Abstract
The centennial olive trees of Tunisia serve as enduring symbols of resilience, having withstood the test of time while witnessing the effects of climate change, rising temperatures, water scarcity, and the emergence of new diseases. Presently, there is a notable lack of research on the genomic analysis of ancient trees. This study investigates the genetic diversity of twenty-eight ancient olive specimens collected from archeological sites in nine governorates from the north to the south of Tunisia. Using nine highly polymorphic microsatellite markers, these ancient olive trees were compared with twenty-five local Tunisian cultivars and sixty olive varieties from other Mediterranean countries (Greece, Italy, and Spain). The ancient olive trees were revealed to have a high genetic diversity, with 67 alleles and a Shannon index of 1.68. The key findings identify the ancient trees M25, M1, M28, and M24 as synonyms for local olive cultivars, while “M10” is noted as a first-generation migrant from Tunisian olives. Cluster analysis methods, including structure, neighbor-joining (NJ), and principal coordinates (PCoA), show that these ancient trees share a common genetic background and ancestry with varieties from Tunisia, Italy, Spain, and Greece. The conservation and evaluation of these genotypes will increase the genetic diversity available for breeding programs and strengthen the resilience of agriculture, which is currently facing unprecedented pressure worldwide.
1. Introduction
Olea europaea L. is one of the oldest cultivated plants in the Mediterranean region and worldwide. The species belongs to the Oleaceae family, which includes about 28 genera and around 700 species. Two primary varieties of Olea europaea L. are recognized: Olea europaea var. europaea, and Olea europaea var. sylvestris [1,2,3,4,5,6]. Centuries-old olive trees exist along the Aegean Sea between Greece and eastern Turkey. On Crete, the largest Greek island, there is a 3000-year-old olive tree of exceptional dimensions in Kavousi, with a circumference of 14.2 m and a diameter of almost 5 m [7]. There are also centenary olive trees on Mount Tabor and in Urla, a small Turkish peninsula, as well as in Spain and Italy [8]. In Tunisia, the olive tree was an integral part of the Berber, Carthaginian, and Roman civilizations and served many purposes, such as providing oil for food, medicine, and lighting, and wood for the construction of ships and tools. The olive tree also played an important role in shaping the landscape, preserving the environment and contrasting desertification and climate change [9].
The Carthaginians and the Romans played a significant role in promoting olive cultivation in ancient Tunisia by turning large arid areas into productive land. The trade exchanges between the Phoenicians and the Romans facilitated the introduction of foreign olive varieties into the Tunisian olive germplasm, leading to an impressive diversification of the olive tree [10,11]. The long history of olive cultivation in Tunisia and the genetic flow from other Mediterranean germplasms have produced large panoply of autochthonous varieties, totaling over 200 [12]. Nevertheless, ninety per cent of olive production is accounted for by two highly productive olive varieties: Chetoui in the north of Tunisia and Chemlali in central and southern Tunisia. The remaining ten per cent is accounted for by several minor varieties grown in marginal areas and cultivated by a few farmers in small local groves [13,14]. In Tunisia, it is common to see olive trees that are several hundred years old scattered across the landscape, reflecting the deep respect and admiration that the local communities have for these ancient trees. They can be considered an invaluable reservoir of genetic diversity, and several studies have shown that they could have great potential to improve the olive production, oil quality, and disease resistance of commercial varieties [15,16,17].
Research into the genetic diversity of ancient olive trees has uncovered unique traits that are potentially lost in modern cultivars due to selective breeding. In Tunisia, research has focused on commercial varieties, whereas there have been relatively few studies examining millennial olive trees. A significant study investigating the genetic diversity of ancient olive trees in the governorate of the Sousse region used RAPD and SSR markers [15]. More recently, Tunisian millennial olive trees were evaluated using morphological and oil quality parameters [15,17,18]. Similar research has been carried out in countries with rich olive-growing traditions, such as Turkey [19,20], Cyprus [21], Lebanon [22], Sicily [18], Malta [23], and Spain [24,25], revealing that ancient trees preserve interesting traits such as resistance to disease and tolerance to drought and salinity, and have specific characteristics that influence their oil’s composition and flavor [26]. Understanding the genetic makeup of ancient olive trees is essential for preserving diversity for future breeding applications. As the global demand for high-quality olive oil increases, that provided by these millennia-old trees could be crucial for the development of new olive tree varieties that can meet consumer demands and withstand the challenges of climate change.
Accordingly, interest in olive heritage is increasing in Mediterranean countries, and more and more initiatives are being introduced to preserve and promote its conservation and valorization [18,19,20,21,22,23,24,25,26,27]. In Tunisia, a research team from the National Gene Bank of Tunisia searched throughout the country for centenary olive trees from Roman and Carthaginian times, and found several giant olive trees with a circumference of 15 m, a diameter of 0.50 m, and gray trunks with knots [15,28]. These centuries-old olive trees produce oils of a good quality which is sometimes even higher than that of commercially available varieties in Tunisia, suggesting that they may have a distinctive genetic background [19]. To fully understand and exploit to the fullest extent the historical and agronomic value of these trees, it is crucial to identify the most valuable specimens and carry out genetic characterization. To achieve this goal, a set of nuclear SSR markers were used to genotype 26 historically important olive tree cultivars from different regions of Tunisia. Subsequently, these accessions were compared with local cultivars and other Mediterranean varieties. The results provide important insights into the origin of these valuable genetic resources, and shed light on their historical distribution and migration patterns in the southern Mediterranean.
2. Materials and Methods
2.1. Plant Material
Leaf samples were collected from 28 ancient olive trees found in Tunisian archeological sites with olive oil presses from the Punic and Roman periods. These sites are located across nine governorates, extending from the north to the south of Tunisia (Table 1, Figure 1). The growth pattern, structure, and trunk diameter of the olive trees were used as approximations of their age [4,29]; only trees with a diameter of 3 to 8 m were selected (Figure 2). The freshest leaves were collected from branches produced in the previous year at the four cardinal points of the tree, immediately placed in ice, and brought to the laboratory for DNA extraction.

