Abstract
Cucumber (Cucumis sativus L.) is a vital economic vegetable crop, and the TONNEAU1 Recruiting Motif (TRM) gene plays a key role in cucumber organ growth. However, the pan-genomic characteristics of the TRM gene family and their expression patterns under different stresses have not been reported in cucumber. In this study, we identified 29 CsTRMs from the pan-genomes of 13 cucumber accessions, with CsTRM29 existing only in PI183967. Most CsTRM proteins exhibited differences in sequence length, except five CsTRMs having consistent protein sequence lengths among the 13 accessions. All CsTRM proteins showed amino acid variations. An analysis of CsTRM gene expression patterns revealed that six CsTRM genes strongly changed in short-fruited lines compared with long-fruited lines. And four CsTRM genes strongly responded to salt and heat stress, while CsTRM14 showed responses to salt stress, powdery mildew, gray mold, and downy mildew. Some CsTRM genes were induced or suppressed at different treatment timepoints, suggesting that cucumber TRM genes may play different roles in responses to different stresses, with expression patterns varying with stress changes. Remarkably, the expression of CsTRM21 showed considerable change between long and short fruits and in responses to abiotic stresses (salt stress and heat stress), as well as biotic stresses (powdery mildew and gray mold), suggesting a dual role of CsTRM21 in both fruit shape determination and stress resistance. Collectively, this study provided a base for the further functional identification of CsTRM genes in cucumber plant growth and stress resistance.
1. Introduction
TONNEAU1 Recruiting Motif (TRM) family genes are crucial for the growth and development of plants, exerting significant functions in various plant species. In Arabidopsis, 34 TRM proteins were identified, and half of them are putative microtubule-associated proteins [1]. AtTRM1 and AtTRM2 regulate leaf morphology by positively promoting longitudinal polar cell elongation [2]. The Attrm5 mutant causes slow leaf growth, delayed flowering, and shortened root length [3]. AtTRM61 has a conserved functional structure and possesses conserved binding motifs for cofactor S-adenosyl-L-methionine (AdoMet), affecting embryo arrest and seed abortion [4]. Additionally, TRMs can interact with TONNEAU1 (TON1) and Protein Phosphatase 2A (PP2A) through their M2 and M3 domains, respectively, forming the TTP (TON1-TRM-PP2A) protein complex. This complex is targeted to microtubules (MTs) [1], regulating microtubule organization and preprophase band (PPB) formation, thus influencing cell division and/or growth. This regulation ultimately affects the size and shape of plant organs [1,5,6,7,8,9]. In tomatoes, TRMs can interact with OVATE FAMILY PROTEINS (OFP) through their M8 domain. The OFP-TRM protein complex undergoes relocalization between the cytoplasm and microtubules, maintaining a dynamic balance to regulate cell division and organ growth, ultimately affecting fruit shape [10,11]. SlTRM5 enhances fruit elongation by influencing cell division [12]. In the LA1589 genetic background, although SlTRM3/4 minimally influenced the fruit shape, the absence of SlTRM5 led to a slight flattening of the fruit [13]. The fruit shape of the double mutant lacking both SlTRM3/4 and SlTRM5 closely resembles that of the single mutant lacking only SlTRM5 [13]. Introducing the non-functional versions of either SlTRM3/4 or SlTRM5 into ovate/sov1 near-isogenic lines (NILs) partially restored the pear shape of the fruit. Moreover, when both non-functional alleles of SlTRM3/4 and SlTRM5 were combined in ovate/sov1 NILs, the fruit shape index (FSI) was similar to wild-type (WT) fruits [11,13], suggesting that SlTRM3/4 and SlTRM5 have additive effects in regulating fruit elongation. The fruit shape analyses of the SlTRM17/20a, SlTRM19, or SlTRM26a null mutants in the LA1589 genetic background, generated using CRISPR/Cas9, revealed an interesting finding, indicating that SlTRM17/20a and SlTRM19 act synergistically to regulate fruit elongation, whereas SlTRM26a has a limited impact on fruit shape. The null alleles of SlTRM5 and SlTRM19, in both the LA1589 and ovate/sov1 backgrounds, were observed to mutually balance their effects on fruit elongation. This suggests that SlTRM5 and SlTRM19 have opposing effects on fruit elongation [13]. In rice, the TRM homologous genes OsGW7/GL7/SLG7 interact with TON1 and PP2A through their M2 and M3 domains, respectively, and target them to the cortical microtubules. By influencing the cell length and width, they regulate the grain size and quality [14,15,16]. In cucumber, CsTRM5 plays a role in shaping fruits by influencing the direction of cell division and expansion. Additionally, ABA was involved in regulating cucumber fruit elongation through CsTRM5-mediated cell expansion [17].
