Abstract
Fresh-cut lettuce rapidly deteriorates during storage, and developing safe strategies to maintain its quality is critical. This study evaluated the effects of 2 mM salicylic acid (SA), 100 mg L−1 calcium oxide nanoparticles (CaNP), and their combination (CaNP + SA), and an untreated control on the physiological and biochemical quality of fresh-cut Lactuca sativa L. var. crispa stored for 18 days at 5 ± 1 °C. Treatments were assessed through measurements of total soluble phenolics (TSP), membrane stability index (MSI), chlorophyll components (Chl a, Chl b, Total Chl), chlorophyll stability index (CSI), relative chlorophyll content (SPAD), weight loss (WL), cutting resistance (CR), and color parameters. Both SA and CaNP + SA significantly delayed senescence and oxidative degradation compared with the control. The CaNP + SA combination showed the most comprehensive protection, exhibiting the lowest WL (3.91%) and the highest MSI and CSI values, indicating enhanced membrane integrity and pigment stability. SA alone maintained elevated TSP, SPAD, and chlorophyll contents, supporting its antioxidative and metabolic regulatory roles. Correlation analysis revealed strong positive relationships among MSI, CSI, SPAD, and chlorophyll variables, while WL and CR were negatively associated with these traits. PCA clearly separated treatments, with SA and CaNP + SA clustering with stability-related parameters. Overall, CaNP + SA effectively preserved membrane function, pigment structure, and water balance, representing a promising postharvest strategy for extending the shelf life of fresh-cut lettuce.
1. Introduction
The fresh-cut fruit and vegetable industry has shown considerable growth over the past decade, with annual expansion rates between 5 and 10%. Within this market, lettuce-based products represent nearly 50% of the total volume, reflecting their popularity and consumer demand. However, the industry continues to face persistent quality challenges, particularly physiological disorders such as tissue browning, which compromise product appearance and shorten shelf life. In fresh-cut lettuce, browning of cut surfaces is a major postharvest concern, primarily triggered by mechanical injury during processing. This injury activates polyphenol oxidase (PPO), leading to the oxidation of phenolic compounds and the subsequent accumulation of brown or pink discolorations that reduce consumer appeal and marketability [,,].
Various postharvest interventions have been developed to mitigate enzymatic browning and quality degradation in leafy vegetables. These include modified atmosphere packaging (MAP), chemical antioxidants, enzyme inhibitors, UV and visible light treatments, and thermal interventions []. While effective to a degree, some of these methods may introduce residues or require specialized infrastructure. Consequently, recent studies have turned toward sustainable and safe alternatives, such as salicylic acid (SA) and calcium-based treatments, which show promise due to their biological roles in plant defense and structural integrity.
Salicylic acid, a naturally occurring phenolic compound, functions as a plant signaling molecule that modulates growth, development, and responses to biotic and abiotic stress. Its exogenous application during postharvest storage has been reported to delay senescence, reduce tissue softening, and enhance antioxidant enzyme activity. For example, SA-treated lettuce maintained higher levels of phenolic compounds and visual quality during cold storage [,,]. Similarly, calcium plays a crucial role in maintaining cell wall structure and membrane integrity by cross-linking with pectin in the middle lamella. Calcium treatments have been shown to reduce weight loss, delay chlorophyll degradation, and preserve overall postharvest quality [].
Nanotechnology has introduced a new dimension to calcium-based preservation methods. Calcium oxide nanoparticles (CaONPs), due to their high surface area and reactivity, may enhance penetration and efficacy compared to bulk calcium salts. Recent research has demonstrated the effectiveness of CaONPs in maintaining firmness, reducing oxidative stress, and preserving membrane stability in horticultural products []. Moreover, there is emerging interest in the potential synergistic use of SA and CaONPs. While the separate benefits of these treatments have been documented, very limited research has explored their combined application in fresh-cut lettuce.
The present study aims to investigate the individual and synergistic effects of 2 mM SA and 100 mg L−1 CaONPs on the postharvest quality of fresh-cut lettuce stored at 5 ± 1 °C for 18 days. The focus is on physiological and biochemical parameters including pigment retention, membrane stability, water balance, and phenolic content. To our knowledge, this is one of the first studies to evaluate the combined influence of SA and CaONPs on minimally processed lettuce, offering new insights into environmentally friendly strategies to extend shelf life and preserve visual and nutritional quality during cold storage.
2. Materials and Methods
2.1. Plant Material
In this study, the curly lettuce (Lactuca sativa L. var. crispa) cultivar was used as the plant material. The lettuce plants were grown under soilless culture conditions using the deep flow technique (DFT) hydroponic system. A nutrient solution specifically formulated for lettuce, whose composition is presented in Table 1, was used throughout the cultivation period. During the experiment, the pH of the nutrient solution was maintained at 6.5 by adjusting with diluted hydrochloric acid (HCl).

Table 1.
The chemical composition and concentrations of the nutrient solution used for hydroponic lettuce cultivation under deep flow technique (DFT) conditions [].
2.2. Chemicals Used
Calcium oxide nanoparticles (CaNPs) used in this study were purchased as a ready-made product from PARS Chemical Co. (Turkey), with a manufacturer-stated particle size range of 10–70 nm and a purity of 99.95%. Because these nanoparticles were not synthesized in-house, no further particle characterization such as scanning electron microscopy (SEM) or dynamic light scattering (DLS) was performed. Instead, the study relied on the technical specifications and characterization data provided by the manufacturer regarding nanoparticle size and other properties. We acknowledge that this reliance on manufacturer-provided data is a limitation of the study, and the reported particle size range (10–70 nm) was not independently verified. Salicylic acid (SA) with 99% purity (CAS No: 69-72-7; EC No: 200-712-3) was used for the treatments.
2.3. Fresh-Cutting Process and Treatments
After harvest, the lettuce heads were immediately transported to the laboratory. The outer yellowish leaves at the basal part of the plants were carefully removed (Figure 1a). Subsequently, the outer leaves surrounding the developing inner leaves were transversely cut at approximately 4 cm intervals (Figure 1b). Following the cutting process, the lettuce leaves were subjected to the treatments described in Table 2. The concentrations of 2 mM salicylic acid (SA) and 100 mg L−1 calcium nanoparticles (CaNP) were selected based on a review of relevant literature and preliminary experimental trials. Finally, the treated leaves were air-dried at room temperature (Figure 1c).
Figure 1.
Appearance of lettuce samples: (a) Harvesting, (b) Cutting process, (c) Air-drying process.

Table 2.
Description of postharvest treatments applied to fresh-cut lettuce samples prior to cold storage.
2.4. Packaging and Cold Storage Conditions
After air-drying at room temperature, fresh-cut lettuce leaves from all treatments were placed into lidded PET containers (dimensions: 8.5 × 9.0 × 6.5 cm), each containing 50 g of sample (Figure 2). The containers were then stored in a cold room at 5 ± 1 °C and 85–90% relative humidity for 18 days. During the storage period, samples were taken on days 0, 3, 6, 9, 12, 15, and 18 for subsequent quality analyses.
Figure 2.
Packaging of fresh-cut lettuce samples in lidded PET containers prior to cold storage.
2.5. Total Soluble Phenolic Content (TSP)
After extracting the juice from the lettuce leaves, 150 µL of the extract was mixed with 2400 µL of distilled water and 150 µL of Folin–Ciocalteu reagent (1:10 dilution). The mixture was vortexed for 30–40 s and allowed to stand for 4 min. Subsequently, 300 µL of 1 N sodium carbonate (Na2CO3) was added, and the samples were incubated for 2 h at room temperature in the dark. The absorbance was measured at 725 nm using a UV–Vis spectrophotometer. The total phenolic content was expressed as mg caffeic acid equivalent (CAE) per 100 mL of extract [].