Table 1.
Origin and use of millennium olives analyzed.
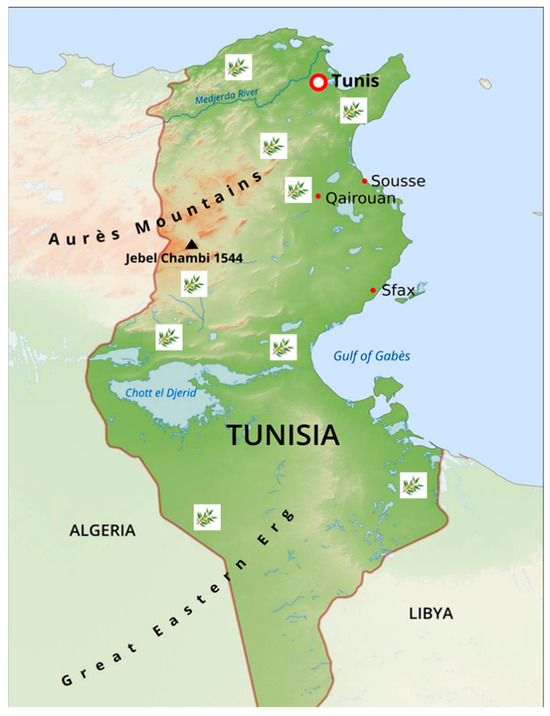
Figure 1.
Map of Tunisia with sites of collection of centenary olive trees.
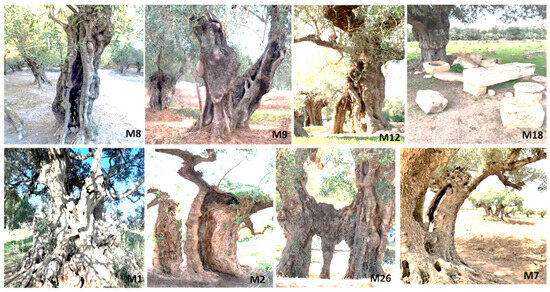
Figure 2.
Examples of millennium olive trees sampled in archeological sites of Kesra (M8-M9-M12), Sbeitla (M18), Haouaria (M1), Mednine (M2, M26), and Zahret Medyen (M7).
2.2. Molecular Analyses
2.2.1. DNA Extraction
Three leaves from each olive sample were freeze-dried, lyophilized, and ground to a fine powder. DNA extraction was performed using 50 mg of this material, according to [30]. To avoid contamination across samples, all the equipment, including mortars and pestles, were thoroughly cleaned with 70% ethanol between uses. Grinding was carried out in the laboratory using disposable materials to avoid contamination between samples. DNA quantity and quality were assessed on 1% agarose gel, using the NanoDrop TM ND 2000c (Thermo Scientific, Waltham, MA, USA). The DNA was then diluted to 50 ng/µL and stored at −20 °C until further use.
2.2.2. Olive Genotyping
The genetic profile of each olive sample was established by PCR, using 9 highly polymorphic microsatellite markers pre-selected for their efficiency, high polymorphism, and reproducibility [31] (Table S1). Amplifications were carried out in a final volume of 12.5 µL containing 50 ng of genomic DNA, 0.25 µL of Dream Taq buffer (10×), 0.6 µL of dNTP (2 M), 1.25 µL of a labeled primer mix (2.5 M), 0.2 µL of Dream Taq, and 7.7 µL of H2O. The thermal cycles consisted of an initial denaturation at 94 °C for 15 min, followed by ten rounds of the following sequence: denaturation at 94 °C for 30 s, annealing at a temperature between 50 °C and 60 °C for 1 min 30 s, depending on the primer, and extension at 72 °C for 1 min; and 25 cycles of the following sequence: denaturation at 94 °C for 30 s, annealing at 50 °C for 1 min 30 s, and extension at 72 °C for 1 min. The amplifications were performed in a C1000TM thermal cycler (Bio-Rad, Hercules, CA, USA). Negative controls were included to detect potential contamination of the reagents. Amplicons were separated using the ABI PRISM 3100 Avant Genetic Analyzer automatic capillary sequencer, using GeneScan Liz 600 dye (Applied Biosystems, Foster City, CA, USA) as an internal molecular weight standard. The allele size of each amplicon was estimated using the genotyping software GeneMapper v.3.7 (Applied Biosystems, Foster City, CA, USA). We performed an in-depth comparative study of the genetic profiles of 28 centenary olive varieties. This process included aligning the SSR size bands of these varieties with those of 25 popular Tunisian olive varieties that have been previously studied [12], and with those of a selection of 60 Mediterranean olive varieties from the Global Olive Genetic Database [32], including 8 varieties from Greece, 32 from Italy, and 20 from Spain (Table S4).
2.3. Statistical Analysis of Data
The results of the molecular analysis were recorded as bands of precisely determined sizes (bp). GenAlEx v. 6.501 software [33] was used to calculate allele frequency, number of alleles (Na), effective number of alleles (Ne), Shannon information index (I), fixation index (F), number of private alleles, marker-based relatedness (LRM), probability of identity (PI), and observed and expected heterozygosity rates (Ho, He). The software was also used to calculate the molecular variance between and within populations (AMOVA) and to perform a principal coordinate analysis (PCoA) based on inter-individual relationships, using Nei’s unbiased genetic distance pairwise population matrix.
We used CERVUS version 3.0.6 [34] to calculate the polymorphic information content (PIC) and estimate the occurrence of null alleles based on [35]. Furthermore, we performed a migrant detection analysis and assignment tests using GENECLASS2 software [36] to understand the dispersal patterns between centenary olives and olive cultivars that are prevalent in Tunisia. We also analyzed the dispersal patterns between the Tunisian gene pool and olive tree populations from Spain, Italy and Greece to identify the ’source’ genotypes among the populations studied.
