Abstract
This study investigated dynamic pollen–stigma coordination to optimize interspecific hybridization in Dendrobium using D. ‘Burana Jade’ as the maternal parent and eight wild species as pollen donors. Stigma receptivity was comprehensively evaluated using a multi-indicator approach, including morphological characterization (crystal secretion and bulging papillae), histochemical benzidine-H2O2 staining, and enzymatic activity profiling (esterase and superoxide dismutase). Concurrently, pollen viability was assessed through TTC testing coupled with ultrastructural observations. Results identified a critical synchronization window: pollen viability peaked at 1–3 days post anthesis (DPA) or during the mid-anthesis phase, while stigmas exhibited maximal receptivity when secretory activity and antioxidant enzyme levels significantly increased. Using stage-specific pollination criteria, 8.4% of crosses (8/95) produced viable fruits, outperforming empirical methods by 2.8-fold. D. ‘Burana Jade’ showed cross-compatibility with four Dendrobium species (D. aphyllum, D. chrysotoxum, D. hercoglossum, D. thyrsiflorum), with D. thyrsiflorum hybrids achieving 54.81% embryogenesis and 22.38% germination. Three compatible combinations germinated successfully in vitro within 45–55 days on 1/4 MS medium supplemented with 20 g/L sucrose, 1 g/L tryptone, 180 mL/L coconut water, and 2.2 g/L Phytagel. Our findings establish that synchronizing pollen viability windows with stigma receptivity phases significantly enhances fruit set and hybrid seed viability, providing a phenology-driven strategy to overcome reproductive barriers in orchid breeding programs. This study provides key physiological criteria for Dendrobium hybridization, though their applicability to other orchids needs validation. Future multi-omics studies should explore cross-species compatibility mechanisms.
1. Introduction
Currently, hard cane Dendrobium is the most important source of potted flowers or cutflower in orchid crop production [1]. D. ‘Burana Jade’, a hybrid of Hard Cane Dendrobium, has a greater ornamental value but has poor cold resistance and branching. However, some desirable traits such as rich branches, fragrance, excellent color, and particular shape could be improved to increase market competitiveness and climatic adaptation [2]. For instance, interspecific hybridization between Chrysanthemum and Ajania resulted in hybrid offspring exhibiting significantly enhanced cold tolerance [3]. Therefore, interspecific hybridization with wild species with desirable characteristics native to China is considered a feasible way to solve these problems [4].
The production of hybrid progeny for novel Dendrobium varieties necessitates fully developed offspring, which can only be achieved through hybrid seeds with well-coordinated embryo and endosperm development. The critical role of embryo–endosperm coordination in hybrid viability has been well documented in Solanum [5] and Mimulus [6], where defective endosperm development led to seed abortion. All of these factors are determined by fertilization completion and parents’ genetic relationships [7,8]. However, in contrast with many other orchid genera, the genus Dendrobium has exhibited high interspecific cross-incompatibility because of its complicated geographic affinity [9,10].
Improving the fruit set is a key issue in hand pollination techniques [11]. In general, traditional hand-pollination techniques are based on empirical judgment. Successful pollination is guaranteed prior to fertilization, which principally depends on pollen vitality and stigma maturation [12,13]. Stigma receptivity and pollen vigor are significantly associated with floral age [14,15]. However, Dendrobium has a long duration of anthesis, making it difficult to guarantee its precision especially when the duration of pollination is short [4]. Determining the optimal pollination time is difficult. Therefore, scientific analysis should be performed to determine the duration of stigmas and pollen viability to improve the success rates of manual Dendrobium pollination [16,17].
Typically, pollen viability can be determined via instant staining with 2,3,5-triphenyltetrazolium chloride (TTC) [18,19]. Stigma maturity can be characterized by high enzymatic activity in addition to morphological performance, such as that of esterase isozymes, superoxide dismutase (SOD), and catalase, which are involved in pollen—pistil interactions and antioxidation to help the pollen tube penetrate the stigmatic tissues and enter the style [20,21,22]. Extensive research has been documented in this field across multiple plant species, including Chaenomeles speciosa [16], Monochasma savatieri [23], and Vaccinium corymbosum [24]. However, there are no related studies on Dendrobium stigma and pollen physiology.
This research aims to explore the development characteristics of some Dendrobium species stigmas and pollens for the determination of the optimal hand-pollination scheme to improve interspecific compatibility performance. The biochemical activities of esterase, SOD, and H2O2 were used to measure the stigma maturity of D. ‘Burana Jade’. TTC staining helped to indicate the pollen viability of eight wild species. On the basis of effective pollination, fruit-set, seed morphology, embryo vigor, and sterile germination were evaluated to reveal the affinity of Dendrobium interspecific hybridization. The aim of this study lies in exploring the pollen and stigma characteristics of hybrid Dendrobium from native species in Yunnan, which will help improve the propagation efficiency of Dendrobium and provide technical support for biodiversity conservation. The key innovation is a multi-parametric evaluation of D. ‘Burana Jade’ stigma maturity (via esterase/SOD activity and H2O2 levels) combined with TTC-based pollen viability assessment, enabling evidence-based determination of optimal pollination timing through synchronized physiological profiling.
2. Materials and Methods
2.1. Parent Materials
Eight species were used as male parents. Dendrobium orchid sections such as Dendrobium, Formosae, and Callista have several unique characteristics, such as a sweet scent of D. aphyllum and D. cariniferum; a fragrant flower of D. chrysotoxum, glossy and bright lip color of D. crystallinum, D. devonianum and D. thyrsiflorum; compactness and an attractive lip of D. hercoglossum (shown in Figure 1). D. ‘Burana Jade’ as a female parent was purchased from Oriental Shangcai Modern Agriculture Co. Ltd. (Shanghai, China). Wild specimens of additional species were collected from their native habitats in southern Yunnan Province, China (geographic range: 22° N–25° N, 99° E–102° E). All experimental materials were subsequently cultivated in the orchid nursery at Southwest Forestry University (SWFU), China (25°02′34″ N, 102°44′12″ E), following a standardized acclimatization protocol.
