea, 13% SO3 and 0.01% of Zn. All fertilized treatments received the same volume of drip irrigation, determined using the FAO method with a crop coefficient (Kc) derived from previous research on the same crop, variety and environment [7]. All cultural practices have been consistent with local commercial crop management using integrated pest management, including regular monitoring and preventive measures [8]. Weed control was effectively managed by hand-weeding. The final yield was recorded at harvest, i.e., 4 September 2023 and 29 August 2024 for the first (105 DAT) and second (94 DAT) growing seasons, respectively.
2.2. Measurements
2.2.1. Plant Sampling
Various morphological traits, including above-ground dry matter (DM: Mg ha−1), total yield (Mg ha−1), marketable yield (Mg ha−1) and leaf area index (LAI), were assessed at multiple time points (approximately 30, 45, 60, 75, and 90 DAT) across both growing seasons. DM accumulation in processed tomato was monitored by destructive sampling conducted at two-week intervals by randomly selecting 4 to 8 plants per plot. After sampling, the plants were separated into individual parts, i.e., stems, leaves and fruits, to determine fresh biomass, and then they were put in a ventilated oven at 80 °C for the dry biomass observation until a consistent weight was observed by using a weight balance (Radwag PM 20.5Y, Radom, Poland). The LAI was measured using a leaf area meter (LI-3100C, LI-COR Biosciences, Lincoln, NE, USA). Subsamples of the dried plant material from each sampling date were finely ground and stored for a subsequent analysis of total nitrogen concentration (N%).
At harvest (carried out when about 80% of the fruits were ripe), 20 plants per plot were harvested by hand, and we divided them into vegetative parts and fruits. The fruits were then further divided into ripe fruit representing marketable yield and rotten fruit/overripen fruit representing non-marketable yield that were combined to calculate the total yield, with fruit quality attributes determined including pH values (using a pH meter, Radiometer Analytical SAS, near Lyon, France), color and total soluble solid contents (TSS: °Brix) using a digital refractometer (Atago Co., Ltd., Tokyo, Japan).
2.2.2. Crop N Monitoring
Tomato crop N nutritional status was evaluated by three “on-field” quick tests carried out on the same sampling day of crop growth analysis until the maximum vegetative growth stage (i.e., about 75 DAT): (i) petiole sap nitrate analysis was conducted on the sap extracted from 15–20 most recently fully matured leaves and analyzed by an ion-specific electrode meter (Cardy, Spectrum Technologies, Inc., Plainfield, IL, USA) for the determination of NO3-N concentration; (ii) optical measurements using chlorophyll meter readings by Chlorophyll Meter SPAD 502 (Minolta Camera Co., Ltd., Osaka, Japan), taking the apical leaflet of the uppermost fully expanded 15–20 leaves (3rd–4th from the top); and (iii) canopy reflectance measured by a spectroradiometer (Rapidskan, Holland Scientific, Lincoln, NE, USA). Moreover, the crop N contents were analyzed by following Dumas’s method [23] using an elemental analyzer (Flash 2000, Thermo Fisher Scientific, Cambridge, UK).
2.2.3. Anatomical Analysis
During the first growing season, stomatal morphological traits were observed by following the nail polish imprint method [24]. Clear nail polish was applied onto the middle of the abaxial leaf surface and left to dry; dry nail polish was then pulled off by using clear tape and mounted onto a glass slide. Stomatal density (SD: mm−2) was determined from digital images using a Leica DMRD light microscope (Leica Mikroskopie & Systeme GmbH, Wetzlar, Germany) equipped with a camera (Leica DFC 420). Stomatal morphology, including guard cell length (Ls, µm), guard cell width (Ws, µm), stomatal pore aperture length (La, µm), and stomatal pore aperture width (Wa, µm), were measured using Cell Sens Standard software v3.1 (Olympus, Tokyo, Japan); these measures were then used to calculate the stomatal size (SZ: µm2).
Additionally, during the second growing season, leaf portions were collected from 5 leaves/plot. Plant material was fixed in 5% (w/v) glutaraldehyde in 0.075 M cacodylate buffer, pH 7.2, for 24 h. The samples were then washed three times for 7 min in 0.075 M cacodylate buffer, pH 7.2, post-fixed in 1% (w/v) OsO4 in the same buffer for 1 h, dehydrated with increasing concentrations of ethanol and embedded in epoxy resin (Epon, 2-dodecenylsuccinic anhydride and methylnadic anhydride mixture) [25]. Semi-thin sections (1–2 μm), obtained with an ultramicrotome (OmU2, Reichert, Heidelberg, Germany) equipped with a glass blade, were stained with toluidine blue 0.1% w/v and observed under a light microscope (BX53; Olympus, Tokyo, Japan). Images were captured with a camera (XC50, Olympus, Tokyo, Japan). Through the software CellSens (Olympus, Tokyo, Japan), in the leaf transversal sections, we measured the width of the section considered in the measurement (W: µm), the thickness between the two epidermises (tmes: µm), and the palisade and spongy cell area. These values were combined to calculate the fraction of intercellular air space (fias) [26], the palisade parenchyma ratio (p-ratio), the spongy parenchyma ratio (s-ratio) and the fraction of palisade cells (L-tmes).
2.2.4. Photosynthetic Pigment Analysis
The samples were cut into small pieces and extracted with 80% acetone. Extracts were maintained at 4 °C until analysis and all manipulations were performed in dim green safe light to avoid photo-degradation. Absorption spectra (400 and 750 nm range) of extracts were recorded at room temperature (25 °C) by a spectrophotometer. For Chl’s and carotenoid determinations, the extracts were measured at 663 nm (Chl a: mg/g fresh weight: FW), 646 nm (Chl b: mg/g FW), and 470 nm (carotenoids: mg/g FW). Pigment concentrations were evaluated according to the equations proposed by Wellburn [27].
