Abstract
Plastic film mulching is widely used in protected agriculture. However, the residues of various types of plastic films, as a consequence, severely affect soil quality. The most widely promoted alternative strategy is the use of biodegradable plastic films. Nevertheless, the research on the effects of different types of plastic films on soil properties remains insufficient. This study explored the impacts of different plastic film mulching on the physicochemical properties and microbial communities of soils for pepper cultivation, with three treatments: traditional polyethylene film (PE-Ctr), PBAT biodegradable film (PBAT bio), and reinforced polyethylene film (RPE). The results showed that the soil pH value was the highest in PE-Ctr treatment, and the soil organic matter content was higher in the biodegradable film treatment, while the electrical conductivity (EC), nitrate, and some cations (Ca2+, Mg2+) were higher in the RPE treatment. The contents of available trace element Zn, Fe, and Mn increased in the PBAT bio treatment. The bacterial richness and evenness indices were higher in PBAT bio treatment than those of other treatments. The fungal community had a relatively high richness, but a lower evenness, compared to the PE-Ctr and PBAT bio treatments. The use of different plastic films significantly affected the composition of soil bacteria, while differences in the composition of soil fungi were only observed between the PBAT bio and RPE treatments. Proteobacteria, Acidobacteriota, and Actinobacteriota were the most dominant bacterial phyla, and Ascomycota and Mortierellomycota were the dominant fungal phylum across all treatments. FAPROTAX functional prediction showed that the abundances of multiple functions of soil bacteria were higher in the RPE treatment, and the chemoheterotrophy function was higher in the PE treatment. FUNGuild analysis indicated that the trophic types and ecological function groups of soil fungi were more abundant in the PBAT bio treatment.
1. Introduction
Plastic film mulching is an economic agricultural practice that has been widely used in facility agriculture. Currently, China ranks first in the world in both the production and use of plastic agricultural films [1]. Plastic film mulching plays an important role in improving the soil environment and increasing crop yields [2,3]. Most of the agricultural plastic films in use are made mainly of polyethylene (PE), which is highly stable, but with a long half-life. Affected by factors such as sunlight, weathering, and tillage, the plastic films’ residues will continuously degrade into smaller fragments and exist in the soil in the form of microplastics, which will affect the normal growth of crops. The environmental problems and ecological risks brought about by plastic film residues are becoming more and more prominent [4,5,6].
Among the alternative strategies, the most widely promoted one is to use fully biodegradable films instead of traditional common PE films. Most biodegradable plastic films are made of degradable materials, such as polybutylene adipate terephthalate (PBAT), poly butylene adipate-co-succinate (PBSA), etc. These films can be decomposed into water and CO2 by microorganisms under natural conditions [7,8]. However, fully biodegradable plastic films have strong degradability; some studies have shown that the degradation components contain microplastics, which might become a new threat [2,9]. Moreover, the mechanical properties of fully biodegradable plastic films are relatively poor, which is not conducive to preventing the growth of weeds [10].
The use of different types of plastic films has a significant impact on soil properties. As one of the main functions, the increase of soil temperature might increase the decomposition rate of organic matter, and lead to lower soil organic matter content than that of the soil covered with biodegradable films [11]. Due to differences in the composition of the plastic films, soil properties, and crops, the experimental results may also vary.
The soil microbial community is crucial for maintaining the health of the soil system and the nutrient absorption of crops. On the one hand, plastic film mulching directly affects the micro-environmental climate of the soil surface. On the other hand, it will lead to an increase in the amount of debris on the soil surface. The synthetic agents, additives [12], and microplastics contained in the plastic film can provide some nutrients for microorganisms, thus enriching specific functional microorganisms, such as plastic-degrading microbes and pathogens [13]. On the other hand, research shows that the use of PE films can reduce the activity and richness of the soil microbial community [14,15]. It was also found that biodegradable films can reduce microbial diversity to a certain extent but inhibit the abundance of antibiotic-resistant genes as compared with PE film [16,17]. This indicates that the impact of plastic film mulching on the soil microbial community varied due to many factors, and further research is still needed to elucidate this [12].
According to the statistics of the FAO, China’s pepper cultivating area reached 759,820 hectares, with a total pepper yield of 16.8374 million tons in 2022. The use of plastic films has greatly increased the pepper yield. However, there is a lack of research on the impact of plastic film use on the soils. This experiment compared the differences in soil physicochemical and microbiological properties under the coverage of traditional plastic films, reinforced PE films, and fully biodegradable plastic films. The objective was to compare the influence of different types of plastic films on soil physiochemical parameters and bacterial and fungal community structure characteristics. The findings are expected to provide theoretical basis and practical experience for the selection and promotion of plastic films in production practice.
2. Materials and Methods
2.1. Experimental Design
The experimental area is located in the Modern Agriculture Industrial Park, Wuqiao Town, Jiangdu District, Yangzhou City, Jiangsu Province (32°28′ N, 119°43′ E), with a subtropical humid monsoon climate. The experiment was carried out from September 2023 to January 2024. Three treatments were set up: covering with traditional polyethylene film (PE-Ctr), PBAT fully biodegradable film (PBAT bio), and polyethylene reinforced film (RPE), with three replicates for each treatment prepared. The thicknesses of the plastic films were as follows: PE-Ctr (0.01 mm), PBAT bio (0.01 mm), and RPE (0.02 mm). All plastic films were black in color.
For all treatments, the interval rows were formed by piling up soil at the edge of the experimental area and each treatment. All plastic films were fixed by soil. The plot was 40 m long and 0.8 m wide. The tested pepper variety was “Sujiao No.5”. The peppers were uniformly nursed and then transplanted into the greenhouse. The plastic film was laid first, and then holes were dug. The plant spacing of the peppers was set at 0.2 m, and 1 plant was planted in each hole.
2.2. Sample Collection and Analysis
2.2.1. Soil Physicochemical Determination
During the fruiting period of the peppers (70 days), soil samples at a depth of 0–20 cm were collected near the roots of the peppers using a soil drill, in November 2023. For three treatments (each with three replicates) a total of 9 soil samples were taken. A portion of the soil samples was immediately frozen for the determination of microbial diversity, and the other part was air-dried, ground, and sieved for the determination of physicochemical properties. The determined items included pH (measured using Leici PHS-SE pH meter (Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China), soil/water ratio at 1:25 m/v), organic matter (by the K2Cr2O7 oxidation–external heating method), nitrate nitrogen (spectrophotometrically), ammonium nitrogen (Indophenol blue method), available phosphorus (Mo−Sb colorimetric assay), available potassium (extracted by CH3COONH4 solution and determined by flame photometry), water-soluble calcium and water-soluble magnesium (extracted with H2O and determined by Atomic absorption spectrophotometry), available iron (AFe), available manganese (AMn), available copper (ACu), available zinc (Azn), available boron (AB), and available molybdenum (AMo). For the determination of AFe, AMn, Acu, AZn the soils were extracted by DTPA, for AB and AMo, by MgSO4 and H2C2O4-(NH4)2C2O4, respectively, followed by determination using ICP-OES. The detailed procedures of the analytical methods for each index were described in the following textbook [18].
2.2.2. Determination of Soil Microbial Composition
The DNA kit used was the MoBio PowerSoil DNA Isolation DNA kit (QIAGEN Inc., Valencia, CA, USA). Soil DNA samples were extracted according to the instructions.
The V3–V4 region of the bacterial 16S rRNA gene was amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR reaction system included: 10 ng of soil DNA, 0.25 μL of bovine serum albumin, 10 μL of ultrapure water, 1 μL of each of the two primers, 5 μL of 5 × FastPfu buffer, 2 μL of 2.5 mM dNTPs, and 0.5 μL of FastPfu polymerase. The amplification steps were as follows: denaturation at 94 °C for 3 min, 30 amplification cycles (denaturation at 94 °C for 45 s, annealing at 50 °C for 45 s, extension at 72 °C for 45 s), and a final extension at 72 °C for 10 min.
For fungi, the primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS1R (5′-GCTGCGTTCTTCATCGATGC-3′) were used. The amplification steps were as follows: denaturation at 98 °C for 1 min, 30 amplification cycles (denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, extension at 72 °C for 60 s), and a final extension at 72 °C for 5 min.
Each sample had 3 replicates. The PCR products of the same sample were mixed and detected by 2% agarose gel electrophoresis. The AxyPrep DNA Gel Extraction Kit (AXYGEN company, Union City, CA, USA) was used to cut the gel and recover and purify the PCR products. According to the standard procedures provided by the company, the PCR products were purified and quantified, and then sequenced on the Illumina MiSeq platform (Shanghai Majorbio Biomedical Technology Co., Ltd., Shanghai, China).