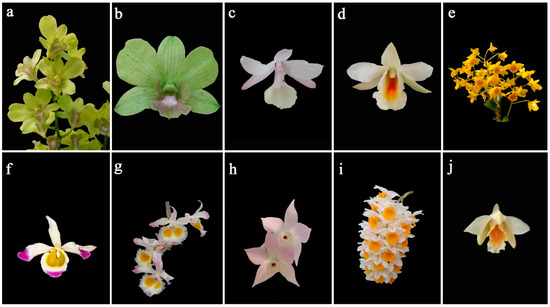
Figure 1.
Flower morphology of eight breeding parents including one cultivar (a,b) and seven wild species (c–i). (a) inflorescence of D. ‘Burana Jade’; (b) floral morphology of D. ‘Burana Jade’; (c) floral morphology of Dendrobium aphyllum; (d) floral morphology of D. christyanum; (e) inflorescence of D. chrysotoxum; (f) floral morphology of D. crystallinum; (g) floral morphology of D. devonianum; (h) floral morphology of D. hercoglossum; (i) floral morphology of D. thyrsiflorum; (j) floral morphology of D. cariniferum.
2.2. Measurement of Pollen Viability at Different Stages
The pollen grain viability of these plants at different stages was examined to ensure pollination effectiveness. The duration of a single flower was observed from the flower opening to fading for 10 random samples, which determined the pollinia sampling time of the male parent. Pollinia collection from Dendrobium aphyllum, D. cariniferum, D. christyanum, D. cilia, D. hercoglossum, and D. thyrsiflorum was conducted at 1, 3, 5, 7, 10, 15, and 20 days post anthesis (DPA), where “anthesis″ (Day 0) was defined as the onset of full flower opening with complete exposure of the labellum and column. The former five stages represent the early anthesis phase, and the latter (10 DPA, 15 DPA) and 20 DPA represent the mid-anthesis and late-anthesis phases, respectively. The experiments were conducted in March 2020 and 2021. Five pollinia were removed from the flowers at the same anthesis phase at 9:00 a.m. The mixed pollinia were subsequently squashed and dyed with 0.5% TTC (Shanghai Zhanyun Chemical Co., Ltd., Shanghai, China) in an incubator at 37 °C for 15 h [25]. Pollen morphology was observed under an Olympus optical microscope via an objective lens at 10 × magnification. The number of pollen grains exhibiting red (indicative of high viability) and pink (indicative of moderate viability) staining was quantified. At least 10 fields under a microscope were observed and recorded. For each pollen donor, a minimum of 300 pollen grains were counted. Pollen viability was calculated as the number of dyed (red and pink) pollen grains divided by the total number of pollen grains. TTC is enzymatically reduced by mitochondrial dehydrogenases in viable pollen grains, generating insoluble red formazan deposits proportional to cellular metabolic activity. Red or pink coloration indicates viable grains with active dehydrogenase systems, while colorless grains demonstrate metabolic inactivation and non-viability.
2.3. Estimation of Stigma Development
A receptive stigma is dependent on many metabolic enzymes, such as esterase, SOD, and H2O2, to enable pollen to stick, be recognized, and to engulf pollinia. Receptivity of stigma was evaluated at three flower phases represented by early anthesis phase (T1) (stigma depression was filled with milky-white secretion), mid-anthesis phase (T2) (secretion bulged beyond the surface of the stigma depression and became crystalline), and late-anthesis phase (T3) (stigma secretion shrank and slightly dried) separately. Esterase and SOD activities were checked with ELISA Kit (Shanghai FANKEL Industrial Co., Ltd. Shanghai, China) with 3 replicates of 10 uniform stigmas in each treatment. The specific activity was calculated as units per gram fresh weight per hour (U·g−1 FW·h−1). The benzidine (H2O2) method was used to evaluate the H2O2 vigor of the stigma. The 10 uniform stigmas were immersed in culture dishes containing benzidine (Shanghai Jinban Biotechnology Co., Ltd. Shanghai, China) and H2O2 solution (1% benzidine: 3% H2O2: H2O = 4:11:22) for 10 min [26]. The intensity of effervescence on the stigma and the proportion of blue stigma were observed with a magnifier and photographed.
2.4. Precise Artificial Pollination
The optimal artificial crossing conditions were determined on the basis of the pollen viability of eight male parents, stigma receptivity, and stigma enzyme dynamic data. The pollinia of D. christyanum, D. devonianum, D. chrysotoxum, D. aphyllum, D. Crystallinum, D. hercoglossum, and D.cariniferum were picked at 5–7 DPA. D. thyrsiflorum was picked at 3 DPA. The receptive stigma of D. ‘Burana Jade’ was spongy and filled with sticky, crystalline, and viscous. The interspecific hybridization between D. ‘Burana Jade’ (♀) and D. aphyllum (♂), D. ‘Burana Jade’ (♀) and D. christyanum (♂), D. ‘Burana Jade’ (♀) and D. chrysotoxum (♂), D. ‘Burana Jade’ (♀) and D. crystallinum (♂), D. ‘Burana Jade’ (♀) and D. devonianum (♂), D. ‘Burana Jade’ (♀) and D. hercoglossum (♂), D. ‘Burana Jade’ (♀) and D. thyrsiflorum (♂), D. ‘Burana Jade’ (♀) and D. cariniferum (♂) was conducted in the Southwest Forestry University Department of Landscape and Horticulture research greenhouses in Kunming, China from March to April 2020 and 2021. For each of the 8 wild species × D. ‘Burana Jade’ combinations, a minimum of 10 flowers were hand-pollinated between 09:00 and 11:00 a.m. after stigma depollination (using sterile tweezers).