2.3. Statistical Analysis
All the experimental data were first checked for normality and the homogeneity of variance followed by a two-way ANOVA using the statistical software RStudio version 4.2.0 [28], considering B and N application rates as the main factors. Mean comparisons among treatment groups, where found significant, were performed using Tukey’s Honestly Significant Difference (HSD) test at a significance level of p ≤ 0.05.
3. Results
3.1. Crop Growth, N Uptake and Yield
The crop growth in terms of above-ground dry matter content was more than 50% higher in the second growing season compared with the first one (6.4 Mg ha−1 and 9.8 Mg ha−1, respectively, averaged for all treatments). Nitrogen (N) application significantly influenced crop growth (p < 0.001) at all sampling dates, whereas the biostimulant application and the two-factor interaction (N × B) had no significant effects. Due to consistent trends across all time points, only data recorded at harvest are presented in Table 3, while other data are shown in Supplementary File Figures S1 and S2. The DM accumulation recorded at harvest exhibited the highest values in both 2023 and 2024 with plants cultivated under optimal (STD) and reduced N supply (RED) treatments (Table 3). Indeed, in both years, the RED treatment showed similar DM values to those measured in STD N doses. In the first growing season, the application of biostimulants increased the DM by 27% and 14% in unfertilized control (N0) and RED treatments, respectively (as an average over the three biostimulants), although not significantly (Table 3). Among the biostimulants under N0, BIOS2 in both growing seasons and BIOS3 only in 2023 showed the highest above-ground biomass increase. At the optimal N availability, none of the biostimulants increased DM accumulation.

Table 3.
Above-ground dry matter accumulation (DM, Mg ha−1), maximum leaf area index (LAImax), N uptake in the above-ground biomass (kg N ha−1), total fruit yield (as fresh weight, Mg ha−1), marketable yield (as fresh weight, Mg ha−1) of processed tomatoes treated with different biostimulants and nitrogen doses recorded at harvest.
Likewise, the LAI was measured at different time points until the maximum vegetative growth phase (LAImax) was recorded at about 80 DAT in both growing seasons (Table 3, Supplementary File Figure S2). Consistent with DM accumulation, plants grown in 2024 produced a higher LAI (+40%) compared to the ones grown in 2023. Moreover, plants with RED and STD nitrogen application exhibited similar values and the significantly highest LAI compared to those under N0, while B and N × B had no significant effects. Interestingly (but not significant), plants grown with BIOS2 showed an increment of about 80% and 30% in LAI in 2023 and 2024, respectively, compared to BIOS0 (Table 3).
Additionally, the N uptake at harvest also revealed the same trend as the above-mentioned morphological traits by showing significant increases only with RED and STD N availability (p < 0.001) compared to the N0 conditions in both growing seasons (Table 3). From 2023, the N uptake in 2024 was enhanced by 45%. On the contrary, B and N × B did not affect N uptake in both growing seasons. Among the biostimulants, BIOS2_N0 showed a slightly higher N uptake value, but limited to the first growing season (+22%), than BIOS0_N0, followed by BIOS3_N0 (+12%), respectively.
Similarly to crop growth traits, total yield and marketable yield were highest in the second growing season (+75% and +120%, respectively, as an overall average) and were significantly affected by N (p < 0.001), with plants under the RED and STD treatment producing the highest yield and marketable yield in 2024 on average, i.e., 109 Mg ha−1 and 92 Mg ha-1, respectively (Table 3). However, the biostimulants and N × B were not significant for either of these traits. Although the effect of biostimulants was not significant, during first growing season under N0, BIOS2 slightly improved the yield and marketable yield by 32% and 10%, respectively, compared to BIOS0. Moreover, a prominent increase in marketable yield was 20% with plants grown in BIOS3_N0 compared to BIOS0_N0. The most pronounced yield increase was observed in 2023 for BIOS2, where BIOS2_N0 treatment exhibited a +30% higher DM and yield and +22% of N uptake compared to BIOS0_N0 (Table 3).
3.2. Crop Monitoring
N-NO3 concentrations in petiole sap, SPAD readings and NDVI values were recorded in roughly two-week intervals starting from 30 DAT during the crop cycle. All data for these time points are presented in Supplementary Figures S3–S5. However, in order to provide a clear and meaningful representation of treatment effects, the data are presented only for the date corresponding to maximum vegetative plant development (LAImax, i.e., 80 DAT) because of its significance in reflecting the peak of canopy development, which is a critical phase for evaluating plant nitrogen status and vegetative growth. Focusing on this time point allows for a more concise and agronomically relevant interpretation of the physiological indicators. As an average over the whole sampling period, the values were almost similar in both growing seasons for the above-mentioned traits (Table 4). More specifically, at LAImax, N-NO3 in petiole sap showed significant differences for N (p < 0.05) but not B and N × B in both growing seasons. The plants grown with STD uptake exhibited an increase in N-NO3 by 120% followed by RED at 72% compared to N0 during 2023, while in the second growing season, these values were 16 and 70%, respectively. Likewise, at LAImax, the SPAD and NDVI values were significantly influenced by N (p < 0.001), while B and N × B had no significant effects in both seasons. At all sampling dates in both growing seasons, the highest SPAD was recorded for the STD treatment, but the values obtained were not different to the RED treatment. Compared to 2023, 2024 was more pronounced in SPAD value improvement in fertilized treatment (on average, 18%) compared to the non-treated one. The similar results were also found for the NDVI value where STD and RED showed comparable results at all sampling dates in both years. Similarly to growth-related traits, the effects of biostimulants were not found to be significant, but BIOS2 and BIOS3 grown with N0 improved NDVI values by 23% and 14%, respectively, in first growing season compared to BIOS0_N0.

Table 4.