2.6. Membrane Stability Index (MSI)
The Membrane Stability Index (MSI) was determined to assess the degree of cell membrane injury caused by freezing–thawing stress, based on the method described by Nabati et al. []. Leaf discs were taken from each treatment and rinsed twice with 50 mL of distilled water. The samples were then incubated in 50 mL of distilled water for 2 h at room temperature, and the initial electrical conductivity (C1) was recorded. Subsequently, the samples were frozen at −18 °C for 24 h, thawed at room temperature, and when the solution temperature exceeded 18 °C, the second conductivity (C2) was measured. The MSI (%) was calculated using the following formula:
The obtained MSI values were evaluated as an indicator of membrane permeability. Higher MSI values indicate better preservation of membrane integrity, while lower values correspond to increased membrane injury. All measurements were conducted in triplicate, and the results are presented as mean ± standard deviation (SD).
2.7. Cutting Resistance (N)
To evaluate the effect of treatments on maintaining leaf turgor at the beginning and throughout the storage period, the cutting resistance of fresh-cut lettuce leaves was measured. For each replicate, three leaf samples were analyzed using a texture analyzer (Shimadzu EZ-LX, Shimadzu Corp., Kyoto, Japan) equipped with a Warner–Bratzler cutting blade. Cutting resistance was always measured on the middle section of each leaf—specifically the central “stem” region (not the leaf edge or base). The force required to cut the leaf surface was recorded in Newtons (N) and used as an indicator of tissue firmness or crispness.
2.8. Weight Loss (%)
Weight measurements were performed on the same samples at each sampling interval for all treatments. The percentage of weight loss during storage was calculated using the following equation:
where W0 is the initial sample weight and Wt is the sample weight at each storage interval.
2.9. Total Soluble Solids (TSS) Content
For the determination of total soluble solids (TSS), juice extracted from lettuce leaves of each treatment group was analyzed using a digital refractometer (Atago Co., Ltd., Tokyo, Japan). The TSS content was expressed as a percentage (%).
2.10. Chlorophyll a, b, and Total Chlorophyll Content
Fresh lettuce samples (0.5 g) were homogenized with 10 mL of 80% acetone. The homogenate was centrifuged at 10,000 rpm for 15 min at 4 °C, and the supernatant was collected for analysis. Absorbance was measured at 645 nm and 663 nm using a spectrophotometer, and the chlorophyll a, chlorophyll b, and total chlorophyll contents were calculated using the equations described by Ngcobo et al. [].
The Chl a, Chl b and total Chl concentrations (mg g−1 FW) were calculated using spectrophotometric absorbance readigns according to the following equation:
where: C represents the Chl a, Chl b, and total Chl content (mg g−1 FW), V is the volume of extract (L), and FW denotes the fresh weight of the sample (g).
2.11. Chlorophyll Stability Index (CSI, %)
The Chlorophyll Stability Index (CSI) was determined to evaluate the extent of degradation of chlorophyll pigments during the postharvest storage period, following the method of Ngcobo et al. []. The CSI was calculated based on the difference between the chlorophyll content of freshly cut samples and those measured at each storage interval (3, 6, 9, 12, 15, and 18 days). A higher CSI value indicates greater chlorophyll stability and, consequently, better maintenance of green color during storage.
2.12. Relative Chlorophyll Content (SPAD)
The relative chlorophyll content of fresh-cut lettuce was determined using a SPAD-502 Plus chlorophyll meter (Konica Minolta, Inc., Osaka, Japan). Measurements were taken from one point on each of five leaves per replicate, and the mean SPAD value was recorded for each treatment.
2.13. Color Measurements
The surface color of lettuce leaves was measured using a colorimeter (Minolta CR-400, Minolta Co., Osaka, Japan). Measurements were taken from the adaxial surface of five leaf samples per replicate, and color parameters were expressed as L* (lightness), a* (red–green), and b* (yellow–blue) values. Based on these primary color coordinates, hue angle (°), Chroma (C), total color difference (ΔE), and yellowness index (YI) were calculated using the following equation:
where: L* (lightness), a* (red–green), and b* (yellow–blue) values.
2.14. Statistical Analysis
The experiment was conducted in a completely randomized design (CRD) with three replicates. For each replicate, three packages of fresh-cut lettuce were used, and each package contained a constant weight of 50 g of lettuce leaves. The obtained data were subjected to analysis of variance (ANOVA) using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). Differences among treatment means were determined using Duncan’s Multiple Range Test (DMRT) at a significance level of p < 0.05. Additionally, Principal Component Analysis (PCA) and Pearson correlation analyses were performed using Python software (v.3.13) to evaluate the relationships among quality parameters and treatment effects throughout the storage period.
3. Results and Discussion
According to the analysis of variance (ANOVA), treatments, storage durations, and their interaction significantly affected most of the physicochemical and physiological parameters of fresh-cut lettuce (p < 0.001). Notable differences were observed, particularly in TSP, MSI, Chl-a, Chl-b, total Chl, and CSI (Table 3).

Table 3.
Analysis of variance (ANOVA) for the effects of treatment, storage time, and their interaction (Treatment × Time) on the physicochemical and physiological quality parameters of fresh-cut lettuce during 18 days of cold storage.
3.1. Total Soluble Fenolic Content (TSP)
The effects of SA, CaNP, and their combination (CaNP + SA) on the TSP of fresh-cut lettuce are presented in Table 4. Both treatment and storage duration showed significant differences (p < 0.001), and the interaction between treatment and time was also significant. Among the treatments, the highest TSP values were recorded in lettuce treated with SA, followed by the control (261.6 mg/100 mL), CaNP + SA (254.9 mg/100 mL), and CaNP (243.4 mg/100 mL). The enhanced TSP in SA-treated samples can be attributed to the stimulation of the phenylpropanoid pathway, which activates key enzymes such as phenylalanine ammonia-lyase (PAL) and chalcone synthase (CHS), promoting phenolic biosynthesis [,]. Similar SA-induced increases in total phenolics and antioxidant capacity have been reported in fresh-cut Chinese cabbage, lettuce and broccoli, supporting the elicitor role of SA in postharvest vegetables [,,]. This is in line with the view of SA as an elicitor that enhances antioxidant metabolism and improves tolerance to oxidative stress.

Table 4.
Changes in total soluble phenolic content (mg GAE 100 g−1 FW) of fresh-cut lettuce as influenced by treatments and storage duration at 4 ± 1 °C.
A dynamic trend was observed throughout storage: TSP increased during the early storage period, peaking on day 9 (366.4 mg/100 mL), and subsequently declined. This biphasic pattern is consistent with wound-induced activation of phenolic metabolism in fresh-cut produce, where the initial increase results from PAL activation, and the later decline reflects phenolic oxidation and polymerization mediated by polyphenol oxidase (PPO) and peroxidase (POD). Similar trends, characterized by an early rise followed by a decrease in phenolic content associated with changes in PAL and PPO activities, have been reported in fresh-cut lettuce and other minimally processed vegetables [,,].
The treatment × time interaction indicated that SA-treated samples maintained the highest TSP values particularly between days 3 and 12 (e.g., 331.1 and 401.0 mg/100 mL on days 3 and 12, respectively), confirming a pronounced elicitor effect during mid-storage. In contrast, CaNP-treated samples exhibited generally lower TSP values, suggesting that CaNP enhanced membrane stability and reduced stress signaling, thereby limiting phenolic synthesis. Calcium-based nanoparticles have been reported to strengthen cellular membranes, reduce electrolyte leakage and mitigate oxidative stress, which can result in moderated phenolic accumulation and better preservation of visual quality [,].