2.2.3. Data Analysis
Microsoft Excel 2016 and Origin 2021 were used for data organization and drawing analysis. IBM SPSS Statistics 19 software was used for significance testing, and the LSD test method was used to compare the differences between treatments. The significance level was set at p < 0.05.
The analysis of high-throughput microbial data was carried out on the Majorbio Scientific Research Service Platform (https://www.majorbio.com/, accessed on 15 February 2025). The species classification databases were silva138/16s_bacteria and unite8.0/its_fungi. The clustering method was the USEARCH11-uparse algorithm. Based on the original data of the bacteria and fungi, the Alpha and Beta diversities, community composition, non-metric multidimensional scaling analysis (NMDS), redundancy analysis (RDA), correlation analysis heat map, and FAPROTAX (version 1.2.4) and FUNGuild (version 1.1) prediction for bacterial and fungal functions of each treatment were analyzed.
3. Results and Analysis
3.1. Effects on Soil Physicochemical Properties
Table 1 shows the changes in soil pH values over time under different plastic film mulching treatments. The soils of all treatments were slightly alkaline, with the highest value in the common film treatment. The pH value of PE-Ctr was significantly higher than that of PBAT bio and RPE (p < 0.05). The content of soil surface organic matter ranged from 15.40 to 16.21 g/kg, with the highest value in the biodegradable film treatment. The RPE treatment had high salinity (EC), nitrate, and cations (Ca, Mg), while the PBAT bio treatment had elevated Zn, Fe, and Mn; soil-available P and K might require supplementation. The PE-Ctr treatment had a higher available P and K with moderate salinity but lower nitrate and Zn contents.

Table 1.
Changes in soil nutrient contents under different plastic film mulching treatments.
3.2. Characteristics of Soil Microbial Communities Under Different Plastic Film Mulching
3.2.1. Alpha Diversity of Soil Microorganisms
Table 2 shows the calculation results of the Alpha diversity indices of soil microorganisms for each treatment. The Ace, Sobs, and Chao indices represent community richness, while the Shannon and Simpson indices represent community evenness. Overall, the richness and evenness indices of the biodegradable film (PBAT bio) treatment were higher than those of the other two treatments. The Chao index of the soil in the PBAT bio treatment was significantly higher than that of the RPE treatment, and the Shannon index of the soil in the PBAT bio treatment was also significantly higher than that of the other two treatments.

Table 2.
Bacterial and fungal diversity of soils under different plastic film mulching treatments.
The soil fungal richness in the PBAT bio treatment was higher than that of the other two treatments, even though it was not statistically significant, while the evenness was lower than that of the PE-Ctr and the RPE treatment, specifically manifested as a higher Shannon index and a lower Simpson index.
3.2.2. Beta Diversity of Soil Microorganisms
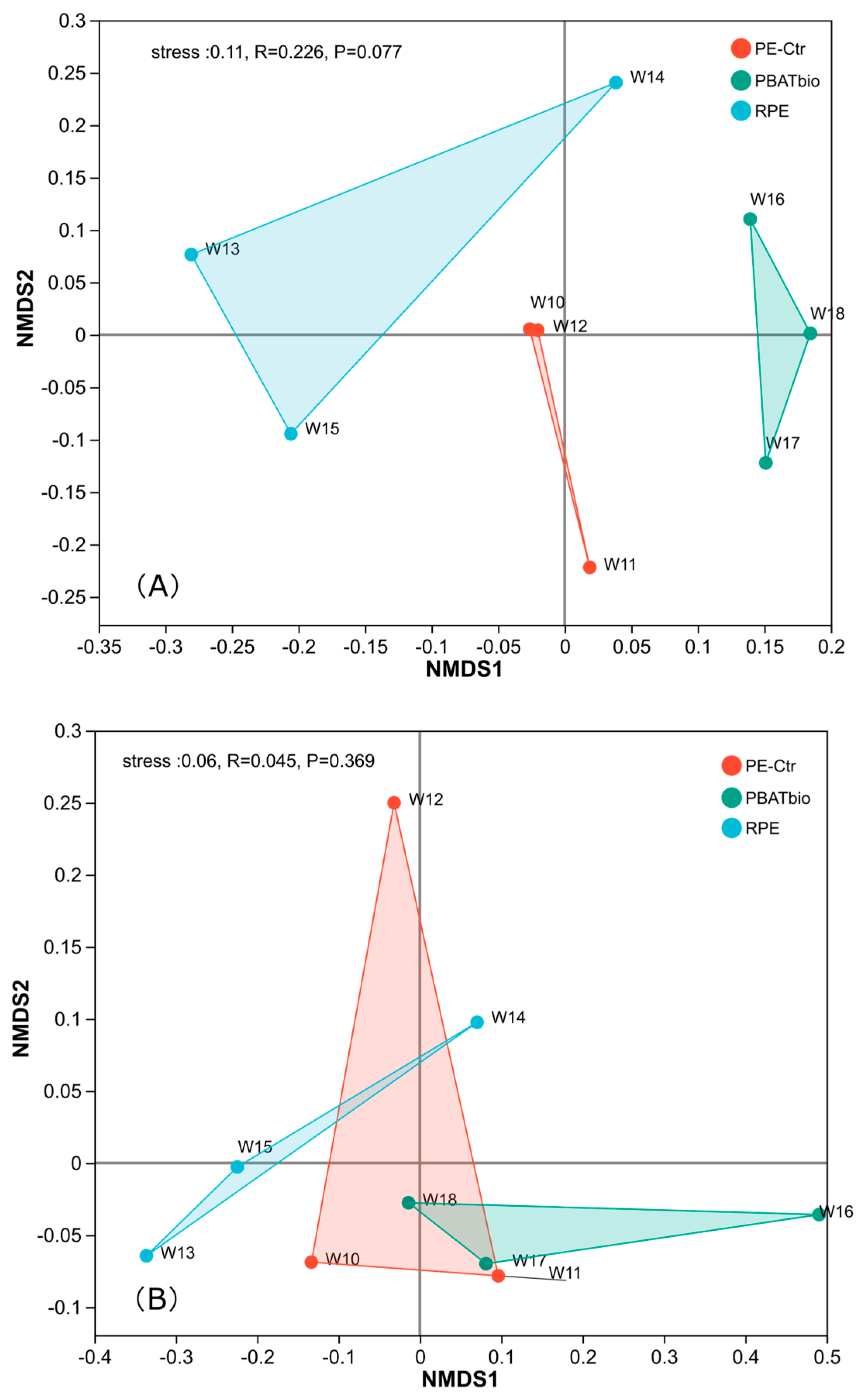
The NMDS plot was used to more intuitively represent the changes in the composition of soil bacterial communities after treatment. Figure 1 shows the non-metric multidimensional scaling analysis (NMDS) of the three plastic film mulching treatments, which can more intuitively display the changes in the composition of soil microbial communities under the three treatments. It can be seen that there are obvious differences in the composition of bacterial communities among the three treatments (Figure 1A), indicating that the use of different plastic films has a significant impact on the composition of soil bacteria. For soil fungi (Figure 1B), there are significant differences in the composition of soil fungi between the degradable plastic film (PBAT bio) and the RPE treatment, while the fungal community composition in PE-Ctr was not distinctively separated from PBAT bio or RPE.
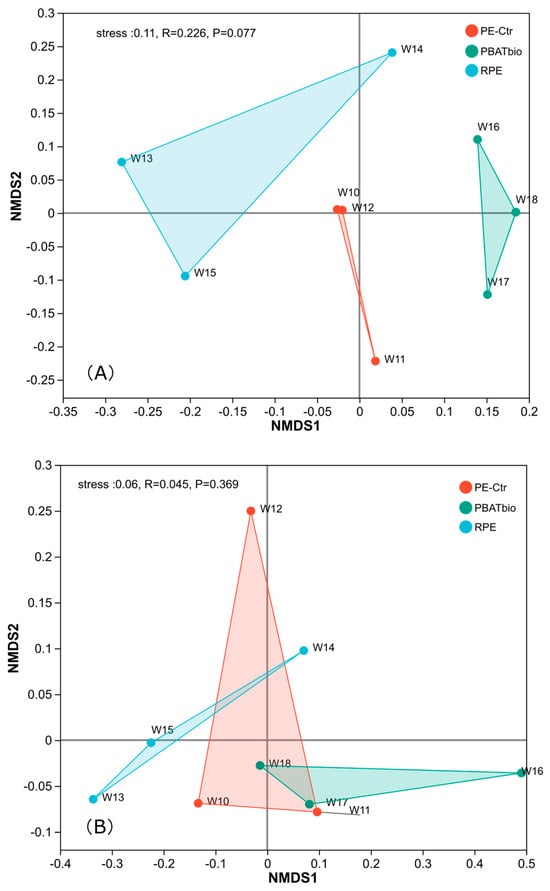
Figure 1.