TRM gene family members are often localized to microtubules [1,2,12]. Microtubules are crucial components of the plant cell skeleton, and they play vital roles in maintaining cell shape, adapting to growth, development, and environmental changes, as well as in processes such as cell division, intracellular transport, immune responses, and stress tolerance [18,19,20,21,22,23,24,25,26,27]. MICROTUBULE-DESTABILIZING PROTEIN 25 (MDP25) is a hydrophilic cation-binding protein of the plant-specific developmentally regulated plasma membrane polypeptide (DREPP) family [28]. It is postulated that AtMDP25 similarly modulates stomatal closure, root hydrotropic response, and immune responses by influencing microtubule dynamics [29,30,31]. OsDREPP2 exhibits an affinity for microtubules and, in vitro, it inhibits microtubule polymerization [32], and MtDREPP induces the fragmentation of microtubules within membrane nanodomains during rhizobial infections [33]. Ethylene signaling regulates microtubule reassembly by upregulating microtubule-stabilizing protein WAVE-DAMPENED2-LIKE5 (WDL5) expression in response to salt stress [34]. Katanin1 (KTN1) acts as a microtubule-severing protein. It aids in maintaining the organized microtubule structure. Under hypersalinity, the microtubule-associated protein KTN1 regulates hypersalinity-induced microtubule disassembly/assembly, thereby enhancing salinity tolerance [35]. Microtubules under high temperature stress undergo depolymerization [36]. High temperature stress (35–37 °C) disrupts the formation of microtubule-organizing centers, leading to changes in microtubule dynamics, including their elongation and the shortening of microtubule arrays [37]. The changes in microtubule dynamics impact vesicular transport, protein trafficking, and cell wall deposition [38,39,40,41]. Currently, there are no reports on the involvement of cucumber TRM family genes in biotic or abiotic stress.
Pan-genomics aims to encompass the entire range of genetic diversity within a species by assembling and comparing genome sequences from multiple individuals and displayed a powerful potential in discovering novel genes or gene novel function [42,43]. Pan-genomes have been constructed for major crops like maize, rice, wheat, and soybean, utilizing high-quality genomes from various samples, leading to significant advances in the study of plant genome evolution and the identification of key genes linked to important agronomic traits [44,45,46,47,48]. In 2014, a pan-genome for wild soybean was developed, offering a valuable potential resource for enhancing the genetic diversity of cultivated soybean, which was reduced during domestication. This effort also identified numerous variations associated with agronomic traits, including seed composition, flowering, maturity time, and biotic resistance [49]. The most comprehensive rice graph-based pan-genome constructed was based on the high-quality genomes of 33 genetically diverse rice accessions [47], which not only provide detailed insights into genomic variations and their mechanisms, but also systematically assessed their effects on genome evolution, gene expression, crop domestication, and environmental adaptability for the first time. Pan-genomes have also been developed for several key vegetable crops, including tomato, cucumber, eggplant, and rapeseed, and have also been released, providing a valuable foundation for future biological studies and breeding programs [50,51,52,53,54]. The first cucumber pan-genome was constructed using genome data from 12 representative accessions, employing PacBio sequencing technology and a graph-based assembly strategy. This study elucidated the karyotype evolution of cucumber during domestication and identified several potentially significant genes associated with agronomic traits. These findings provide a foundation for key gene discovery, breeding, and the improvement of cucumber.
In this study, we identified 29 CsTRM genes in the cucumber pan-genome and discovered that the majority exhibit variations in protein length across the 13 accessions, and all CsTRM proteins showed amino acid variations. Additionally, we analyzed the expression patterns of the CsTRM genes with RNA sequencing (RNA-seq) data in fruit and under different stresses, which may have distinct roles in response to these stresses. Consequently, our study offers a basis for exploring the potential role of TRMs for fruit shape and stress tolerance in cucumber.