2.5. Assessment of Fruit Set
Successful pollination in Dendrobium is indicated by the swelling of the ovary and peduncle, followed by capsule formation. Unsuccessfully pollinated flowers, on the other hand, naturally withered within 2 or 3 weeks. After hand pollination, ovary morphology, seedpod development, fruit drop, and fruit ripening were observed until maturity. When the seedpods matured, which was physiologically characterized by constant growth in fruit length and width as well as a slightly yellowish skin color, the fruit was ready to be harvested. The determination of fruit set began at the beginning of ovary swelling. The fruit set rate was calculated as the number of successful pollinations divided by the total number of pollinations. The fruit maturity rate was calculated as the ratio of the number of mature pods to the number of fruits set.
2.6. Evaluation of Cross-Seed Development and Viability
After the pod was harvested, it was weighed and measured and then stored in a sealed container with a drying agent (Andydrous Calcium chloride, Hunan BKMAM Biotechnology Co., Ltd. Hunna, China) until the capsule cracked. Dried cross-seeds were collected in a tube for further study. Seed development was estimated in two ways: (1) Seed length and round size were measured under an Olympus optical microscope at 1 × magnification. Thirty seeds were observed in one capsule and randomly selected for observation and their width and length were measured under a microscope, (2) Thirty visual fields were calculated by counting the percentage of seeds containing an embryo. Seed viability was evaluated in two ways. (1) Seeds were dyed with a solution of 1% modified tetrazolium (TZ) and the seeds were incubated in the dark at 37 °C for 17 h [27]. Seeds with a rose-red hue were considered viable, whereas white and yellow seeds were considered nonviable. Each treatment above included three replicates of 50 seeds (each unit) in one pod;.(2) Sterile germination tests were carried out with 1/4 Murashige and Skoog (MS) medium containing 20 g/L sucrose (Sigma Chemical Co., St. Louis, MO, USA), 1 g/L tryptone (Merck KGaA, Darmstadt, Germany), 180 mL/L coconut water, 2.2 g/L Phytagel (Sigma Chemical Co.), and 1 g/L activated charcoal. A 1% sodium hypochlorite solution containing two drops of a wetting agent (Tween-20; Sigma, MO, USA) was used to disinfect the seeds. Seeds were washed twice with sterile distilled water and added to the medium. Three culture bottles were used per species, sealed with polyvinylchloride film and transferred to a growth room at 25 ± 1 °C with a 16 h light /dark cycle at 30 µmol/m−2/s.
2.7. Statistical Analysis
The significance of esterase, SOD, and pollen viability was subjected to one-way analysis of variance (ANOVA) with EXCEL (Microsoft Corporation, Redmond, WA, USA) via SPSS 17.0 software. For significance tests of differences, a comparison of means from all treatments was performed by the least squares difference (LSD) test method at a significance level of 5% (p = 0.05). Prior to ANOVA, data normality was confirmed using Shapiro–Wilk tests (p > 0.05 for all datasets). Homogeneity of variance was verified through Levene’s test.
3. Results
3.1. Dynamic Changes in and Duration of Pollen Viability
The staining patterns of pollen viability for the eight male parents are shown in Figure 2. There were two changing patterns of Dendrobium pollen vigor: a bell-shaped curve ‘weak vigor–strong vigor–weak vigor″ (Figure 3a–d,f–h), and a slash shape ‘strong vigor–weak vigor’ (Figure 3e).
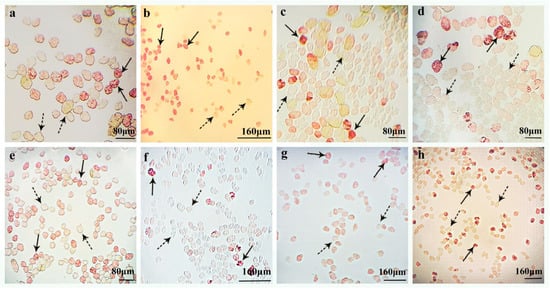
Figure 2.
Pollen viability was assessed in eight parental lines using 0.5% TTC staining. Viable pollen grains exhibiting red formazan deposition are indicated by solid arrowheads, while non-viable pollen (white/yellow coloration lacking formazan) is marked with dashed arrowheads. (a) D. aphyllum; (b) D. cariniferum; (c) D. christyanum; (d) D. chrysotoxum; (e) D. crystallinum; (f) D. devonianum; (g) D. hercoglossum; (h) D. thyrsiflorum.
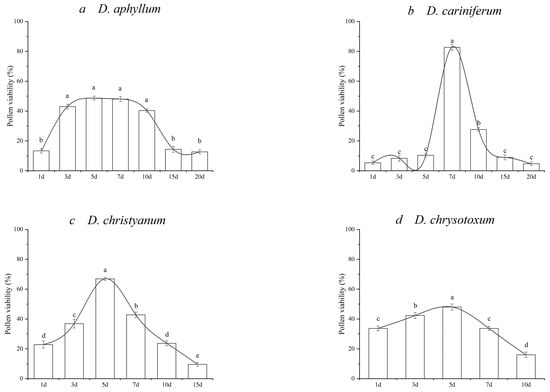
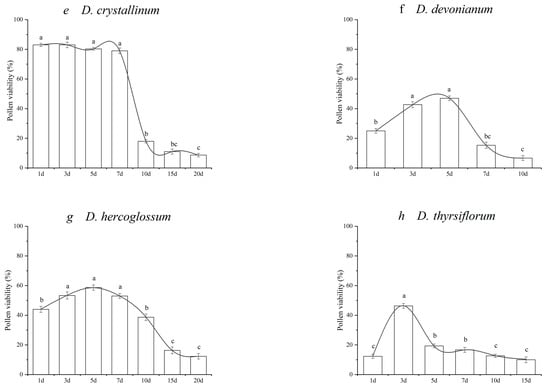
Figure 3.