Interestingly, the combined CaNP + SA treatment induced a more moderate increase in phenolics compared to SA alone. This suggests that CaNP’s stabilizing effect counterbalanced the SA-induced metabolic activation, potentially preventing excessive phenolic accumulation and enzymatic browning. This interpretation aligns with the color analysis results, where the combination treatment showed slower changes in ΔE and yellowness index values, indicating delayed discoloration. This pattern suggests that the stabilizing effect of CaNP may partially counterbalance the SA-induced metabolic activation, potentially avoiding excessive phenolic accumulation and associated enzymatic browning. Similar synergistic or complementary effects of SA and calcium formulations on maintaining pigment content, antioxidant capacity and marketable quality have been observed in minimally processed lettuce and other horticultural commodities []. This interpretation is compatible with the color measurements in the present study, in which the combination treatment showed slower changes in ΔE and yellowness index values, indicative of delayed discoloration.
Descriptive correlation analysis further indicated that samples with higher Membrane Stability Index (MSI) and Chlorophyll Stability Index (CSI) tended to show elevated Total Chlorophyll (Total-chl), SPAD, and TSP values, while weight loss (WL) and ΔE tended to be inversely related to these parameters. Likewise, PCA mainly served to summarize these multivariate relationships by placing CaNP + SA samples closer to stability- and pigment-related variables in the score–loading space. Although correlation coefficients and PCA clustering do not establish causal or mechanistic links, these associative patterns are consistent with the broader literature, in which multivariate analyses are widely used to visualize how postharvest treatments jointly influence pigment retention, phenolic content and visual quality in fresh-cut products []. Overall, the combined evidence from the univariate ANOVA, together with these descriptive multivariate patterns, supports the conclusion that treatments helping to maintain membrane and pigment stability are also those in which phenolic levels remain relatively high and discoloration is attenuated during cold storage.
3.2. Membrane Stability Index (MSI)
The effects of treatment and storage duration on the membrane stability index (MSI) of fresh-cut lettuce were found to be significant (p < 0.05) (Table 5). At the beginning of storage, all treatments showed similarly high MSI values (97.7%), indicating that cutting affected all samples uniformly. By day 3, however, a clear decline was evident in every treatment. This early reduction is consistent with the post-cutting oxidative burst described for leafy vegetables, where the accumulation of reactive oxygen species (ROS) promotes lipid peroxidation and increased ion leakage across membranes [,,].

Table 5.
Effects of salicylic acid (SA, 2 mM) and calcium nanoparticles (CaNP, 100 mg L−1) on the membrane stability index (MSI, %) of fresh-cut lettuce during 18 days of cold storage (4 ± 1 °C).
As storage advanced, treatment-dependent differences became more pronounced, particularly after day 9. The highest MSI values were observed in the SA (98.93%) and CaNP + SA (98.51%) treatments. Similar improvements in MSI or related membrane integrity indices under SA treatment have been reported in lettuce under drought–salinity stress and in other vegetable species, where SA enhanced antioxidant enzyme activity and mitigated membrane damage []. MSI is a key physiological indicator reflecting the resistance of cell membranes to electrolyte leakage and, consequently, the integrity of cellular structure during storage []. In our study, MSI values later stabilized around 95–97% during the final storage stages, suggesting that tissues progressively shifted from an acute stress phase to a more moderate, quasi-steady state. A comparable biphasic response—initial decline followed by partial recovery—has also been observed in leafy vegetables during prolonged stress or storage []. Similarly, Yang et al. [] reported an initial membrane stress response in fresh-cut lettuce followed by an adaptive recovery phase during prolonged storage.
Based on treatment means, SA, CaNP + SA and the control belonged to the same statistical group, whereas CaNP alone consistently exhibited lower MSI values. This pattern indicates that CaNP at 100 mg L−1, when applied alone, provided a more limited degree of membrane protection than SA-containing treatments. Similar variability in the effectiveness of calcium-based treatments has been noted in postharvest and preharvest studies, where the outcome depends on dose, formulation and tissue type [,].
In contrast, the CaNP + SA combination showed MSI values comparable to SA alone and generally higher than CaNP alone, suggesting complementary effects of the two components. Calcium is known to contribute to cell wall and membrane stabilization, whereas SA enhances ROS-scavenging systems and stress signalling; recent work on cucumbers treated with CaNPs blended with SA demonstrated reduced tissue breakdown, lower weight loss and better textural retention compared with CaNPs alone []. Likewise, studies on minimally processed lettuce have reported that combined treatments with SA and calcium salts help extend shelf life and maintain overall quality, supporting the plausibility of such synergistic behavior [].
In the present work, correlation analysis showed that higher MSI values were generally associated with higher CSI, Total Chlorophyll and SPAD, and with lower weight loss and cutting resistance. These relationships reflect expected postharvest patterns—samples that better preserve membrane integrity also tend to retain pigments and water status—yet they should be regarded as associative. Similarly, PCA was used solely as a descriptive multivariate tool to visualize how MSI grouped with other quality attributes: MSI, CSI and SPAD loaded in similar directions, and CaNP + SA samples clustered closer to these variables in score space. While PCA cannot by itself validate physiological mechanisms or treatment effects, its use to summarize and visualize complex postharvest datasets is well established in the recent literature on fresh-cut and minimally processed produce. Taken together, the ANOVA results, supported by these descriptive multivariate patterns, indicate that SA and CaNP + SA treatments are more effective than CaNP alone in maintaining membrane stability and, consequently, the overall physiological status of fresh-cut lettuce during cold storage.
3.3. Cutting Resistance (CR)
According to the analysis of variance (ANOVA), treatment, storage duration, and their interaction significantly affected the cutting resistance (CR) of fresh-cut lettuce (p < 0.05) (Table 6). At the beginning of storage, CR values were 13.8 N across all treatments. However, as the storage period progressed, a general decreasing trend was observed. Between days 3 and 9, CR values declined, indicating that the lettuce leaves remained crisp and fresh during this period. Conversely, in the control group, CR values increased notably on days 15 and 18, suggesting that leaf tissues had lost turgidity due to water loss and became less crisp at the end of storage. Based on treatment averages, the lowest CR values were recorded in the CaNP group (9.9 N), followed by CaNP + SA (11.5 N) and SA (12.1 N). Salicylic acid mitigates this process by inhibiting ethylene biosynthesis and reducing the activities of cell wall–degrading enzymes such as polygalacturonase, pectin methylesterase, and β-galactosidase, thereby improving water retention capacity [,]. In contrast, CaNP reinforces Ca2+–pectate bridges within the cell wall matrix, enhancing structural firmness [,]. The combined SA + CaNP treatment amplified this effect by integrating SA-induced activation of stress signaling with CaNP-mediated ionic stabilization, resulting in synergistic reinforcement of cell wall integrity.

Table 6.
Effect of salicylic acid (SA) and calcium oxide nanoparticles (CaNP) on cutting resistance (N) of fresh-cut lettuce during cold storage.
The PCA results further supported the distribution patterns in the dataset: CR was positioned in the same loading direction as weight loss (WL) and the opposite direction of MSI, CSI, and SPAD. This reflects the tendency higher CR values to appear in samples with greater WL and lower membrane- or pigment-related stability. PCA provides a visual summary of how variables co-vary within the dataset. The clustering of CaNP + SA samples toward MSI, CSI, and SPAD vectors indicates that this treatment aligned more closely with parameters reflecting better physiological stability, similar to postharvest multivariate patterns reported in lettuce and spinach [,].
Correlation analysis showed parallel associations: CR was positively correlated with WL (r = 0.64) and negatively correlated with MSI and SPAD. These relationships demonstrate that samples with greater water loss tended to show higher resistance to cutting, whereas samples with higher membrane and pigment stability maintained lower CR values. This pattern is consistent with earlier work showing that dehydration accelerates tissue hardening in fresh-cut leafy vegetables [] and that chlorophyll integrity is often associated with improved textural stability under cold storage []. As with PCA, these correlations describe co-variation among parameters.