2. Materials and Method
2.1. Identification of TRM Genes in Cucumber
The cucumber pan-genome assembly and annotation files were download from https://www.ncbi.nlm.nih.gov/ (accessed on 3 March 2024), and the ‘PI183967’ genome assembly from http://www.cucurbitgenomics.org/ (accessed on 3 March 2024). CDS sequences were extracted and we translated them into protein sequences using TBtools software (v2.031). The AtTRM family members were retrieved from https://www.arabidopsis.org/ (accessed on 20 March 2024), and these sequences were employed as queries in TBtools to predicted TRM family members in cucumber. A conserved motif analysis was performed using MEME (https://www.omicsclass.com/article/432) (accessed on 20 March 2024). The results were visualized with TBtools, and the final CsTRM family members were screened based on the conserved M2 motif.
2.2. Protein Length, Motif Composition, and Gene Structure Analysis Amino Acid Variations
The protein sequences of CsTRMs from various cucumber accessions were obtained, and their lengths were measured with TBtools. The amino acid variations were assessed with the DNAman6.0 program. The conserved motifs were characterized with TBtools. The locations of CDSs and UTRs were retrieved from the genomic annotation database and visualized with TBtools [55].
2.3. Gene Duplication and Synteny Analysis
The genomic databases for cucumber, maize, rice, Arabidopsis, and tomato were obtained from http://cucurbitgenomics.org/organism/20 (accessed on 25 March 2024) and http://plants.ensembl.org/index.html (accessed on 25 March 2024). The gene duplication events and the syntenic relationships were identified using the Multiple Collinearity Scan toolkit (MCScan X) [56] employing the standard settings. The results were visualized and constructed used by TBtools [55].
2.4. Transcriptome Profiling of CsTRMs in Fruit
The publicly available transcriptomic data of cucumber fruit at anthesis from inbred lines 32X and Gui Fei Cui carpel numbers (SPR182933) [57], long fruit 408 and short fruit 409 (SPR045470) [58], and fruit of WT and CsFUL1A-OX-29 (SPR117025) [59] were acquired from NCBI (https://www.ncbi.nlm.nih.gov/geo/browse) (accessed on 25 May 2024) to analyze the expression patterns of CsTRMs in fruit. The genome-wide expression patterns of the CsTRM gene were displayed on a heatmap generated with TBtools [55]. For analyzing the transcriptome of the CsTRMs, a threshold of p-value (or FDR) ≤ 0.05, and a value of log2 (fold-change) ≤ −1 or log2 (fold-change) ≥ 1 were employed to define DEGs.
2.5. Transcriptome Profiling of CsTRMs in Response to Abiotic and Biotic Stresses
We used the publicly accessible transcriptomic data of cucumber seedlings exposed to salt under 75 mM NaCl and 0.3 mM Si (GSE116265) [60], heated at 42 °C in Chinese Long 9930 (GSE151055) [61], and cucumber leaves were inoculated by powdery mildew (PM), Pm5.1 (GSE81234) [62], and gray mold (GM) (SRP062592) [63]; and powdery mildew (DM) (SRP009350) [64] were acquired from https://www.ncbi.nlm.nih.gov/ (accessed on 25 May 2024) to investigate CsTRM expression profiles in response to different stresses. After aligning the gene IDs to the cucumber genome, the genome-wide expression of the CsTRM gene was displayed on a heatmap generated with TBtools [55]. For analyzing the transcriptome of the CsTRMs, a level of p-value (or FDR) ≤ 0.05, and a value of log2 (fold-change) ≤ −1 or log2 (fold-change) ≥ 1 were employed to define DEGs.
2.6. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
To acquire samples for the purpose of expression analysis, the ovary was collected from cucumber plants 4 days before anthesis (4 DBA) and 0 day after anthesis (0 DAA) of the long fruit line CSSL2-7 and the round fruit line RNS7 [65], and the cotyledons of cucumber seedlings inoculated with GM 0 h, 6 h, 24 h, and 72 h for RNA extraction. Total RNA was extracted using TRIzol (Accurate Biotechnology, AG21102, Changsha, China), and then we verified quality and measured concentration. And, for cDNA Synthesis with the Evo M-MLV RT Mix Kit with gDNA Clean for qPCR (Accurate Biotechnology, AG11728, Changsha, China), qRT-PCR was performed using the 2X SYBR Green Pro Taq HS Premix (Accurate Biotechnology, AG11718) with CFX Opus 96 real-time PCR system (BIO-RAD). The gene of cucumber Actin served as reference gene. Three biological replicates were included in the expression analysis. The relative expression levels of CsTRMs were calculated using the 2−∆∆Ct approach. Primers were listed in Table S5.
