Systematic Analysis of Nutritional Components and Characteristics in Red-Fleshed Dragon Fruit from Different Origins Using Non-Targeted Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of Vitamin C, Total Acidity, and Soluble Sugars
2.3. Determination of Total Phenolic and Total Flavonoid Content
2.4. Determination of Betacyanin Content
2.5. Based on Non-Targeted UPLC-MS/MS Analysis
2.6. Statistical Analysis
3. Results
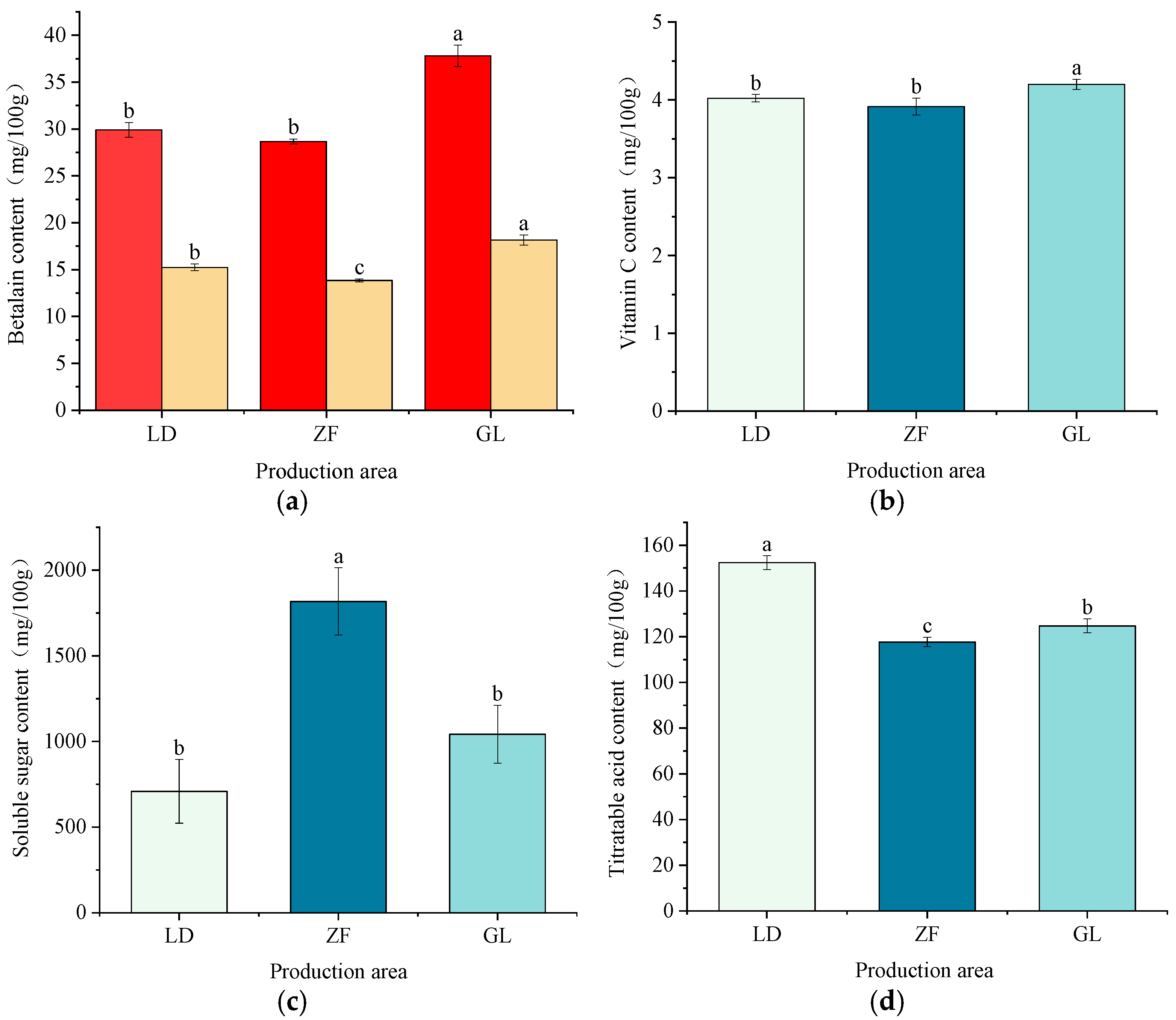
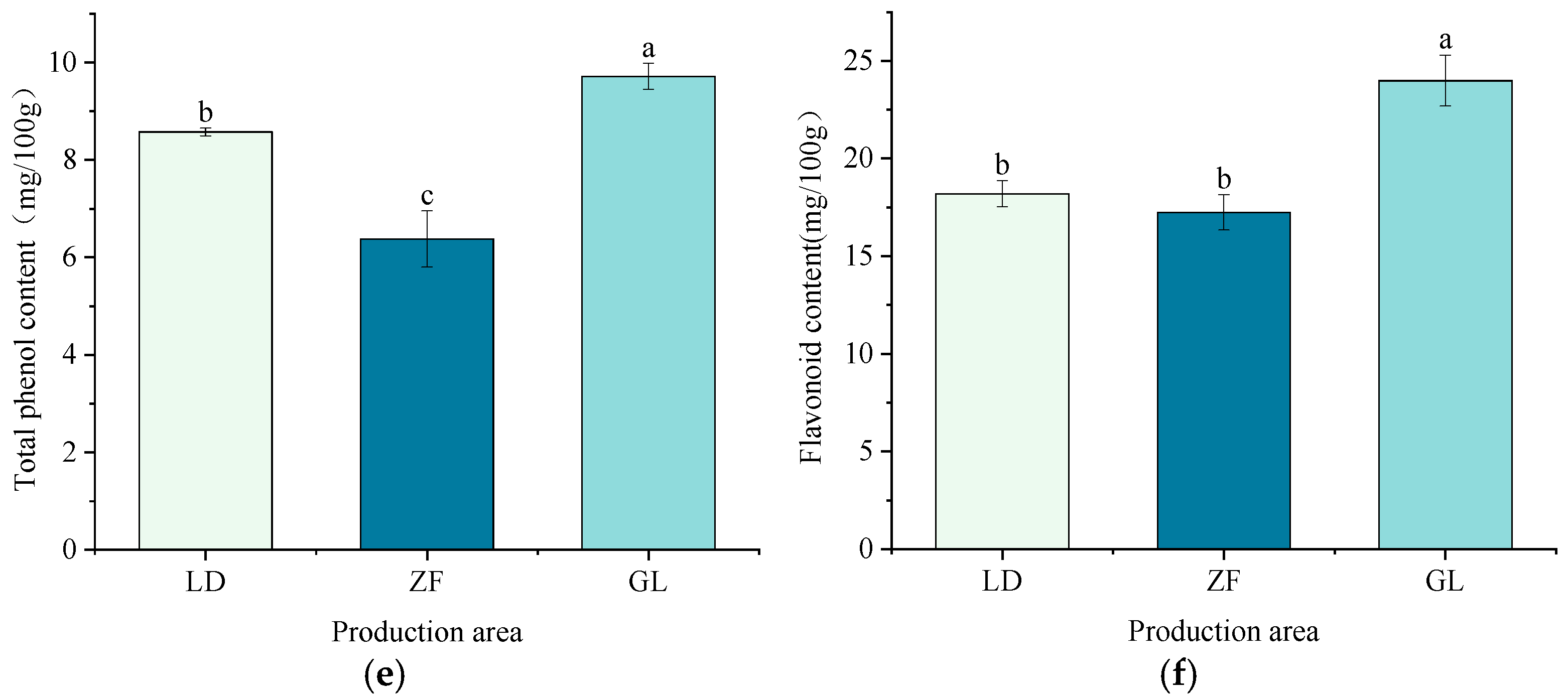
3.1. Nutritional Component Analysis
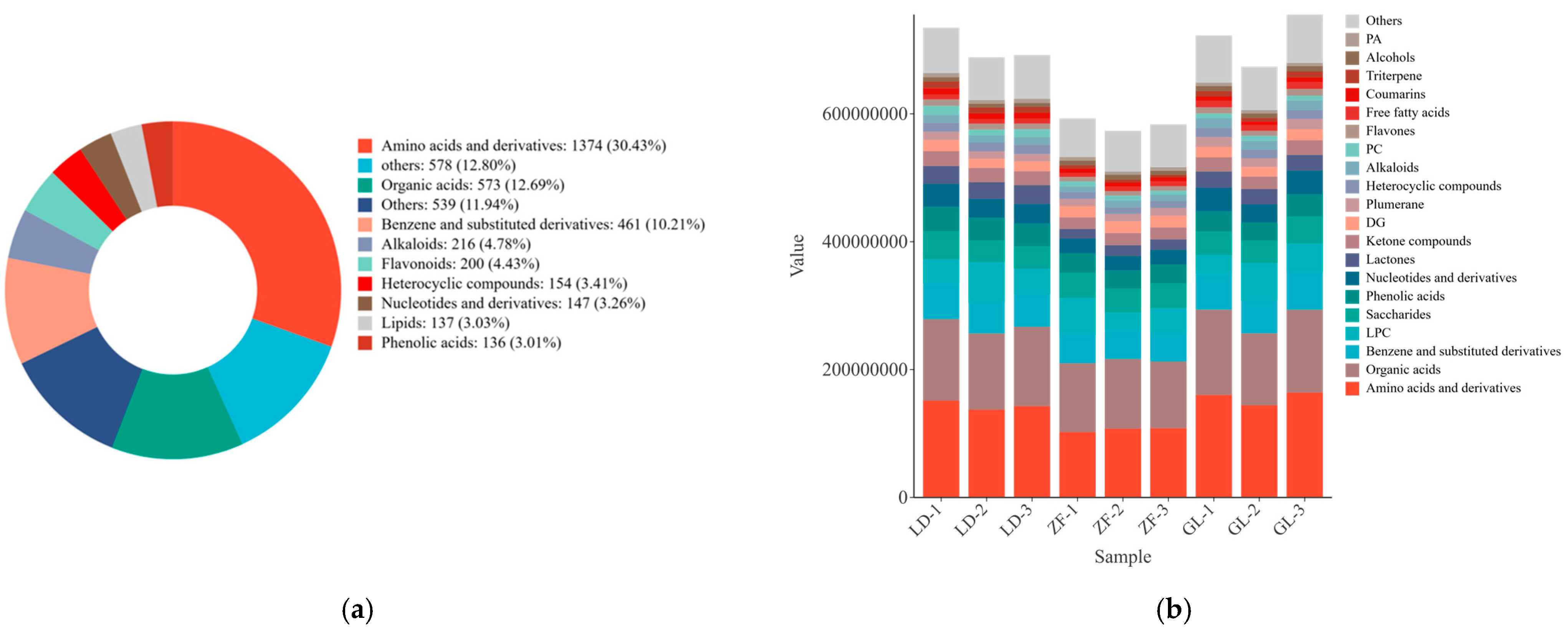
3.2. Non-Targeted Metabolite Analysis
3.3. PCA and OPLS-DA Analysis of Dragon Fruit from Different Origins
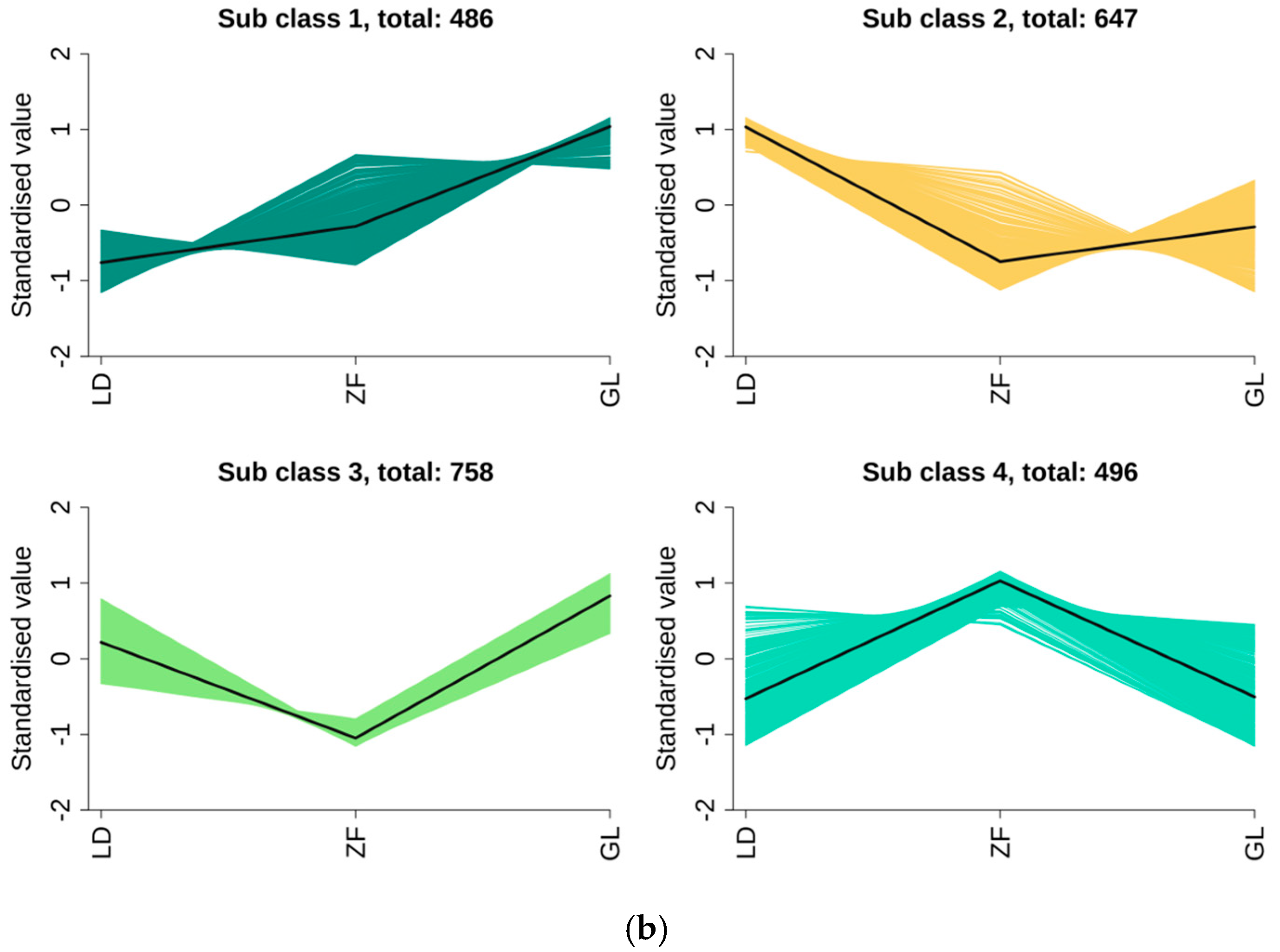
3.4. Cluster Analysis of Red-Flesh Dragon Fruit from Different Origins
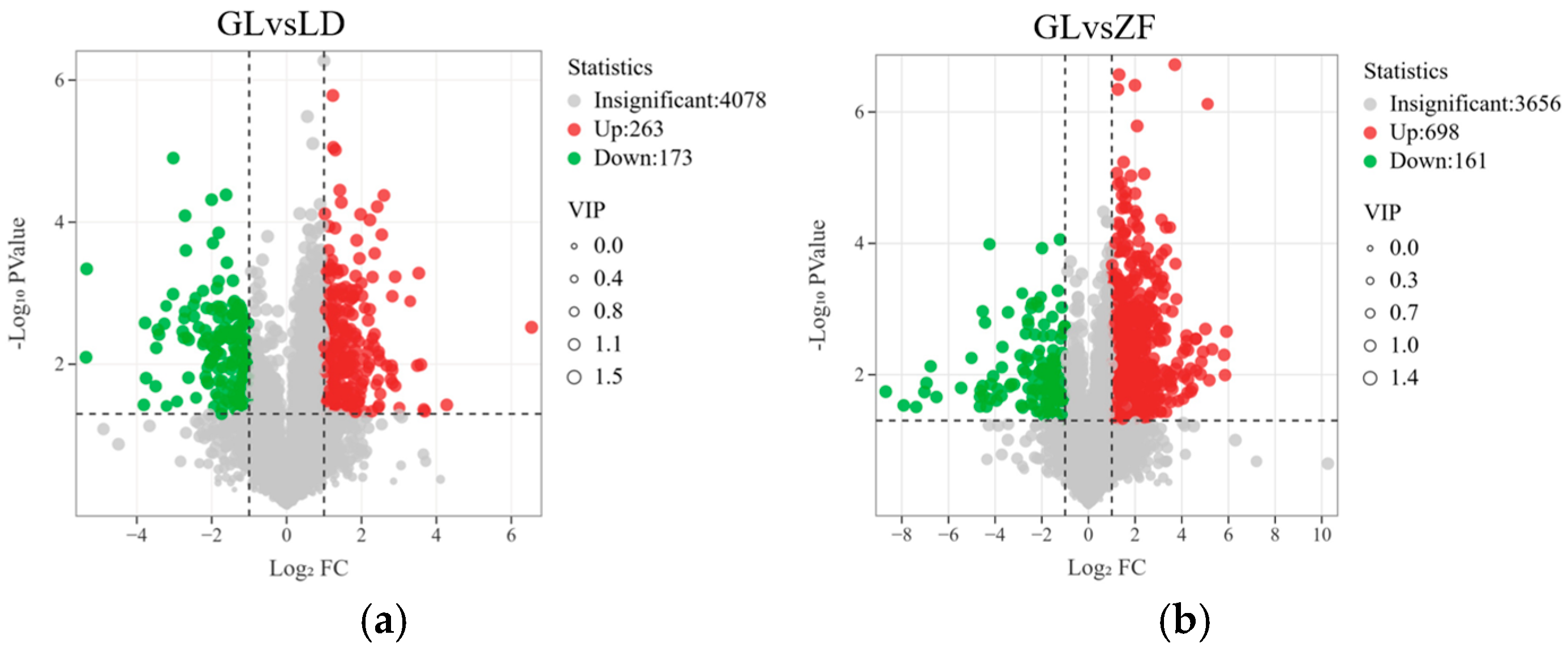
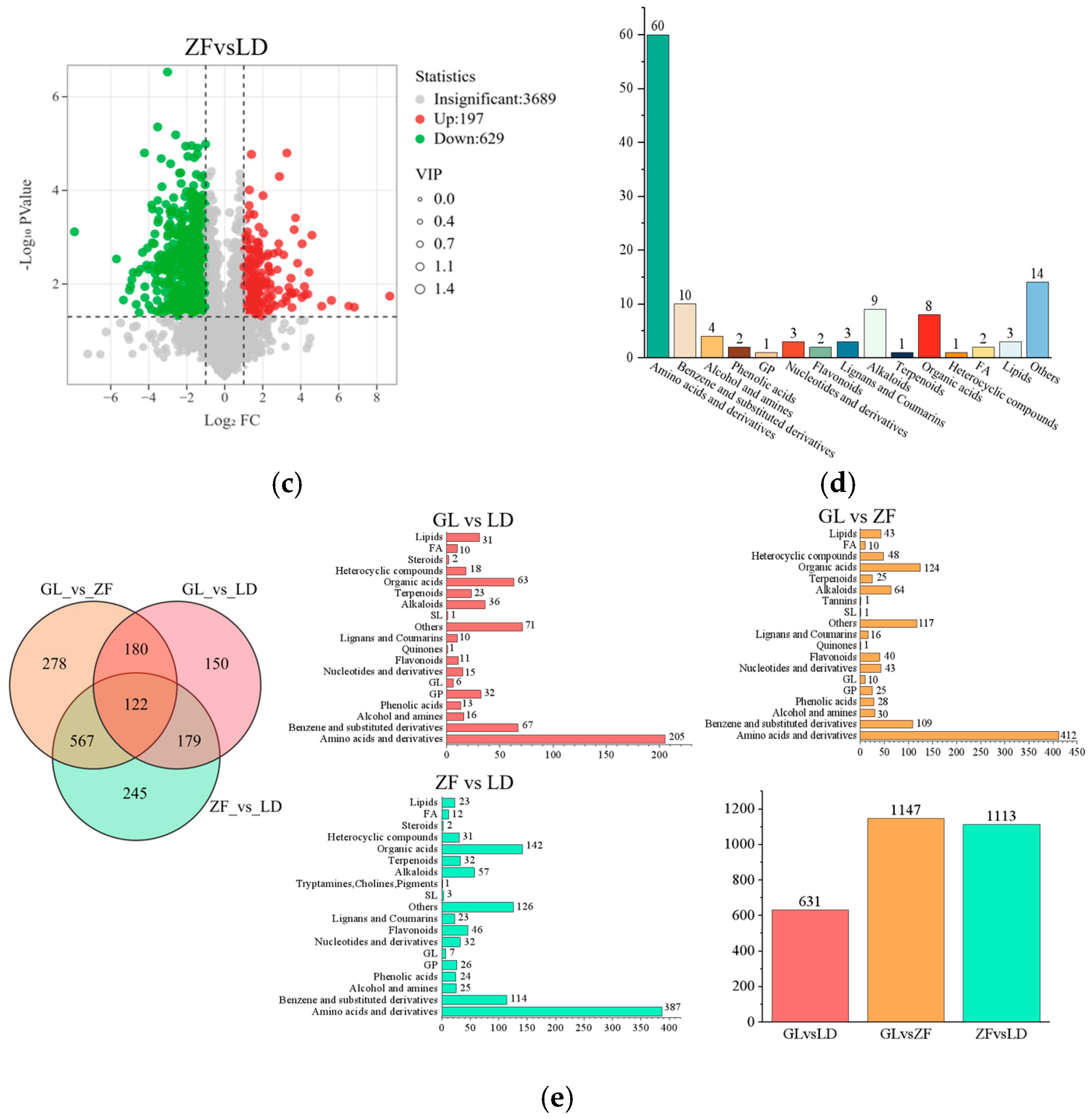
