Metabolomic and Transcriptomic Insights into Quality Formation of Orange-Red Carrot (Daucus carota L.) During Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Widely Targeted Metabolomics Analysis
2.2.1. Sample Preparation and Extraction
2.2.2. Chromatographic and Mass Spectrometric Conditions
2.2.3. Metabolite Identification and Quantification
2.3. RNA Extraction and RNA-Seq Analysis
- (1)
- mRNA enrichment via oligo (dT) magnetic beads;
- (2)
- RNA fragmentation and first-strand cDNA synthesis with random hexamers;
- (3)
- Second-strand synthesis using DNA Polymerase I/RNase H;
- (4)
- Purification (QiaQuick PCR Extraction Kit, Canspec, Shanghai, China) and size selection (agarose gel electrophoresis).
- Removing adapter sequences;
- Discarding reads with > 10% N bases or Q20 < 50%;
- Filtering rRNA and low-quality reads to obtain clean data (Q30 > 90%).
2.4. Statistical Analysis
3. Results
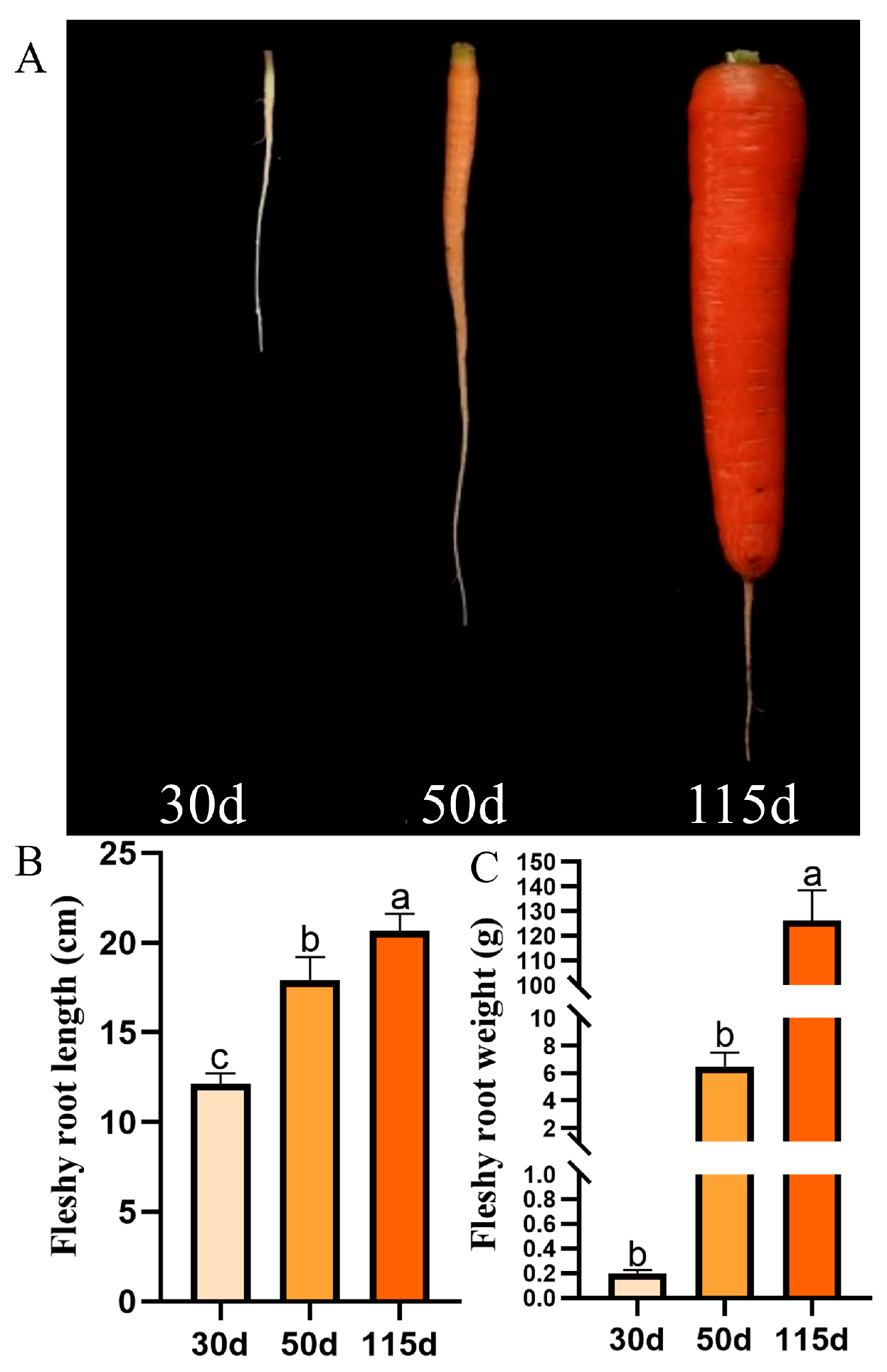
3.1. Morphological Physiology of Carrots
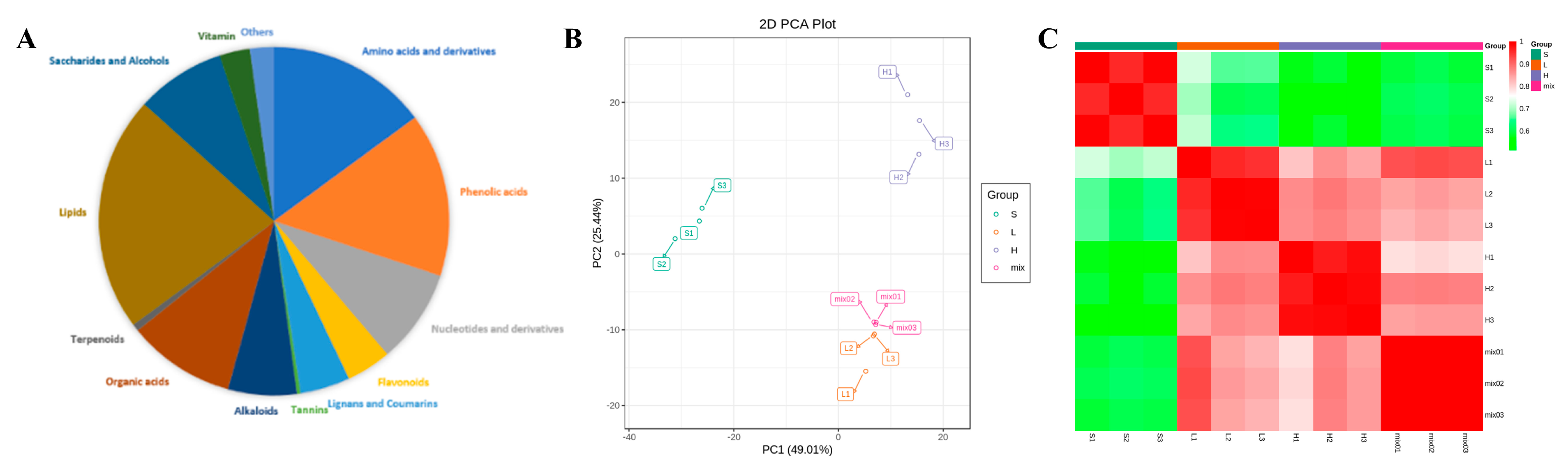
3.2. Analysis of Carrot Metabolites
3.3. Characteristics of Primary Metabolism During Carrot Maturation
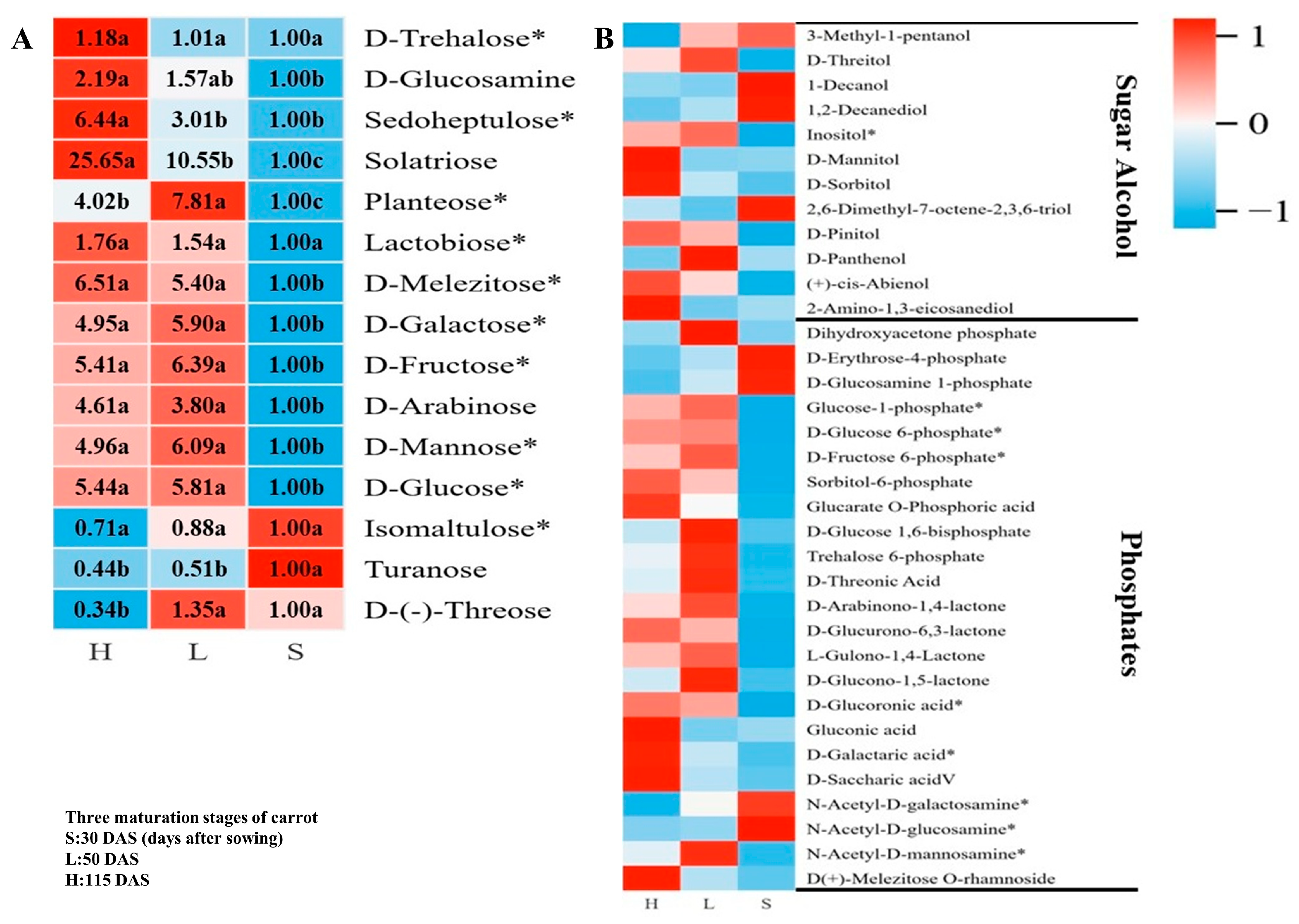
3.3.1. Metabolism of Sugar, Sugar Alcohols, and Their Phosphates
3.3.2. Organic Acid Metabolism
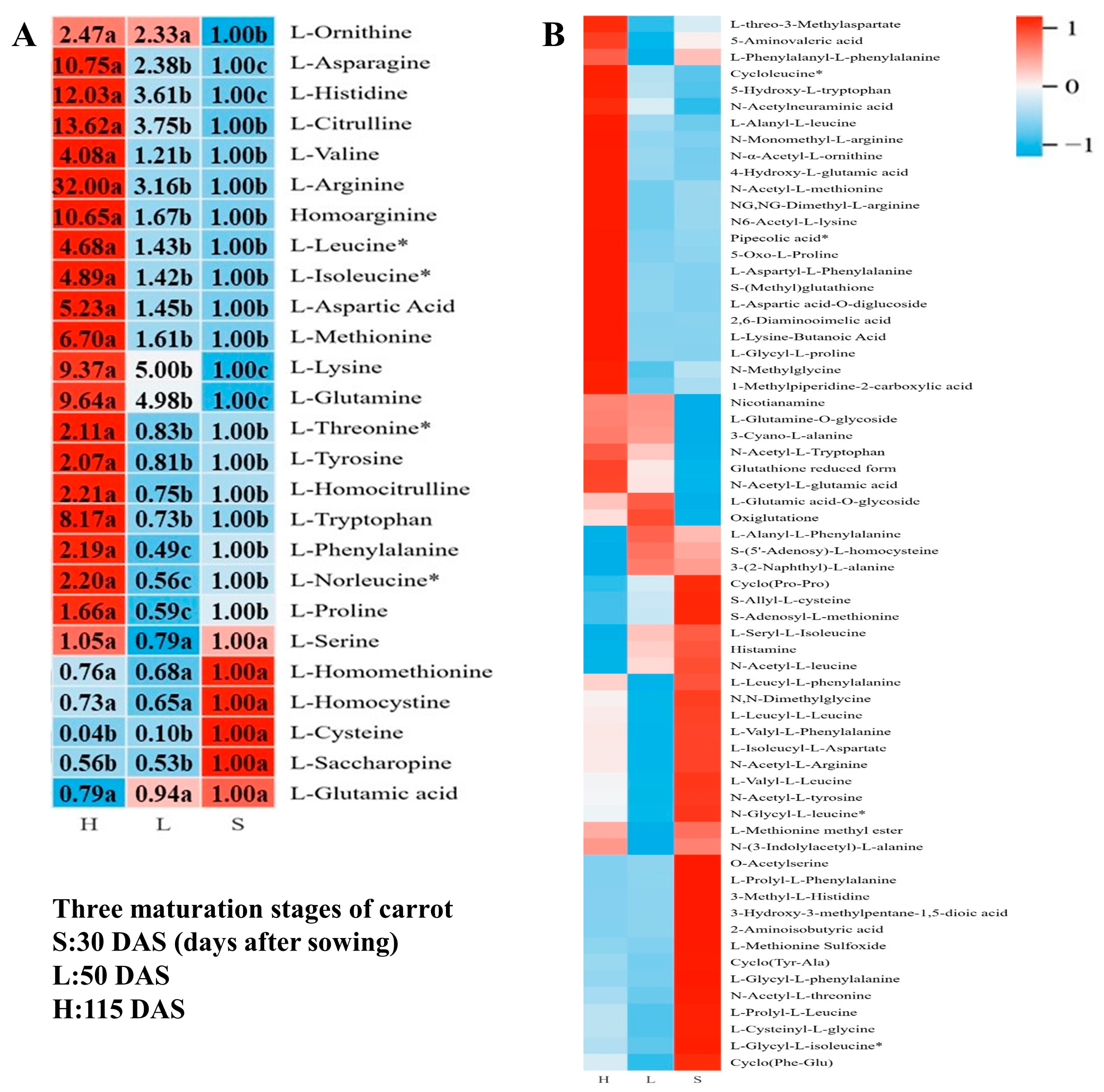