MDS plot of soil microbial community under different plastic film mulching treatments (A) bacteria; (B) Fungi.
3.2.3. Composition of Soil Microbial Communities
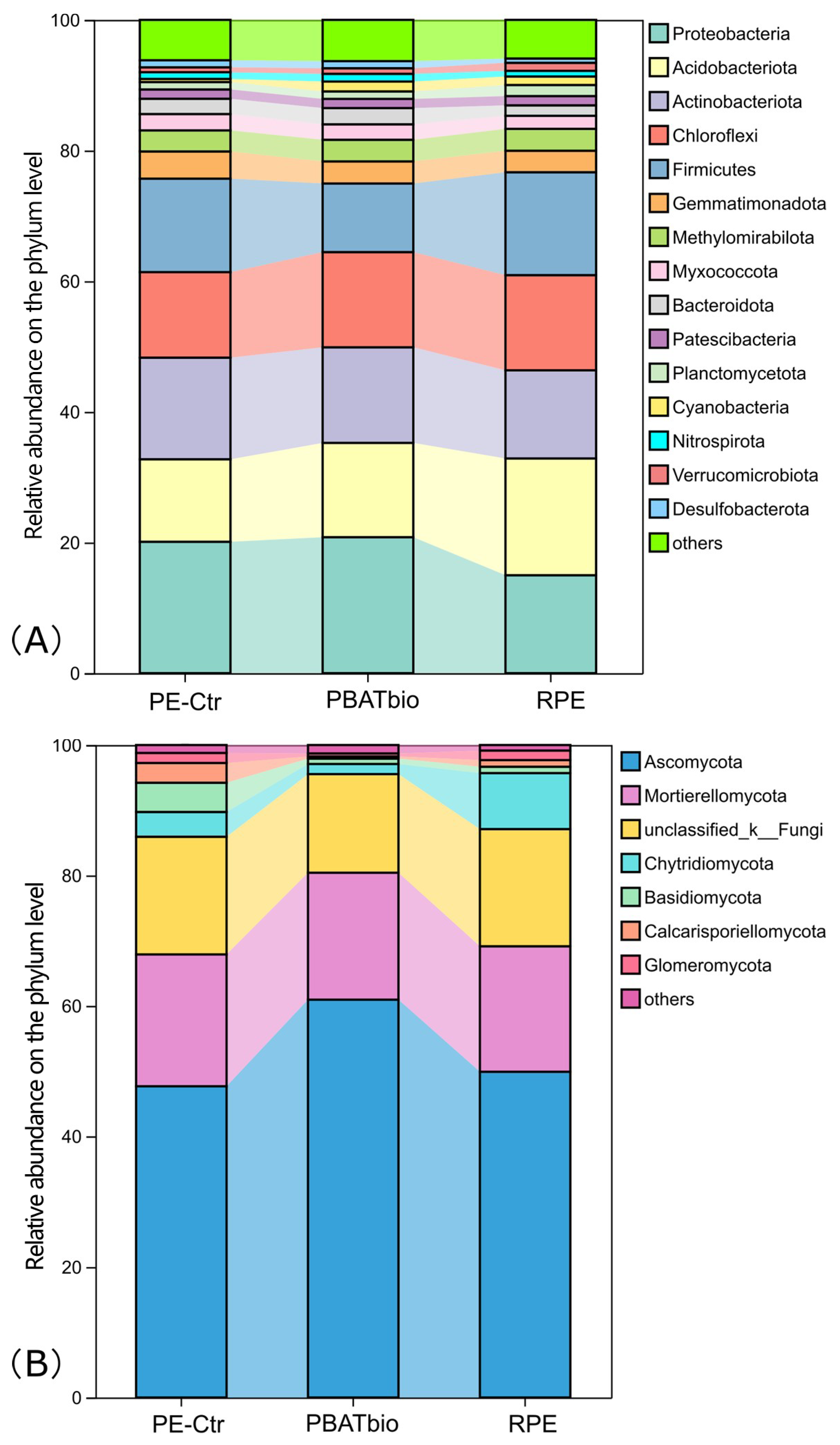
The relative abundances of bacterial taxa at the phylum level (>1%) are shown in Figure 2A. There are 15 bacterial phyla with a relative abundance higher than 1%. The dominant phyla were Proteobacteria (15.0–20.8%), Acidobacteriota (12.6–17.9%), Actinobacteriota (13.5–15.6%), Chloroflexi (13.1–14.6%), and Firmicutes (10.5–15.8%), in sequence. Among them, the relative abundance of Proteobacteria in the PBAT bio treatment was the highest, reaching 20.8%, which is significantly higher than that in the reinforced film treatment (15.0%). The relative abundance of Acidobacteriota in the RPE treatment was higher than that in the other two treatments. The Chloroflexi in the soil under the traditional plastic film mulching (PE-Ctr) had the highest relative abundance, but the difference was not significant.
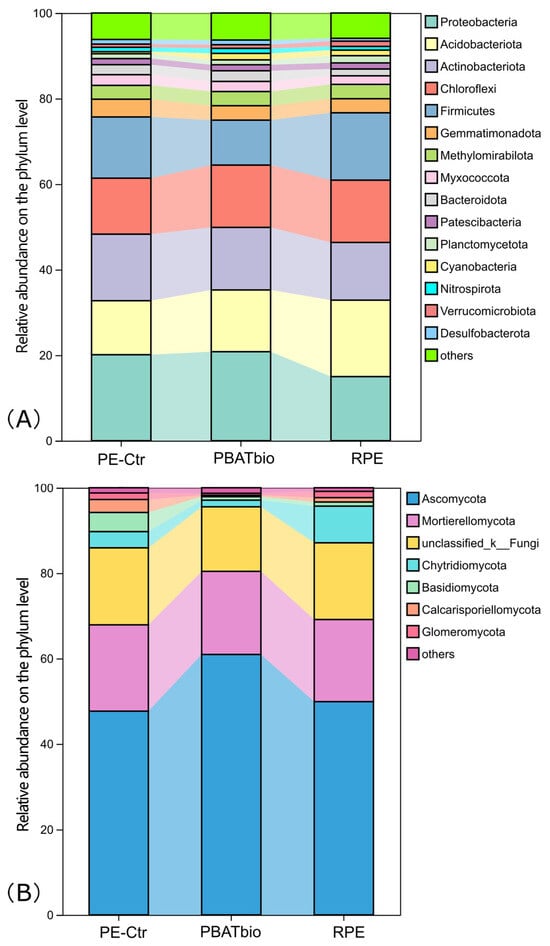
Figure 2.
Relative abundances of bacterial (A) and fungal (B) taxa at the phylum level under different plastic film mulching treatments (>1%).
The analysis results based on the fungal ITS sequence are shown in Figure 2B. It can be seen from the figure that Ascomycota was the most dominant fungal phylum in the soils of all treatments, accounting for 47.7–60.9%, which was the highest in the PBAT biodegradable film treatment (PBAT bio). Mortierellomycota ranked second in the relative abundances in all three periods, accounting for 19.2–20.2%, whose distribution was similar among the treatments. Chytridiomycota, which ranked fourth in abundance, was significantly the highest in the RPE treatment.