3.5. Metabolite Analysis of Red-Fleshed Dragon Fruit from Different Origins
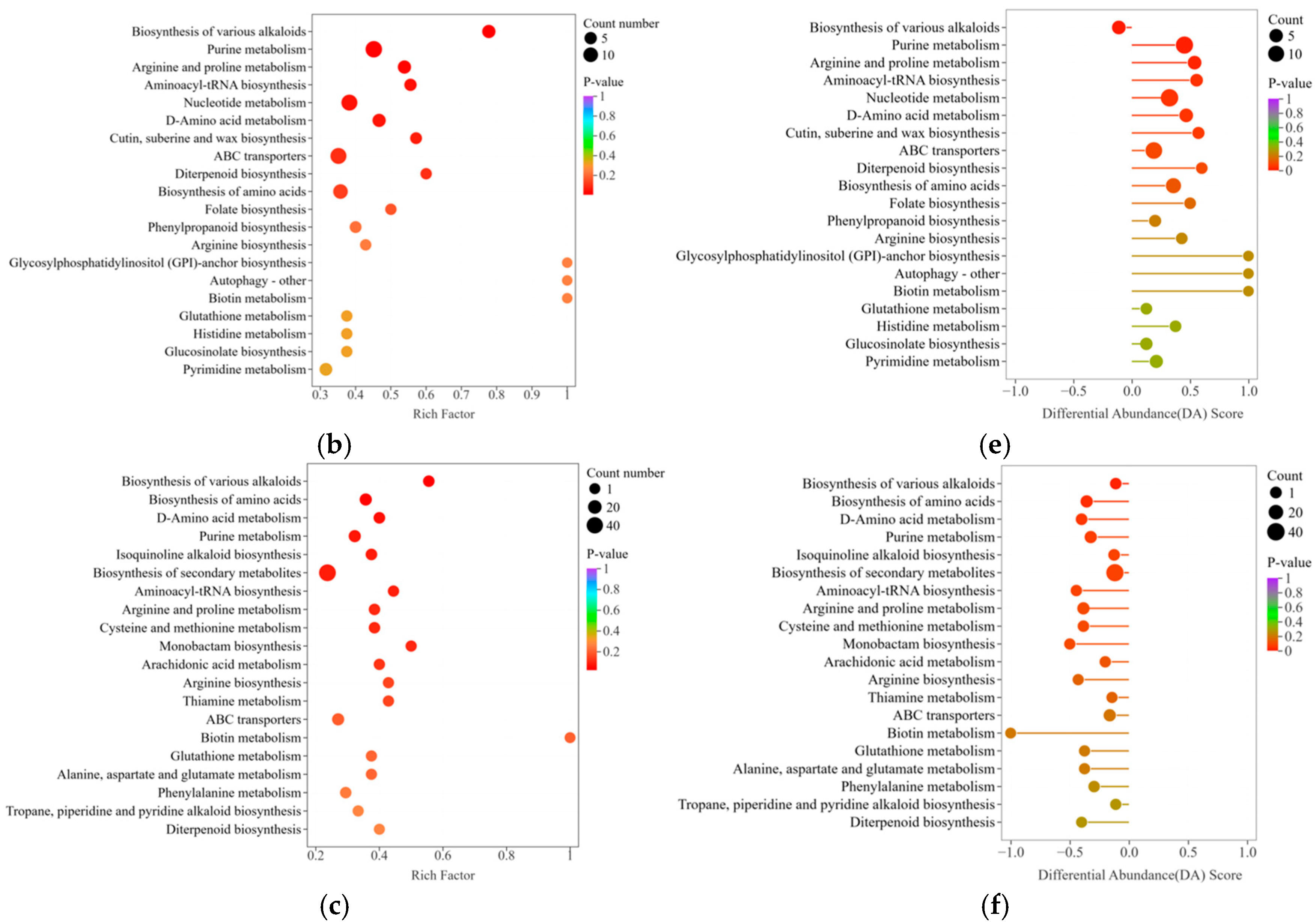
3.6. Annotation and Analysis of Metabolite Differences in Red-Fleshed Dragon Fruit from Different Origins
4. Discussion
4.1. Effects of Different Origins on the Nutritional Composition of Red-Fleshed Dragon Fruit
4.2. Effects of Metabolite Differences in Red-Fleshed Dragon Fruit from Different Origins on Fruit Quality
4.3. Regulatory Mechanisms of Dragon Fruit Quality Under Different Production Environments
4.4. Feasibility of Origin Tracing and Potential Markers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GL | Guanling |
| LD | Luodian |
| ZF | Zhenfeng |
| KNN | K-nearest neighbor |
| PCA | Linear dichroism |
| CMDC | China Meteorological Data Service Center |
| TCA | Trichloroacetic acid |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| HCA | Hierarchical cluster analysis |