2.3.1. Cluster Analysis
Using Darwin software, version 6.0.21 [37] (http://darwin.cirad.fr, accessed on 26 April 2019), the genotypes of centenary olive trees, together with those of the commercial olive varieties and the Spanish, Italian, and Greek olive germplasm, were hierarchically classified by applying the neighbor-joining (NJ) method based on a dissimilarity matrix, with bootstrapping of 1000 replicates to determine the support for each node [38].
2.3.2. Structure Analysis
The SSR profiles of the Tunisian monumental trees were compared with those of local cultivars and varieties from Spain, Italy, and Greece using STRUCTURE 2.3.4 software [39]. The nine microsatellite loci were first analyzed using the linkage disequilibrium (LD) test [40,41] to assess their association and to determine whether they met the necessary conditions for the application of the Bayesian approach. Subsequently, the Markov chain Monte Carlo (MCMC) algorithm [42] was used to explore the genetic structure of the populations. To determine the optimal number of subpopulations (K), ten separate iterations were performed for each value of K (from 1 to 10), using 100,000 MCMC repetitions and 100,000 burn-in periods. Harvester software (0.6.93) was used to determine the ideal number of subpopulations, as determined by the ad hoc statistical ∆K test developed by [43]. The membership coefficient (qi), which determines whether genotypes belong to the same population, was chosen as qi > 0.8; otherwise, genotypes were considered admixed (qi < 0.8). GenALEx software (6.503) was used to calculate the pairwise Fst between the groups identified by the STRUCTURE analysis.
3. Results
3.1. Genetic Diversity of Olive Genotypes
The molecular diversity analysis of the Tunisian centenarian olive trees revealed 67 bands, with an average of 7.44 alleles per locus (Table 2). The effective alleles (Ne) ranged from 2.53 for DCA15 to 6.67 for DCA09, with a mean of 4.78. The Shannon information index (I) ranged from 1.08 to 2.06 for the same markers (mean of 1.65). The polymorphism information content (PIC) was minimal for DCA15 (0.58), and reached its maximum for DCA09 (0.84). The highest observed heterozygosity (Ho) was found for GAPU101 (mean of 0.97), while the expected heterozygosity (He) was highest for DCA09 (mean 0.84). The mean of the inbreeding coefficient (F) was −0.049, and it ranged from −0.2 (GAPU101) to 0.26 (DCA18).

Table 2.
The global diversity indices of nine simple sequence repeat (SSR) markers, detected in twenty-eight historical and twenty-five commercialized olive trees in Tunisia, and sixty Mediterranean olive genotypes.
The analysis of genetic diversity in the five olive populations revealed the highest number of alleles (80) for the Italian germplasm (Table 3). The 28 centenary olives had a number of alleles (67) that was comparable to other groups and outnumbered the Tunisian varieties (60). The observed heterozygosity was higher than the expected heterozygosity in all the groups. Notably, the probability of identity (PI) was very low, at 7.1 × 10−11, indicating unique genotypes within the centenary germplasm overall, and a low probability of identity.

Table 3.
The global diversity indices obtained nine SSR markers in the five Mediterranean olive populations analyzed: number of alleles (Na), number of effective alleles (Ne), Shannon’s information index (I), observed heterozygosity (Ho), expected heterozygosity (He), fixation index (F).
PI = 10−10 indicates that the selected markers were highly informative, enabling clear differentiation among the five Mediterranean olive populations. Pairwise LRM relatedness identified five pairs of identical instances (LRM = 0.50) between centenarian olives and local Tunisian cultivars: M25/Chemlali Tataouine, M1/Barouni, Chemlali Sfax/Zalmati, M28/Meski, and M24/JEMRI_BC. In addition, the LRM cut-off at 0.35 revealed a dense network of close relationships between several ancient genotypes and cultivars, including M1, BAROUNI, and Besbessi; M13 and Neb Jemal Tataouine; and M10 and Chemlali Jerba in the Tunisian germplasm (Table S2).
3.2. Genetic Relationships Between Olive Genotypes
The AMOVA analysis revealed that only 4% of the genetic variation exists between populations, while 96% arises from within-population variance (at a significance level of 0.01%). This finding suggests limited genetic diversity between the groups and emphasizes a substantial genetic exchange among the Mediterranean O. europaea L. cultivars. This conclusion is bolstered by the F < 0 values, which indicate high heterozygosity within the population (Table S3).
A multi-locus genotype analysis was carried out to individuate unique combinations of alleles across multiple loci, to study the dispersal patterns among the analyzed Mediterranean olive populations, and to determine the origins of genotypes [36]. With a few exceptions, most of the samples could be assigned to their respective populations. The analysis revealed four potential first-generation migrants among the five olive populations (p < 0.01). Specifically, the centenary olive M10 was identified as a first-generation migrant of the local olive cultivars in Tunisia. In addition, the local Tunisian genotype Neb-Jemal-Tataouine was found to be a first-generation migrant from old olive trees. Similarly, the Spanish cultivars Arbequina and Sevillenca were recognized as potential first-generation migrants from the Italian olive tree population (Table 4 and Table S4).

Table 4.
Assignment of 113 Mediterranean olive cultivars to five predefined populations, using the algorithm of GeneClass2.
The genetic structure of the entire olive collection, including the centenarian genotypes, was analyzed using non-parametric principal coordinate analysis (PCoA) based on Nei’s unbiased genetic distance matrix. In this analysis, 21.2% of total diversity was assigned to the first two principal coordinates, PCo1 and PCo2 (Figure 3). The plot revealed two main clusters along the PCo1 axis, one including the samples from Italy, Spain, and Greece clustered on the right side, and the other collecting most of the Tunisian olive varieties, together with some European varieties, on the left side.