N-NO3 concentration in petiole SAP (mg L−1), SPAD readings and NDVI values of processed tomatoes treated with different biostimulants and nitrogen doses recorded at the maximum vegetative growth stage, i.e., LAImax at 80 DAT.
3.3. Fruit Quality Traits
Fruit quality parameters, including TSS (°Brix), pH and fruit color, were evaluated at harvest (Table 5). N significantly influenced TSS during 2023 (p < 0.001), but not in 2024. However, B did not have a significant effect on these parameters in both growing seasons. Although the effect was not significant, BIOS3 during the first season and BIOS2 during the second season resulted in higher °Brix values compared to the other treatments. Conversely, fruit pH remained unaffected by any experimental factor in both seasons. Fruit color exhibited a similar trend to °Brix, with no significant effect by B, whereas N influenced significantly (p < 0.05) in both growing seasons. No significant interaction of N × B was observed for any of the measured fruit quality traits in either growing season.

Table 5.
Total soluble solid contents (TSS, °Brix), pH and fruit color (5 for red fruits) of processed tomatoes treated with different biostimulants and nitrogen doses recorded at harvest.
3.4. Photosynthetic Pigmentation
Photosynthetic pigment concentrations, including chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl tot) and total carotenoids, were quantified for 50 days post transplantation (Table 6). Statistical analysis revealed a significant impact of varying nitrogen application rates (p < 0.001) on all measured photosynthetic pigment traits across both growing seasons. In contrast, biostimulant application and their two-way interaction did not affect these parameters. Specifically, plants treated with RED and STD nitrogen application rates exhibited comparable levels of all photosynthetic pigments, while the absence of nitrogen application either with or without biostimulants consistently resulted in the lowest pigmentation levels. BIOS2 consistently enhanced photosynthetic pigment levels across both seasons, suggesting a positive trend despite the lack of statistical significance.

Table 6.
Chlorophyll (a, b and total: mg g−1 fresh weight; FW) and carotenoid contents of processed tomatoes treated with different biostimulants and nitrogen doses recorded at 50 days after transplanting.
3.5. Anatomical Attributes
During the second growing season, the anatomical parameters of tomato leaves, including leaf thickness (L), thickness between the two epidermises (tmes), intercellular air space (fias), the palisade parenchyma ratio (p-ratio), the spongy parenchyma ratio (s-ratio) and epidermis thickness (L-tmes) were recorded at two time points, i.e., 36 and 64 DAT (Table 7). L and mesophyll thickness tmes were significantly affected by B and N × B at both 34 and 64 DAT (p < 0.05) (Table 7), but not by N application rates alone. Specifically, at 36 DAT, BIOS3_RED resulted in the highest L, while BIOS1_STD became higher at 64 DAT. For tmes, BIOS0_STD and BIO1_STD have higher values at 36 and 64 DAT, respectively. However, fias and p-ratio were not significantly affected by any experimental factor at any time. Moreover, s-ratio except for N application was not affected by B and N × B at both time points. Additionally, regarding L-tmes, it was only found different at 36 DAT among all experimental factors, i.e., B (p < 0.05), N (p < 0.05) and N × B (p < 0.01), but not by any factor at 64 DAT. BIOS0_N0 and BIOS1_N0 showed maximum values for s-ratio at 36 and 64 DAT, respectively. For L-tmes, BIOS2_N0 revealed higher values at both time points.

Table 7.
Leaf thickness (L: μm), thickness between two epidermises (tmes: μm), fraction of intercellular air space (fias), palisade parenchyma ratio (p-ratio) spongy parenchyma ratio (s-ratio) and fraction of palaside cells (L-tmes) of processed tomatoes treated with different biostimulants and nitrogen doses recorded at 34 and 64 days after transplanting.
3.6. Stomatal Morphology
During the first growing season, stomatal density (SD) and stomatal size (SZ) were assessed on both the abaxial and adaxial surfaces of tomato leaves at 36 and 50 DAT (Figure 2 and Figure 3). On the abaxial side, no significant differences were observed for SD by B and N at both time points. N × B was found non-significant at 36 DAT; however, at 50 DAT, it was found to be significant (p < 0.01). The highest SD was recorded in plants grown without biostimulants under standard nitrogen application (STD), although this value was not significantly different from that of plants under BIOS2_RED. On the adaxial side, significant N × B (p < 0.01) values were observed at both time points. At 36 DAT, plants treated with BIOS1_N0 exhibited a marked increase in SD. Conversely, at 50 DAT, this same treatment resulted in the lowest SD among all combinations, indicating a temporal shift in response dynamics.
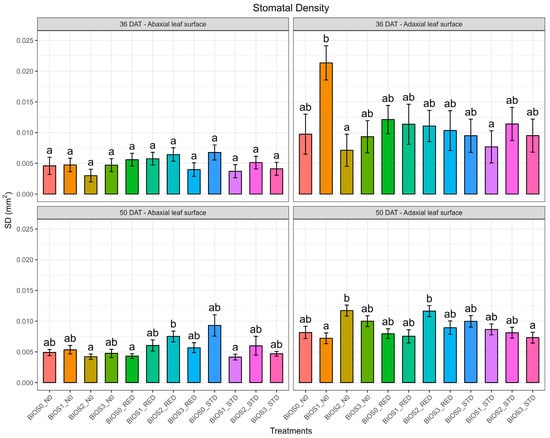
Figure 2.
Stomatal density (SD, mm2) of processed tomatoes treated with different biostimulants and nitrogen doses recorded at 36 and 50 days of transplanting (DAT). Values are means (n = 3) ± S.E. In each graph, different letters indicate significant differences among treatments (p < 0.05, Tukey’s test).