The increasing CR values in the control group during late storage stages corresponded with elevated WL, while SA-treated samples showed more stable CR values throughout storage. SA has been shown to modulate water status and delay senescence in lettuce and Brassica vegetables [,]. CaNP-treated samples showed the lowest CR values overall, and similar behavior has been reported in produce where calcium application influenced tissue firmness dynamics depending on dose and nanoparticle properties [,]. The intermediate CR levels observed under the CaNP + SA combination suggest a complementary effect, aligning with studies showing that SA-based elicitors combined with calcium treatments support both structural and physiological quality in stored vegetables [].
Overall, these findings emphasize that CR changes during storage were closely associated with variations in WL, membrane stability, and pigment condition. The combined application of SA and CaNP moderated CR development more effectively than either treatment alone, and descriptive multivariate analyses (PCA and correlations) consistently showed that treatments preserving physiological stability were also associated with lower CR values.
3.4. Weight Loss (WL)
The changes in weight loss (WL) of fresh-cut lettuce during storage are presented in Figure 3. WL significantly increased with storage duration across all treatments (p < 0.001). Clear differences were also observed among treatments; notably, salicylic acid (SA) and the CaNP + SA combination exhibited substantially lower WL compared with the control. By day 18, WL in the control exceeded 5%, whereas the CaNP + SA treatment maintained losses around 3.91%. These results indicate that both SA and CaNP + SA effectively moderated water loss during storage. Similar reductions in WL with SA-based coatings or calcium treatments have been reported for fresh-cut lettuce and leafy vegetables [,].
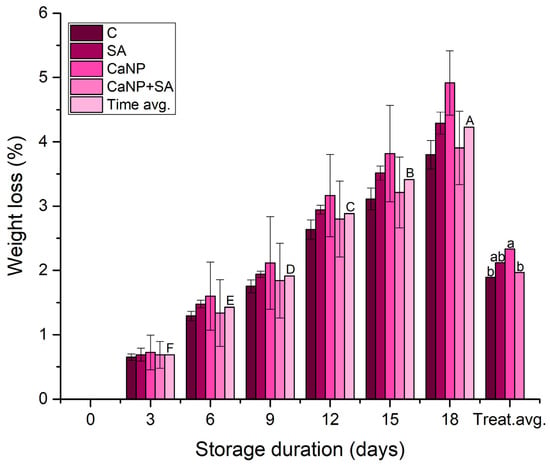
Figure 3.
Changes in weight loss (%) of fresh-cut lettuce as influenced by calcium nanoparticles (CaNP) and salicylic acid (SA) treatments during cold storage (4 ± 1 °C). Different uppercase letters indicate significant differences among storage days (p < 0.05), and different lowercase letters indicate significant differences among treatments (p < 0.05) according to DMRT (n = 3).
SA-treated samples maintained comparatively lower WL than the control, consistent with previous showing that SA enhances water status and delays dehydration in minimally processed produce []. CaNP-treated samples showed higher WL than SA and CaNP + SA, indicating that CaNP alone was less effective in limiting moisture loss. This aligns with studies demonstrating that calcium nanoparticles can improve structural stability but their impact on WL depends on dose and formulation [,]. When SA and CaNP were applied together, WL remained the lowest among treatments, suggesting a complementary interaction that may support both membrane structure and stress modulation.
According to the correlation matrix, WL showed negative associations with SPAD (r = −0.58), MSI (r = −0.52), and CSI (r = −0.39), indicating that samples with higher WL tended to exhibit lower membrane and pigment stability. WL was positively correlated with total soluble phenolics (TSP) (r = 0.76), which reflects a typical postharvest pattern in which phenolic accumulation increases as tissues experience stress. Correlations represent co-variation and illustrate how WL progressed in parallel with changes in pigment content and membrane integrity. These associative patterns are consistent with previous observations in parsley and Chinese flowering cabbage stored under cold conditions [,].
The PCA results further summarized these multivariate relationships. In PC1, WL, TSP, and color change (ΔE) loaded positively, while SPAD, MSI, and CSI loaded negatively, illustrating how water loss and discoloration varied together with membrane-related parameters. PCA biplot positioning placed CaNP + SA closer to SPAD, MSI, and CSI, indicating that samples under this treatment grouped with variables representing higher stability. In contrast, control samples aligned with WL and ΔE, reflecting greater dehydration and discoloration. PCA does not imply physiological mechanisms but offers a visual representation of how variables and treatments are clustered within the dataset. Similar PCA-based quality groupings have been reported in fresh-cut leafy vegetables []
Overall, the combined application of SA and CaNP minimized WL more effectively than either treatment alone and maintained both physiological and biochemical stability during cold storage. Correlation and PCA analyses consistently illustrated an inverse association between WL and membrane/pigment stability, supporting the potential of combined SA and CaNP-based strategies for enhancing postharvest quality retention.
3.5. Total Soluble Solids (TSS)
Slight fluctuations were observed in the total soluble solids (TSS) values of fresh-cut lettuce during storage (Figure 4). According to the analysis of variance, the treatment factor had a statistically significant effect on TSS (p < 0.01), whereas the effect of storage duration was marginally significant (p ≈ 0.059). This indicates that the variations in TSS were primarily treatment-dependent. During the 3rd and 6th days of storage, salicylic acid (SA) treatment exhibited the highest TSS values (approximately 3.1–3.2%), while the control and CaNP groups showed lower levels (2.6–2.8%), and the CaNP + SA combination maintained intermediate values. These findings may be attributed to the regulatory role of SA in carbohydrate metabolism. Previous studies have reported that SA can influence carbohydrate balance and contribute to sugar retention in stored vegetables by modulating enzymes associated with carbohydrate turnover [,].
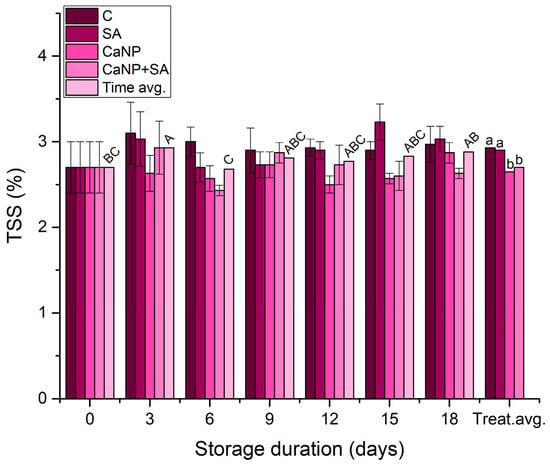
Figure 4.
Changes in TSS (%) of fresh-cut lettuce as influenced by calcium nanoparticles (CaNP) and salicylic acid (SA) treatments during cold storage (4 ± 1 °C). Different uppercase letters indicate significant differences among storage days (p < 0.05), and different lowercase letters indicate significant differences among treatments (p < 0.05) according to DMRT (n = 3).
In the present study, CaNP alone did not significantly increase TSS, which is consistent with the fact that calcium treatments generally act by supporting structural integrity rather than directly altering sugar metabolism [,]. The similarity between SA and CaNP + SA suggests that both treatments contributed to maintaining moderate TSS levels during storage.
According to the correlation matrix (Figure 3), TSS was positively correlated with TSP (r = 0.61) and WL (r = 0.62), indicating that samples with higher soluble solids also tended to exhibit higher phenolic content and moisture loss during storage. These correlations describe statistical associations among quality parameters. In contrast, negative correlations were found between TSS and SPAD (r = −0.04) and MSI (r = −0.52), suggesting that increases in TSS coincided with decreases in pigment- and membrane-related indices as storage progressed [].