| UPLC-MS/MS | Ultra-performance liquid chromatography-tandem mass spectrometry |
| ANOVA | Analysis of variance |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| CL(i-13:0/i-13:0/i-12:0/18:2(9Z,11Z)) | 1,1′-bis(iso-13-carbon saturated acyl)-2-(iso-12-carbon saturated acyl)-3-[(9Z,11Z)-octadecadienoyl] cardiolipin |
References
- Attar, Ş.H.; Gündeşli, M.A.; Urün, I.; Kafkas, S.; Kafkas, N.E.; Ercisli, S.; Ge, C.; Mlcek, J.; Adamkova, A. Nutritional analysis of red-purple and white-fleshed pitaya (Hylocereus) species. Molecules 2022, 27, 808. [Google Scholar] [CrossRef]
- Chen, J.; Sabir, I.A.; Qin, Y. From challenges to opportunities: Unveiling the secrets of pitaya through omics studies. Sci. Hortic. 2023, 321, 112357. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, Z.; Zhang, Z.; Zhu, M.; Yin, L.; Peng, R.; Zhang, Y.; Zhang, W. Recognition and counting of pitaya trees in karst mountain environment based on unmanned aerial vehicle RGB images. J. Appl. Remote Sens. 2021, 15, 042402. [Google Scholar] [CrossRef]