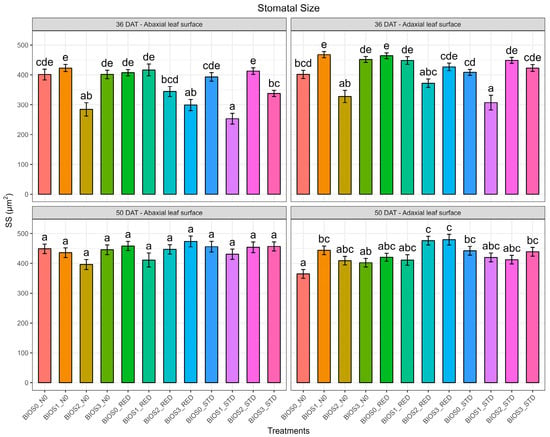
Figure 3.
Stomatal size (SZ, µm2) of processed tomatoes treated with different biostimulants and nitrogen doses recorded at 36 and 50 days of transplanting (DAT). Different letters indicate differences among treatments. Values are means (n = 3) ± S.E. In each graph, different letters indicate significant differences among treatments (p < 0.05, Tukey’s test).
Additionally, at 36 DAT, the SZ measured at the abaxial side of the tomato leaves showed a significant difference for both N and B and interaction factor N × B (p < 0.001) but became non-significant at 50 DAT (Figure 3). The SZ revealed a significant increase in the plants grown under BIOS1_N0 but was not different to BIOS1_RED, BIOS3_N0 and BIOS2_STD. Similarly, the SZ recorded on the adaxial side of leaves showed a significant difference for N × B at both time points (p < 0.001). In particular, plants grown under BIOS1_N0 consistently showed the largest SZ values at both 36 and 50 DAT.
4. Discussion
The above-ground biomass and nitrogen accumulation of processed tomatoes during the two-year study clearly depended upon weather seasons for its different effects on soil N availability and crop growth. In the first year of experimentation, the heavy rain that occurred soon after the transplanting probably hindered a good root setting and decreased base N fertility in soil while reducing crop growth and development. The observed stronger effects of biostimulants in 2023 compared to 2024 during the present investigation can be partially attributed to differences in climatic conditions between the two growing seasons, as described in Figure 1. Notably, higher precipitation, with even higher-than-average rainfall in this zone during 2023 compared to 2024, probably hindered root development, although root-related traits were not observed during this study, but it is evident in the above-ground biomass observation at the early growth stages (Supplementary File Figure S1) that this may have contributed to mild environmental stress, enhancing plant responsiveness to biostimulant treatments. This stress-induced sensitivity may have amplified physiological and morphological improvements under biostimulant application, as reported in other studies [29,30,31,32]. Although specific stress indices were not quantified in this study, incorporating such measures in future research could provide a clearer understanding of how environmental factors modulate tested biostimulant efficacy.
Furthermore, the present investigation consistently showed the significant effects of N application rates on crop growth. Moreover, the reduced N application (−30%) produced comparable results without compromising the wide range of morphophysiological, anatomical and yield aspects of processed tomatoes. Similar kinds of results were also observed for the other studies [16,17]. In particular, the plants grown with reduced N supply produced the highest biomass accumulation, leaf area index and total commercial yield, similar to the standard N doses applied. This standard dose (200 kg N/ha) was based on continuous previous experimentation under the same environmental conditions and similar cultivar. In the current study, we changed in the variety PS1296 from previous studies [7,33] to Heinz 5108, and while they have the same growth behavior and crop cycle, this change might affect the results, which need to be further validated with the actual optimization of the N dose for this specific variety. We have to consider not only the effect change according to plant varieties but also the moment in which there are applied and environmental conditions [34]. These outcomes signify the importance of reduced N input that does not necessarily compromise yield in comparison to the standard dose that happen likely due to improved nitrogen use efficiency (NUE) and reduced physiological stress associated with overfertilization. The results from different studies revealed that NUE can be enhanced by reducing N supply, which may delay leaf senescence, resulting in no loss in yield [35]. A study on cotton showed that a 20% reduction in N supply compared to a standard dose did not affect the yield [36]. The findings from another study on tomato demonstrated that excessive N inputs lead to reduced returns, lower NUE and potentially imbalanced nutrients. Therefore, it should be noted that adjusting N inputs is necessary for the crop to optimize its yield and resource allocation [37].
In the current investigation, biostimulants alone did not significantly affect tomato growth and N uptake, contrasting with the already available literature in terms of biostimulant contribution towards adjustments in nutrient supply [38], but the literature is often fragmented and unconvincing [39]. However, a positive effect was recorded when biostimulants were coupled with low N availability, even if this effect was probably masked by the high variability observed in our samples. Moreover, soil pH denotes mildly alkaline conditions, which can significantly influence nutrient bioavailability, particularly nitrogen forms, and thereby impact nutrient uptake efficiency. Alkaline soils often affect the solubility and mobility of essential nutrients, potentially altering biostimulant effectiveness, as evident in other studies [40,41]. These interactions between soil chemical properties and biostimulant activity merit consideration, as they may modulate nutrient assimilation and physiological responses in tomato plants under different fertilization regimes. As evident in our study, biostimulant efficiency is more prominent under stress conditions (the first growing season) compared to non-stressed ones (the second growing season), which we observed when examining the differences among the two growing seasons. These findings are in line with previous studies that have highlighted biostimulants’ potential to enhances plant performance by modulating hormonal activities, promoting root growth and development that helps in enhancing nutrient uptake and improving tolerance in abiotic stress conditions [42].