PCA analizi (Şekil 4), değişkenler arasındaki çok değişkenli ilişkilere dair tamamlayıcı bir genel bakış sağlamıştır. TSS, PC1 ekseninde TSP ve WL ile aynı yönde yüklenmiş olup, bu parametrelerin işlemler ve depolama süreleri boyunca birlikte değiştiğini göstermektedir. PCA ikili grafiğinde, SA ile işlem görmüş numuneler PC1’ in pozitif bölgesinde kümelenmiş olup, bu da nispeten daha yüksek TSS ve TSP değerleriyle ilişkili olduklarını göstermektedir.
3.6. Chlorophyll Content (Chl a, Chl b, Total Chl), Chlorophyll Stability Index (CSI), and Relative Chlorophyll (SPAD)
The chlorophyll content (Chl a, Chl b, and total Chl), chlorophyll stability index (CSI), and SPAD values of fresh-cut lettuce significantly decreased during cold storage (Table 7). Chlorophyll degradation became more evident after day 12, leading to highly significant differences among treatments (p < 0.001). Both SA and CaNP + SA treatments maintained higher Chl a and Chl b levels than the control and CaNP alone. The highest mean total Chl content (0.891 mg g−1 FW) was recorded in the CaNP + SA treatment, while CaNP alone produced comparatively lower values, indicating a more limited impact on pigment retention. Similar improvement in pigment preservation by SA on Ca-based treatments have been reported for spinach and lettuce under storage conditions [,]. Also, the improved pigment retention under SA treatment can be attributed to its inhibitory effect on chlorophyll catabolic enzymes such as chlorophyllase, Mg-dechelatase, and pheophorbide a oxygenase, which delay chlorophyll degradation [,].

Table 7.
Effect of salicylic acid (SA) and calcium oxide nanoparticles (CaNP) on Chl a, chl b, total Chl, CSI and SPAD values of fresh-cut lettuce during cold storage.
Throughout storage, SA and CaNP + SA consistently retained greater pigment levels, and these statistical differences suggest that SA contributed more markedly to chlorophyll preservation than CaNP when applied alone. CSI showed a significant treatment effect (p < 0.001), peaking around day 9 before gradually declining. The CaNP + SA combination exhibited the highest overall CSI mean (96.6%), whereas CaNP alone maintained lower CSI, reflecting weaker protection against pigment loss. Similar trends have been reported in leafy vegetables treated with calcium-based coatings, where membrane integrity was linked to delayed chlorophyll deterioration []. Correlation analysis positive associations between CSI and Chl-a (r = 0.75), Chl-b (r = 0.74), and Total Chl (r = 0.62). These relationships indicate that samples with higher membrane stability tended to retain mora chlorophyll.
SPAD values also declined significantly (p < 0.001). By day 18, the control reached the lowest SPAD value (21.1), while SA and CaNP + SA maintained higher values (24.9 and 24.2), consistent with their observed chlorophyll retention. CaNP alone exhibited intermediate or inconsistent SPAD patterns, in line with its pigment results.
PCA illustrated the multivariate distribution of variables. Chl-a, Chl-b, total Chl, CSI, SPAD, and MSI loaded in a similar direction, indicating that these quality parameters tended to vary together across treatments. This pattern does not imply mechanistic relationships but reflects shared variability. CaNP + SA samples were located near these pigment- and membrane-related variables, whereas CaNP alone appeared more scattered, consistent with its variable performance in maintaining pigment and membrane stability. Similar PCA clustering patterns have been observed in fresh-cut vegetables subjected to SA and calcium treatments [].
Overall, SA and CaNP + SA were more effective in preserving chlorophyll content, CSI, and SPAD values than CaNP alone or the control. CaNP alone provided limited and less consistent protection, whereas the combined treatment produced more stable outcomes across pigment- and membrane-related traits. These findings indicate that treatments involving SA—either alone or with CaNP—better supported chlorophyll preservation and pigment stability during extended cold storage.
3.7. Color Parameters
Significant changes were observed in the color parameters (L, Hue°, ΔE, Chroma, and Yellowness Index, YI*) of fresh-cut lettuce throughout the storage period (Table 8). As storage progressed, L and Hue° values generally decreased, while ΔE and YI increased, indicating progressive darkening and yellowing of the leaves. Treatment differences were statistically significant (p < 0.001). The CaNP + SA combination maintained the highest L (53.03), Hue° (119.3), and Chroma (33.8) values during storage, reflecting better preservation of brightness and color saturation. In contrast, the control exhibited pronounced declines in L and Hue°, accompanied by marked increases in ΔE and YI, indicating advanced discoloration and senescence.

Table 8.
Effect of salicylic acid (SA) and calcium oxide nanoparticles (CaNP) on color parameters of fresh-cut lettuce during cold storage.
The reduction in L after day 12 corresponded with increased pigment deterioration. SA helped maintain higher L values compared with the control, previous findings that SA delays chlorophyll degradation in leafy vegetables []. The CaNP + SA treatment yielded the lowest overall ΔE (2.40), indicating smallest perceptible color change. Although Hue° declined in all treatments, the decrease was minimal under CaNP + SA, suggesting slower yellowing. Higher Hue° values correspond to better retention of green coloration, a pattern also reported in SA- or calcium-treated leafy vegetables stored under cold conditions []. Chroma gradually decreased in all treatments but remained significantly higher in SA and CaNP + SA (p < 0.05), indicating better color saturation and pigment integrity.
YI values showed that the control experienced pronounced yellowing (83.2 to 77.9), whereas CaNP + SA maintained higher values (80.6), consistent with reduced yellowing. These differences align with the color and chlorophyll results obtained earlier for the same treatments.
Correlation analysis revealed positive associations between L and physiological parameters such as CSI (r = 0.59) and MSI (r = 0.55). ΔE and YI showed negative correlations with Chl-a, Chl-b, and total Chl (r = −0.79 to −0.83), indicating that greater discoloration tended to accompany lower chlorophyll levels. PCA further illustrated these multivariate patterns: L, Hue°, and Chroma loaded in the same direction as CSI, SPAD, and total Chl, indicating that samples with better pigment and membrane stability generally clustered together. CaNP + SA samples appeared near these color-related variables, while the control samples were associated with ΔE and YI, reflecting more advanced discoloration. These PCA patterns provide an overview of how color and physiological traits varied jointly but should not be interpreted as causal evidence.
Collectively, these results show that SA and especially CaNP + SA treatments were more effective than CaNP alone or the control in preserving color quality. The combined treatment provided the most consistent protection against darkening and yellowing, supporting overall visual stability in fresh-cut lettuce during cold storage.
3.8. Multivariate Analysis (PCA & Correlation)
The multivariate analysis comprehensively revealed the effects of 2 mM salicylic acid (SA) and 100 mg L−1 calcium oxide nanoparticles (CaNP) on the quality parameters of fresh-cut lettuce.
3.8.1. Principal Component Analysis (PCA)
Three principal components (PC1, PC2, and PC3) explained 59.18% of the total variance (Figure 5). PC1, PC2, and PC3 accounted for 18.8%, 20.2%, and 14.1%, respectively, indicating that the measured quality variables were well separated according to treatment effects. On the PCA loading plot, SPAD, Chl a, Chl b, Total Chl, chlorophyll stability index (CSI), and membrane stability index (MSI) loaded in the same direction, highlighting the strong interdependence between pigment stability and membrane integrity. In contrast, weight loss (WL) and cutting resistance (CR) vectors were oriented oppositely, signifying that increased water loss and tissue stiffness were inversely related to chlorophyll retention and cell-membrane stability. The distribution of treatment groups was clearly differentiated within the PCA model. Samples treated with SA and CaNP + SA clustered toward the vectors of SPAD, CSI, and MSI, confirming that these treatments effectively maintained pigment and membrane stability. CaNP alone applied samples were aligned with WL and CR vectors, indicating greater moisture loss and higher tissue rigidity. The control group exhibited a broader dispersion, reflecting greater fluctuations in quality attributes during storage.