3.3.3. Accumulation Profiles of Amino Acids and Their Derivatives
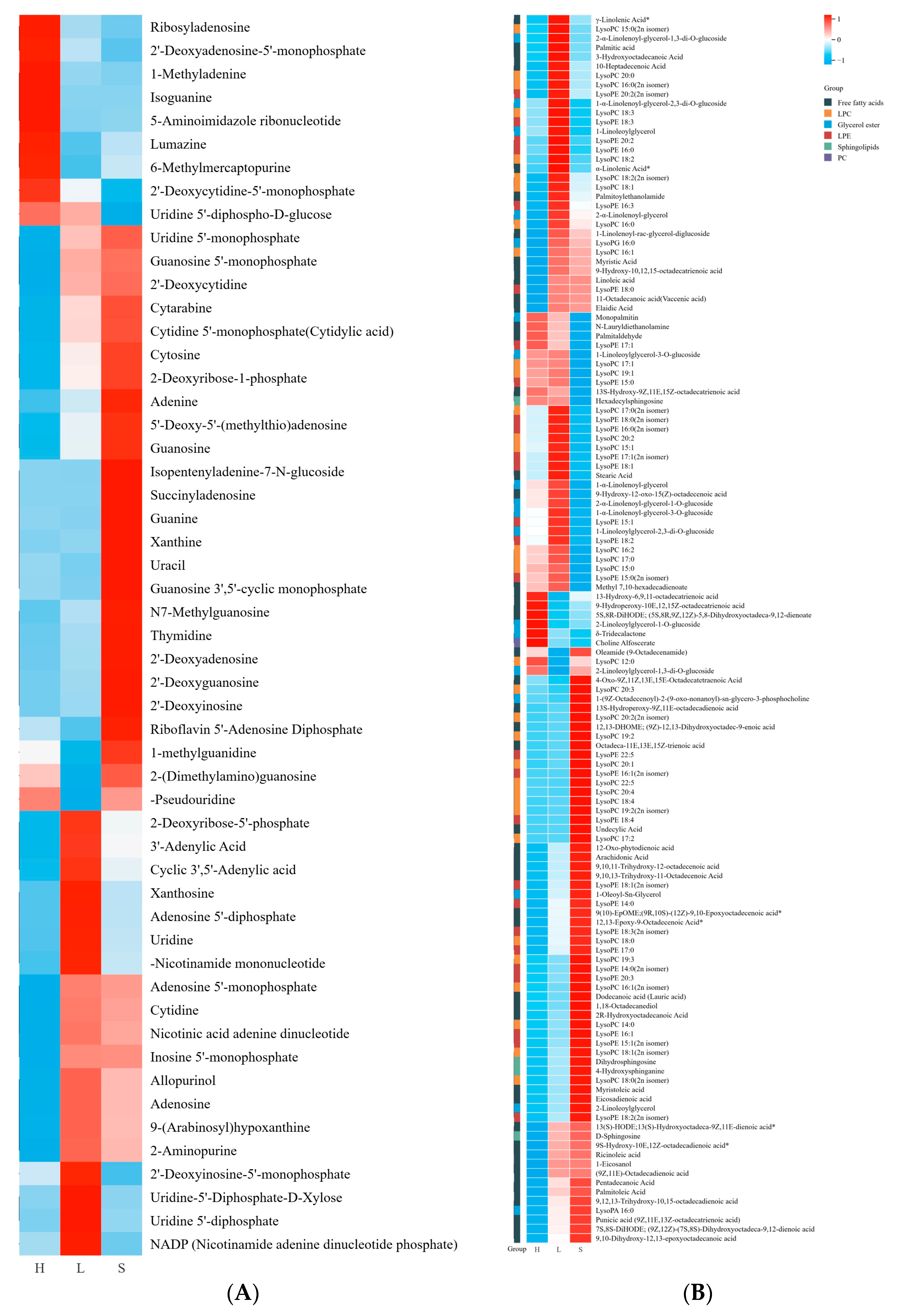
3.3.4. Other Primary Metabolites
3.4. Secondary Metabolism Characteristics During Carrot Maturation
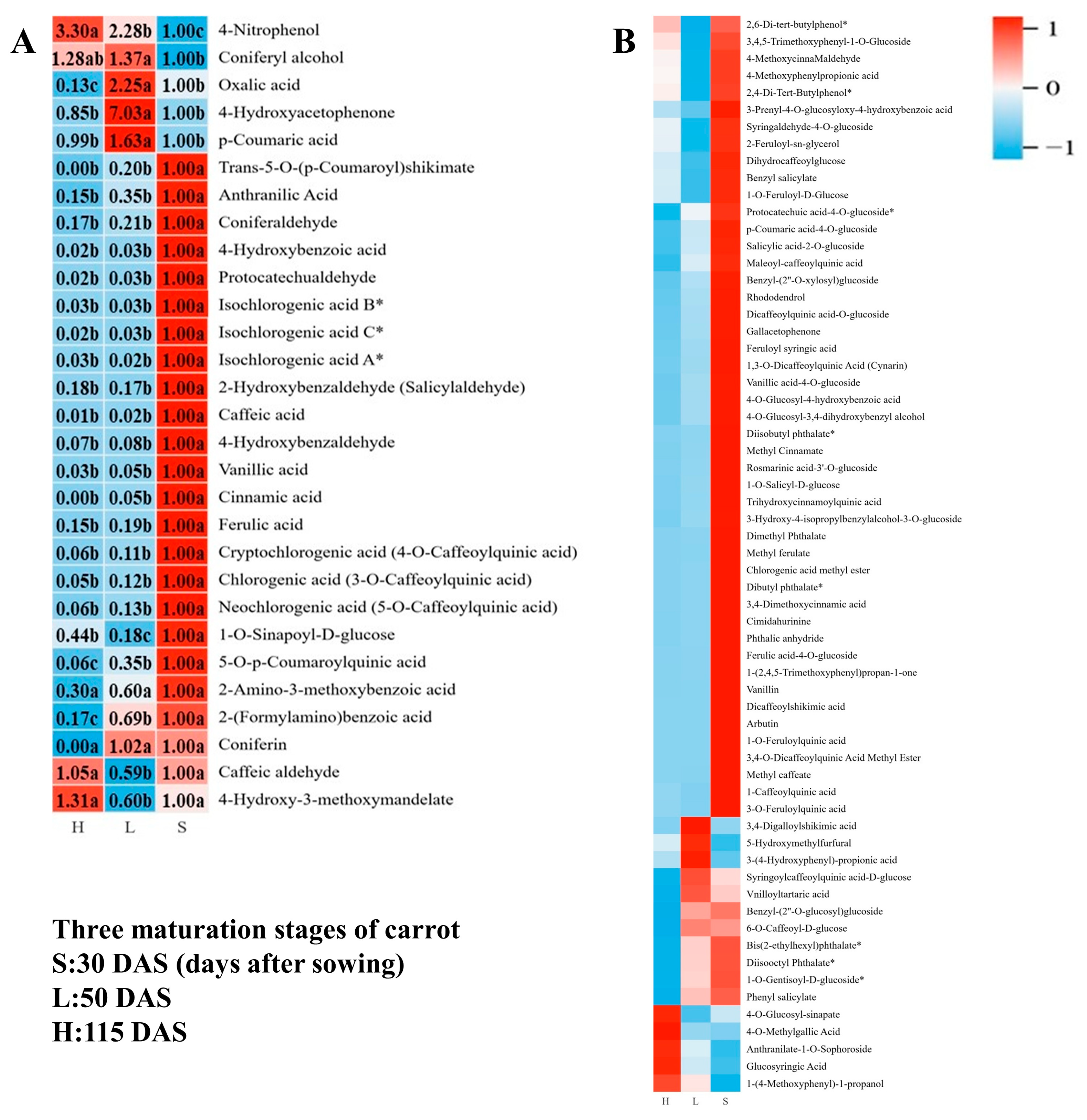
3.4.1. Metabolism of Phenolic Acid
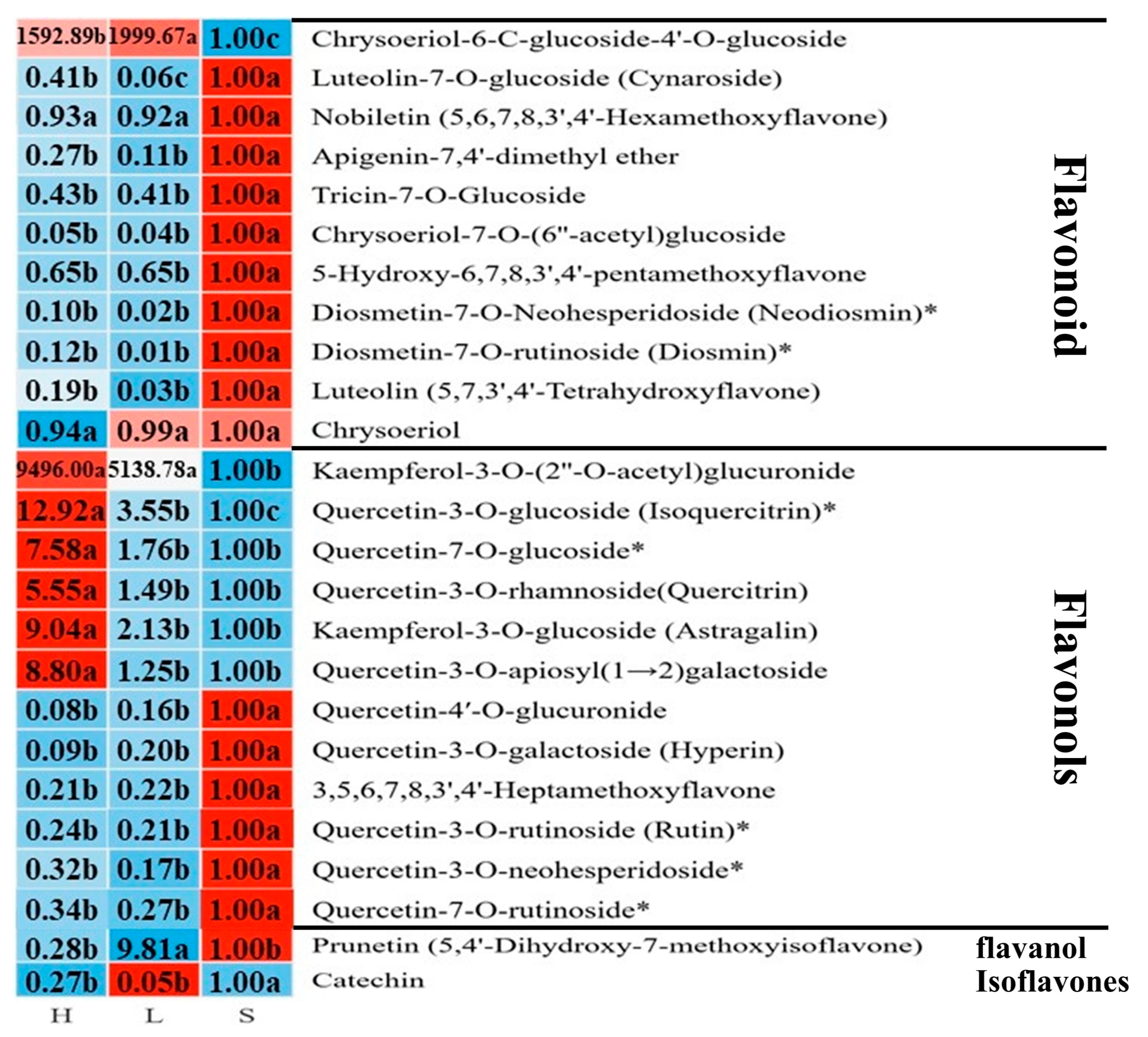
3.4.2. Flavonoid Metabolism
3.4.3. Accumulation of Other Secondary Metabolites
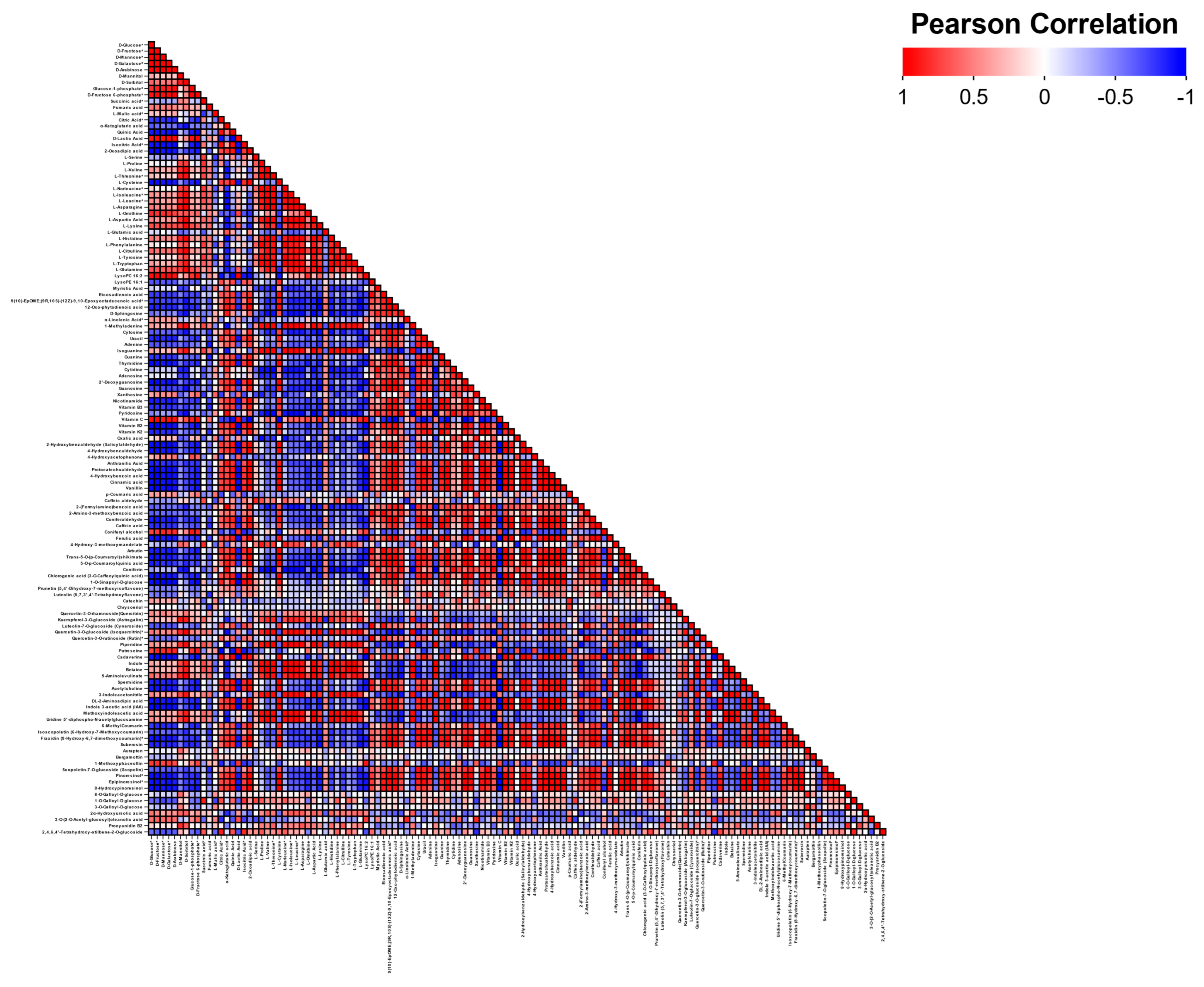
3.5. Metabolite–Metabolite Correlation During Carrot Maturation
3.6. Differentially Accumulated Metabolites Among Different Carrot Maturation
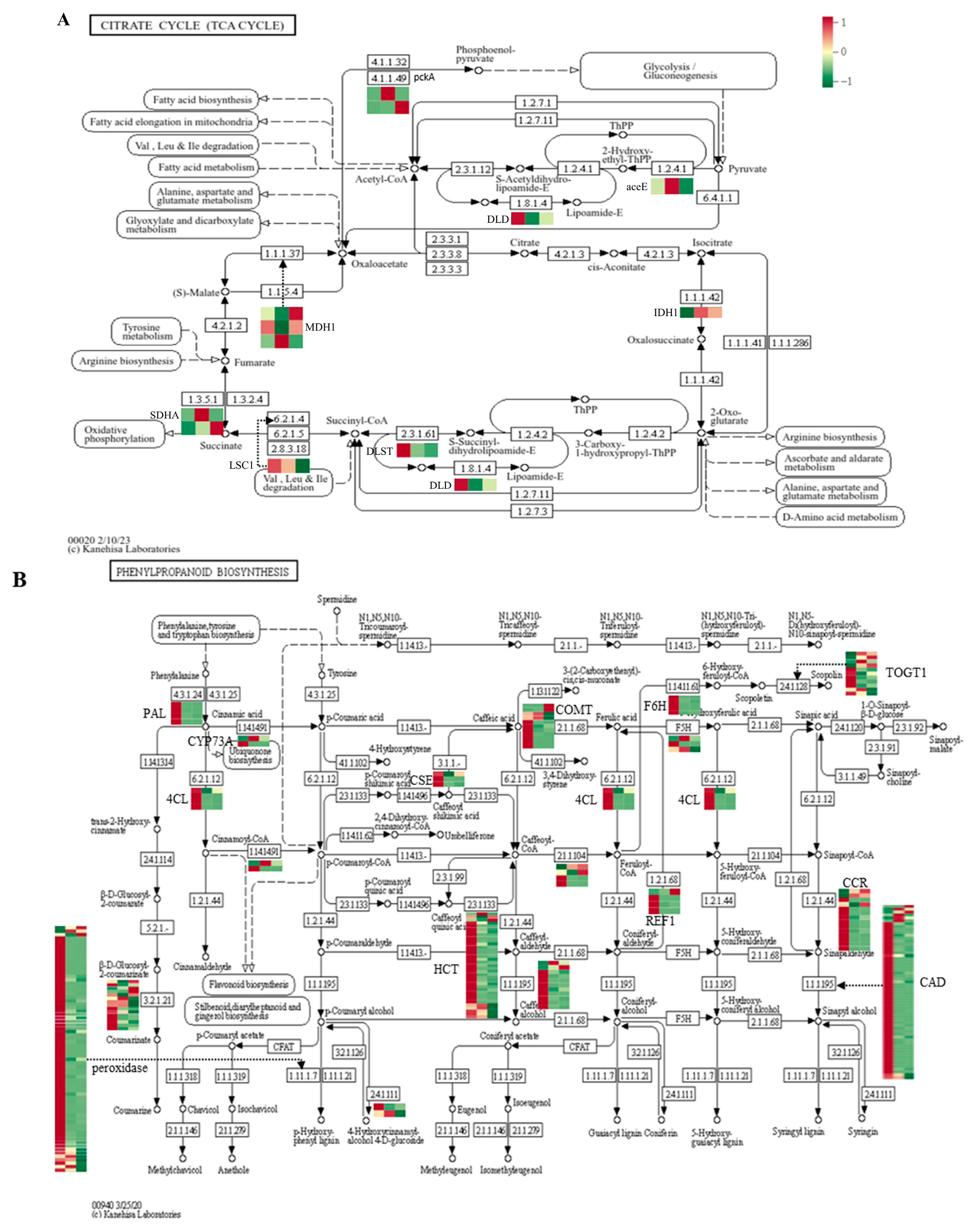
3.7. Expression Patterns of Metabolic Pathway-Related Genes During Carrot Development and Maturation
4. Discussion
4.1. Primary Metabolites
4.2. Secondary Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Que, F.; Hou, X.-L.; Wang, G.-L.; Xu, Z.-S.; Tan, G.-F.; Li, T.; Wang, Y.-H.; Khadr, A.; Xiong, A.-S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2011, 49, 22–32. [Google Scholar] [CrossRef]
- Riaz, N.; Yousaf, Z.; Yasmin, Z.; Munawar, M.; Younas, A.; Rashid, M.; Aftab, A.; Shamsheer, B.; Yasin, H.; Najeebullah, M.; et al. Development of Carrot Nutraceutical Products as an Alternative Supplement for the Prevention of Nutritional Diseases. Front. Nutr. 2022, 8, 787351. [Google Scholar] [CrossRef]