3. Result
3.1. Identification of CsTRM Genes Based on the Cucumber Pan-Genome
To investigate the variation of the TRM genes across cucumber accessions, we identified CsTRM genes from the pan-genome, which includes 13 cucumber accessions [43]. A total of 29 putative TRM genes were identified among the genomes of the 13 cucumber accessions (Table 1, Table S1). We renamed them CsTRM01–CsTRM29 according to their chromosomal order to avoid confusion in this study (Table 1). Additionally, CsTRM04 exhibits multiple copies in W4. There were 28 CsTRM genes identified from ‘9930’, being consistent with the previous study [54], and from PI183967, lacking CsTRM03 and possessing a unique CsTRM29 (Table 1); 27 from ‘Cu2’, ‘Cuc64’, ‘W4’, ‘Hx14’, ‘Hx17’ ‘Cuc37’, ‘Gy14’, and ‘9110gt’; 26 from ‘XTMC’; and 25 from ‘Cuc80’ and ‘W8’ (Table 1). For CsTRM01, 02, 05, 07, 09, 11, 12, 13, 14, 15, 17, 18, 19, 21, 23, 24, 25, 26, 27, and 28, all are present in the 13 cucumber accessions. CsTRM3 is absent in Cuc80 and PI; CsTRM04 is absent in Cuc80 and W8; CsTRM05 is absent in XTMC and W8; CsTRM08 is absent in XTMC; CsTRM10 is absent in Cu2 and Cuc80; CsTRM16 is absent in Cuc64, W4, W8, Hx14, Hx17, and Cuc37; CsTRM20 is absent in Gy14; CsTRM22 is absent in 9110gt. CsTRM29 only existing in PI183967 is identified as a new member of the CsTRM gene family in the 13 cucumber accessions.

Table 1.
Identification of TRM genes in the 13 cucumber accessions.
3.2. Analysis of Protein Length and Amino Acid Variations in the CsTRM Proteins
To further understand the protein length variation of CsTRMs among the cucumber accessions, the lengths of the identified CsTRM proteins are presented in Table 2. Five CsTRMs showed the same protein lengths across 13 cucumber accessions, CsTRM04, 11, 14, 15, and 21, respectively. The length of CsTRM01, 02, 05, 06, 13, 18, 22, 24, and 26 varied by just one of the accessions. And others exhibited variations in protein length across different accessions. The data highlighting these length differences are shown in red in Table 2.

Table 2.
The predicted lengths of TRM proteins (amino acid residues) in the 13 cucumber accessions.
Among the proteins differing in lengths, CsTRM01 in ‘W4’; CsTRM02 in ‘9930’; CsTRM03 in ‘9930’; CsTRM05 in ‘9930’; CsTRM07 in ‘XTMC’; CsTRM09 in ‘9930’; CsTRM13 in ‘Gy14’; CsTRM18 in ‘Cuc64’; CsTRM19 in ‘9930’ and ‘XTMC’; CsTRM20 in ‘Cuc37’; CsTRM22 in ‘Gy14’; CsTRM23 and CsTRM24 in ‘9110gt’; CsTRM25 in ‘PI183967’; CsTRM27 in ‘Hx117’; and CsTRM28 in ‘Hx14’ were shorter in length compared to those in other accessions, whereas CsTRM16 in ‘Cu2’, ‘Cuc80’, and ‘Gy14’; CsTRM19 in ‘Cu2’, ‘Cuc80’, and ‘Cuc64’; and CsTRM26 in ‘W4’ were longer compared to those in other accessions. Furthermore, the lengths of certain proteins exhibited multiple instances of polymorphism. For example, the protein length of CsTRM17 showed no difference in ‘9930’, ‘Cu2’, ‘Cuc80’, ‘PI’, ‘Cuc64’, and ‘Cuc37’, but was totally different in other accessions, furthermore, dramatically shortened in ‘W4’, ‘W8’, ‘Hx14’, ‘Hx117’, and ‘9110gt’ (Table 2).