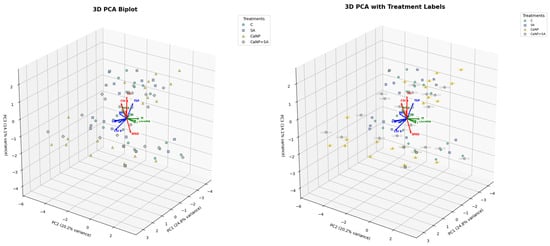
Figure 5.
Principal Component Analysis (PCA) biplot showing the relationships among quality parameters of fresh-cut lettuce subjected to different treatments (Control, SA, CaNP, CaNP + SA) during 18 days of cold storage. The PCA model explained 59.18% of the total variance (PC1: 24.8%, PC2: 20.2%, PC3: 14.1%). Vectors pointing in the same direction indicate strong positive correlations among variables such as SPAD, Chl a, Chl b, Total Chl, CSI, and MSI, while opposite directions represent negative relationships with weight loss (WL) and cutting resistance (CR). SA and CaNP + SA treatments were clustered close to stability-related parameters, indicating improved pigment and membrane integrity compared to other treatments.
3.8.2. Correlation Matrix
The correlation analysis supported the PCA findings, revealing consistent interrelationships among physiological and biochemical quality parameters of fresh-cut lettuce (Figure 6). Strong positive correlations (r > 0.70) were detected among SPAD, Chl-a, Chl-b, Total-Chl, MSI, and CSI, confirming that pigment retention is tightly linked to the maintenance of membrane integrity. In particular, the positive correlation between CSI and MSI (r = 0.73) demonstrates that chlorophyll stability and membrane durability develop concurrently during cold storage. Similarly, the high correlations between SPAD and both Chl-a and Chl-b (r > 0.80) confirm that the greenness of lettuce leaves directly depends on chlorophyll content. Conversely, weight loss (WL) showed negative correlations with SPAD (r = −0.58), CSI (r = −0.60), and MSI (r = −0.52), indicating that increasing water loss led to reductions in pigment stability and cell-membrane integrity. The negative relationship between cutting resistance (CR) and MSI supports the interpretation that increased tissue rigidity is associated with cellular water imbalance and membrane leakage. In contrast, total soluble phenolics (TSP) exhibited positive correlations with both MSI and CSI (r ≈ 0.75), suggesting that phenolic compounds contribute to the attenuation of oxidative stress during storage and help preserve membrane stability. Taken together, the correlation and PCA results indicate that SA and CaNP + SA treatments mitigated water loss while maintaining membrane stability, chlorophyll pigments, and phenolic compounds, whereas CaNP alone had a limited protective effect. This multivariate approach demonstrates that the combined application of salicylic acid and calcium oxide nanoparticles exerts complementary and synergistic effects on preserving the physiological quality of fresh-cut lettuce during storage.
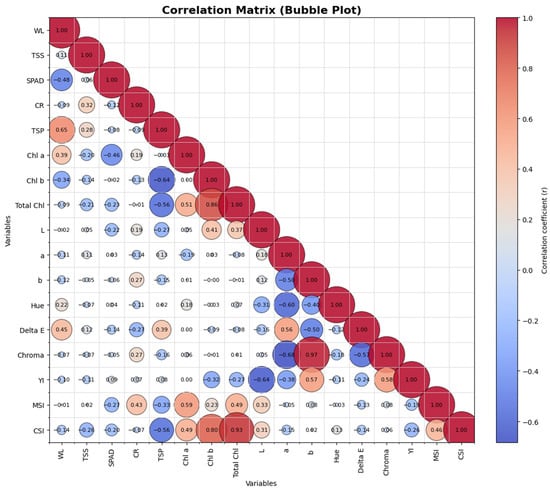
Figure 6.
Correlation matrix (bubble plot) illustrating Pearson correlation coefficients among physicochemical and physiological parameters of fresh-cut lettuce stored for 18 days under different treatments. Positive correlations are indicated in red and negative in blue. Strong positive associations were observed among SPAD, Chl a, Chl b, Total Chl, CSI, and MSI (r > 0.70), whereas weight loss (WL) and cutting resistance (CR) showed significant negative correlations with these parameters (r < −0.50). The results confirm the consistency between PCA and correlation analysis in identifying the synergistic effects of SA and CaNP + SA treatments on maintaining leaf quality.
4. Conclusions
This study evaluated the effects of 2 mM salicylic acid (SA) and 100 mg L−1 calcium oxide nanoparticles (CaNP), applied individually or in combination, on the postharvest quality of fresh-cut lettuce during 18 days of cold storage. SA and CaNP + SA consistently supported key quality attributes, including phenolic content, pigment retention, and membrane-related parameters, whereas CaNP alone showed more variable outcomes across different quality indicators.
SA treatment maintained higher total soluble phenolic content and contributed to better preservation of chlorophyll pigments and SPAD values, helping delay senescence-related discoloration. The combined CaNP + SA treatment resulted in the lowest weight loss and relatively high membrane stability index (MSI), indicating improved capacity to limit water loss and maintain cellular integrity. In contrast, CaNP applied alone had weaker performance in MSI and produced intermediate or fluctuating responses in several parameters, suggesting that its effectiveness was more limited under the tested conditions.
Correlation analysis revealed consistent relationships among physiological variables—SPAD, CSI, and MSI were positively associated with each other and negatively associated with weight loss and cutting resistance—indicating that improved membrane stability and pigment retention coincided with better textural and hydration status. These associations describe shared response patterns among variables, without implying mechanistic causality. Similarly, PCA provided an overview of multivariate distribution patterns, showing that SA and CaNP + SA were situated near variables linked to pigment and membrane stability, whereas CaNP alone aligned more variably with mechanical or dehydration-related axes.
Overall, the combined application of SA and CaNP delayed chlorophyll degradation, moderated water loss, and supported the maintenance of phenolic content, resulting in improved postharvest performance of fresh-cut lettuce. The complementary effects observed for CaNP + SA suggest that integrating elicitor-based (SA) and structural support (Ca-based) treatments may represent a practical approach to sustaining the visual and physiological quality of minimally processed leafy vegetables. Future work should evaluate different CaNP concentrations and application modes to clarify the conditions under which CaNP can perform optimally, as well as validate these findings under commercial processing scenarios.