Pollen viability of the investigated species of Dendrobium during flower lifespan. (a) D.aphyllum; (b) D. cariniferum; (c) D. christyanum; (d) D. chrysotoxum; (e) D. crystallinum; (f) D. devonianum; (g) D. hercoglossum; (h) D. thyrsiflorum. The means followed by different letters are significantly different according to the least squared difference at p ≤ 0.05 (LSD0.05).
The slash model included D. crystallinum, with the greatest value occurring at the initial flowering of 83.00% (Figure 3e). The former four stages were significantly greater than the other stages (p ≤ 0.05) and then gradually declined to 11.00% at 15 DPA. Conversely, the other seven species presented different patterns, with the maximum pollen vigor occurring at the mid-anthesis phase. The greatest pollen viability of D. aphyllum, D. cariniferum, D. christyanum, D. chrysotoxum, D. devonianum, and D. hercoglossum occurred at 5–7 DPA, with 48.67%, 82.67%, 63%, 48.00%, 58.67%, 47.00%, and 46.33%, respectively. D. thyriflorum had great vigor at 3 DPA.
According to the statistical analysis of pollen vigor at different stages, D. aphyllum and D. crystallinum maintained greater pollen vigor nearly at 7 d. The average duration of pollen viability was approximately 2–3 d for most species. Overall, D. aphyllum, D. crystallinum, and D. hercoglossum maintained a relatively long duration of pollen vigor. The pollen viability of D. thyrsiflorum decreased within two days.
3.2. Stigma Receptivity at the Different Development Stages
The activities of fatty acyl esterase and SOD significantly increased from T1 to T2 with increasing stigma secretion (Figure 4b,c). These values peaked at the T2 stage and then moderately decreased at the T3 stage. The most intense SOD and esterase activities reached 1908.40 μ∙g−1·FW∙h−1, and 6.21 μ∙g−1·FW∙h−1, respectively, at T2 (Figure 5). H2O2 activity at different phases was visually evaluated by color and bubble occurrence (Figure 4e–g). More than 2/3 of the stigmas were stained blue at T2, and abundant effervescence was observed (Figure 4g). There was a similar tendency toward decreasing stigma receptivity at both the T1 stage and the T3 stage. Stigma secretion performance at the floral development stages was consistent with enzyme activity.
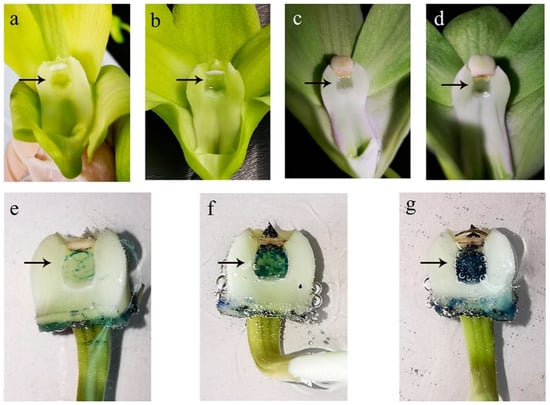
Figure 4.
Stigma secretion performance and receptivity at four anthesis phases. (a) Secretory activity of the stigma on the first day of anthesis; (b) Secretory activity of the stigma on early anthesis phase-T1; (c) Secretory activity of the stigma on mid-anthesis phase-T2; (d) Secretory activity of the stigma on late-anthesis phase-T3 (arrows show stigma secretion); (e) Stigma stain at early anthesis phase-T1; (f) Stigma stain at late-anthesis phase-T3; (g) Stigma stain at mid-anthesis phase-T2 (arrows indicate color and bubble occurrence).
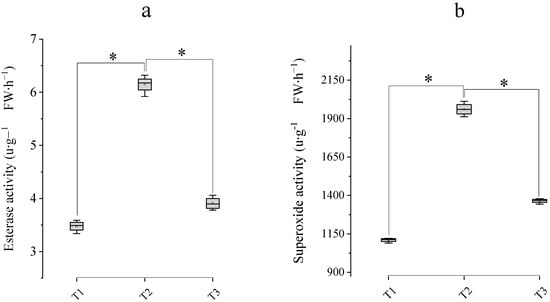
Figure 5.
Temporal dynamics of stigma enzyme activities in D. ‘Burana Jade’ at the early flowering stage, full-bloom stage, and fading stage. (a) Esterase activity (μmol·min−1·mg−1 protein); (b) Superoxide dismutase. Note: * indicates a significant difference according to the LSD test (α = 0.05). T1: early-anthesis phase; T2: mid-anthesis phase; T3: late-anthesis phase.
Morphological evidence as a useful sampling indicator could be used to assess stigma receptivity for an optimal hand-pollination period. In our research, Dendrobium stigmas were relatively receptive at a typical phase with crystals and bulging secretions.
3.3. Fruit Set of Eight Cross Combinations
Precision pollination: Targeted application of pollen to stigmas during peak receptivity windows, optimized by synchronizing pollen viability and stigma secretion status. Compared with random pollination, a total of eight precision combinations were pollinated 95 times to obtain eight fruits. The male parent was compatible with four wild species (D. aphyllum, D. chrysotoxum, D. hercoglossum and D. thyriflorum). The maximum fruit set (30.77%) was recorded across D. ‘Burana Jade’ × D. hercoglossum followed by D. ‘ Burana Jade’ × D. aphyllum (18.18%). D. ‘Burana Jade’ × D. chrysotoxum (10%) was equal to D. ‘Burana Jade’× D. thyriflorum (Table 1).