Moreover, biostimulants have the ability to maintain photosynthetic capacity and pigment concentration, especially evident in the current study where plants treated with protein hydrolysates outperformed the other treatments, further reinforcing biostimulants’ role in maintaining photosynthetic capacity and metabolic efficiency under sup-optimal N conditions [43]. The improved performance observed with BIOS2 and BIOS3 under reduced nitrogen conditions, also demonstrated by the increase in leaf thickness, may be attributed to distinct mechanisms. BIOS2, being a protein hydrolysate, likely enhances nutrient uptake and assimilation through hormone-like activity (e.g., auxin and cytokinin stimulation) and the upregulation of nitrogen transporters [44]. On the other hand, BIOS3, containing endophytic nitrogen-fixing bacteria, may contribute to increased nitrogen availability through biological N fixation and the modulation of the rhizosphere microbial community, thereby supporting plant growth under reduced nitrogen supply [45]. As observed by Zhang et al. [46] in Cassava (Manibot esculenta Crantz), the ability of some nitrogen-fixing endophytic bacteria increases with an increase in the content of nitrogen under a specific range of concentration; conversely, above this range, the nitrogenase activity of these bacteria and their positive effect decrease. This behavior could contribute to explaining why the effects of this kind of biostimulant is evident above all under reduced nitrogen supply. A study reported that the application of protein hydrolysates along with reduced N supply to the tomato improved its growth compared to non-treated ones through upregulating the gene expression of amino acid transporters and glutamine synthetase, resulting in higher N uptake that ultimately helped improve plant growth [47]. Based on the results of the current study, protein hydrolysates and N-fixing bacteria demonstrated enhanced crop growth that ultimately enhanced the yield-contributing traits and N uptake of processed tomatoes, in line with another study [17]. On the contrary, the biostimulant’s effect was not evident in improving the marketable yield and fruit quality parameters, in line with other studies [39,48]. However, from our findings, it is noted that the effect of seaweed extract remains unchanged for processed tomatoes.
Likewise, fruit quality in terms of total soluble solids was slightly changed by N application rates, but interestingly, they were not different in RED and STD. This effect might be due to moderate nitrogen limitations that may favor the accumulation of soluble carbohydrates, a key determinant of tomato processing quality [49]. Fruit quality was not influenced by the presence of biostimulants; these results disagree with what was observed by other authors [48,50]. This discrepancy could be explained by the use of different kinds of biostimulants and their sensitivity to environmental and soil conditions [40].
As expected, the photosynthetic pigments including chlorophyll a, chlorophyll b, total chlorophyll and carotenoid analysis confirmed the strong effect of nitrogen application rates due to N’s integral role in chlorophyll biosynthesis [51]. However, chlorophyll content further increased in the presence of BIOS2, although the differences were not statistically significant and were detected only in 2024. This last observation confirms that the tomato response is strictly season-dependent, as also observed by Patanè et al. [39]. A season-dependent effect was also observed in the N-NO3 concentrations in petiole sap [10]. At LAImax, N-NO3 concentrations in petiole sap were primarily influenced by nitrogen rates, with significantly higher values under the standard control, followed by the reduced and unfertilized control. This approach is evidently suitable in detecting the crop N status in relation to SPAD and NDVI. However, the sap test could not identify minor changes in the processed tomatoes’ N status related to biostimulant application, in line with the results of other studies [52,53].
Anatomical and stomatal morphology analysis revealed, instead, a biostimulant potential effect on leaf development under different N application rates. Mesophyll and leaf thickness were significantly affected due to the presence of biostimulants, especially under reduced N supply. The effects changed according to the biostimulant used and depending on N supply. At the standard N dose, the presence of biostimulants determined a decrease in leaf thickness, but at the reduced N dose, plants treated with BIOS3 showed thicker leaves than the control. The observed increase in leaf thickness may suggest an adaptive anatomical response aimed at enhancing physiological performance. Thicker leaves often indicate increased mesophyll tissue, which can improve internal CO2 diffusion and potentially support higher photosynthetic activity or water retention [54]. While direct correlations with photosynthetic efficiency or water use were not assessed in this study and are recommended for further studies, these anatomical adaptations may contribute to improved stress resilience, particularly under reduced nitrogen availability. Biostimulants can increase the concentration of certain compounds like tocopherols and fatty acids in plant tissues, potentially affecting leaf structure [55]. The observed stomatal traits, i.e., density and size, showed that notable changes occurred over time, highlighting the variability of treatment combinations and dynamic acclimation processes. For instance, biostimulant-treated plants showed higher stomatal dimensions that can enhance water use efficiency and stress tolerance [43]. Biostimulants like BIOS2 and BIOS3 may enhance nitrogen uptake and photosynthesis by modulating molecular key pathways, including the upregulation of nitrate transporters (NRT1 and NRT2), ammonium transporters (AMT1), and peptide transporters (PTR), as reported by [56]. Additionally, enzymes involved in nitrogen assimilation such as nitrate reductase (NR) and glutamine synthetase (GS), along with key chlorophyll biosynthesis enzymes like glutamyl-tRNA reductase (GluTR), may be upregulated [57]. These molecular changes could also contribute to structural adaptations such as increased leaf thickness, thereby potentially improving photosynthetic efficiency under reduced nitrogen conditions.