Aside from changes in protein length, amino acid substitutions can also impact a protein’s function [66]. The analysis focused on the amino acid variations in CsTRMs across various cucumber accessions (Table S1). Amino acid variations were annotated using the CsTRM protein sequence of 9930 as reference, and all CsTRM proteins exhibit amino acid variations. CsTRM04, 11, 14, 15, and 21 have six, five, three, seven, and two amino acid variations, respectively, but these do not lead to changes in protein length (Table 2). Some CsTRMs exhibit amino acid insertions leading to an increase in protein length. For example, CsTRM02 has 27 amino acid insertions in accessions other than 9930. In CsTRM06, 17, 26, 27, and 28, there are frame shifts leading to amino acid variations. Some amino acid variations are quite significant, such as CsTRM07, 17, and 24.
Some amino acid variations are quite significant, such as CsTRM07, 17, and 24 (Table 2). Further comparisons of the CsTRM07, 17, and 24 gene structures and gene conservative motifs are conducted (Figure 1). CsTRM07 in XTMC has only 478 amino acids, which is significantly shorter than that in the other 12 accessions (Table 2), and its gene structure underwent changes along with alterations in some conserved motifs, experiencing an increase in gene length, but not leading to the loss of conserved motifs in Gy14 and PI183967 (Figure 1A). For CsTRM17, the protein length varied from 210 amino acids to 1091 amino acids across the 13 accessions (Table 2), with corresponding changes in the gene structure and some conserved motifs; especially in W4, there are only two conserved motifs (Figure 1B). In CsTRM24 of 9110gt, alterations in the gene structure caused the decreased protein length, but without a reduction in conserved motifs.
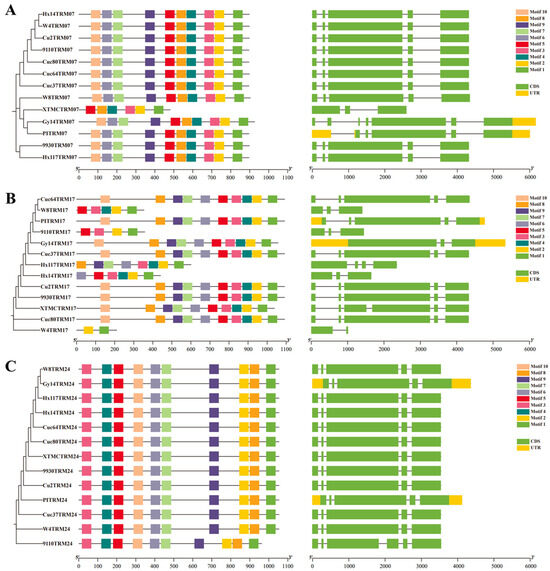
Figure 1.
Comparison of the conserved motifs and gene structures of CsTRM07 (A), CsTRM17 (B), and CsTRM24 (C) in the 13 cucumber accessions.
3.3. Synteny Analysis of CsTRM Genes
The phylogenetic relationship of the cucumber TRM family was further examined by creating comparative syntenic maps of cucumber and comparing them with four representative species, containing two dicots (Arabidopsis and tomato) and two monocots (rice and maize) (Figure 2). One, three, eight, and nineteen CsTRM genes showed syntenic relationships with those in the other four species: maize, rice, Arabidopsis, and tomato, respectively (Figure 2). Only one TRM collinear gene pair was found between cucumber and maize, followed by cucumber and rice (four), cucumber and Arabidopsis (nine), and cucumber and tomato (twenty) (Table S2). It is evident that dicotyledonous plants exhibit a notably higher number of homologous genes compared to those shared between dicotyledonous and monocotyledonous plants. This observation aligns with the patterns expected in biological evolution. CsTRM18 and its collinear gene pairs with maize are observed in rice and tomato, but not in Arabidopsis, indicating differences in the evolutionary process of CsTRM18. Additionally, collinear gene pairs between cucumber and rice, maize, and Arabidopsis are observed in cucumber and tomato, suggesting that cucumber and tomato may have undergone a common evolutionary history.