Author Contributions
Conceptualization, R.K. and M.U.K.; methodology, R.K.; M.U.K. and H.S. software, R.K.; validation, R.K. and H.S.; formal analysis, Y.G. and M.D.; investigation, R.K., Y.G. and M.D.; resources, Y.G., M.D., R.K., M.U.K. and H.S.; data curation, R.K.; writing—original draft preparation, R.K.; writing—review and editing, R.K., M.U.K. and H.S.; visualization, R.K.; supervision, R.K., M.U.K. and H.S.; project administration, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors would like to acknowledge the administrative and technical support provided by the Department of Horticulture at Kocaeli University. The contributions of the laboratory staff during sample preparation and analytical measurements are gratefully appreciated. During the preparation of this manuscript, the authors used OpenAI ChatGPT (GPT-5) for language refinement and improvement of textual clarity. The authors have reviewed and edited all generated content and take full responsibility for the final version of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Charles, F.; Nilprapruck, P.; Roux, D.; Sallanon, H. Visible Light as a New Tool to Maintain Fresh-Cut Lettuce Postharvest Quality. Postharvest Biol. Technol. 2018, 135, 51–56. [Google Scholar] [CrossRef]
- Peng, H.; Simko, I. Extending Lettuce Shelf Life Through Integrated Technologies. Curr. Opin. Biotechnol. 2023, 81, 102951. [Google Scholar] [CrossRef]
- Nogales-Delgado, S. Polyphenoloxidase (PPO): Effect, current determination and inhibition treatments in fresh-cut produce. Appl. Sci. 2021, 11, 7813. [Google Scholar] [CrossRef]
- Choi, I.L.; Lee, J.H.; Choi, D.H.; Wang, L.X.; Kang, H.M. Evaluation of the Storage Characteristics in Maintaining the Overall Quality of Whole and Fresh-Cut Romaine Lettuce during MA Storage. Horticulturae 2021, 7, 461. [Google Scholar] [CrossRef]
- Morillo, D.M.C.; Delgado, E.T.; Flórez, L.F.V.; Vásquez, L.L.; Escobar, D.T.; España, D.F.M. Minimally Processed Lettuces: Extending Shelf Life Through Packaging and Treatment with Salicylic Acid and Sodium Chloride Solutions. Braz. J. Food Technol. 2025, 28, e2023116. [Google Scholar] [CrossRef]
- Abdelkader, M.F.; Mahmoud, M.H.; Lo’ay, A.A.; Abdein, M.A.; Metwally, K.; Ikeno, S.; Doklega, S.M. The Effect of Combining Postharvest Calcium Nanoparticles with a Salicylic Acid Treatment on Cucumber Tissue Breakdown via Enzyme Activity during Shelf Life. Molecules 2022, 27, 3687. [Google Scholar] [CrossRef]
- Nawarathna, H.M.K.C.; Eeswara, J.P. The Effect of Salicylic Acid on Shelf Life and Bio-active Compounds in Lettuce (Lactuca sativa L.). Ceylon J. Sci. 2025, 54, 599–605. [Google Scholar] [CrossRef]
- De Corato, U. Improving the Shelf-Life and Quality of Fresh and Minimally-Processed Fruits and Vegetables for a Modern Food Industry: A Comprehensive Critical Review from the Traditional Technologies into the Most Promising Advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef]
- Cid-López, M.L.; Soriano-Melgar, L.D.A.A.; García-González, A.; Cortéz-Mazatán, G.; Mendoza-Mendoza, E.; Rivera-Cabrera, F.; Peralta-Rodríguez, R.D. The Benefits of Adding Calcium Oxide Nanoparticles to Biocompatible Polymeric Coatings during Cucumber Fruits Postharvest Storage. Sci. Hortic. 2021, 287, 110285. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil, 2nd ed.; California Agricultural Experiment Station: Davis, CA, USA, 1950; Volume 347, p. 32. [Google Scholar]
- Chavez-Santiago, J.O.; Rodríguez-Castillejos, G.C.; Montenegro, G.; Bridi, R.; Valdes-Gomez, H.; Alvarado-Reyna, S.; Castillo-Ruiz, O.; Santiago-Adame, R. Phenolic Content, Antioxidant and Antifungal Activity of Jackfruit Extracts (Artocarpus heterophyllus Lam.). Food Sci. Technol. 2021, 42, e02221. [Google Scholar] [CrossRef]
- Nabati, J.; Nezami, A.; Hasanfard, A.; Nemati, Z.; Kahrom, N. Prolonged Exposure to Freezing Stress Reduces the Ability of Chickpea Seedlings to Effectively Tolerate Extremely Low Temperatures. Front. Plant Sci. 2023, 14, 1239008. [Google Scholar] [CrossRef]
- Ngcobo, S.; Bada, S.O.; Ukpong, A.M.; Risenga, I. Optimal Chlorophyll Extraction Conditions and Postharvest Stability in Moringa (M. oleifera) Leaves. Food Meas. Charact. 2024, 18, 1611–1626. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Sotelo, H.; Figueroa-Pérez, M.G. Use of Salicylic Acid during Cultivation of Plants as a Strategy to Improve its Metabolite Profile and Beneficial Health Effects. Ital. J. Food Sci. 2023, 35, 79–90. [Google Scholar] [CrossRef]
- Yu, L.; Lu, K.; Yi, Y.; Wang, L.; Hou, W.; Ai, Y.; Wang, H.; Min, T. Effects of Salicylic Acid on the Quality of Fresh-Cut Chinese Water Chestnuts. J. Agric. Food Res. 2025, 22, 102028. [Google Scholar] [CrossRef]
- Chen, C.; Sun, C.; Wang, Y.; Gong, H.; Zhang, A.; Yang, Y.; Guo, F.; Cui, K.; Fan, X.; Li, X. The Preharvest and Postharvest Application of Salicylic Acid and its Derivatives on Storage of Fruit and Vegetables: A Review. Sci. Hortic. 2023, 312, 111858. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Bornhorst, E.R.; Zhou, B.; Simko, I.; Trouth, F. Identification of Romaine Lettuce (Lactuca sativa var. longifolia) Cultivars with Reduced Browning Discoloration for Fresh-Cut Processing. Postharvest Biol. Technol. 2019, 156, 110931. [Google Scholar] [CrossRef]
- Singh, S. Salicylic Acid Elicitation Improves Antioxidant Activity of Spinach Leaves by Increasing Phenolic Content and Enzyme Levels. Food Chem. Adv. 2023, 2, 100156. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Y.; Xu, W.; Wang, S.; Zhu, J.; Xu, Y.; Xu, M. Quality Changes in Fresh-Cut Lettuce When Subjected to Ultrasound Combined with Zinc Oxide Nanoparticle (ZnO NP) Treatment. Coatings 2024, 14, 943. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Esmaeili, S.; Eskandarzade, P.; Sharifani, M.; Lastochkina, O. Nanotechnology in Regulation of Growth and Stress Tolerance in Vegetable Crops. In Growth Regulation and Quality Improvement of Vegetable Crops: Physiological and Molecular Features; Springer Nature: Singapore, 2025; pp. 653–690. [Google Scholar]
- Veeramani, C.; El Newehy, A.S.; Aloud, A.A.; Alsaif, M.A.; Al-Numair, K.S. Effect of Calcium Oxide Nanoparticles Produced from Lavetira critica Leaf Extract on the Freshness of Fresh-Sliced Fruits. J. Food Process Preserv. 2022, 46, e17234. [Google Scholar] [CrossRef]
- Akter, J.; Hassan, J.; Rahman, M.M.; Biswas, M.S.; Khan, H.I.; Rajib, M.M.R.; Ahmed, M.M.; Khan, M.N.E.A.; Hasan, M.F.A. Colour, Nutritional Composition and Antioxidant Properties of Dehydrated Carrot (Daucus carota var. sativus) using Solar Drying Techniques and Pretreatments. Heliyon 2024, 10, e24165. [Google Scholar] [CrossRef]
- Iturralde-García, R.D.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Cornejo-Ramirez, Y.I.; Bernal-Mercado, A.T.; Del-Toro-Sánchez, C.L. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae 2022, 8, 731. [Google Scholar] [CrossRef]