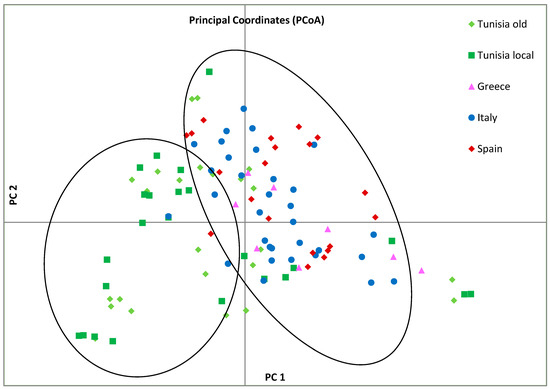
Figure 3.
Principal coordinate analysis (PCoA) of analyzed olive samples. Different colors represent geographic groups of olive varieties: Spanish: red, Italian: blue, Tunisian millennium olives: light green, Greek: pink, and Tunisian olive varieties: dark green. Principal component analysis (PCA) was performed, as a non-parametric alternative, to study genetic structure. PCA plot based on first two principal axes (PC1 and PC2) clearly separates individuals belonging to Tunisian population from other populations, which fall in different quadrants.
A neighbor-joining dendrogram (Figure 4) confirmed the results of the PCoA analysis, dividing the 113 genotypes into three distinct clusters. Cluster I contained a combination of types from the five populations, classified into two subclusters. The subcluster CI-1 consisted of most of the centenarian olive trees and Tunisian local cultivars, while the subcluster CI-2 included olive varieties from Spain, Greece, and Italy. Cluster II contained the Italian variety Leccino and the Spanish varieties Manzanilla de Sevilla and Villalonga. Cluster III comprised a mixture of olive varieties from the five populations.
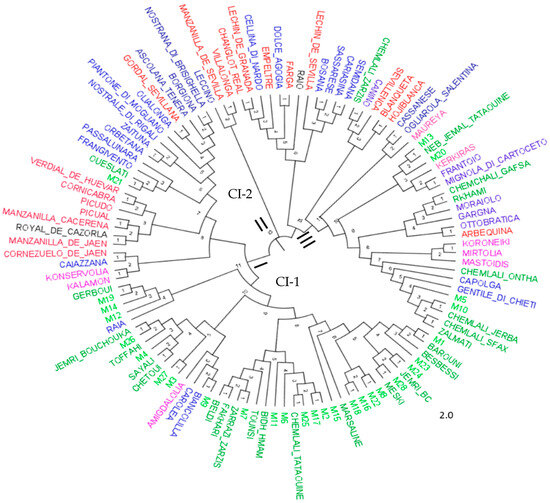
Figure 4.
Neighbor-joining tree of analyzed olives derived from genetic distance generated by 09 SSR markers, according to Dice’s genetic coefficient. Branches of different colors indicate the five Mediterranean populations: Spanish: red, Italian: blue, Greek: pink, Tunisian centennial trees: light green, and Tunisian olive varieties: dark green.
3.3. Genetic Structure
The microsatellites used revealed no significant associations in the linkage disequilibrium (LD) test, and thus fulfilled the requirements for the application of the Bayesian approach in the STRUCTURE analysis (Figure 5). Using an ad hoc measure derived from the second-order rate of variation of the likelihood function (∆K), we identified the best K = 3 (∆K = 168.97) (Supplementary Figure S1). Three distinct subpopulations were observed, represented by different colors, in addition to a few mixed genotypes (Figure S1). Each individual was assigned to a subpopulation if its membership exceeded 0.8. The red population (Q1) comprised mainly Spanish olive trees, some Italian and Greek varieties, and the centenarian olive trees M9 and M14. The green population (Q2) consisted mainly of the centenarian olive trees and the commercial Tunisian varieties. The third, blue-striped population (Q3) consisted mainly of Italian olive trees, Greek and Spanish olive varieties, four Tunisian cultivars, and the centenary trees M5 and M10. The presence of admixed genotypes (ADM), represented by two or three colors with memberships (<0.8), was also detected, which included the centenarian samples M7, M11, M12, M13, M15, and M20, and several Italian varieties.
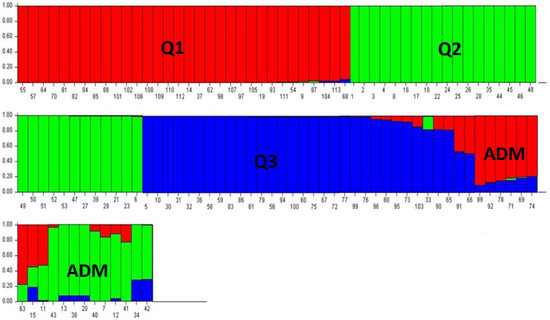
Figure 5.
The genetic structure of the 113 analyzed olive genotypes, identified by the STRUCTURE algorithm at K = 3. Each bar refers to an individual and is colored according to the proportion of the genome (qi) associated with each K detected. Centenary olives (1–28); autochthonous Tunisian olive varieties (29–53); Greek olive varieties (54–61); Italian olive varieties (62–93); Spanish olive varieties (94–113).
4. Discussion
The importance of millenary olive trees as genetic resources carrying crucial traits for robustness and adaptability has only recently been recognized in the face of climate change, rising temperatures, water scarcity, and the spread of new diseases such as Xylella fastidiosa subsp. pauca “ST53” [44]. These trees have attracted attention due to their exceptional ability to withstand the effects of climate change [14,20,21].
The practice of olive cultivation in Tunisia has deep historical roots, dating back to the Punic, Roman, and Arab-Muslim eras [45]. The country is characterized by a rich heritage of ancient olive trees that have thrived for centuries. In this study, the genetic diversity of 28 ancient Tunisian olive trees was analyzed for the first time using SSR markers. The nine SSR markers exhibited a high level of polymorphic information content (PIC) (>0.5), with seven of them exceeding a PIC value of 0.7 [46], thus proving to be efficient for the study of Tunisian germplasm.