- Haq, R.-U.; Prasad, K. Nutritional and processing aspects of carrot (Daucus carota)—A review. South Asian J. Food Technol. Environ. 2015, 1, 1–14. [Google Scholar] [CrossRef]
- Nothnagel, T.; Ulrich, D.; Dunemann, F.; Budahn, H. Sensory Perception and Consumer Acceptance of Carrot Cultivars Are Influenced by Their Metabolic Profiles for Volatile and Non-Volatile Organic Compounds. Foods 2023, 12, 4389. [Google Scholar] [CrossRef]
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Schmid, C.; Sharma, S.; Stark, T.D.; Günzkofer, D.; Hofmann, T.F.; Ulrich, D.; Dunemann, F.; Nothnagel, T.; Dawid, C. Influence of the Abiotic Stress Conditions, Waterlogging and Drought, on the Bitter Sensometabolome as Well as Agronomical Traits of Six Genotypes of Daucus carota. Foods 2021, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Muchlinski, A.; Ibdah, M.; Ellison, S.; Yahyaa, M.; Nawade, B.; Laliberte, S.; Senalik, D.; Simon, P.; Whitehead, S.R.; Tholl, D. Diversity and function of terpene synthases in the production of carrot aroma and flavor compounds. Sci. Rep. 2020, 10, 9989. [Google Scholar] [CrossRef]
- Prasad, K.; Haq, R.