5. Conclusions
The present study findings highlight the potential of integrating biostimulants into reduced nitrogen management systems to maintain processed tomato performance. All studied parameters responded well to the N application rates imposed during the experiment. While the main effects of biostimulants were not consistently significant across all parameters, their contribution under low/no-nitrogen inputs was evident, particularly in terms of physiological efficiency, pigment concentration and anatomical adaptation. This multifactorial study underscores the feasibility of reducing nitrogen inputs in tomato production without compromising yield or quality, especially when biostimulants are employed together. The investigation showed that plants grown with biostimulants at low/no nitrogen availability outperformed to the ones grown without them in terms of biomass accumulation, the LAI and total yield. Based on the outcomes of biostimulant effects, they were strictly season-dependent because they were more evident in the first year compared to the second one, as interannual variations were observed in the current experiment, likely due to the occurrence of stress conditions. Among the biostimulants tested, protein hydrolysates and endophytic N-fixing bacteria performed better than the seaweed extract in processed tomato. Future research should aim to clarify the mechanisms by which different biostimulants influence plant hormonal pathways and microbial interactions under low-input conditions. Long-term field trials across diverse climates, soil types and tomato varieties are essential to assess the scalability and economic viability of this approach. Additionally, further investigation is needed to evaluate greater nitrogen reductions, particularly beyond the 30% reduction tested in the current study, to determine the optimal rate for the variety used.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11080931/s1, Figure S1: Total plant dry weight (Mg ha−1) of tomato treated with different biostimulants and nitrogen doses recorded at different time-points during the experiment in both growing seasons i.e., 2023: on left and 2024: on right. Black squares: no-nitrogen without biostimulant; white squares: no-nitrogen with biostimulants. Black circles: 30% reduced nitrogen dose without biostimulants; white circles: 30 reduced nitrogen dose with biostimulants. Black triangles: standard nitrogen dose without biostimulants; white triangles: standard nitrogen dose with biostimulants. Solid lines—no biostimulant, dash lines—biostimulants. Figure S2. Leaf area index (LAI) of tomato treated with different biostimulants and nitrogen doses recorded at different time-points during the experiment in both growing seasons i.e., 2023: on left and 2024: on right. Black squares: no-nitrogen without biostimulant; white squares: no-nitrogen with biostimulants. Black circles: 30% reduced nitrogen dose without biostimulants; white circles: 30 reduced nitrogen dose with biostimulants. Black triangles: standard nitrogen dose without biostimulants; white triangles: standard nitrogen dose with biostimulants. Solid lines—no biostimulant, dash lines—biostimulants. Figure S3. Nitrate-nitrogen concentration in petiole sap (N-NO3 SAP (mg L-1)) of tomato treated with different biostimulants and nitrogen doses recorded at different time-points during the experiment in both growing seasons i.e., 2023: on left and 2024: on right. Black squares: no-nitrogen without biostimulant; white squares: no-nitrogen with biostimulants. Black circles: 30% reduced nitrogen dose without biostimulants; white circles: 30 reduced nitrogen dose with biostimulants. Black triangles: standard nitrogen dose without biostimulants; white triangles: standard nitrogen dose with biostimulants. Solid lines—no biostimulant, dash lines—biostimulants. Figure S4. SPAD index of tomato plants treated with different biostimulants and nitrogen doses recorded at different time-points during the experiment in both growing seasons i.e., 2023: on left and 2024: on right. Black squares: no-nitrogen without biostimulant; white squares: no-nitrogen with biostimulants. Black circles: 30% reduced nitrogen dose without biostimulants; white circles: 30 reduced nitrogen dose with biostimulants. Black triangles: standard nitrogen dose without biostimulants; white triangles: standard nitrogen dose with biostimulants. Solid lines—no biostimulant, dash lines—biostimulants. Figure S5. NDVI vegetation index of tomato plants treated by different biostimulants and nitrogen doses recorded at different time-points during the experiment in both growing seasons i.e., 2023: on left and 2024: on right. Black squares: no-nitrogen without biostimulant; white squares: no-nitrogen with biostimulants. Black circles: 30% reduced nitrogen dose without biostimulants; white circles: 30 reduced nitrogen dose with biostimulants. Black triangles: standard nitrogen dose without biostimulants; white triangles: standard nitrogen dose with biostimulants. Solid lines—no biostimulant, dash lines—biostimulants.
Author Contributions
Conceptualization, M.F., L.R., E.P., M.Z.A. and F.T.; methodology, M.F., L.R., E.P. and F.T.; formal analysis, M.F., M.Z.A., L.R., S.C., E.C. and M.P.; investigation, M.F., M.Z.A., L.R., E.C., M.P., F.C. and B.F.; resources, M.F.; data curation, M.F., L.R., M.Z.A., S.C. and F.C.; writing—original draft preparation, M.F., L.R. and M.Z.A.; writing—review and editing, M.F., L.R., M.Z.A., F.C. and B.F.; supervision, M.F. and L.R.; project administration, M.F. and E.P.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out within the framework of the project “Innovative farm strategies that integrate sustainable N fertilization, water management and pest control to reduce water and soil pollution and salinization in the Mediterranean, Safe-H2O-Farm”. The Safe-H2O-Farm is part of the PRIMA Program supported by the European Union with co-funding from the Italian Ministry of University and Research (MUR).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We gratefully acknowledge all workers of the FieldLab of the Department of Agricultural, Food and Environmental Sciences, the University of Perugia, Perugia, Italy, for managing the field experiments. We would also like to say thanks to Fomet Spa and Corteva Agriscience srl, for providing protein hydrolysates and endophytic N-fixing bacteria, respectively, for the current experimentations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schneider, S.H.; Semenov, S.; Patwardhan, A.; Burton, I.; Magadza, C.H.D.; Oppenheimer, M.; Pittock, A.B.; Rahman, A.; Smith, J.B.; Suarez, A.; et al. Assessing key vulnerabilities and the risk from climate change. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., Linden, P.J.v.d., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 779–810. [Google Scholar]
- McCarthy, J.J.; Canziani, O.F.; Leary, N.A.; Dokken, D.J.; White, K.S. Climate Change 2001: Impacts, Adaptation and Vulnerability; University of Cambridge: Cambridge, UK, 2001. [Google Scholar]
- Albou, E.M.; Abdellaoui, M.; Abdaoui, A.; Boughrous, A.A. Agricultural practices and their impact on aquatic ecosystems–a mini-review. Ecol. Eng. Environ. Tech. 2024, 25, 321–331. [Google Scholar] [CrossRef]
- Valenzuela, H. Optimizing the nitrogen use efficiency in vegetable crops. Nitrogen 2024, 5, 106–143. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAOSTAT) Statistical Database. 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 2 July 2025).