Table 1.
Different pollination type revealed the results of interspecific cross-compatibility.
The pods needed 121 d to 252 d to ripen, and the longest development period was observed in the D. ‘Burana Jade’ × D. chrysotoxum cross. Only D. ‘Burana Jade’ crossed with D. thyrsiflorum or D. aphyllum produced a relatively short maturity process (121 d, 138 d). The cross combination (D. ‘Burana Jade’ × D. aphyllum) had the heaviest capsule of 4.01 g despite a shorter maturity period.
3.4. Hybrid Seed Development and Viability
Naturally matured hybrid capsules were dissected to reveal internally distributed white or yellow seeds (Figure 6a–d). Seed arrangement varied significantly among cross combinations: In D. ‘Burana Jade’ × D. aphyllum, D. ‘Burana Jade’ × D. chrysotoxum, and D. ‘Burana Jade’ × D. thyrsiflorum, seeds remained embedded within protuberant flocculent placental tissues, forming aggregated powdery masses without dispersal. Crosses D. ‘Burana Jade’ × D. aphyllum and D. ‘Burana Jade’ × D. chrysotoxum showed preferential seed accumulation in the proximal (calyx end) and median regions of the fruit, with sparse distribution toward the distal (pedicel end). Conversely, hybrids D. ‘Burana Jade’ × D. hercoglossum and D. ‘Burana Jade’ × D. thyrsiflorum displayed uniform seed distribution throughout the locules. Seeds of D. ‘Burana Jade’ × D. hercoglossum exhibited a dispersed morphology, appearing as distinct granular particles. The cross D. ‘Burana Jade’ × D. hercoglossum exhibited markedly higher seed density compared to other hybrids. The greatest length of seed was 450.80 μm in cross D. ‘Burana Jade’ × D. thyrsiflorum followed by D. ‘Burana Jade’ × D. aphyllum (442.56 μm) (Table 2). The minimum length was observed in the hybrid D. ‘Burana Jade’ × D. chrysotoxum (238.96 μm), which was significantly different from the others. The maximum width of the seeds was D. ‘Burana Jade’ × D. thyrsiflorum with a size of 79.65 μm. The smallest width was 28.84 μm in D. ‘Burana Jade’ × D. chrysotoxum. Seed morphology also exhibited distinct variation among different cross combinations (Figure 6e–h).
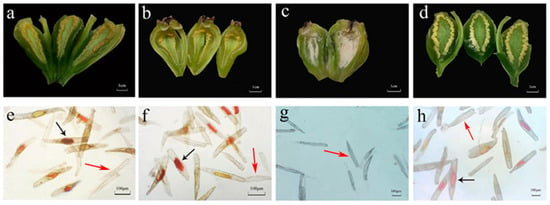
Figure 6.
Hybrid seeds in their seedpod and its viability as determined by TZ staining. Figure (a–d) shows the internal seed arrangement within seedpod of four hybrid cross combinations. Figure (e–h) shows the seed vigor of the four hybrids. (a,e) Cross D. ‘Burana Jade’ × D.aphyllum; (b,f) cross D. ‘Burana Jade’ × D.chrysotoxum; (c,g) cross D. ‘Burana Jade’ v D. hercoglossum; (d,h) cross D. ‘Burana Jade’ × D. thyrsiflorum (viable embryos exhibiting red coloration are denoted by black arrowheads, while non-viable embryos displaying white or yellow pigmentation are marked with red arrowheads).

Table 2.
The morphological characteristics of the seeds from the four interspecific crosses of Dendrobium included the size of the seeds, percentage of seeds with visible embryos, and amount of viability. The hybrid seed vigor was estimated with a 1% TZ test. The means followed by different letters are significantly different according to the least squared difference at p ≤ 0.05 (LSD0.05).
When D. ‘Burana Jade’ was crossed with D. thyrsiflorum and D. aphyllum, a greater percentage of visible embryos was observed in the hybrid seeds (54.81% and 42.78%, respectively). The embryo was located regularly in the center of the seed. However, the percentage of vigorous seeds in all four crosses was low and less than 25% (Table 2). Hybrid seeds of all the cross combinations except cross D. ‘Burana Jade’ × D. hercoglossum turned green up to 45–60 d, germinated in vitro, and produced protocorms on 1/4 Murashige and Skoog (MS) media. The germination frequency did not increase after 60 d (Figure 7).

Figure 7.
Germination performance of seeds from three hybrid cross combinations cultured on 1/4 MS (Murashige and Skoog) media. (a) D. ‘Burana Jade’ × D. thyrsiflorum; (b) D. ‘Burana Jade’ × D. chrysotoxum; (c) D. ‘Burana Jade’ × D. aphyllum.
4. Discussion
4.1. Vigor Dynamics of the Pollen and Stigma
The variation in pollen vigor during the different flowering periods was significantly associated with floral age. Balloon flowers (Platycodon grandiflorum A.DC.) could bear fruits if they were fertilized at the time of highest pollen vigor within 2 d of flowering [28,29]. The pollen viability of marijuana (Cannabis sativa L.) was greatest between 0 and 3 d of anther dehiscence [30]. Because of the long flowering period, the determination of Dendrobium pollen activity is helpful for determining the optimum sampling time. Our findings revealed that Dendrobium has variable patterns of pollen viability, which might occur during early anthesis or mid-anthesis phase. Few varieties, such as Dendrobium lindleyi Stendel, have high vigor during early flowering [31]. Consequently, the use of randomly selected pollen grains without prior viability assessment (e.g., via TTC staining) significantly elevates the risk of fertilization failure during artificial pollination.