-U.; Bansal, V.; Siddiqui, M.W.; Ilahy, R. Carrot: Secondary metabolites and their prospective health benefits. In Plant Secondary Metabolites; Apple Academic Press: Palm Bay, FL, USA, 2017; Volume 2, pp. 133–220. [Google Scholar]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Velu, G.; Palanichamy, V.; Rajan, A.P. Phytochemical and Pharmacological Importance of Plant Secondary Metabolites in Modern Medicine. In Bioorganic Phase in Natural Food: An Overview; Springer: Cham, Switzerland, 2018; pp. 135–156. [Google Scholar]
- Clausen, M.R.; Edelenbos, M.; Bertram, H.C. Mapping the Variation of the Carrot Metabolome Using 1H NMR Spectroscopy and Consensus PCA. J. Agric. Food Chem. 2014, 62, 4392–4398. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, W.; Moussa, S.-A.; Ottoni, M.M.N.; Dubois-Laurent, C.; Huet, S.; Aubert, C.; Desnoues, E.; Navez, B.; Cottet, V.; Chalot, G.; et al. Evaluation of pedoclimatic factors and cultural practices effects on carotenoid and sugar content in carrot root. Eur. J. Agron. 2022, 140, 126577. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Wang, G.-L.; Ma, J.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Transcript profiling of sucrose synthase genes involved in sucrose metabolism among four carrot (Daucus carota L.) cultivars reveals distinct patterns. BMC Plant Biol. 2018, 18, 8. [Google Scholar] [CrossRef]