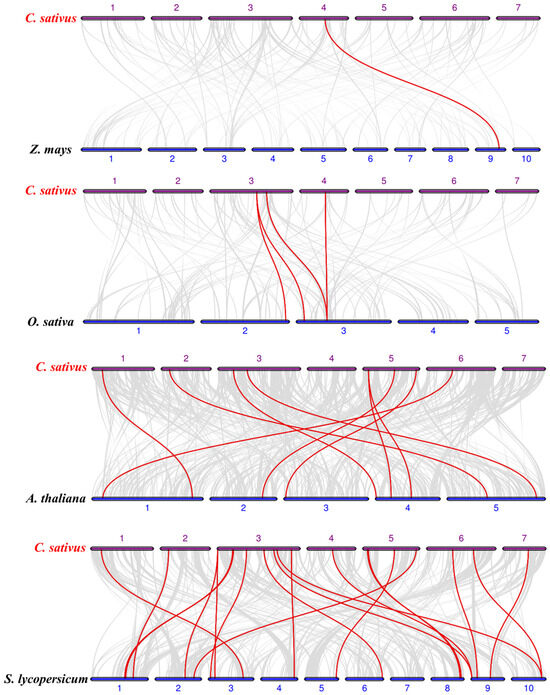
Figure 2.
Synteny analysis of TRMs among cucumber and other plant species: Gray lines indicate the collinear blocks, while red lines highlight the collinear gene pairs involving TRM genes. ‘C. sativus’, ‘Z. mays’, ‘O. sativa’, ‘A. thaliana’, and ‘S. lycopersicum’ indicate Cucumis sativus, Zea mays, Oryza sativa, Arabidopsis thaliana, and Solanum lycopersicum, respectively.
3.4. Expression Profiles of CsTRM Genes in the Fruit
In cucumber and tomato, some TRM genes can regulate the fruit shape [11,13,18]. To investigate the function of CsTRMs in fruit shape, we conducted the expression analysis of CsTRMs using published RNA-seq data on fruits with different carpel numbers and lengths [57,58,59]. Relative to the South China-type cucumber 32X (carpel number = 3), the transcription levels of CsTRMs in the mutant Gui Fei Cui (GFC, carpel number = 5) from 32X showed no significant changes (Figure 3A, Table S3), indicating that CsTRMs might not play a crucial role in regulating the cucumber fruit carpel number. Compared to long fruit 408, there were eight genes downregulated in short fruit 409, namely, CsTRM5, 6, 10, 11, 14, 21, 26, and 27 (Figure 3B). Compared to empty vector/control transgenic plants WT, CsFUL1A-OX-29 had a total of 12 genes downregulated, namely, CsTRM1, 2, 5, 6, 7, 10, 12, 13, 16, 20, 21, and 27; and 4 genes upregulated, namely, CsTRM8, 17, 25, and 26 (Figure 3C). In CsFUL1A-OX-29 versus empty vector/control plants and 409 versus 408, CsTRM5, 6, 10, 21, and 27 were significantly downregulated (Figure 3B,C), indicating that these genes play a crucial role in regulating the fruit shape. However, the expression trend of CsTRM26 in the two groups of long and short fruit materials is opposite (Figure 3B,C), which may be due to the different genetic backgrounds of the materials.
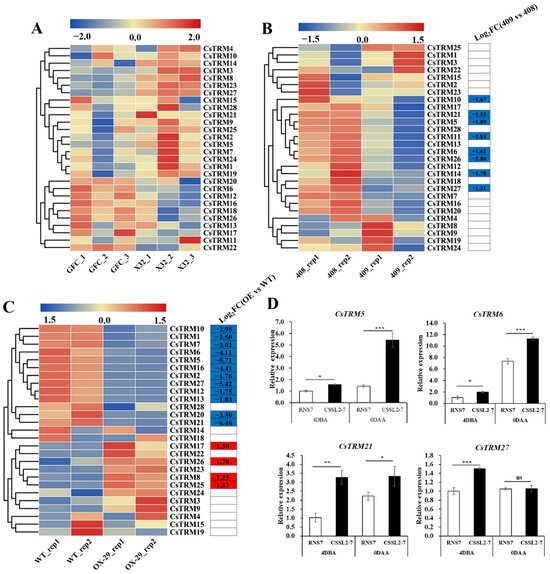
Figure 3.