The optimal stigma receptivity affects the recognition of pollen and its germination [32]. Some species can be judged by visual evidence. The proper period of Chinese jujube (Zizyphus jujuba ‘Zhongqiu Sucui’) development is from the sepal flattening stage to the petal flattening stage [33]. Swainsona formosa was found to be receptive from 1 d before anther dehiscence until 4 d after anther dehiscence [34]. Lagerstroemia indica is accompanied by a columnar style, an upwards stigma, green and wet papillae, and copious exudates. The stigmas exhibited prolonged receptivity [35]. The mucilage secretion phenotype of Dendrobium stigma was consistent with that of H2O2, SOD, and esterase activity.
4.2. Determinators of the Creation of Interspecific Hybrids
Fertilization depends not only on pollen and stigma viability, but also on parental relationships. Phylogenetic divergence strongly impacts the intersectional cross-compatibility of Dendrobium [36,37]. The relatively greater compatibility of the intersectional cross (Phalaenanthe vs Spatulata) created all of the current generations of cutflower or potted cultivars of Dendrobium [38]. In addition, Section Phalaenanthe showed compatibility from high to low in sequence with Sections Formosae, Callista, and Dendrobium [39]. Devadas et al. [40] reported that the pod set across combinations of species × species was dramatically lower than that of hybrids × species as well as D. phalaenopsis hybrids (Section Phalaenanthe) crossed with Breviflore (100%), Callista (85.7%) and Dendrobium (33.3%) species. In our experiment, D. ‘Burana Jade’, a hybrid of Section Phalaenopsis, exhibited maximum pod formation when crossed with D. hercoglossum from the Brevifloes section (30.77%). The mid-range was observed in cross combination (D. ‘Burana Jade’ and D. aphyllum of Section Dendrobium) (18.18%). When D. ‘Burana Jade’ was crossed with D. chrysotoxum and D. thyrsiflorum from the Callista section, the average fruit set was 10%. However, D. ‘Burana Jade’ did not cross successfully with D. cariniferum from the Formosae section. These findings suggest that there were different degrees of cross-compatibility among the four hybrids. These results support Devadas’ points on the intersectional compatibility of hybrids with the species of different sections mentioned above.
In previous studies on Dendrobium cross compatibility, in vitro germination was measured as an important and classic indicator at the postzygotic stage [9,40,41]. Seed germination confirmed the postzygotic compatibility of the three hybrids except for the cross of D. ‘Burana Jade’ × D. hercoglossum. Compared with self-crossed seeds of D. hercoglossum, the ratio of seeds with visible embryos from the cross D. ‘Burana Jade’ × D. hercoglossum was relatively lower and the hybrid embryo appeared malnourished because of parent gamete incompatibility. In our research, hybrid embryo in Dendrobium (visible embryo, embryo vigor) is reported for the first time. The results reveal that an abortive embryo, a malnutrition barrier, and weak viability of the embryo are characteristic features of interspecific incompatibility.
5. Conclusions
This is the first study showing a precision Dendrobium artificial pollination by pollen viability and stigma receptivity. A precise sampling criterion for morphological characteristics can be confirmed by means of relative physiological measures of the stigma and pollen vigor associated with accurate hand pollination. Dendrobium stigmas exhibit peak receptivity during the secretory phase, characterized by distinct crystalline deposits and pronounced exudate formation, which facilitates pollen adhesion and hydration. At this stage, the enzyme activities of esterase and superoxide dismutase (SOD) reach their peak levels.
Dendrobium pollen vigor shows two changing patterns: ‘weak vigor-–strong vigor–weak vigor’ and slash shape ‘strong vigor–weak vigor’. Identification of the changing patterns of Dendrobium pollen viability and its duration would be beneficial for determining the optimal sampling time for a successful fertilization. These results provide a valuable and user-friendly pollination technique for manual pollination of Dendrobium for hybridization. It is also revealed that the abortive embryo, malnutrition barrier, and weak viability of the embryo are characteristic features of interspecific incompatibility. Nevertheless, certain limitations inherent to this investigation warrant consideration. First, the sample size (n = 95 crosses) may limit generalizability across diverse Dendrobium genotypes. Second, while enzymatic markers show predictive value, their molecular regulatory mechanisms require functional validation.
Author Contributions
Conceptualization, Z.L. and S.Y.; methodology, J.Y. (Jianwei Yang); formal analysis, Y.Y. and A.G.; resources, Y.L. and J.Y. (Junmei Yin); data curation, Q.W.; writing—original draft preparation, Q.W. and B.D.; writing—review and editing, Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from the following sources: the National Key Research and Development Project of the China Ministry of Science and Technology (2019YFD1001003), the Hainan Major Science and Technology Program (ZDKJ2021015), the Hainan Natural Science Foundation (321RC648) and the Earmarked Fund for CARS-23-G60. The authors thank Dr. Jun Ming of the Chinese Academy of Agricultural Science for valuable suggestions on the manuscript. The authors also thank anonymous reviewers for their comments on the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Zongyan Li, upon reasonable request. Please contact lizyan@swfu.edu.cn for data access.