- Machaj, G.; Bostan, H.; Macko-Podgórni, A.; Iorizzo, M.; Grzebelus, D. Comparative Transcriptomics of Root Development in Wild and Cultivated Carrots. Genes 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, Y.; Wu, J.; Chen, Y.; An, Y.; Hu, Z.; Xiong, A. Integrated metabolome analysis and transcript profiles revealed a potential role of SWEETs in sugar accumulation during Carrot taproot development. BMC Plant Biol. 2025, 25, 470. [Google Scholar] [CrossRef]
- Ibdah, M.; Muchlinski, A.; Yahyaa, M.; Nawade, B.; Tholl, D. Carrot Volatile Terpene Metabolism: Terpene Diversity and Biosynthetic Genes. In The Carrot Genome; Compendium of Plant Genomes; Springer: Cham, Switzerland, 2019; pp. 279–293. [Google Scholar]
- Xu, J.; Yan, J.; Li, W.; Wang, Q.; Wang, C.; Guo, J.; Geng, D.; Guan, Q.; Ma, F. Integrative Analyses of Widely Targeted Metabolic Profiling and Transcriptome Data Reveals Molecular Insight into Metabolomic Variations during Apple (Malus domestica) Fruit Development and Ripening. Int. J. Mol. Sci. 2020, 21, 4797. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, R.; Guo, R.; Nong, H.; Qin, Y.; Dong, N. Integrative analysis of metabolome and transcriptome reveals molecular insight into metabolomic variations during hawthorn fruit development. Metabolites 2023, 13, 423. [Google Scholar] [CrossRef]
- Song, H.; Lu, Q.; Song, T.; Gao, C.; Zhu, W.; Guo, X. Study on the Mechanism of Carotenoid Production and Accumulation in Orange Red Carrot (Daucus carota L.). Sci. Hortic. 2024, 327, 112825. [Google Scholar] [CrossRef]