Expression analysis of CsTRMs in the fruit: The transcriptional levels of CsTRM genes in GFC (carpel number = 5) and 32X (carpel number = 3) (A), 408 (long fruit) and 409 (short fruit) (B), and WT and CsFUL1A-OX (C) are shown on the heatmaps. A color scale range of −2.0 to 2.0 and −1.5 to 1.5 was applied, based on the normalized values. The color gradient, from blue to red, represents increasing expression levels. GFC, mutant Gui Fei Cui (GFC) from South China-type cucumber 32X. The carpel number changed from 3 in 32X to 5 in GFC, despite the number of other floral organs, such as sepal, petal, and stamen, remaining unchanged. WT, empty vector/control transgenic plants. FC, fold-change. (D) qRT-PCR analysis of CsTRM expression of the cucumber ovary at 4 days before anthesis (4 DBA) and 0 days after anthesis (0 DAA) at the long fruit CSSL2-7 and the round fruit RNS7. The gene of cucumber Actin served as reference gene. The standard error of the mean is represented by the error bars (n = 3). Significance analysis was performed with the two-tailed Student’s t-test (ns p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001).
To further verify the reliability of the RNA-seq results, we conducted qRT-PCR analyses of CsTRM gene expression in the ovary of the long-fruited line CSSL2-7 and the round-fruited line RNS7 at 4 days before anthesis (4DBA) and on the day of anthesis (0DAA). Collectively, the qRT-PCR results corresponded well with the transcriptomic data, confirming the accuracy of the datasets (Figure 3D). In RNS7, the expression levels of CsTRM5, CsTRM6, and CsTRM21 were consistently lower than those in CSSL2-7 at both 4DBA and 0DAA. The CsTRM27 expression in RNS7 was lower than in CSSL2-7 at 4DBA but showed no significant difference at 0DAA, suggesting that gene expression levels change during fruit development.
3.5. Expression Patterns of CsTRM Genes under Abiotic and Biotic Stresses
TRM gene family members are often localized to microtubules; microtubules are involved in immune responses and stress tolerance. To further investigate the roles of CsTRM genes under various stresses, we analyzed their comprehensive expression patterns in response to various stresses, including salt, heat, downy mildew (DM, Pseudoperonospora cubensis), gray mold (GM, Botrytis cinerea), and powdery mildew (PM, Podosphaera fusca) based on public RNA-seq data [60,61,62,63,64].
Initially, we examined the roles of CsTRM genes in response to salt stress (Table S4). The transcriptomic data were visualized using a heatmap (Figure 4A). We observed that the expression levels of CsTRM4, 8, and 14 considerably increased in response to NaCl stress, and four genes exhibited the opposite trend with exposure to NaCl stress: they are CsTRM5, 11, 21, and 24 (Figure 4A). Under the conditions treated with Silicon (Si) only, the expression of CsTRM3 and CsTRM14 was upregulated, whereas the expression of CsTRM11, 21, and 24 was downregulated. The expression of CsTRM14 was upregulated under both individual NaCl treatment and individual Si treatment, while the expression of CsTRM11, 21, and 24 was downregulated. Previous research has demonstrated that the application of Silicon (Si) can enhance plant growth when subjected to salt stress. After treatment with Si, the gene expression levels of CsTRM11, 14, and 24, which exhibited significant changes under salt stress, returned to normal levels; CsTRM5, 8, and 21 showed only slight regression, while the expression level of upregulated CsTRM4 showed a slight increase. We also investigated the responses of CsTRM genes to heat tolerance (Figure 4B, Table S4). At three hours after high-temperature treatment, CsTRM1, 11, 16, 18, 21, 22, and 26 were downregulated, while CsTRM3, 8, and 20 were upregulated. The expression of CsTRM16 and CsTRM22 at six hours after heat stress showed no significant difference compared to the 0 h heat treatment, while the changes in other differentially expressed genes were consistent with the 3 h heat treatment. Specifically, at three and six hours after heat stress, the genes upregulated were nearly identical (Figure 4B), indicating their potential significant roles in conferring thermostolerance. Additionally, CsTRM3, 8, 11, and 21 were differentially expressed in responding to heat and NaCl treatments, with consistent trends.
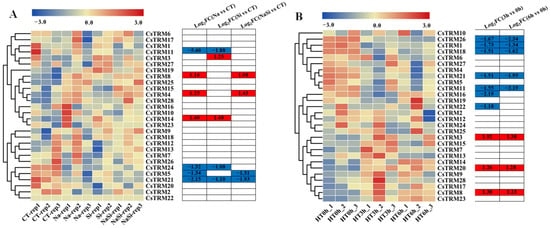
Figure 4.