- Shah, K.; Chen, J.; Chen, J.; Qin, Y. Pitaya nutrition, biology, and biotechnology: A review. Int. J. Mol. Sci. 2023, 24, 13986. [Google Scholar] [CrossRef]
- Taharuddin, N.; Jumaidin, R.; Mansor, M.; Hazrati, K.; Tarique, J.; Asyraf, M.; Razman, M. Unlocking the potential of lignocellulosic biomass dragon fruit (Hylocereus polyrhizus) in bioplastics, biocomposites and various commercial applications (en línea). Polymers 2023, 15, 2654. [Google Scholar] [CrossRef]
- Angonese, M.; Motta, G.E.; de Farias, N.S.; Molognoni, L.; Daguer, H.; Brugnerotto, P.; de Oliveira Costa, A.C.; Müller, C.M.O. Organic dragon fruits (Hylocereus undatus and Hylocereus polyrhizus) grown at the same edaphoclimatic conditions: Comparison of phenolic and organic acids profiles and antioxidant activities. LWT 2021, 149, 111924. [Google Scholar] [CrossRef]
- Magalhães, D.S.; da Silva, D.M.; Ramos, J.D.; Pio, L.A.S.; Pasqual, M.; Boas, E.V.B.V.; Galvão, E.C.; de Melo, E.T. Changes in the physical and physico-chemical characteristics of red-pulp dragon fruit during its development. Sci. Hortic. 2019, 253, 180–186. [Google Scholar] [CrossRef]
- Yang, H.; Garousi, F.; Wang, J.; Cao, J.; Xu, X.; Zhu, T.; Müller, C. Land use effects on gross soil nitrogen transformations in karst desertification area. Plant Soil 2022, 475, 61–77. [Google Scholar] [CrossRef]
- Guanling Bouyei and Miao Autonomous County People’s Government. Available online: https://www.guanling.gov.cn (accessed on 18 September 2025).
- Luodian County People’s Government. Available online: https://www.gzluodian.gov.cn (accessed on 18 September 2025).
- Zhenfeng County People’s Government. Available online: https://www.gzzf.gov.cn (accessed on 18 September 2025).