- WPTC. World Processing Tomato Council. 2024. Available online: https://www.wptc.to (accessed on 2 July 2025).
- Farneselli, M.; Benincasa, P.; Tosti, G.; Simonne, E.; Guiducci, M.; Tei, F. High fertigation frequency improves nitrogen uptake and crop performance in processing tomato grown with high nitrogen and water supply. Agric. Water Manag. 2015, 154, 52–58. [Google Scholar] [CrossRef]
- Farneselli, M.; Tosti, G.; Onofri, A.; Benincasa, P.; Guiducci, M.; Pannacci, E.; Tei, F. Effects of N sources and management strategies on crop growth, yield and potential N leaching in processing tomato. Eur. J. Agron. 2018, 98, 46–54. [Google Scholar] [CrossRef]
- Farneselli, M.; Benincasa, P.; Tosti, G.; Guiducci, M.; Tei, F. Combining green manuring and fertigation maximizes tomato crop yield and minimizes nitrogen losses. Agronomy 2020, 10, 977. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, H.; Fan, J.; Xiang, Y.; Tang, Z.; Pei, S.; Zeng, H.; Zhang, C.; Dai, Y.; Li, Z. Effects of nitrogen supply on tomato yield, water use efficiency and fruit quality: A global meta-analysis. Sci. Hortic. 2021, 290, 110553. [Google Scholar] [CrossRef]
- Simonne, A.; Fuzere, J.; Simonne, E.; Hochmuth, R.; Marshall, M. Effects of nitrogen rates on chemical composition of yellow grape tomato grown in a subtropical climate. J. Plant Nutr. 2007, 30, 927–935. [Google Scholar] [CrossRef]
- Kaniszewski, S.; Kosson, R.; Grzegorzewska, M.; Kowalski, A.; Badełek, E.; Szwejda-Grzybowska, J.; Tuccio, L.; Agati, G. Yield and quality traits of field grown tomato as affected by cultivar and nitrogen application rate. J. Agric. Sci. Tech. 2019, 21, 683–697. Available online: https://jast.modares.ac.ir/article-23-14381-en.html (accessed on 2 July 2025).
- Truffault, V.; Ristorto, M.; Brajeul, E.; Vercambre, G.; Gautier, H. To stop nitrogen overdose in soilless tomato crop: A way to promote fruit quality without affecting fruit yield. Agronomy 2019, 9, 80. [Google Scholar] [CrossRef]
- Paradiso, R.; Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Pelosi, M.E.; Rippa, M.; Mormile, P.; Mori, M. Integrating smart greenhouse cover, reduced nitrogen dose and biostimulant application as a strategy for sustainable cultivation of cherry Tomato. Plants 2024, 13, 440. [Google Scholar] [CrossRef]
- Ganugi, P.; Fiorini, A.; Tabaglio, V.; Capra, F.; Zengin, G.; Bonini, P.; Caffi, T.; Puglisi, E.; Trevisan, M.; Lucini, L. The functional profile and antioxidant capacity of tomato fruits are modulated by the interaction between microbial biostimulants, soil properties, and soil nitrogen status. Antioxidants 2023, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Mauro, R.P.; Distefano, M.; Steingass, C.B.; May, B.; Giuffrida, F.; Schweiggert, R.; Leonardi, C. Boosting cherry tomato yield, quality, and mineral profile through the application of a plant-derived biostimulant. Sci. Hortic. 2024, 337, 113597. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar application of plant-based biostimulants improve yield and upgrade qualitative characteristics of processing tomato. Ital. J. Agron. 2021, 16, 1825. [Google Scholar] [CrossRef]
- Matthews, S.; Siddiqui, Y.; Ali, A. Unleashing the power of bio-stimulants for enhanced crop growth, productivity, and quality: A comprehensive review. J. Plant Nutr. 2025, 48, 703–725. [Google Scholar] [CrossRef]
- Malécange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant properties of protein hydrolysates: Recent advances and future challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef]
- Mughunth, R.; Velmurugan, S.; Mohanalakshmi, M.; Vanitha, K. A review of seaweed extract’s potential as a biostimulant to enhance growth and mitigate stress in horticulture crops. Sci. Hortic. 2024, 334, 113312. [Google Scholar] [CrossRef]
- Lau, S.-E.; Lim, L.W.T.; Hamdan, M.F.; Chan, C.; Saidi, N.B.; Ong-Abdullah, J.; Tan, B.C. Enhancing Plant Resilience to Abiotic Stress: The Power of Biostimulants. Phyton-Int. J. Exp. Bot. 2025, 94, 1–31. [Google Scholar] [CrossRef]
- Asif, A.; Ali, M.; Qadir, M.; Karthikeyan, R.; Singh, Z.; Khangura, R.; Di Gioia, F.; Ahmed, Z.F. Enhancing crop resilience by harnessing the synergistic effects of biostimulants against abiotic stress. Front. Plant Sci. 2023, 14, 1276117. [Google Scholar] [CrossRef]
- Dumas, J. Procédés de l’analyse organique. Ann. Chim. Phys 1831, 47, 198–205. Available online: https://cir.nii.ac.jp/crid/1370865816771910273 (accessed on 2 July 2025).
- Zhao, W.L.; Chen, Y.J.; Brodribb, T.J.; Cao, K.F. Weak co-ordination between vein and stomatal densities in 105 angiosperm tree species along altitudinal gradients in Southwest China. Funct. Plant Biol. 2016, 43, 1126–1133. [Google Scholar] [CrossRef]
- Cerri, M.; Reale, L.; Roscini, F.; Fornaciari da Passano, M.; Orlandi, F. Fibers development in a dioecious hemp cultivar: The role of plant sex and cultivation conditions. Plant Biosyst. 2023, 157, 140–146. [Google Scholar] [CrossRef]
- Tosens, T.; Niinemets, U.; Vislap, V.; Eichelmann, H.; Castro Diez, P. Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: How structure constrains function. Plant Cell Environ. 2012, 35, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Team, R.C.; R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2013. Available online: https://cir.nii.ac.jp/crid/1370298755636824325 (accessed on 2 July 2025).