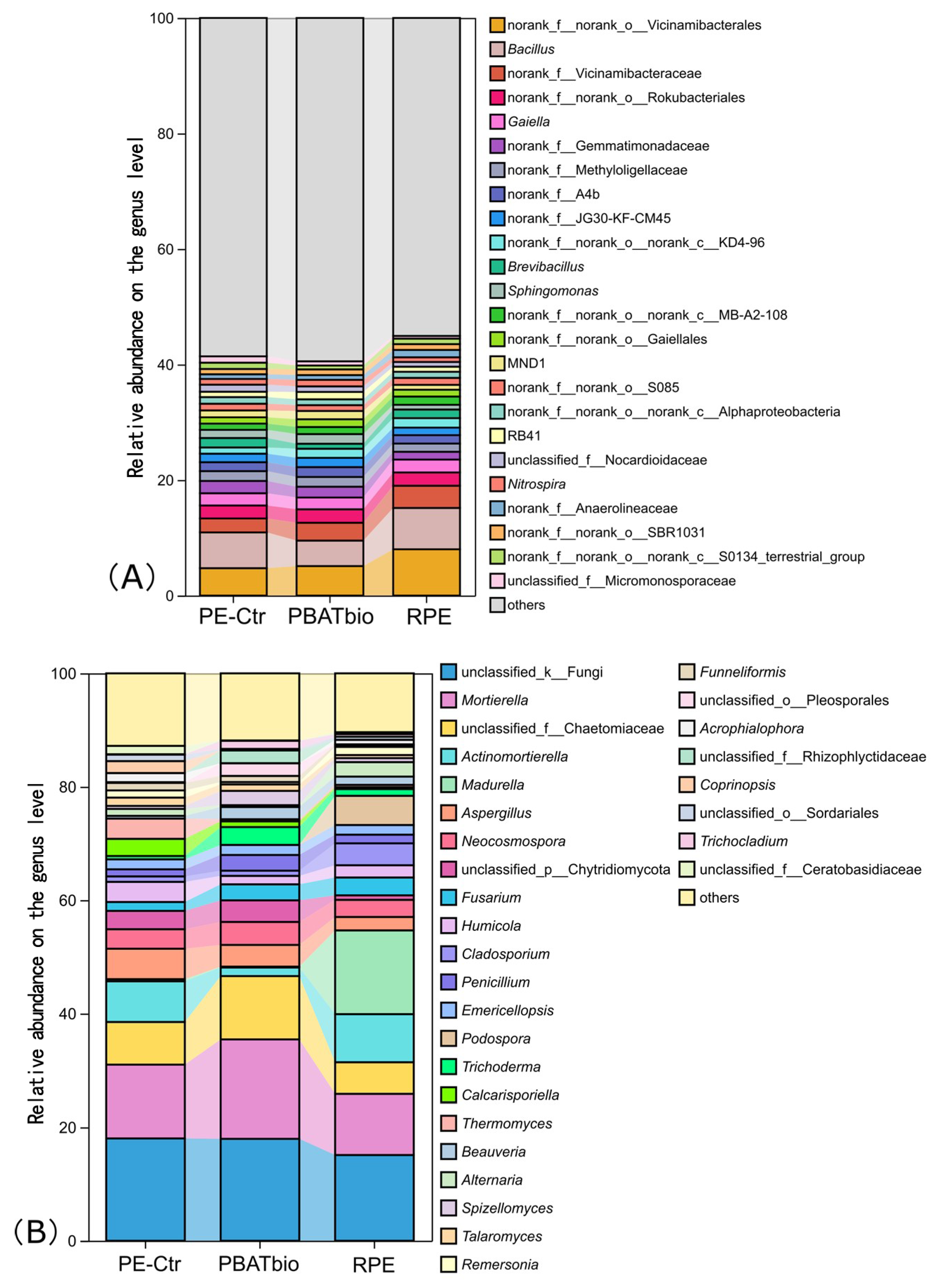
At the genus level (Figure 3A), the top three dominant genera include norank_f_norank_o_Vicinamibacterales, Bacillus, and rank_f_Vicinamibacteraceae. The relative abundances of these three bacterial genera are significantly higher in the RPE treatment than those in the other two treatments. The biodegradable film treatment, however, increased the relative abundances of some rare genera (relative abundance < 1%) in the soil.
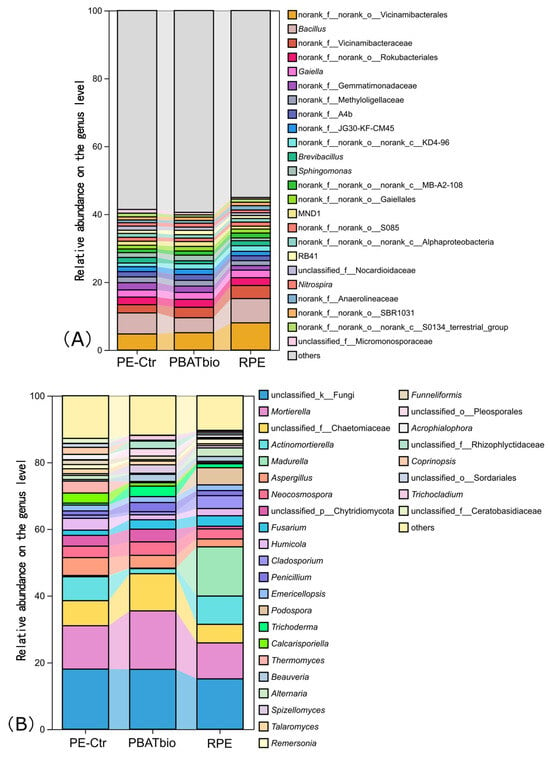
Figure 3.
Relative abundances of bacterial (A) and fungal (B) taxa at the genus level under different plastic film mulching treatments (>1%).
The top three fungal genera in terms of relative abundance are unclassified_k_Fungi, Mortierella, and unclassified_f_Chaetomiaceae (Figure 3B), accounting for 15.1–8.0%, 10.7–17.5%, and 5.6–11.2% respectively. Among them, the relative abundances of Mortierella and unclassified_f_Chaetomiaceae in RPE are the highest, while those in PBAT bio are the lowest. It is worth noting that the relative abundance of Madurella in the soil of the T2 treatment reaches 14.8%, which is significantly higher than that in the PE-Ctr (0.4%) and RPE treatments (0.1%). The relative abundance of Actinomortierella in the RPE treatment is significantly lower than that in the other two treatments.
3.2.4. The Correlation Between Soil Physicochemical Properties and Microbial Community Compositions
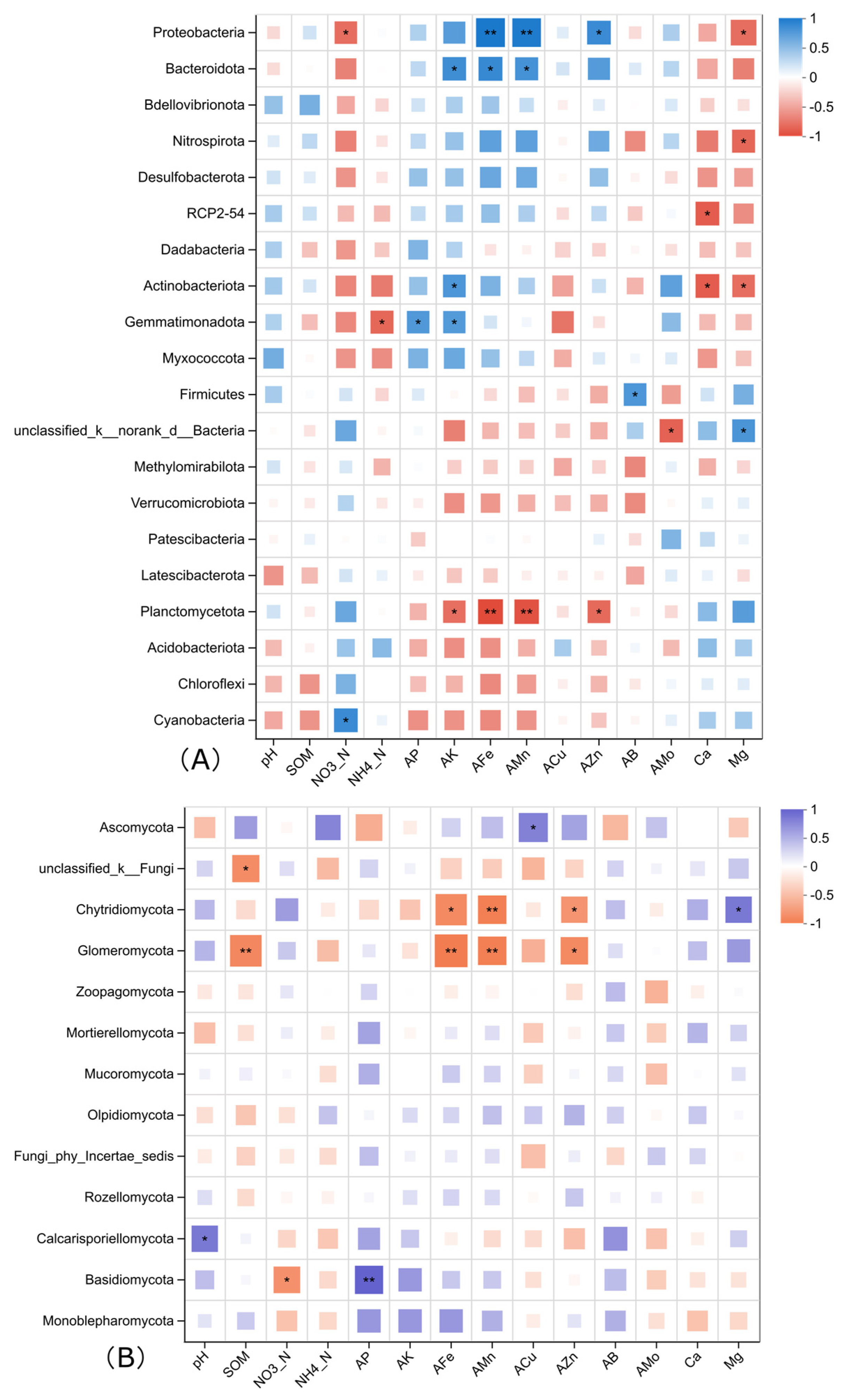
Based on the Spearman correlation analysis, the relationships between the relative abundances of different bacterial phyla in the soil under different treatments and the main soil physicochemical factors were obtained, and the results are shown in Figure 4. Among the top 20 dominant bacterial phyla, Proteobacteria, which has the highest relative abundance, is significantly positively correlated with available iron, available manganese, and available zinc, and significantly negatively correlated with nitrate nitrogen and water-soluble magnesium. Actinobacteriota, ranking third in abundance, is significantly positively correlated with available potassium and significantly negatively correlated with water-soluble calcium and magnesium. Firmicutes is significantly positively correlated with available boron in the soil; Gemmatimonadota is significantly positively correlated with available phosphorus and available potassium and negatively correlated with soil ammonium nitrogen; Bacteroidota is significantly positively correlated with available potassium, available iron, and available manganese; Planctomycetota is significantly negatively correlated with available potassium, available iron, available manganese, and available zinc; and Nitrospirota is significantly negatively correlated with water-soluble calcium and magnesium.