- Tian, J.; Wen, H.; Liu, B.; Tian, X.; Wu, Y.; Yang, J.; Zhang, B.; Li, H. Fruit quality evaluation of different mulberry varieties. Front. Plant Sci. 2025, 15, 1500253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yi, H.; Gao, X.; Bai, T.; Ni, Z.; Chen, Y.; Wang, M.; Zhang, Y.; Pan, J.; Yu, W.; et al. Effect of different altitudes on morpho-physiological attributes associated with mango quality. Diversity 2022, 14, 876. [Google Scholar] [CrossRef]
- Wang, Z.; Erasmus, S.W.; Liu, X.; van Ruth, S.M. Study on the relations between hyperspectral images of bananas (Musa spp.) from different countries, their compositional traits and growing conditions. Sensors 2020, 20, 5793. [Google Scholar] [CrossRef]
- Okoro, U.K.; Akalazu, J.N.; Nwulu, N.C. Near-term impact of climate variability on yam rot incidence over a humid tropical region: Projections in CORDEX-Africa scenarios. Renew. Agric. Food Syst. 2021, 36, 477–490. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Xiang, Q.; Kim, J.; Dufresne, C.; Liu, Y.; Li, T.; Chen, S. Creation of a plant metabolite spectral library for untargeted and targeted metabolomics. Int. J. Mol. Sci. 2023, 24, 2249. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, D.; Zou, W.; Wang, J.; Luo, R.; Wang, M.; Yu, H. Comparison of widely targeted metabolomics and untargeted metabolomics of wild Ophiocordyceps sinensis. Molecules 2022, 27, 3645. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, H.; Shi, J.; Li, J.; Nie, X.; Yang, G. Effects of, 2, 4-dichlorophenoxyacetic acid on cucumber fruit development and metabolism. Int. J. Mol. Sci. 2019, 20, 1126. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, Z.; Shi, Q.; Du, J.; Xue, Q.; Li, X. Active compound analysis of Ziziphus jujuba cv. Jinsixiaozao in different developmental stages using metabolomic and transcriptomic approaches. Plant Physiol. Biochem. 2022, 189, 14–23. [Google Scholar] [CrossRef]
- Darwish, A.G.; Das, P.R.; Ismail, A.; Gajjar, P.; Balasubramani, S.P.; Sheikh, M.B.; Tsolova, V.; Sherif, S.M.; El-Sharkawy, I. Untargeted metabolomics and antioxidant capacities of muscadine grape genotypes during berry development. Antioxidants 2021, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Nadi, R.; Golein, B.; Gómez-Cadenas, A.; Arbona, V. Developmental stage-and genotype-dependent regulation of specialized metabolite accumulation in fruit tissues of different citrus varieties. Int. J. Mol. Sci. 2019, 20, 1245. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Qiu, J.; Qian, Y.; Wang, M. Comparative metabolomic analysis of the nutritional aspects from ten cultivars of the strawberry fruit. Foods 2023, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Guo, Y.; Wang, Y.; Li, M.; Li, K.; Liu, X.; Fang, C.; Luo, J. Metabolomic analysis reveals nutritional diversity among three staple crops and three fruits. Foods 2022, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Madden, R.T.; Sung, J.; Chambers, A.H.; Crane, J.; Wang, Y. Pathway-based metabolomics analysis reveals biosynthesis of key flavor compounds in mango. J. Agric. Food Chem. 2021, 70, 10389–10399. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Chen, J.; Lamu, Y.; Qi, X.; Lei, L.; Mao, K.; Tso, S. Analysis of metabolic differences in Tibetan medicinal plant Phlomoides rotata leaves in different habitats based on non-targeted metabolomics. Front. Plant Sci. 2025, 16, 1503218. [Google Scholar] [CrossRef] [PubMed]
- Pegiou, E.; Engel, J.; Mumm, R.; Hall, R.D. Unravelling the seasonal dynamics of the metabolome of white asparagus spears using untargeted metabolomics. Metabolomics 2023, 19, 23. [Google Scholar] [CrossRef]
- China Meteorological Data Service Center. Available online: https://data.cma.cn/ (accessed on 23 May 2025).
- Arakawa, N.; Tsutsumi, K.; Sanceda, N.G.; Kurata, T.; Inagaki, C. A rapid and sensitive method for the determination of ascorbic acid using 4, 7-diphenyl-l, 10-phenanthroline. Agric. Biol. Chem. 1981, 5, 1289–1290. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, W.; Di, H.; Yang, S.; Tian, Y.; Tong, Y.; Huang, H.; Escalona, V.H.; Tang, Y.; Li, H.; et al. Variation in nutritional components and antioxidant capacity of different cultivars and organs of Basella alba. Plants 2024, 13, 892. [Google Scholar] [CrossRef]
- Hua, Q.; Chen, C.; Zur, N.T.; Wang, H.; Wu, J.; Chen, J.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. Metabolomic characterization of pitaya fruit from three red-skinned cultivars with different pulp colors. Plant Physiol. Biochem. 2018, 126, 117–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Xing, Y.; Xie, F.; Chen, C.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. Quality analysis and evaluation of different batches of pitaya fruit (Hylocereus) in south china. Int. J. Fruit Sci. 2024, 24, 33–43. [Google Scholar] [CrossRef]
- Maiwei Cloud. Available online: https://cloud.metware.cn (accessed on 17 September 2025).