- Quandoh, E.; Albornoz, K. Fresh-Cut Watermelon: Postharvest Physiology, Technology, and Opportunities for Quality Improvement. Front. Genet. 2025, 16, 1523240. [Google Scholar] [CrossRef] [PubMed]
- Kiremit, M.S.; Akınoğlu, G.; Mitrovica, B.; Rakıcıoğlu, S. Enhancing Drought-Salinity Stress Tolerance in Lettuce: Synergistic Effects of Salicylic Acid and Melatonin. S. Afr. J. Bot. 2024, 172, 212–226. [Google Scholar] [CrossRef]
- Dawood, M.F.; Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Abdel Latef, A.A.H. Role of Acetic Acid and Nitric Oxide Against Salinity and Lithium Stress in Canola (Brassica napus L.). Plants 2023, 13, 51. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, L.; Shao, P.; Sun, P. Phase-Change Materials and Exogenous Melatonin Treatment Alleviated Postharvest Senescence of Agaricus bisporus by Inhibiting Browning and Maintaining Cell Membrane Integrity. Postharvest Biol. Technol. 2022, 192, 112009. [Google Scholar] [CrossRef]
- Azarmi-Atajan, F.; Sayyari-Zohan, M.H. Alleviation of Salt Stress in Lettuce (Lactuca sativa L.) by Plant Growth-Promoting Rhizobacteria. JHPR 2020, 3, 67–78. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Liu, J.; Dong, X.; Zhang, H.; Jeong, B.R. Prolonged Post-harvest Preservation in Lettuce (Lactuca sativa L.) by Reducing Water Loss Rate and Chlorophyll Degradation Regulated through Lighting Direction-Induced Morphophysiological Improvements. Plants 2024, 13, 2564. [Google Scholar] [CrossRef]
- Rasheed, Y.; Khalid, F.; Ashraf, H.; Asif, K.; Maqsood, M.F.; Naz, N.; Shahbaz, M.; Zulfiqar, U.; Rana, S. Enhancing Plant Stress Resilience with Osmolytes and Nanoparticles. J. Soil. Sci. Plant Nutr. 2024, 24, 1871–1906. [Google Scholar] [CrossRef]
- Wang, J.; Allan, A.C.; Wang, W.Q.; Yin, X.R. The Effects of Salicylic Acid on Quality Control of Horticultural Commodities. N. Z. J. Crop. Hortic. Sci. 2022, 50, 99–117. [Google Scholar] [CrossRef]
- Asrey, R.; Vinod, B.R.; Menaka, M.; Ahamed, S.; Kumar, A. Recent Trends in Postharvest Treatments for Fruits and Vegetables. In Advances in Postharvest and Analytical Technology of Horticulture Crops; Springer Nature: Singapore, 2024; pp. 35–64. [Google Scholar]
- Chinnaswamy, S.; Rudra, S.G.; Sharma, R.R. Texturizers for Fresh-Cut Fruit and Vegetable Products. In Fresh-Cut Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020; pp. 121–149. [Google Scholar]
- Singh, A.; Upadhyay, P.; Rami, E.; Singh, S.K. Nanotechnology Interventions for Sustainable Plant Nutrition and Biosensing. J. Soil. Sci. Plant Nutr. 2024, 24, 1775–1798. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Clarkson, G.J.; Rothwell, S.D.; Fry, S.C. Novel Insights into Ascorbate Retention and Degradation during the Washing and Post-Harvest Storage of Spinach and Other Salad Leaves. Food Chem. 2017, 233, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging postharvest technologies to enhance the shelf-life of fruit and vegetables: An overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Bhandari, B. Fresh-Cut Vegetables. In Handbook of Vegetables and Vegetable Processing; Wiley: Hoboken, NJ, USA, 2011; pp. 219–242. [Google Scholar] [CrossRef]
- Zhang, H.; Cun, Y.; Wang, J.; Wu, M.; Li, X.; Liang, Q.; Wang, C.; Zhao, L.; Deng, J. Acetylsalicylic Acid and Salicylic Acid Alleviate Postharvest Leaf Senescence in Chinese Flowering Cabbage (Brassica rapa var. parachinensis). Postharvest Biol. Technol. 2022, 194, 112070. [Google Scholar] [CrossRef]
- Abdel-Kader, H.A.; Yousef, N.; Hossain, M.A.; Dawood, M.F. Lipid Production, Oxidative Status, Antioxidant Enzymes and Photosynthetic Efficiency of Coccomyxa chodatii SAG 216-2 in Response to Calcium Oxide Nanoparticles. Phyton 2023, 92, 1955–1974. [Google Scholar] [CrossRef]
- Jacuinde-Guzman, J.K.; Escalona-Buendía, H.B.; Barbosa-Martínez, C.; Rivera-Cabrera, F.; Raddatz-Mota, D.; Soriano-Melgar, L.D.A.A. The Potential of Calcium Nanoparticles in Posthaverst Conservation of Fresh-Cut Seedless Watermelon (Citrullus lanatus). Postharvest Biol. Technol. 2024, 216, 113069. [Google Scholar] [CrossRef]
- MR Elshawa, G.R.; Ibrahim, F.A.; Arafat, L.A. Effect of Nano Calcium, Calcium Chloride, and Salicylic Acid on Bent Neck in Cut Roses. J. Hortic. Sci. Biotechnol. 2023, 98, 233–245. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms In Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Ranjbar, S.; Rahemi, M.; Ramezanian, A. Comparison of Nano-Calcium and Calcium Chloride Spray on Postharvest Quality and Cell Wall Enzymes Activity in Apple cv. Red. Delic. Sci. Hortic. 2018, 240, 57–64. [Google Scholar] [CrossRef]
- Üner Öztürk, K.; Koyuncu, M.A. Effects of Ozone and Salicylic Acid on Post-Harvest Quality of Parsley During Storage. Biol. Agric. Hortic. 2021, 37, 183–196. [Google Scholar] [CrossRef]
- Tonto, T.C.; Cimini, S.; Grasso, S.; Zompanti, A.; Santonico, M.; De Gara, L.; Locato, V. Methodological Pipeline for Monitoring Post-Harvest Quality of Leafy Vegetables. Sci. Rep. 2023, 13, 20568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic Acid Treatment Mitigates Chilling Injury in Peach Fruit by Regulation of Sucrose Metabolism and Soluble Sugar Content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, C.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic Acid and Salicylic Acid Induce the Accumulation of Sucrose and Increase Resistance to Chilling İnjury in Peach Fruit. J. Sci. Food Agric. 2021, 101, 4250–4255. [Google Scholar] [CrossRef] [PubMed]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Zhang, C.; Li, Y.; Gou, H.; Liang, G.; Ma, Z.; Mao, J.; Chen, B. Glucose Enhanced Lignin Accumulation in Grapevine Stems cia Promoting Phenylpropanoid Biosynthesis. Chem. Biol. Technol. Agric. 2024, 11, 152. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Asghari, M.; Babalar, M.; Sarcheshmeh, M.A.A. Impact of Salicylic Acid on Postharvest Physiology of Fruits and Vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Academic Press: Cambridge, MA, USA, 2016; pp. 243–268. [Google Scholar] [CrossRef]
- Chepngeno, J.; Owino, W.; Kinyuru, J.; Nenguwo, N. Effect of Calcium Chloride and Hydrocooling on Postharvest Quality of Selected Vegetables. J. Food Res. 2016, 5, 23. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wang, T.; Fang, C.; Wang, J. Methyl Salicylate Delays Peel Yellowing of ‘Zaosu’pear (Pyrus bretschneideri) during Storage by Regulating Chlorophyll Metabolism and Maintaining Chloroplast Ultrastructure. J. Sci. Food Agric. 2019, 99, 4816–4824. [Google Scholar] [CrossRef]
- Tanaka, A.; Ito, H. Chlorophyll Degradation and its Physiological Function. Plant Cell Physiol. 2025, 66, 139–152. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Y.; Dong, S.; Shao, X.; Wang, H.; Zheng, Y.; Yang, Z. Reducing Yellowing and Enhancing Antioxidant Capacity of Broccoli in Storage by Sucrose Treatment. Postharvest Biol. Technol. 2016, 112, 39–45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).