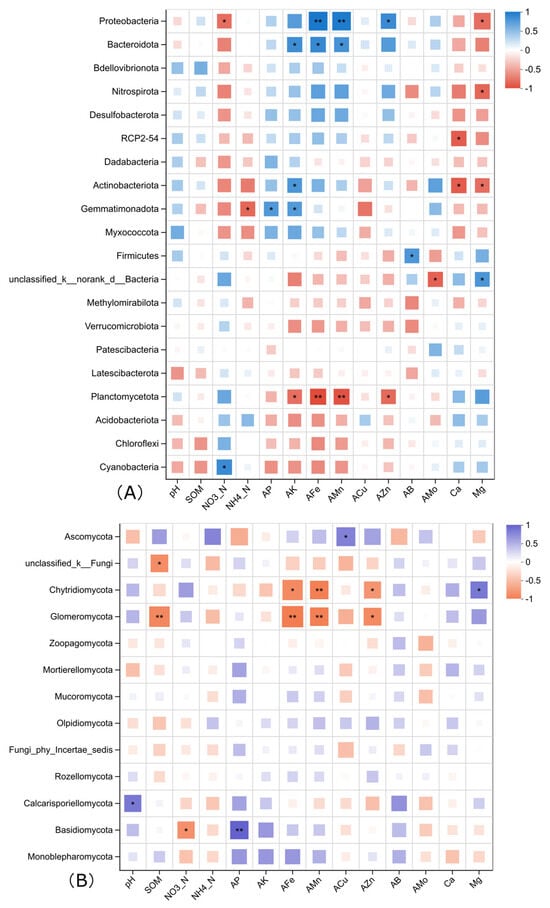
Figure 4.
Correlation heatmap of soil physicochemical factors and the abundant bacterial (A) and fungal (B) species in the soil of each treatment under plastic film mulching. Asterisks represent a significant difference detected by analysis of variance (* 0.01 < p ≤ 0.05, ** 0.001 < p ≤ 0.01).
Among fungal phyla, the most abundant, Ascomycota, is only positively correlated with soil available Cu, while the third and fourth abundant group, Chytridiomycota and Glomeromyctoa are both negatively correlated with soil available Fe, Mn, and Zn. Basidiomycota is positively correlated to soil available P and negatively correlated with soil nitrate.
3.2.5. Prediction of Potential Functions in Different Treatments
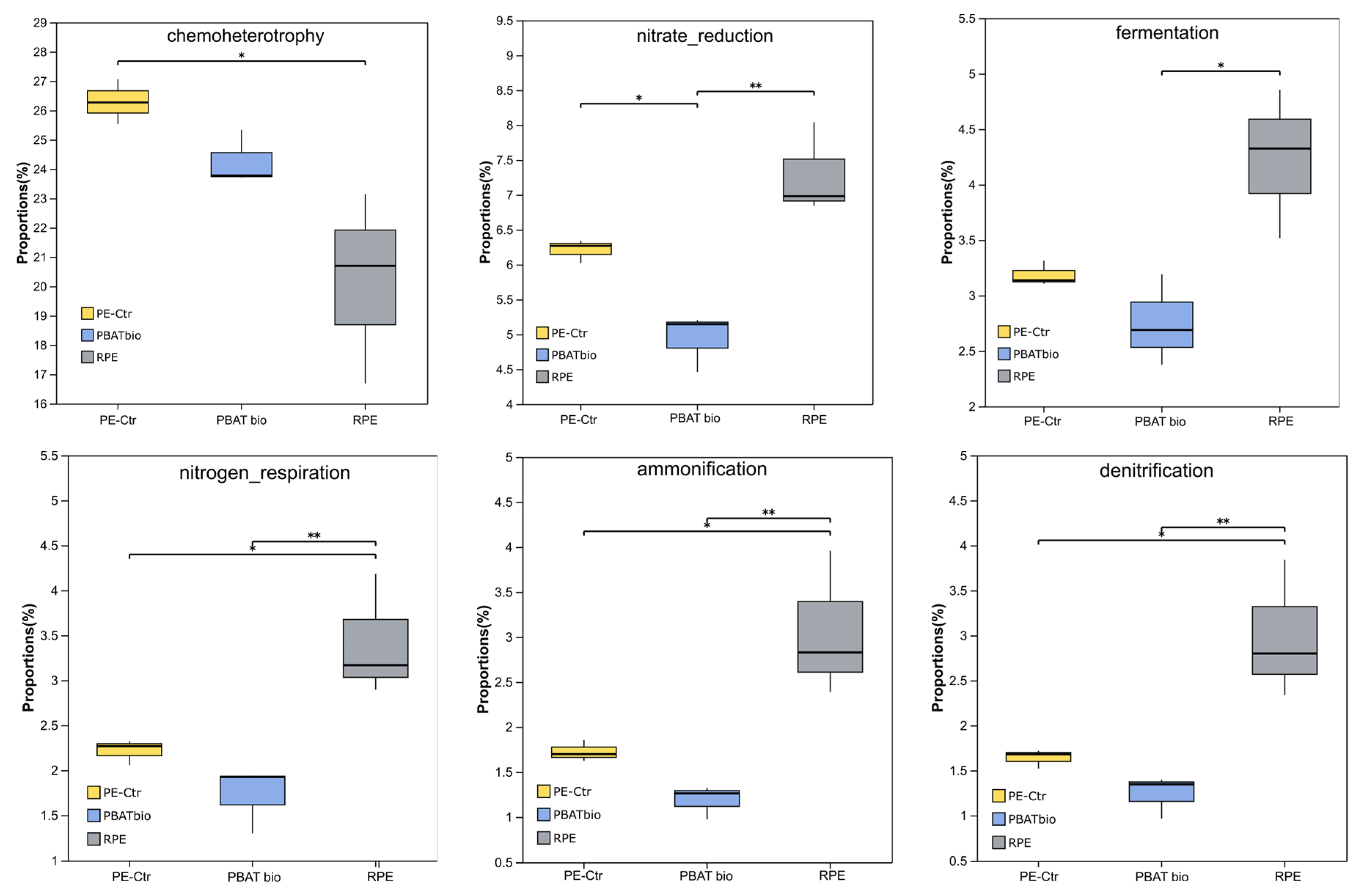
Based on the FAPROTAX function prediction, the metabolism of bacterial functions and related ecological functions in the soil of each treatment were analyzed (see Figure 5). The results show that in the RPE treatment, the soils had six upregulated functions, and the functional abundances of nitrate reduction, nitrogen respiration, ammonification, denitrification, and fermentation were significantly higher than those in PE-Ctr and PBAT bio, while the PE-Ctr treatment had a significantly higher ratio of chemoheterotrophy function than RPE. The PBAT bio treatment reduced the above functions to a certain extent, especially nitrate reduction.
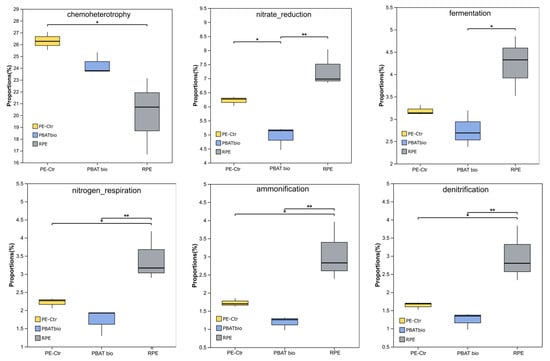
Figure 5.