- Causin, H.F.; Cá, F.D.; Spotorno, V.G.; Palacios, M.B.; Tosar, L.M.; Burrieza, H.P.; Tossi, V.E. Comparative role of betalains and other key antioxidant metabolites in the photoprotection against acute exposure to UV-B radiation in Chenopodium quinoa and C. berlandieri seedlings. Plant Physiol. Biochem. 2025, 221, 109580. [Google Scholar] [CrossRef] [PubMed]
- Paunović, S.M.; Mašković, P.; Milinković, M. Bestimmung von Primärmetaboliten, Vitaminen und Mineralien in Schwarzen Maulbeeren (Morus nigra), angebaut in verschiedenen Höhenlagen. Erwerbs Obstbau 2020, 62, 355–360. [Google Scholar] [CrossRef]
- Boyarskikh, I.G.; Artemov, I.A.; Kuznetsov, A.A.; Kostikova, V.A. Changes in Profiles of Classes and of Individual Polyphenols in Leaves of Spiraea chamaedryfolia and Spiraea media along an Altitudinal Gradient. Plants 2023, 12, 2977. [Google Scholar] [CrossRef]
- Rao, M.J.; Wang, H.; Lei, H.; Zhang, H.; Duan, X.; Bao, L.; Yang, C.; Han, D.; Zhang, Y.; Yang, S.; et al. LC-MS/MS-based metabolomic study provides insights into altitude-dependent variations in flavonoid profiles of strawberries. Front. Plant Sci. 2025, 15, 1527212. [Google Scholar] [CrossRef] [PubMed]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, H.; Li, S.; Zhong, X.; Wang, H.; Liu, X. Comparative metabolic profiling of Ophiocordyceps sinensis and its cultured mycelia using GC–MS. Food Res. Int. 2020, 134, 109241. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Khatoon, S.; Pandey, M.; Rawat, A. Altitudinal variation of berberine, total phenolics and flavonoid content in Thalictrum foliolosum and their correlation with antimicrobial and antioxidant activities. J. Ayurveda Integr. Med. 2018, 9, 169–176. [Google Scholar] [CrossRef]
- Kuźniak, E.; Gajewska, E. Lipids and lipid-mediated signaling in plant–pathogen interactions. Int. J. Mol. Sci. 2024, 25, 7255. [Google Scholar] [CrossRef]
- Arabsalehi, F.; Rahimmalek, M.; Sabzalian, M.R.; Ghanadian, M.; Matkowski, A.; Szumny, A. Changes in polyphenolic composition, physiological characteristics, and yield-related traits of Moshgak (Ducrosia anethifolia Boiss.) populations in response to drought stress. Protoplasma 2023, 260, 967–985. [Google Scholar] [CrossRef]
- Hu, L.; Yang, C.; Zhang, L.; Feng, J.; Xi, W. Effect of light-emitting diodes and ultraviolet irradiation on the soluble sugar, organic acid, and carotenoid content of postharvest sweet oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef]
- Qin, Y.; Gong, A.; Liu, X.; Li, N.; Ji, T.; Li, J.; Yang, F. Testing a Simulation Model for the Response of Tomato Fruit Quality Formation to Temperature and Light in Solar Greenhouses. Plants 2024, 13, 1662. [Google Scholar] [CrossRef]
- Ma, W.-F.; Li, Y.-B.; Nai, G.-J.; Liang, G.-P.; Ma, Z.-H.; Chen, B.-H.; Mao, J. Changes and response mechanism of sugar and organic acids in fruits under water deficit stress. PeerJ 2022, 10, e13691. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Rocha, D.S., Jr.; Silva, G.C.; Santos, M.G.; Camillo, L.R.; De Oliveira, P.H.; Costa, M.G. Dynamics of the sucrose metabolism and related gene expression in tomato fruits under water deficit. Physiol. Mol. Biol. Plants 2023, 29, 159–172. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, M.; Yang, X.; Xu, J.; Yu, Z. Effect of cutting and storage temperature on sucrose and organic acids metabolism in postharvest melon fruit. Postharvest Biol. Technol. 2020, 161, 111081. [Google Scholar] [CrossRef]
- Mulas, M.; Fadda, A.; Angioni, A. Effect of maturation and cold storage on the organic acid composition of myrtle fruits. J. Sci. Food Agric. 2013, 93, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiao, W.; Cui, K.; Fan, X.; Shu, C.; Zhang, W.; Cao, J.; Jiang, W. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chem. 2019, 289, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, C.; Zhang, Y.; Chu, Y.; Yang, W.; Cui, Y.; Kou, G.; Chen, H.; Song, H.; Gong, R. Effect of low-light stress on sugar and acid accumulation during fruit development and ripening of sweet cherry. Horticulturae 2023, 9, 654. [Google Scholar] [CrossRef]
- Zhou, X.; Obel, H.O.; Liu, S.; Yang, Y.; Liu, J.; Zhuang, Y. Comparative Analysis of Metabolic Variation in Eggplant Fruit of Different Varieties Reveals Metabolites Important for Quality Traits. Foods 2023, 12, 4383. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, P.; Chen, J.; Li, L.; Tang, L.; Huang, W.; Wei, X.; Xu, J. The accumulation pattern of sugars and organic acids in diverse fruit pulps coordinates sweet-sour taste of passion fruit. LWT 2025, 225, 117948. [Google Scholar] [CrossRef]
- Wei, J.; Chenlei, L.; Yi, S.W. Analysis of quality changes in mulberry fruit at three developmental stages using targeted metabolomics based on lcms. Trop. Plant Biol. 2023, 16, 287–306. [Google Scholar] [CrossRef]
- Jiang, D.; Han, Q.; Su, Y.; Cao, X.; Wu, B.; Wei, C.; Chen, K.; Li, X.; Zhang, B. Glycoside hydrolase ppgh28bg1 modulates benzaldehyde metabolism and enhances fruit aroma and immune responses in peach. Plant Physiol. 2024, 196, 1444–1459. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Han, J.; Chen, C.; Yao, L.; Chen, J.; Yuan, T. Monosubstituted benzene derivatives from fruits of Ficus hirta and their antifungal activity against phytopathogen Penicillium italicum. J. Agric. Food Chem. 2016, 64, 5621–5624. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, C.; Wang, X.; Zhu, F.; Wang, X.; Zhang, M.; Duan, Y. Alternative splicing analysis revealed the role of alpha-linolenic acid and carotenoids in fruit development of Osmanthus fragrans. Int. J. Mol. Sci. 2023, 24, 8666. [Google Scholar] [CrossRef]
- Li, X.; Qi, L.; Zang, N.; Zhao, L.; Sun, Y.; Huang, X.; Wang, H.; Yin, Z.; Wang, A. Integrated metabolome and transcriptome analysis of the regulatory network of volatile ester formation during fruit ripening in pear. Plant Physiol. Biochem. 2022, 185, 80–90. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Maoz, I.; Feygenberg, O.; Maurer, D.; Alkan, N. Chilling stress upregulates α-linolenic acid-oxidation pathway and induces volatiles of C6 and C9 aldehydes in mango fruit. J. Agric. Food Chem. 2017, 65, 632–638. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, S.; Ma, C.; Gao, X.; Ji, J.; Luo, J.; Hua, H.; Cui, J. Integrated Omics Analysis Reveals Key Pathways in Cotton Defense against Mirid Bug (Adelphocoris suturalis Jakovlev) Feeding. Insects 2024, 15, 254. [Google Scholar] [CrossRef]