The study revealed a remarkably high genetic diversity among these ancient olive trees, which displayed a total of 67 alleles and a Shannon index (I) of 1.68, which was higher than that of Tunisian cultivars (I = 1.57), confirming the preciousness of this ancient germplasm, as already noted by [9]. It also provided valuable insights into the Italian, Spanish, and Greek germplasm. In particular, the Italian varieties displayed the highest degree of polymorphism, the highest Shannon index value, and the highest observed heterozygosity, which is consistent with previous studies on the Italian olive germplasm [47,48,49] and Spanish olive germplasm [26].
Several studies have emphasized the great genetic diversity of ancient olive trees and their relationships with local cultivars in different Mediterranean countries, such as Cyprus [21], Turkey [20], Spain [24,25], Lebanon [22], Sicily [18], Malta [23], and Greece [50,51]. In all of these studies, the results pointed out the particularity of the ancient germplasm, and they led to the registration of some genotypes in the International IFAPA’s World Germplasm Bank of Olive Varieties, to ensure their conservation in the future. These old trees have been cultivated for hundreds of years and have survived against all sorts of adversities, and they were probably selected to enhance the flavor and aroma of oil for a wide range of use [19]. In Tunisia, the Romans cultivated Olea europaea subsp. europaea var. europaea in challenging environments to support nomadic communities, and contributed to the development of resilient agricultural systems in arid regions by identifying robust olive cultivars [45]. In addition, the Hellenistic era witnessed a proliferation of olive varieties due to extensive trade that led to the spread of different olive cultivars through grafting and the exchange of knowledge about these plants [26,51,52].
Among the centenary trees, pairwise relatedness analysis identified samples M25, M1, M28, and M24 as synonyms of the local cultivars Chemlali Tataouine, Barouni, Meski, and JEMRI_BC, respectively, revealing the antiquity of these important Tunisian varieties. The migrant detection analysis revealed that accession M10 from the old Tunisian olive population is a potential first-generation migrant of local olive cultivars in Tunisia, and the local variety Neb-Jemal-Tataouine was identified as a first-generation migrant from ancient olive trees. In addition, the Spanish varieties Arbequina and Sevillenca were assessed as potential first-generation migrants from the Italian olive population. Overall, these results indicate genetic exchange between olive populations and the transfer of genetic material from older varieties to cultivars. The assignment test data also show potential genetic exchange in both directions, which is consistent with previous studies demonstrating the introgression of Western European olive cultivars from native olive trees from the East [53]. According to [18], ancient olive trees found at archeological sites in Agrigento, Italy, include well-known varieties such as Santagatese, Giarraffa, and Cerasuola. Additionally, [54] highlights the close relationship between samples of a medieval Maltese olive and the traditional Maltese variety, Bidni. In their assessment of the significant millenary olive tree ’Throuba Naxos’ and its comparison with 89 olive tree varieties from the Mediterranean region, the authors of [50] note that the domestication of olive trees in Greece may have begun as far back as 3000 years ago. In addition, in their study of the genetic connections between Greek Olea europaea subsp. europaea var. sylvestris populations and Olea europaea subsp. europaea var. europaea using SSR markers, the authors of [55] emphasize that special attention should be paid to centennial olive trees in traditional olive groves. Considering that grafting with Olea europaea subsp. europaea var. sylvestris rootstocks was a common method of establishing olive groves in Greece, these centenary trees serve as vital preservation vessels for potentially extinct Olea europaea subsp. europaea var. sylvestris populations. As a result, they should be carefully studied and protected.
The AMOVA analysis confirmed the genetic exchange between olive populations and the transfer of genes from older cultivars to those grown today. This indicates a complex evolutionary path influenced by local adaptations and environmental factors, and emphasizes the contributions of both natural elements and human activities such as cultivation and breeding to shaping the genetics of olives. These results are bolstered by a recent study [56] involving 90 olive accessions and six varieties from the USDA repository, which applied genotyping-by-sequencing (GBS) and found no correlation between subpopulations, geographical origins, or climatic conditions. Considerable genetic diversity was observed within the populations, highlighting the significance for future breeding initiatives of supporting the selection of a wide range of parent plants and aiding in the discovery of genes for olive breeding. The genetic clustering of the analyzed olives did not correlate exactly with their geographical origin, and there was overlap between local Tunisian cultivars, old varieties, and cultivars from Italy, Spain, and Greece. This conclusion was supported by the results of the structure analysis, which revealed three distinct groups, and one admixed group comprising six centenary samples with intermingled genetic backgrounds showing genetic relatedness with Italian, Spanish, and Greek cultivars, as cited by several authors [11,18,31]. The diverse genetic composition of olive cultivars was emphasized by the authors of [57], who performed a comparative SNP analysis of Jordan’s olive heritage, in comparison with data from the Worldwide Olive Germplasm Bank of Córdoba. This study identified 73 previously unknown Jordan olive genotypes and highlighted a significant degree of genetic admixture, revealing an intricate relationship between olive varieties in the region.
Overall, this study enabled a genetic characterization of endangered Tunisian centenary olive genotypes. These genotypes are currently included in the Tunisian National GENE BANK collection, increasing the number to more than 200 genetic profiles, as well as in an olive DNA repository with more than 75 samples [12]. These ancient genotypes, whose history spans centuries, represent a valuable source of genetic diversity that can be leveraged to combat new emergencies related to climate change and emerging diseases. The conservation of these ancient genotypes not only enriches the genetic base available for breeding programs, but also strengthens the resilience of agriculture, which is facing unprecedented pressure worldwide.