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of biostimulants in tomato plants (Solanum lycopersicum) to enhance plant growth and salt stress tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef]
- Akram, M.Z.; Libutti, A.; Rivelli, A.R. Evaluation of vegetative development of quinoa under water stress by applying different organic amendments. Agronomy 2023, 13, 1412. [Google Scholar] [CrossRef]
- Tei, F.; Benincasa, P.; Guiducci, M. Critical nitrogen concentration in processing tomato. Eur. J. Agron. 2002, 18, 45–55. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O.; Santoyo, G.; Perazzolli, M. The potential role of microbial biostimulants in the amelioration of climate change-associated abiotic stresses on crops. Front. Microbiol. 2022, 12, 829099. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 1–20. [Google Scholar] [CrossRef]
- Rochester, I.; Ceeney, S.; Maas, S.; Gordon, R.; Hanna, L.; Hill, J. Monitoring nitrogen use efficiency in cotton crops. Aust. Cottongrow. 2009, 30, 42–43. Available online: https://search.informit.org/doi/10.3316/informit.950796977920799 (accessed on 2 July 2025).
- Wang, X.; Jia, J.; Lu, C.; Chen, H.; Chen, X.; Peng, X.; Chi, G.; Song, Q.; Hu, Y.; Ma, J. Optimizing nitrogen for sustainable yield and efficiency: Insights from shouguang facility-grown tomatoes. Agronomy 2025, 15, 420. [Google Scholar] [CrossRef]
- Redoy, M.H.; Al Mamun, M.; Cooley, A.L.; Darby, E.; Islam, T. Enhancing nitrogen uptake efficiency and tomato plant growth in soilless substrates using fulvic acids and mycorrhizal biostimulants. Sci. Hortic. 2025, 348, 114212. [Google Scholar] [CrossRef]
- Patanè, C.; Pellegrino, A.; Saita, A.; Calcagno, S.; Cosentino, S.L.; Scandurra, A.; Cafaro, V. A study on the effect of biostimulant application on yield and quality of tomato under long-lasting water stress conditions. Heliyon 2025, 11, e41187. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Della, L.M.C. Characterization of Plant Physiological and Molecular Responses to Biostimulant Applications. Ph.D. Thesis, University of Padova, Padova, Italy, 2023. Available online: https://hdl.handle.net/11577/3478583 (accessed on 2 July 2025).
- Della, L.M.C.; Baghdadi, A.; Mangione, F.; Borella, M.; Zegada-Lizarazu, W.; Ravi, S.; Deb, S.; Broccanello, C.; Concheri, G.; Monti, A. Transcriptional and physiological analyses to assess the effects of a novel biostimulant in tomato. Front. Plant Sci. 2022, 12, 781993. [Google Scholar] [CrossRef]
- Monterisi, S.; Garcia-Perez, P.; Buffagni, V.; Zuluaga, M.Y.A.; Ciriello, M.; Formisano, L.; El-Nakhel, C.; Cardarelli, M.; Colla, G.; Rouphael, Y. Unravelling the biostimulant activity of a protein hydrolysate in lettuce plants under optimal and low N availability: A multi-omics approach. Physiol. Plant. 2024, 176, e14357. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Rhizosphere microbiome modulators: Contributions of nitrogen fixing bacteria towards sustainable agriculture. Int. J. Environ. Res. Public Health 2018, 15, 574. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, J.; Dong, M.; Akhtar, K.; He, B. Isolation, identification and characterization of nitrogen fixing endophytic bacteria and their effects on cassava production. PeerJ 2022, 10, e12677. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Fan, M.; Qin, Y.; Jiang, X.; Cui, N.; Wang, Y.; Zhang, Y.; Zhao, L.; Jiang, S. Proper deficit nitrogen application and irrigation of tomato can obtain a higher fruit quality and improve cultivation profit. Agronomy 2022, 12, 2578. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Peralta-Sánchez, M.G.; Gómez-Merino, F.C.; Tejeda-Sartorius, O.; Trejo-Téllez, L.I. Nitrogen nutrition differentially affects concentrations of photosynthetic pigments and antioxidant compounds in Mexican Marigold (Tagetes erecta L.). Agriculture 2023, 13, 517. [Google Scholar] [CrossRef]
- Farneselli, M.; Benincasa, P.; Tei, F. Validation of N nutritional status tools for processing tomato. In Proceedings of the IV International Symposium on Ecologically Sound Fertilization Strategies for Field Vegetable Production 852, Alnarp, Sweden, 22–29 September 2008; pp. 227–232. [Google Scholar] [CrossRef]
- Padilla, F.M.; Farneselli, M.; Gianquinto, G.; Tei, F.; Thompson, R.B. Monitoring nitrogen status of vegetable crops and soils for optimal nitrogen management. Agric. Water Manag. 2020, 241, 106356. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Díaz-Espejo, A.; Flexas, J.; Galmes, J.; Warren, C.R. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J. Exp. Bot. 2009, 60, 2249–2270. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Nieves-Silva, E.; Sandoval-Castro, E.; Delgado-Alvarado, A.; Castañeda-Antonio, M.D.; Huerta-De la Peña, A. Nitrate reductase and Glutamine synthetase enzyme activities and chlorophyll in sorghum leaves (Sorghum bicolor) in response to organic fertilization. Int. J. Plant Biol. 2024, 15, 827–836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).