Relative abundances of bacterial functions predicted by FAPROTAX under different plastic film mulching treatments. Asterisks represent a significant difference detected by analysis of variance (* 0.01 < p ≤ 0.05, ** 0.001 < p ≤ 0.01).
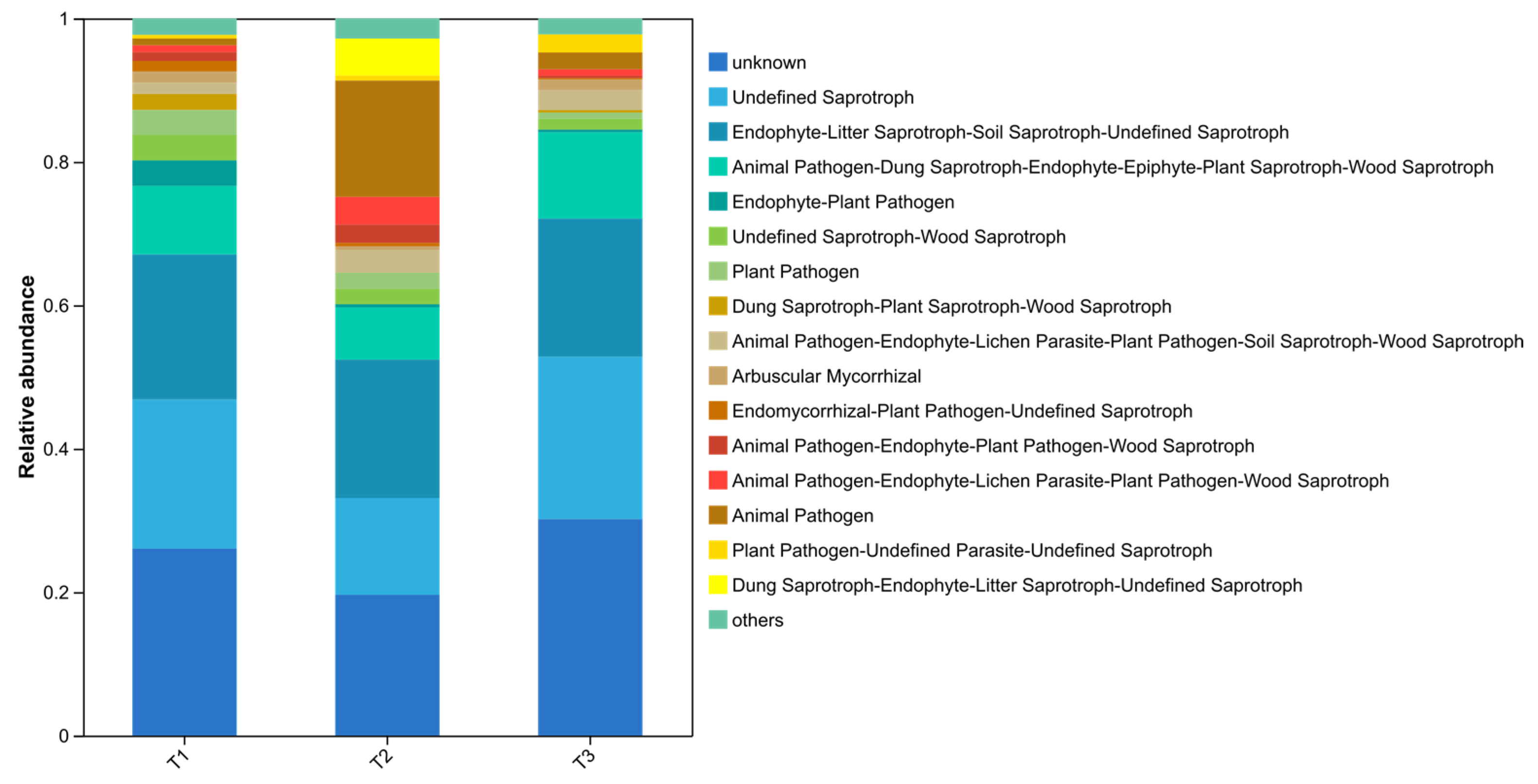
The FUNGuild analysis was carried out on the trophic types and ecological function groups of soil fungi (Figure 6). The results showed that 315 OTUs were successfully annotated. The trophic types included Saprotroph (160 OTUs, 50.8%), Symbiotroph (34 OTUs, 10.8%), Pathotroph (32 OTUs, 10.2%), Saprotroph–Symbiotroph (32 OTUs, 6%), Pathotroph–Saprotroph (11 OTUs, 3.5%), Pathotroph–Saprotroph–Symbiotroph (44 OTUs, 14.0%), Pathotroph–Symbiotroph (8 OTUs, 2.5%), and Pathogen–Saprotroph–Symbiotroph (3 OTUs, 1.0%). In addition to the fact that the unclassified fungal group (unknown) accounted for a certain proportion, the others were Unclassified Saprotroph and Endophyte–Litter Saprotroph–Soil Saprotroph–Unclassified Saprotroph, accounting for 13.5–22.7% and 19.2–20.2% respectively.
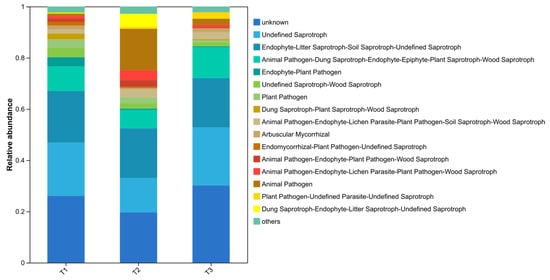
Figure 6.
FUNGuild analysis of the soil fungal functional groups under different plastic film mulching treatments.
The dominant microbial types in each sample varied among treatments. In the PE-Ctr samples, the Animal Pathogen–Dung Saprotroph–Endophyte–Epiphyte–Plant Saprotroph–Wood Saprotroph type accounted for a relatively high proportion; in the PBAT bio samples, the Dung Saprotroph–Plant Saprotroph–Wood Saprotroph type was relatively prominent; in the RPE treatment, Endophyte–Litter Saprotroph–Soil Saprotroph–Unclassified Saprotroph and other types accounted for a relatively large proportion. The prediction results show that the trophic types and ecological function groups of fungi in PBAT bio treatment were more abundant than those in the common film and reinforced film treatments.
4. Discussion
The study found that different plastic film mulching treatments had varying effects on soil physicochemical properties. The soil under the traditional film mulching had higher available phosphorus and available potassium contents. Kong et al. [19] found that plastic film mulching significantly reduced the available potassium content in the soil, which was thought to be connected to the plant depletion. This might also explain the lower soil available K contents in PBAT bio and RPE treatments. Nevertheless, it was also found that the biodegradable film treatment and traditional films had no significant difference in the contents of nitrate nitrogen, available phosphorus, available potassium, and total nitrogen among the treatments [20]. The effects of plastic films on soil nutrients varied greatly with the textures of the plastic films [12], as well as soil and crop types [21]. The soil under the RPE treatment had higher contents of water-soluble calcium and magnesium, as well as a higher EC value. This may be because the coverage of PE film reduced the adsorption of calcium and magnesium elements by the soil and increased their desorption [22,23]. In addition, due to the enhanced heat-preservation and water-retention effects of RPE, the possibility of salt surface accumulation was greatly increased [4]. The organic matter content of the soil in the biodegradable film treatment was higher than that in the other two treatments, which is consistent with the results of previous studies [24]. This may be related to the fact that biodegradable films stimulate microbial activity, which may ultimately affect soil organic matter dynamics [12]. The contents of nitrate nitrogen and trace elements such as available Fe, Mn, and Zn were higher in the biodegradable film treatment, which may have a positive effect on pepper growth. Relevant studies have also shown that the nitrate content in the soil of the biodegradable film treatment is higher than that of common plastic films [2,25]. Yang et al. [23] found that biodegradable film significantly increased the total nitrogen and nitrate nitrogen contents in the soil. The plastic film may also affect the loss of nitrate nitrogen through leaching or surface runoff [26]. These differences in soil physicochemical properties will further affect the living environment and activity of soil microorganisms.
Plastic film mulching increases soil temperature, reduces the activity of most enzymes, and changes the composition of the soil microbial community [12]. Studies have shown that the coverage and incorporation of plastic films can change the abundance of soil microorganisms, largely depending on factors such as soil type, soil environment, crop variety, plastic film material, and agricultural management [27,28]. In this experiment, the biodegradable film (PBAT bio) treatment showed higher bacterial richness and evenness, indicating that biodegradable films are more conducive to maintaining the diversity of the soil microbial community and providing more stable functions for the soil ecosystem. For soil fungi, although the biodegradable plastic film increased the community richness, the evenness was lower, indicating that it had a unique impact on the fungal community structure. Jacquiod et al. [11] also found that the coverage of common polyethylene plastic films reduced the activity of most enzymes in the soil and changed the composition of the soil microbial community, while the coverage of biodegradable films reduced fungal diversity, which is similar to the results of this study.