5. Conclusions
Given the unprecedented pressures facing agriculture worldwide, tapping into the genetic reservoir of old olive varieties can facilitate the development of robust varieties that require fewer resources and are more adaptable. Research into old genotypes offers the opportunity to develop new olive varieties that can effectively meet today’s agricultural challenges. In this work, a first attempt was made to assess the genetic diversity of ancient olive germplasm in Tunisia, in order to take a first step towards the conservation of the genetic resources of olive trees. Using SSR markers, this study successfully identified the genetic profiles of 28 historical olive trees collected from archeological sites in nine different governorates from the north to the south of Tunisia. This study revealed a remarkably high genetic diversity among these ancient olive trees, which exhibited 67 alleles and a Shannon index (I) of 1.68. The analysis revealed that the ancient olive varieties had a higher value than the Tunisian cultivars (I = 1.57), underscoring their significance. Pairwise relatedness analyses identified the old olive varieties M25, M1, M28, and M24 as synonyms for the local cultivars Chemlali Tataouine, Barouni, Meski, and Jemri-bc, thereby highlighting their historical connections. Furthermore, accession M10 was recognized as a potential first-generation migrant of local Tunisian cultivars, while Neb-Jemal-Tataouine can be traced back to ancient trees. Spanish varieties such as Arbequina and Sevillenca were suggested as possible first-generation migrants originating from Italian olives, emphasizing the genetic exchange between olive populations. This information provides valuable insights into the origins, historical distribution patterns, and migration routes of olives in the Mediterranean. The protection of these rare trees, some of which have a history dating back thousands of years, is therefore crucial to preserve our natural heritage and ensure that their ecological benefits are preserved for future generations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11020147/s1. Figure S1: Evanno test based on Delta K values; Table S1: list of microsatellites used for molecular characterization of olive samples; Table S2: list of pairwise relatedness based on LRM estimator [58], Table S3: partitioning of genetic variation within and among groups obtained by AMOVA analysis for the 5 groups of Mediterranean olive accessions: Tunisian centennial trees, Tunisian commercialized varieties, Greek, Italian, and Spanish varieties; Table S4: detection of first-generation migrants among olive trees from five Mediterranean populations based on likelihood ratio (L_origin/L_max), as outlined by [59].
Author Contributions
Conceptualization, S.R.M. and O.S.D.; formal analysis, S.R.M. and M.M.M.; validation, O.S.D. and M.M.M.; writing—original draft, S.R.M. and M.M.M.; writing—review and editing, S.R.M., M.M.M., C.M. and O.S.D.; supervision, O.S.D. and M.M.M.; funding acquisition, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by RIGENERA (Approcci IntegRati per il mIglioramento Genetico, la selezione e l’ottenimento di materiali vegetali Resistenti a Xylella fastidiosa) (CUP: H93C22000750001) and the Agritech National Research Center, European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA 6 (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4-D.D. 1032 17/06/2022, CN00000022).
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; the collection, analyses, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
References
- Besnard, G.; Rubio de Casas, R.; Christin, P.A.; Vargas, P. Phylogenetics of Olea (Oleaceae) based on plastid and nuclear ribosomal DNA sequences: Tertiary climatic shifts and lineage differentiation times. Ann Bot. 2009, 104, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S. A revision of olea L. (Oleaceae). Kew Bull. 2002, 57, 91. [Google Scholar] [CrossRef]
- Fanelli, V.; Mascio, I.; Falek, W.; Miazzi, M.M.; Montemurro, C. Current Status of Biodiversity Assessment and Conservation of Wild Olive (Olea europaea L. subsp. europaea var. sylvestris). Plants 2022, 11, 480. [Google Scholar] [CrossRef]
- Schicchi, R.; Speciale, C.; Amato, F.; Bazan, G. The Monumental Olive Trees as Biocultural Heritage of Mediterranean Landscapes: The Case Study of Sicily. Sustainability 2021, 13, 6767. [Google Scholar] [CrossRef]
- Newton, C.; Lorre, C.; Sauvage, C.; Ivorra, S.; Terral, J.F. On the origins and spread of Olea europaea L. (olive) domestication: Evidence for shape variation of olive stones at Ugarit, Late Bronze Age, Syria: A window on the Mediterranean basin and on the westward diffusion of olive varieties. Veg. Hist. Archaeobotany 2014, 23, 567–575. [Google Scholar] [CrossRef]
- Martínez-Moreno, F.; Guzmán-Álvarez, J.R.; Díez, C.M.; Rallo, P. The Origin of Spanish Durum Wheat and Olive Tree Landraces Based on Genetic Structure Analysis and Historical Records. Agronomy 2023, 13, 1608. [Google Scholar] [CrossRef]
- Kaniewski, D.; Van Campo, E.; Boiy, T.; Terral, J.F.; Khadari, B.; Besnard, G. Primary domestication and early uses of the emblematic olive tree: Palaeobotanical, historical and molecular evidence from the Middle East. Biol. Rev. 2012, 87, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, M. The age of the olive trees in the Garden of Gethsemane. J. Archaeol. Sci. 2015, 53, 43–48. [Google Scholar] [CrossRef]
- Loussert, L.; Brousse, G. Mediterranean Agricultural Techniques of Olive Production; New home and Rose Publishing GP: Paris, France, 1978; pp. 44–111. [Google Scholar]