Acknowledgments
All the authors thank Jun Ming of the Chinese Academy of Agricultural Science for valuable suggestions regarding the manuscript. The authors also thank anonymous reviewers for their comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SOD | superoxide dismutase |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| MS | Murashige and Skoog |
| TZ | modified tetrazolium |
References
- Ketsa, S.; Warrington, I.J. The Dendrobium Orchid: Botany, Horticulture, and Utilization. Crop Sci. 2023, 63, 1829–1888. [Google Scholar] [CrossRef]
- Hayati, N.Q.; Nugrahapsari, R.A.; Sastro, Y. Analysis of Competitive Assessment of Consumer Requirements and Technical Requirements on The Quality Development of Dendrobium Orchid Potted Flowers. In Proceedings of the International Symposia on Horticulture (ISH) on Emerging Challenges and Opportunities in Horticulture Supporting Sustainable Development Goals, Bali, Indonesia, 27–30 November 2018. [Google Scholar]
- Deng, Y.; Chen, S.; Chen, F.; Cheng, X.; Zhang, F. The Embryo Rescue Derived Intergeneric Hybrid between Chrysanthemum and Ajania Przewalskii Shows Enhanced Cold Tolerance. Plant Cell Rep. 2011, 30, 2177–2186. [Google Scholar] [CrossRef]
- Zhu, W.; Lu, M.; He, Q.; Zhu, G. A Review: Orchid Industry and Scientific Research Achievements in China. In Proceedings of the Paper presented at the IV International Orchid Symposium 1414, Guangzhou, China, 9 December 2024. [Google Scholar]
- Lester, R.N.; Kang, J.H. Embryo and Endosperm Function and Failure inSolanumSpecies and Hybrids. Ann. Bot. 1998, 82, 445–453. [Google Scholar] [CrossRef]
- Oneal, E.; Willis, J.H.; Franks, R.G. Disruption of Endosperm Development Is a Major Cause of Hybrid Seed Inviability between Mimulus Guttatus and Mimulus Nudatus. New Phytol. 2016, 210, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Borba, E.L.; Semir, J.; Shepherd, G.J. Self-Incompatibility, Inbreeding Depression and Crossing Potential in Five Brazilianpleurothallis (Orchidaceae) Species. Ann. Bot. 2001, 88, 89–99. [Google Scholar] [CrossRef]
- Ricci, N.A.P.; Bento, J.P.S.P.; Mayer, J.L.S.; Singer, R.B.; Koehler, S. Gametophytic Self-Incompatibility in Maxillariinae Orchids. Protoplasma 2024, 261, 271–279. [Google Scholar] [CrossRef]
- Johansen, B. Incompatibility in Dendrobium (Orchidaceae). Bot. J. Linn. Soc. 1990, 103, 165–196. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Y.; Liu, Y.; Chen, D.; Luo, Y.; Niu, S. Challenges and Perspectives in the Study of Self-Incompatibility in Orchids. Int. J. Mol. Sci. 2021, 22, 12901. [Google Scholar] [CrossRef]
- Eyles, A.; Close, D.C.; Quarrell, S.R.; Allen, G.R.; Spurr, C.J.; Barry, K.M.; Whiting, M.D.; Gracie, A.J. Feasibility of Mechanical Pollination in Tree Fruit and Nut Crops: A Review. Agronomy 2022, 12, 1113. [Google Scholar] [CrossRef]
- Xing, G.; Qu, L.; Zhang, W.; Zhang, Y.; Yuan, X.; Lei, J. Study on Interspecific Hybridization between Tulip Cultivars and Wild Species Native to China. Euphytica 2020, 216, 1–17. [Google Scholar] [CrossRef]
- Liu, P.; Quan, X.; Song, Z.; Li, W.; Wang, Y.; Gu, H.; Xie, D.; Yang, W.; Dresselhaus, T.; Zhong, S.; et al. A Two-Step Self-Pollination Mechanism Maximizes Fertility in Brassicaceae. Cell 2025, 24, 3550–3558. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Yadav, N.S.; Sorokin, A.; Bilichak, A.; Kovalchuk, I. Development and Optimization of a Germination Assay and Long-Term Storage for Cannabis Sativa Pollen. Plants 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Lorne, M.W. Stigma Receptivity over the Lifetime of the Hermaphroditic Flower of Elsholtzia Rugulosa Was Negatively Correlated with Pollen Viability. Plant Signal. Behav. 2016, 11, e1259052. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Sun, D.; Gao, C. Flower Opening Dynamics, Pollen-Ovule Ratio, Stigma Receptivity and Stigmatic Pollen Germination (in-Vivo) in Chaenomeles Speciosa (Sweet) Nakai. Sci. Rep. 2024, 14, 7127. [Google Scholar] [CrossRef]
- Qiu, J.; Gao, C.; Wei, H.; Wang, B.; Hu, Y.; Guo, Z.; Long, L.; Yang, L.; Li, H. Flowering Biology of Rhododendron Pulchrum. Horticulturae 2021, 7, 508. [Google Scholar] [CrossRef]
- Buchner, L.; Eisen, A.-K.; Šikoparija, B.; Jochner-Oette, S. Pollen Viability of Fraxinus Excelsior in Storage Experiments and Investigations on the Potential Effect of Long-Range Transport. Forests 2022, 13, 600. [Google Scholar] [CrossRef]
- Al-Najm, A.; Brauer, S.; Trethowan, R.; Merchant, A.; Ahmad, N. Optimization of in Vitro Pollen Germination and Viability Testing of Some Australian Selections of Date Palm (Phoenix Dactylifera L.) and Their Xenic and Metaxenic Effects on the Tissue Culture—Derived Female Cultivar Barhee. Vitr. Cell. Dev. Biol. Plant 2021, 57, 771–785. [Google Scholar] [CrossRef]