The dominant bacterial groups at the phylum level in each plastic film mulching treatment include Proteobacteria, Acidobacteriota, Actinobacteriota, Chloroflexi, etc., and the dominant fungi are Ascomycota and Mortierellomycota. Studies have found that in soil environments with microplastics, the abundances of Acidobacteria, Proteobacteria, and Chloroflexi increased [10,29,30]. Proteobacteria is highly adaptable to eutrophic environments and is also common under plastic film mulching conditions [31], which is similar to the results of previous studies [32]. The relative abundance of this bacterial phylum is the highest in the biodegradable film treatment, which may be related to the relatively high nitrogen content in this treatment [33]. The degradation characteristics of Actinobacteriota have been proven in many scenarios. Natthicha Butbunchu et al. found that Actinobacteria are promising candidates for the degradation of polylactic acid-type bioplastics [34], and Basik et al. found that Actinobacteria have outstanding abilities in degrading rubber waste [35]. These research results indicate that these bacteria have prominent degradation characteristics and also explain why the relative abundances of Actinobacteria are relatively high under the three plastic film mulching treatments. At the genus level, the ratio of Bacillus related sequences was relatively high. This is a common bacterial genus with the potential to degrade PE [36,37], especially in the reinforced film treatment, which may be related to changes in the micro-environment.
From the perspective of the soil fungal community composition, the relative abundance of Ascomycota, the most abundant phylum, was higher in the PBAT bio treatment and lower in the RPE treatment. Studies have shown that Ascomycota were enriched more on the surface of biodegradable films because they may degrade PBAT through cutinize and play a dominant role in the biodegradation of PBAT [38]. In the experiment, the proportion of Chytridiomycota in the RPE treatment was relatively high. Similarly, Fan et al. found that an increase in microplastic content led to a decrease in the abundance of Chytridiomycota [39]. At the genus level, the relative abundance of Mortierella was higher in the biodegradable film treatment, while the relative abundance of Aspergillus was higher in the PE treatment. Nevertheless, studies have shown that both of these fungi may play important roles in the biodegradation of microplastics [40]. The predicted trophic types and ecological function groups of the fungi were enriched in the biodegradable plastic film treatment, indicating fungal groups involved in the degradation of these plastics [41].
The correlation analysis demonstrated that soil physicochemical factors and the relative abundances of bacterial phyla were related. For example, Proteobacteria was positively correlated with available Fe, Mn, and Zn and negatively correlated with NO3−-N and water-soluble Mg. Studies showed that plastic film application might affect soil micronutrients that are related to microbial functions [42]. This interaction reveals the dynamic balance between the soil physicochemical environment and the microbial community and provides an important basis for understanding the functions of the soil ecosystem.
FAPROTAX function prediction showed that the soil bacteria in the reinforced film treatment had high abundances in functions such as nitrate reduction, denitrification, and fermentation, which play an important role in the soil nitrogen cycle and organic matter decomposition [43], which may be related to the rapid turnover of N under reinforced plastic films [33]. FUNGuild analysis of fungal functional groups shows that the proportion of unclassified saprophytic fungi is the highest. Under plastic film mulching, the microbial types gradually shift towards saprophytic fungi, indicating the response of soil microorganisms to the plastic film mulching environment. However, the trophic types and ecological function groups of fungi in the soil of the biodegradable film treatment are more abundant. This may be because there are more weeds on the soil surface in the biodegradable film treatment, which is of great significance for maintaining the stability and diversity of the soil ecosystem. Fungal groups include a large number of plant pathogens, and their growth and decline are of great significance for maintaining a healthy ecological environment [44]. Further in-depth research on the impact of plastic film mulching on soil fungi is still needed.
5. Conclusions
This experiment found that the soil under the reinforced film mulching had relatively high contents of water-soluble Ca and Mg; the traditional PE film had higher contents of available P, K, and B, while the biodegradable film mulching had higher contents of nitrate nitrogen and trace elements including available iron, manganese, and zinc. The diversity of soil bacteria and fungi communities was relatively higher in biodegradable film treatment. Proteobacteria, Acidobacteriota, Actinobacteriota, Chloroflexi, and Firmicutes were the dominant bacterial phyla, and Ascomycota the dominant fungal phylum in all treatment. Proteobacteria was positively correlated with available Fe, Mn, and Zn and negatively correlated with nitrate nitrogen and water-soluble Mg. Biodegradable plastic film treatment increased the relative abundances of rare genera, as well as the fungal trophic types and ecological functions, while it decreased N-related bacterial functions, as well as fermentation, which were the highest in reinforced plastic film treatment. Collectively, soil physicochemical properties and microbial communities was significantly affected by the different types of plastic film mulching.
Author Contributions
Conceptualization, G.W. and X.Z.; methodology, N.H., Y.L. and W.H.; software, G.W. and J.W.; validation, X.Q. and X.Z.; investigation, N.H., Y.L., W.H. and L.Y.; resources, X.Z.; data curation, J.W., Y.C. and X.Q.; writing—original draft preparation, G.W. and Y.C.; writing—review and editing, J.W. and X.Q.; visualization, N.H.; supervision, X.Q.; project administration, X.Z.; funding acquisition, L.Y. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Yangzhou Science and Technology Plan Project (YZ2024060).
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
Author Yifan Cao was employed by the company Yangzhou Ruihua Environmental and Bioengineering Research Institute Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bulati, A.; Zhan, L.; Xu, Z.; Yang, K. Obtaining the value of waste polyethylene mulch film through pretreatment and recycling technology in China. Waste Manag. 2025, 197, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Flury, M.; Sun, S.; Cai, J.; Zhang, A.; Li, Q.; Jiang, R. In-field degradation of polybutylene adipate-co-terephthalate (PBAT) films, microplastic formation, and impacts on soil health. Environ. Res. 2025, 121086. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Zhu, J.; Li, Y.; Yao, P.; Bi, Z.; Sun, C.; Liu, Y. Effects of different water treatments on the growth, physiological, photosynthesis, and yield of potato under drip irrigation with plastic mulch in northwest China. Sci. Hortic. 2025, 341, 113978. [Google Scholar] [CrossRef]
- Tan, Q.; Yang, L.; Wei, F.; Chen, Y.; Li, J. Comparative life cycle assessment of polyethylene agricultural mulching film and alternative options including different end-of-life routes. Renew. Sustain. Energy Rev. 2023, 178, 113239. [Google Scholar] [CrossRef]
- Li, S.; Ding, F.; Flury, M.; Wang, J. Dynamics of macroplastics and microplastics formed by biodegradable mulch film in an agricultural field. Sci. Total Environ. 2023, 894, 164674. [Google Scholar] [CrossRef]
- Dada, O.I.; Liyanage, T.U.H.; Chi, T.; Chen, S.; Yu, L.; Wasko DeVetter, L. Towards Sustainable Agroecosystems: A Life Cycle Assessment Review of Soil-Biodegradable and Traditional Plastic Mulch Films. Environ. Sci. Ecotechnol. 2025, 24, 100541. [Google Scholar] [CrossRef]
- Yu, Y.; Velandia, M.; Hayes, D.G.; DeVetter, L.W.; Miles, C.A.; Flury, M. Biodegradable plastics as alternatives for polyethylene mulch films. Adv. Agron. 2024, 183, 121–192. [Google Scholar]