- Gabriel, C.; Lancel, S. Hannibal. In Revue des Mondes Musulmans et de la Méditerranée; Persée: Fayard, Paris, 1995; Volume 75–76, pp. 305–306. [Google Scholar]
- Díez, C.; Trujillo, I.; Barrio, E.; Belaj, A.; Diego, B.; Rallo, L. Centennial olive trees as a reservoir of genetic diversity. Ann. Bot. 2011, 108, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Saddoud, D.O.; Miazzi, M.M.; Elloumi, O.; Fendri, M.; Ben Amar, F.; Savoia, M.; Sion, S.; Souabni, H.; Mnasri, R.S.; Ben Abdelaali, S.; et al. Recovery, assessment, and molecular characterization of minor olive genotypes in Tunisia. Plants 2020, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Saddoud, D.O.; Mnasri, R.S.; Ben Amar, F.; Ben Naceur, M.; Montemurro, C.; Miazzi, M.M. Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia. Genes 2021, 12, 286. [Google Scholar] [CrossRef]
- Saddoud, D.O.; Ben Amar, F.; Mnasri, R.S.; Taranto, F.; Montemurro, C.; Miazzi, M.M. The Status of Genetic Resources and Olive Breeding in Tunisia. Plants 2022, 11, 1759. [Google Scholar] [CrossRef] [PubMed]
- Laaribi, L.; Gouta, H.; Ayachi, M.M.; Labidi, F.; Mars, M. Combination of morphological and molecular markers for the characterization of ancient native olive accessions in Central-Eastern Tunisia. C. R. Biol. 2017, 5, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, S.; Omri, A.; Grati-Kamoun, N.; Marra, F.; Caruso, T. Molecular characterization and genetic relationships of cultivated Tunisian olive varieties (Olea europaea L.) using SSR markers. J. N. Sci. 2017, 40, 2175–2185. [Google Scholar]
- Mnasri, R.S.; Saddoud, D.O.; Ben Amar, F.; Dellino, M.; Montemurro, C.; Miazzi, M.M. Exploring the quality and nutritional profiles of mono-varietal oils from millennial olive trees in Tunisia. Eur. Food Res. Technol. 2023, 249, 2807–2820. [Google Scholar] [CrossRef]
- Marchese, A.; Bonanno, F.; Marra, F.P.; Trippa, D.A.; Zelasco, S.; Rizzo, S.; Giovino, A.; Imperiale, V.; Ioppolo, A.; Sala, G.; et al. Recovery and genotyping ancient Sicilian monumental olive trees. Front. Conserv. Sci. 2023, 4, 1206832. [Google Scholar] [CrossRef]
- Yoruk, B.; Taskin, V. Genetic diversity and relationships of wild and cultivated olives in Turkey. Plant Syst. Evol. 2014, 300, 1247–1258. [Google Scholar] [CrossRef]
- Aksehirli-Pakyurek, M.; Koubouris, G.C.; Petrakis, P.V.; Hepaksoy, S.; Metzidakis, I.T.; Yalcinkaya, E. Cultivated and Wild Olives in Crete, Greece Genetic Diversity and Relationships with Major Turkish Cultivars Revealed by SSR Markers. Plant Mol. Biol. Rep. 2017, 35, 575–585. [Google Scholar] [CrossRef]
- Anestiadou, K.; Nikoloudakis, N.; Hagidimitriou, M.; Katsiotis, A. Monumental olive trees of Cyprus contributed to the establishment of the contemporary olive germplasm. PLoS ONE 2017, 12, e0187697. [Google Scholar] [CrossRef]
- Chalak, L.; Haouane, H.; Essalouh, L.; Santoni, S.; Besnard, G.; Khadari, B. Extent of the genetic diversity in Lebanese olive (Olea europaea L.) trees: A mixture of an ancient germplasm with recently introduced varieties. Genet. Resour. Crop Evol. 2015, 62, 621–633. [Google Scholar] [CrossRef]
- Valeri, M.C.; Mifsud, D.; Sammut, C.; Pandolfi, S.; Lilli, E.; Bufacchi, M.; Stanzione, V.; Passeri, V.; Baldoni, L.; Mariotti, R.; et al. Exploring Olive Genetic Diversity in the Maltese Islands. Sustainability 2022, 14, 10684. [Google Scholar] [CrossRef]
- Ninota, A.; Howadb, W.; Aranzanab, J.M.; Senarc, R.; Romeroa, A.; Mariottid, R.; Baldonid, L.; Belaje, A. Survey of over 4,500 monumental olive trees preserved on-farm in the northeast Iberian Peninsula, their genotyping and characterization. Sci. Hortic. 2017, 231, 253–264. [Google Scholar] [CrossRef]
- Serreta-Oliván, A.; Sancho-Cohen, R.; Sánchez-Gimeno, A.C.; Martín-Ramos, P.; Cuchí Oterino, J.A.; Casanova-Gascón, J. Traditional Olive Tree Varieties in Alto Aragón (NE Spain): Molecular Characterization, Single-Varietal Oils, and Monumental Trees. Agriculture 2023, 13, 2204. [Google Scholar] [CrossRef]
- Gago, P.; Boso, S.; Santiago, J.L.; Martínez, M.-C. Identification and Characterization of Relict Olive Varieties (Olea europaea L.) in the Northwest of the Iberian Peninsula. Horticulturae 2024, 10, 175. [Google Scholar] [CrossRef]
- Sion, S.; Savoia, M.A.; Gadaleta, S.; Piarulli, L.; Mascio, I.; Fanelli, V.; Montemurro, C.; Miazzi, M.M. How to Choose a Good Marker to Analyze the Olive Germplasm (Olea europaea L.) and Derived Products. Genes 2021, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Mnasri, R.S.; Saddoud, D.O.; Ferchichi, A. Preliminary characterization and morph-agronomic evaluation of millennium olive varieties in Tunisia. J. Biodiv. Environ. Sci. 2013, 3, 150–155. [Google Scholar]
- Ismaili, H.; Zaim, V. Determining the age of olive trees through morphometric methods. Int. J. Agric. Innov. Res. 2014, 3, 574–578. [Google Scholar]
- Spadoni, A.; Sion, S.; Gadaleta, S.; Savoia, M.A.; Piarulli, L.; Fanelli, V.; Di Rienzo, V.; Taranto, F.; Miazzi, M.M.; Montemurro, C.; et al. A Simple and Rapid Method for Genomic DNA Extraction and Microsatellite Analysis in Tree Plants. J. Agric. Sci. Technol. 2019, 21, 1215–1226. [Google Scholar]
- Di Rienzo, V.; Miazzi, M.M.; Fanelli, V.; Sabetta, W.; Montemurro, C. The preservation and characterization of Apulian olive germplasm biodiversity. Acta Hortic. 2018, 1199, 1–6. [Google Scholar] [CrossRef]
- Olea Databases. 2008. Available online: http://www.oleadb.it/ (accessed on 26 April 2019).