- Picchioni, G.; Watada, A.; Conway, W.; Whitaker, B.; Sams, C. Phospholipid, galactolipid, and steryl lipid composition of apple fruit cortical tissue following postharvest CaCl2 infiltration. Phytochemistry 1995, 39, 763–769. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Zhi, P.; Chang, C. Update on cuticular wax biosynthesis and its roles in plant disease resistance. Int. J. Mol. Sci. 2020, 21, 5514. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Huang, H.; Zheng, M.; Zaman, W.; Li, D.; Saqib, S.; Zhao, H.; Lü, S. Molecular cloning and functional analysis of GmLACS2-3 reveals its involvement in cutin and suberin biosynthesis along with abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 9175. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mesa, P.A.; Roda, F. Alkaloid evolution in the Solanaceae. Curr. Opin. Plant Biol. 2025, 85, 102727. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, Y.; Jia, H.; Wang, K.; Chen, S.; Ma, T.; Niu, Q. Tomato MADS-RIN regulates GAME5 expression to promote non-bitter glycoalkaloid biosynthesis in fruit. Plant J. 2024, 120, 2500–2514. [Google Scholar] [CrossRef]
- Adedayo, B.C.; Oyeleye, S.I.; Okeke, B.M.; Oboh, G. Anti-cholinesterase and antioxidant properties of alkaloid and phenolic-rich extracts from pawpaw (Carica papaya) leaf: A comparative study. Flavour Fragr. J. 2021, 36, 47–54. [Google Scholar] [CrossRef]
- Melo Czekster, C.; Robertson, W.E.; Walker, A.S.; Söll, D.; Schepartz, A. In vivo biosynthesis of a β-amino acid-containing protein. J. Am. Chem. Soc. 2016, 138, 5194–5197. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Esashi, Y. D-Amino-acid-stimulated ethylene production in seed tissues. Planta 1980, 149, 64–68. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Huang, A.; Zhang, H.; Zheng, Y. The metabolism of amino acids, AsA and abscisic acid induced by strigolactone participates in chilling tolerance in postharvest zucchini fruit. Front. Plant Sci. 2024, 15, 1402521. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kurimoto, S.-I.; Kobayashi, J.; Kubota, T. Ishigadine A, a new canthin-6-one alkaloid from an Okinawan marine sponge Hyrtios sp. Tetrahedron Lett. 2018, 59, 4500–4502. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Wu, S.; Chen, J.; Li, A.; Tatsis, E.C. Deciphering and reprogramming the cyclization regioselectivity in bifurcation of indole alkaloid biosynthesis. Chem. Sci. 2022, 13, 12389–12395. [Google Scholar] [CrossRef]
- Senecoff, J.F.; McKinney, E.C.; Meagher, R.B. De novo purine synthesis in Arabidopsis thaliana (II. the PUR7 gene encoding 5 [prime]-phosphoribosyl-4-(N-succinocarboxamide)-5-aminoimidazole synthetase is expressed in rapidly dividing tissues). Plant Physiol. 1996, 112, 905–917. [Google Scholar] [CrossRef]
- Sun, M.; Dai, P.; Cao, Z.; Dong, J. Purine metabolism in plant pathogenic fungi. Front. Microbiol. 2024, 15, 1352. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhong, C.; Xie, J.; Liu, H.; Xie, Z.; Zhang, S.; Jin, J. Geographical region traceability of Poria cocos and correlation between environmental factors and biomarkers based on a metabolomic approach. Food Chem. 2023, 417, 135817. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, F.; Wu, W.; Yu, W.; Zhang, G.; Huang, X.; Hao, Y.; Luo, L. Identification and quality evaluation of Lushan Yunwu tea from different geographical origins based on metabolomics. Food Res. Int. 2024, 186, 114379. [Google Scholar] [CrossRef]




| Area | Daily Average Temperature (°C) | Daily Maximum Temperature (°C) | Daily Minimum Temperature (°C) | Relative Humidity (%) | Annual Rainfall (mm) | Sunshine Duration (h) | Altitude (m) | Monthly Average Temperature (°C) | Monthly Average Relative Humidity (%) | Monthly Sunshine Duration (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| LD | 25.6 | 34.49 | 22.24 | 51 | 596.8 | 11.2 | 440.1 | 27.1 | 78 | 268.7 |
| ZF | 22.6 | 32.01 | 19.01 | 70 | 658.7 | 9.6 | 1063.8 | 23.3 | 78 | 275.6 |
| GL | 24.7 | 32.77 | 19.47 | 60 | 1239.9 | 9.7 | 1138.4 | 24 | 74 | 273.8 |
| Index | Compounds | Class | Formula |
|---|---|---|---|
| MEDP0017 | L-Histidine | Amino acids and derivatives | C6H9N3O2 |
| MEDL00353 | Glu-Leu | Amino acids and derivatives | C11H20N2O5 |
| MW0152601 | Leu-Glu | Amino acids and derivatives | C11H20N2O5 |
| MW0152405 | Leu-Leu-Ser-Pro-Tyr | Amino acids and derivatives | C29H45N5O8 |
| MW0104552 | 2-amino-4-({1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-[(3-hydroxy-2-methyl-4-oxobutan-2-yl)sulfanyl]ethyl}-C-hydroxycarbonimidoyl)butanoic acid | Organic acids | C15H25N3O8S |
| MEDN1093 | (2S)-2-Isopropylmalate | Organic acids | C7H12O5 |
| MEDP0179 | Uridine | Nucleotides and derivatives | C9H12N2O6 |
| MW0043185 | CL(i-13:0/i-13:0/i-12:0/18:2(9Z,11Z)) | GP | C65H122O17P2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Wang, L.; Luo, Y.; Ba, L. Systematic Analysis of Nutritional Components and Characteristics in Red-Fleshed Dragon Fruit from Different Origins Using Non-Targeted Metabolomics. Horticulturae 2025, 11, 1436. https://doi.org/10.3390/horticulturae11121436
Zhao Z, Wang L, Luo Y, Ba L. Systematic Analysis of Nutritional Components and Characteristics in Red-Fleshed Dragon Fruit from Different Origins Using Non-Targeted Metabolomics. Horticulturae. 2025; 11(12):1436. https://doi.org/10.3390/horticulturae11121436
Chicago/Turabian StyleZhao, Zhibing, Lang Wang, Yinmei Luo, and Liangjie Ba. 2025. "Systematic Analysis of Nutritional Components and Characteristics in Red-Fleshed Dragon Fruit from Different Origins Using Non-Targeted Metabolomics" Horticulturae 11, no. 12: 1436. https://doi.org/10.3390/horticulturae11121436
APA StyleZhao, Z., Wang, L., Luo, Y., & Ba, L. (2025). Systematic Analysis of Nutritional Components and Characteristics in Red-Fleshed Dragon Fruit from Different Origins Using Non-Targeted Metabolomics. Horticulturae, 11(12), 1436. https://doi.org/10.3390/horticulturae11121436