- Wu, H.; Liu, Q.; Yang, F.; Hou, M. Degradation of Polyethylene Plastics by Microbial Action of Rhizobium spp. BM Isolated from Soil. JOM 2025, 12, 1–13. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Yan, C.; Chadwick, D.; Jones, D.L.; Liu, E.; Liu, Q.; Bai, R.; He, W. Kinetics of microplastic generation from different types of mulch films in agricultural soil. Sci. Total Environ. 2022, 814, 152572. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, K.; Ren, L.; Peng, A.; Zhou, S. Biodegradable microplastics, A review on the interaction with pollutants and influence to organisms. Bull. Environ. Contam. Toxicol. 2022, 108, 1006–1012. [Google Scholar] [CrossRef]
- Jacquiod, S.; Bouchard, E.; Beguet, J.; Roure, F.; Cheviron, N.; Mougin, C.; Coffin, A.; Blouin, M.; Martin-Laurent, F. Effect of plastic film and hemp canvas mulching on soil properties, microbial diversity and lettuce yield. Plant Soil 2024, 503, 65–83. [Google Scholar] [CrossRef]
- Campanale, C.; Galafassi, S.; Di Pippo, F.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A critical review of biodegradable plastic mulch films in agriculture, Definitions, scientific background and potential impacts. TrAC Trends Anal. Chem. 2024, 170, 117391. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, N.; Zhao, F.; Wang, J. Mulching practices decreased soil microbial carbon degradation potential under spring maize in the Loess Plateau of China. Agric. Ecosyst. Environ. 2025, 381, 109465. [Google Scholar] [CrossRef]
- Shi, J.; Sun, Y.; Wang, X.; Wang, J. Microplastics reduce soil microbial network complexity and ecological deterministic selection. Environ. Microbiol. 2022, 24, 2157–2169. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Graf, M.; Greenfield, L.M.; Reay, M.K.; Bargiela, R.; Golyshin, P.N.; Evershed, R.P.; Lloyd, C.E.; Williams, G.B.; Chadwick, D.R.; Jones, D.L. Field-based assessment of the effect of conventional and biodegradable plastic mulch film on nitrogen partitioning, soil microbial diversity, and maize biomass. Appl. Soil Ecol. 2024, 202, 105595. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Li, Q.; Zhang, L.; Sun, B.; Qin, Y.; Yuan, Y.; Li, C.; Zhang, J.; Liu, H. Biodegradable film drip fertigation is more conducive to reducing the diversity and abundance of antibiotic resistance genes than plastic film drip fertigation. Plant Soil 2025, 1–18. [Google Scholar] [CrossRef]
- Bao, S. Soil Agro-Chemistrical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2007; pp. 268–270. [Google Scholar]
- Kong, M.; Gu, Y.-J.; Han, C.-L.; Shi, X.-P.; Kang, J.; Siddique, K.H.M.; Li, F.-M.; Yuan, Z.-Q. The Prolonged Effect of Film Mulch and P Application on Lucerne Forage Yield in a Semiarid Environment. Front. Plant Sci. 2023, 14, 1331704. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Cui, Z.; Xu, L.; Liu, Q.; Li, J.; Wang, S.; Zeng, X. Effects of biodegradable plastic mulch film on cabbage agronomic and nutritional quality traits, soil physicochemical properties and microbial communities. Agronomy 2023, 13, 1220. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, Y.; Jin, T.; Zhang, K.; Li, Z.; Sun, C.; Mi, Q.; Li, Q. Effect of long-term biodegradable film mulch on soil physicochemical and microbial properties. Toxics 2022, 10, 129. [Google Scholar] [CrossRef]
- Santini, G.; Maisto, G.; Memoli, V.; Di Natale, G.; Trifuoggi, M.; Santorufo, L. Does the element availability change in soils exposed to bioplastics and plastics for six months? Int. J. Environ. Res. Public Health 2022, 19, 9610. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Y.; Long, B.; Wang, F.; Li, F.; Xie, D. Biodegradable mulch films improve yield of winter potatoes through effects on soil properties and nutrients. Ecotoxicol. Environ. Saf. 2023, 264, 115402. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, X.; Riksen, M.; Geissen, V. Effect of different polymers of microplastics on soil organic carbon and nitrogen–A mesocosm experiment. Environ. Res. 2022, 204, 111938. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhang, W.; Dai, Z.; Li, J.; Mao, W.; Yu, F.; Ma, J.; Wang, S.; Zeng, X. Comparative analysis of the effects of plastic mulch films on soil nutrient, yields and soil microbiome in three vegetable fields. Agronomy 2022, 12, 506. [Google Scholar] [CrossRef]
- Nan, W.-G.; Yue, S.-C.; Huang, H.-Z.; Li, S.-Q.; Shen, Y.-F. Effects of plastic film mulching on soil greenhouse gases (CO2, CH4 and N2O) concentration within soil profiles in maize fields on the Loess Plateau, China. J. Integr. Agric. 2016, 15, 451–464. [Google Scholar] [CrossRef]
- Somanathan, H.; Sathasivam, R.; Sivaram, S.; Kumaresan, S.M.; Muthuraman, M.S.; Park, S.U. An update on polyethylene and biodegradable plastic mulch films and their impact on the environment. Chemosphere 2022, 307, 135839. [Google Scholar] [CrossRef]
- Li, Y.; Hou, Y.; Hou, Q.; Long, M.; Wang, Z.; Rillig, M.C.; Liao, Y.; Yong, T. Soil microbial community parameters affected by microplastics and other plastic residues. Front. Microbiol. 2023, 14, 1258606. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere, Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Jin, T.; Li, L.; Peng, K.; Li, W.; Jin, D.; Chen, W.; Peng, J. Comparative Analysis of Biodegradable Mulches on Soil Bacterial Community and Pepper Cultivation. Agronomy 2024, 14, 905. [Google Scholar] [CrossRef]
- Muroi, F.; Tachibana, Y.; Kobayashi, Y.; Sakurai, T.; Kasuya, K.-I. Influences of poly (butylene adipate-co-terephthalate) on soil microbiota and plant growth. Polym. Degrad. Stab. 2016, 129, 338–346. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.; Jiang, J.; Li, R.; Liang, W. Nitrogen conversion and mechanisms related to reduced emissions by adding exogenous modified magnesium ore during aerobic composting. J. Environ. Manag. 2025, 378, 124550. [Google Scholar] [CrossRef] [PubMed]
- Butbunchu, N.; Pathom-Aree, W. Actinobacteria as promising candidate for polylactic acid type bioplastic degradation. Front. Microbiol. 2019, 10, 2834. [Google Scholar] [CrossRef] [PubMed]
- Basik, A.A.; Sanglier, J.-J.; Yeo, C.T.; Sudesh, K. Microbial degradation of rubber, Actinobacteria. Polymers 2021, 13, 1989. [Google Scholar] [CrossRef]
- Akash, K.; Parthasarathi, R.; Elango, R.; Bragadeeswaran, S. Exploring the plastic-fed Indian mealworm (Tenebrio molitor) gut bacterial strain (Bacillus subtilis AP-04)–A potential driver of polyethylene degradation. J. Hazard. Mater. 2025, 486, 137022. [Google Scholar] [CrossRef]
- Zeng, J.; Yao, J.; Zhang, W.; Zhang, M.; Wang, T.; Yu, X.; Liu, Y.; Sun, X.; Li, L. Biodegradation of commercial polyester polyurethane by a soil-borne bacterium Bacillus velezensis MB01B, Efficiency, degradation pathway, and in-situ remediation in landfill soil. Environ. Pollut. 2024, 363, 125300. [Google Scholar] [CrossRef]
- Nikolić, M.A.; Gauthier, E.; Colwell, J.M.; Halley, P.; Bottle, S.E.; Laycock, B.; Truss, R. The challenges in lifetime prediction of oxodegradable polyolefin and biodegradable polymer films. Polym. Degrad. Stab. 2017, 145, 102–119. [Google Scholar] [CrossRef]
- Fan, P.; Tan, W.; Yu, H. Effects of different concentrations and types of microplastics on bacteria and fungi in alkaline soil. Ecotoxicol. Environ. Saf. 2022, 229, 113045. [Google Scholar] [CrossRef]
- Nasrabadi, A.E.; Ramavandi, B.; Bonyadi, Z. Recent progress in biodegradation of microplastics by Aspergillus sp. in aquatic environments. Colloid Interface Sci. Commun. 2023, 57, 100754. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Liquet y González, J.E.; Henderson, K.B.; Anunciado, M.B.; Hayes, D.G.; DeBruyn, J.M. Soil Microbial Communities Associated with Biodegradable Plastic Mulch Films. Front. Microbiol. 2020, 11, 2840. [Google Scholar] [CrossRef]
- Wang, K.; Flury, M.; Kuzyakov, Y.; Zhang, H.; Zhu, W.; Jiang, R. Aluminum and microplastic release from reflective agricultural films disrupt microbial communities and functions in soil. J. Hazard. Mater. 2025, 491, 137891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.D.; Qin, X.R.; Li, T.L.; Cao, H.B.; Xie, Y.H. Effects of planting patterns plastic film mulching on soil temperature, moisture, functional bacteria and yield of winter wheat in the Loess Plateau of China. J. Integr. Agric. 2023, 22, 1560–1573. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Y.-B.; Sui, J.-H.; Liu, C.-S.; Liu, R.; Xu, Z.F.; Han, X.-Y.; Zhang, T.; Zhang, Q.-H.; Chen, C.-B. Response of ginseng rhizosphere microbial communities and soil nutrients to phosphorus addition. Ind. Crops Prod. 2025, 226, 120687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).