- Aubert, C.; Bruaut, M.; Chalot, G. Spatial distribution of sugars, organic acids, vitamin C, carotenoids, tocopherols, 6-methoxymellein, polyacetylenic compounds, polyphenols and terpenes in two orange Nantes type carrots (Daucus carota L.). J. Food Compos. Anal. 2022, 108, 104421. [Google Scholar] [CrossRef]
- Yusuf, E.; Tkacz, K.; Turkiewicz, I.P.; Wojdyło, A.; Nowicka, P. Analysis of chemical compounds’ content in different varieties of carrots, including qualification and quantification of sugars, organic acids, minerals, and bioactive compounds by UPLC. Eur. Food Res. Technol. 2021, 247, 3053–3062. [Google Scholar] [CrossRef]
- Schaller, R.G.; Schnitzler, W.H. Nitrogen nutrition and flavour compounds of carrots (Daucus carota L.) cultivated in Mitscherlich pots. J. Sci. Food Agric. 2000, 80, 49–56. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Gambacorta, G.; Elia, A. Morpho-Biometrical, Nutritional and Phytochemical Characterization of Carrot Landraces from Puglia Region (Southern Italy). Sustainability 2021, 13, 3940. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef]
- Kebede, B.T.; Grauwet, T.; Palmers, S.; Vervoort, L.; Carle, R.; Hendrickx, M.; Van Loey, A.J.F.C. Effect of high pressure high temperature processing on the volatile fraction of differently coloured carrots. Food Chem. 2014, 153, 340–352. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and Nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant Phytochemicals and Antioxidant Capacity of Biofortified Carrots (Daucus carota L.) of Various Colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef]
- Bartley, G.E.; Avena-Bustillos, R.J.; Du, W.-X.; Hidalgo, M.; Cain, B.; Breksa, A.P., III. Transcriptional regulation of chlorogenic acid biosynthesis in carrot root slices exposed to UV-B light. Plant Gene 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). J. Food Agric. Environ. 2004, 2, 95–100. [Google Scholar] [CrossRef]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Czepa, A.; Hofmann, T. Quantitative studies and sensory analyses on the influence of cultivar, spatial tissue distribution, and industrial processing on the bitter off-taste of carrots (Daucus carota L.) and carrot products. J. Agric. Food Chem. 2004, 52, 4508–4514. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Guo, L.; Zhao, H.; Ho, C.-T. Chemistry and bioactivity of nobiletin and its metabolites. J. Funct. Foods 2014, 6, 2–10. [Google Scholar] [CrossRef]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, P.; Gajewski, M.; Radzanowska, J.; Dabrowska, A. Sensory quality and consumer liking of carrot cultivars of different genotype. Veg. Crops Res. Bull. 2007, 67, 163. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Zhang, H.; Wang, J.; Guo, Z.; Shen, R.; Zhu, W.; Song, T.; Song, H. Metabolomic and Transcriptomic Insights into Quality Formation of Orange-Red Carrot (Daucus carota L.) During Maturation. Horticulturae 2025, 11, 542. https://doi.org/10.3390/horticulturae11050542
Gao C, Zhang H, Wang J, Guo Z, Shen R, Zhu W, Song T, Song H. Metabolomic and Transcriptomic Insights into Quality Formation of Orange-Red Carrot (Daucus carota L.) During Maturation. Horticulturae. 2025; 11(5):542. https://doi.org/10.3390/horticulturae11050542
Chicago/Turabian StyleGao, Chongzhen, Hongtao Zhang, Jiayu Wang, Ziqing Guo, Ruixue Shen, Weilong Zhu, Tianyue Song, and Hongxia Song. 2025. "Metabolomic and Transcriptomic Insights into Quality Formation of Orange-Red Carrot (Daucus carota L.) During Maturation" Horticulturae 11, no. 5: 542. https://doi.org/10.3390/horticulturae11050542
APA StyleGao, C., Zhang, H., Wang, J., Guo, Z., Shen, R., Zhu, W., Song, T., & Song, H. (2025). Metabolomic and Transcriptomic Insights into Quality Formation of Orange-Red Carrot (Daucus carota L.) During Maturation. Horticulturae, 11(5), 542. https://doi.org/10.3390/horticulturae11050542