- Breygina, M.; Klimenko, E.; Shilov, E.; Podolyan, A.; Mamaeva, A.; Zgoda, V.; Fesenko, I. Hydrogen Peroxide in Tobacco Stigma Exudate Affects Pollen Proteome and Membrane Potential in Pollen Tubes. Plant Biol. 2021, 23, 592–602. [Google Scholar] [CrossRef]
- Breygina, M.; Luneva, O.; Schekaleva, O.; Lazareva, N.; Babushkina, K.; Kirilyuk, I.A. Pattern of Ros Generation and Interconversion on Wet Stigmas in Basal and Divergent Angiosperms. Plant Growth Regul. 2023, 101, 463–472. [Google Scholar] [CrossRef]
- Schekaleva, O.; Luneva, O.; Klimenko, E.; Shaliukhina, S.; Breygina, M. Dynamics of Ros Production, Sod, Pod and Cat Activity During Stigma Maturation and Pollination in Nicotiana Tabacum and Lilium Longiflorum. Plant Biol. 2024, 26, 1240–1246. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Wei, J. Study on Flowering Dynamics and Pollination Habits of Monochasma Savatieri under Artificial Cultivation Conditions. Plants 2025, 14, 715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yu, Y.; Liu, Y.; Yang, S.; Chen, Y. Gradual Pollen Presentation in Vaccinium Corymbosum ‘Bluecrop’: An Adaptive Mechanism to Improve Pollination Efficiency and Outcrossing. PeerJ 2024, 12, e17273. [Google Scholar] [CrossRef]
- Hu, S. Experimental Methods in Plant Embryology (I) Determination of Pollen Viability. Chin. Bull. Bot. 1993, 10, 60. [Google Scholar]
- Breygina, M.; Schekaleva, O.; Klimenko, E.; Luneva, O. The Balance between Different Ros on Tobacco Stigma During Flowering and Its Role in Pollen Germination. Plants 2022, 11, 993. [Google Scholar] [CrossRef]
- Soares, J.S.; Rosa, Y.B.C.J.; Tatara, M.B.; Sorgato, J.C.; Lemes, C.S.R. Orchid Seeds Viability Identification by Tetrazolium Test. Dir. Open Access J. 2014, 35, 2275–2284. [Google Scholar]
- Kılıç, T.; Sinanoğlu, E.; Kırbay, E.; Kazaz, S.; Ercişli, S. Determining Appropriate Methods for Estimating Pollen Viability and Germination Rates in Lisianthus. Acta Sci. Pol. Hortorum Cultus 2024, 23, 33–42. [Google Scholar] [CrossRef]
- Li, M.; Jiang, F.; Huang, L.; Wang, H.; Song, W.; Zhang, X.; Zhang, Y.; Niu, L. Optimization of in Vitro Germination, Viability Tests and Storage of Paeonia Ostii Pollen. Plants 2023, 12, 2460. [Google Scholar] [CrossRef]
- Wizenberg, S.B.; Dang, M.; Campbell, L.G. Methods for Characterizing Pollen Fitness in Cannabis Sativa L. PLoS ONE 2022, 17, e0270799. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.M.; Zheng, B.Q.; Guo, X.; Wang, Y. Pollen Viability and Preservation of Dendrobium Lindleyi/ Pollen viability and preservation of Dendrobium lindleyi. Lin Ye Ke Xue Yan Jiu 2014, 27, 657. [Google Scholar]
- Hao, Q.; Xu, L.; Wang, H.; Liu, Q.; Wang, K. Evaluation of Pollen Viability, Stigma Receptivity, and the Cross Barrier between Tropical and Hardy Water Lily Cultivars. Flora 2022, 290, 152046. [Google Scholar] [CrossRef]
- Shao, F.; Wang, S.; Chen, J.; Chen, J.; Hong, R.; Tang, Y.; Wang, J. Stigma Shape Development and Receptivity Of’zhongqiu Sucui’chinese Jujube. Acta Hortic. Sin. 2019, 46, 2309–2322. [Google Scholar]
- Zulkarnain, Z.; Eliyanti, E.; Swari, E.I. Pollen Viability Stigma Receptivity in Swainsona Formosa (G. Don) J. Thompson (Fabaceae), an Ornamental Legume Native to Australia. Ornam. Hortic. 2019, 25, 158–167. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.; Wang, X.; Zeng, H.; Qiao, Z. Germination Characteristics of Fruiting Nonfruiting Lagerstroemia Indica Pollen. J. Cent. South. Univ. For. Technol. 2023, 43, 74–81. [Google Scholar]
- Lestari, N.K.D.; Deswiniyanti, N.W.; Sari, N.K.Y.; Murna, I.M.; Rizqy, A.N. Morphological Relationships and Cross Compatibility of Seven Dendrobium Species in Indonesia. Biodiversitas J. Biol. Divers. 2023, 24, 6. [Google Scholar] [CrossRef]
- Maulida, D.; Yusnita, Y.; Hapsoro, d.; Agustiansyah, A.; Karyanto, A. Interspecific Hybridization of Dendrobium Mirbelianum X D. Nindii or D. Discolor, in Vitro Seed Germination, Seedling Growth and Plantlet Acclimatization. Biodiversitas J. Biol. Divers. 2023, 24, 5. [Google Scholar] [CrossRef]
- Yuan, S.-C.; Lekawatana, S.; Amore, T.D.; Chen, F.-C.; Chin, S.-W.; Vega, D.M.; Wang, Y.-T. The Global Orchid Market. In Orchid Genome; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–28. [Google Scholar]
- Kamemoto, H.; Wilfret, G.J. Inter-and multi-sectional Dendrobium hybrids. Proc. 9th World Orchid Conf. 1980, 255, 261. [Google Scholar]
- Devadas, R.; Pattanayak, S.R.; Singh, D.R. Studies on Cross Compatibility in Dendrobium Species Hybrids. Indian J. Genet. Plant Breed. 2016, 76, 344–355. [Google Scholar] [CrossRef]
- Sirilak, I.; Bundithya, W.; Kuanprasert, N.; Apavatjrut, P. Analysis of Intersectional Hybrids of Dendrobiumby RAPD Technique. Kasetsart J. Nat. Sci. 2006, 40, 456–461. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).