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skalnick, M.H.; Davies, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Piry, S.; Alapetite, A.; Cornuet, J.-M.; Paetkau, D.; Baudouin, L.; Estoup, A. GENECLASS2: A Software for Genetic Assignment and First-Generation Migrant Detection. J. Hered. 2004, 95, 536–539. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. 2006. Available online: https://darwin.cirad.fr/ (accessed on 26 April 2019).
- Felsentein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstraps. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Delvin, B.; Risch, N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics 1995, 29, 311–322. [Google Scholar]
- Jorde, L.B. Linkage disequilibrium and the search for complex disease genes. Genome Res. 2000, 10, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.P.; George, C. Monte Carlo Statistical Methods, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Savoia, M.A.; Fanelli, V.; Miazzi, M.M.; Taranto, F.; Procino, S.; Susca, L.; Montilon, V.; Potere, O.; Nigro, F.; Montemurro, C. Apulian Autochthonous Olive Germplasm: A Promising Resource to Restore Cultivation in Xylella fastidiosa-Infected Areas. Agriculture 2023, 13, 1746. [Google Scholar] [CrossRef]
- Camps-Fabrer, H. La culture de l’olivier en Afrique du Nord, Evolution et histoire. In Encyclopédie Mondial de l’Olivier; International Olive Oil Council: Madrid, Spain, 1997; pp. 30–33. [Google Scholar]
- Hearne, C.M.; Ghosh, S.; Todd, J.A. Microsatellites for link-age analysis of genetic traits. Trends Genet. 1992, 8, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, A.; Ganino, T.; Beghè, D.; DiVirgilio, N.; Morrone, L.; Fabbri, A.; Neri, L. Genetic and landscape characterization of ancient autochthonous olive trees in northern Italy. Plant Biosyst. 2018, 152, 1067–1074. [Google Scholar] [CrossRef]
- Bracci, T.; Sebastiani, L.; Busconi, M.; Fogher, C.; Belaj, A.; Trujillo, I. SSR markers reveal the uniqueness of olive cultivars from the Italian region of Liguria. Sci. Hortic. 2009, 122, 209–215. [Google Scholar] [CrossRef]
- Sion, S.; Taranto, F.; Montemurro, C.; Mangini, G.; Camposeo, S.; Falco, V.; Gallo, A.; Mita, G.; Saddoud Debbabi, O.; Ben Amar, F.; et al. Genetic Characterization of Apulian Olive Germplasm as Potential Source in New Breeding Programs. Plants 2019, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Bazakos, C.; Alexiou, K.G.; Ramos-Onsins, S.; Koubouris, G.; Tourvas, N.; Xanthopoulou, A.; Mellidou, I.; Moysiadis, T.; Vourlaki, I.T.; Metzidakis, I.; et al. Whole genome scanning of a Mediterranean basin hotspot collection provides new insights into olive tree biodiversity and biology. Plant J. 2023, 116, 303–319. [Google Scholar] [CrossRef]
- Tourvas, N.; Ganopoulos, I.; Koubouris, G.; Kostelenos, G.; Manthos, I.; Bazakos, C.; Stournaras, V.; Molassiotis, A.; Aravanopoulos, F. Wild and cultivated olive tree genetic diversity in Greece: A diverse resource in danger of erosion. Front. Genet. 2023, 14, 1298565. [Google Scholar] [CrossRef]
- Vildan, U.; Gökçen, Y. The Historical Development and Nutritional Importance of Olive and Olive Oil Constituted an Important Part of the Mediterranean Diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef]
- Myles, J.D. The University of Louisville Department of Architecture. Ohio Val. Hist. 2024, 24, 17–25, Project MUSE. Available online: https://muse.jhu.edu/article/925127 (accessed on 26 April 2019).
- Miazzi, M.M.; Pasqualone, A.; Zammit-Mangion, M.; Savoia, M.A.; Fanelli, V.; Procino, S.; Gadaleta, S.; Aurelio, F.L.; Montemurro, C. A Glimpse into the Genetic Heritage of the Olive Tree in Malta. Agriculture 2024, 14, 495. [Google Scholar] [CrossRef]
- Vanwalleghem, T.; Infante Amate, J.; De Molina, G.M.; Fernández, S.D.; Gómez, A.J. Quantifying the effect of historical soil management on soil erosion rates in Mediterranean olive orchards. Agric. Ecosyst. Environ. 2011, 142, 341–351. [Google Scholar] [CrossRef]
- Islam, A.F.; Sanders, D.; Mishra, A.K.; Joshi, V. Genetic diversity and population structure analysis of the USDA olive germplasm using genotyping-by-sequencing (GBS). Genes 2021, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Julca, I.; Marcet-Houben, M.; Cruz, F.; Gómez-Garrido, J.; Gaut, B.S.; Díez, C.M.; Gut, I.G.; Alioto, T.S.; Vargas, P.; Gabaldón, T. Genomic evidence for recurrent genetic admixture during the domestication of Mediterranean olive trees (Olea europaea L.). BMC Biol. 2020, 18, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lynch, M.; Ritland, K. Estimation of pairwise relatedness with molecular markers. Genetics 1999, 152, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Paetkau, D.; Calvert, W.I.S.; Strobeck, C. Microsatellite analysis of population structure in Canadian polar bears. Mol. Ecol. 1995, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).