Expression patterns of CsTRM genes in response to abiotic stress: The heatmap displays the gene expression levels of CsTRM genes in response to salt (A) and heat (B) tolerance. A color scale range of –3.0 to 3.0 was applied, based on the normalized values. The color gradient, from blue to red, represents increasing expression levels. Abbreviations include CT for control treatment; HT for heat treatment; HT0h for HT at 0 h; HT3h for HT at 3 h; and HT6h for HT at 6 h. FC, fold-change.
To investigate the possible roles of CsTRMs in resisting biotic stresses, we analyzed the expression of CsTRMs using RNA-Seq data from cucumber seedlings infected with PM for 48 h, GM for 96 h, and DM for 8 days [62,63,64]. Four genes were found to be differentially expressed after PM inoculation in the susceptible cucumber line D8 leaves compared with the control. The expression of CsTRM14, 21, and 27 were upregulated, while CsTRM20 were downregulated; and four genes were differentially expressed in the resistant cucumber line SSL508-28 leaves compared with the control. The expression of CsTRM4, 14, and 27 were upregulated, while CsTRM21 were downregulated (Figure 5A). In the susceptible and the resistant cucumber line affected by PM, CsTRM14, and CsTRM27 had similar expression trends, while CsTRM21 had opposite expression trends (Figure 5A). After 96 h of GM inoculation, cucumber seedlings showed a significant downregulation of 14 CsTRM genes compared to the uninoculated control, namely, CsTRM1, 2, 5, 6, 7, 10, 11, 13, 14, 16, 20, 21, 27, and 28, and significant upregulation of three genes, namely, CsTRM3, 18, and 26 (Figure 5B). In the transcriptomic analysis of cucumber seedlings infected with DM, only five TRMs genes exhibited significant changes in expression (Figure 5C). CsTRM1, 7, 14, and 28 were upregulated at least at one time point during treatment, while CsTRM8 was downregulated at 6 days post inoculation (dpi) and 8 dpi. CsTRM28 was upregulated at 2 dpi, 3 dpi, 4 dpi, 6 dpi, and 8 dpi (Figure 5C), indicating its significant role in responding to the DM. In summary, the expression of CsTRM14 was significantly upregulated in cucumber seedlings inoculated with PM, BC, and DM, indicating its broad-spectrum role in responding to biotic stress.
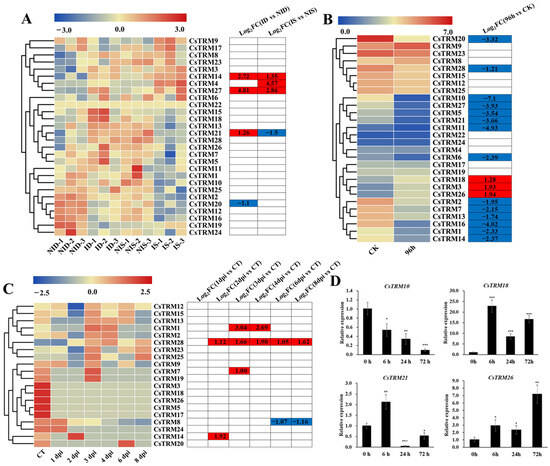
Figure 5.
Expression analysis of CsTRMs under biotic stresses: The heatmaps displays the transcriptional levels of CsTRM genes in response to powdery mildew (PM) for 48 h (A), gray mold (GM) for 96 h (B), and downy mildew (DM) for 1–8 days post inoculation (C). A color scale range of –3.0 to 3.0 was applied, based on the normalized values. The color gradient, from blue to red, represents increasing expression levels. Abbreviations include ID for PM-inoculated susceptible cucumber line D8 leaves; NID for non-inoculated D8 leaves; IS for PM-inoculated resistant cucumber line SSL508-28 leaves; NIS for non-inoculated SSL508-28 leaves; CT for without inoculation; DPI for days post inoculation; and FC for fold-change. (D) qRT-PCR analysis of CsTRM expression of the cotyledons of cucumber seedlings inoculated with gray mold (GM) at 0 h, 6 h, 24 h, and 72 h, and maintaining environmental humidity after inoculation was necessary. The gene of cucumber Actin served as reference gene. The standard error of the mean is represented by the error bars (n = 3). Significance analysis was performed with the two-tailed Student’s t-test (* p < 0.05, ** p < 0.01, *** p < 0.001).
To further confirm the reliability of the RNA-seq results, we conducted qRT-PCR analyses of the CsTRM gene expression of the cotyledons of cucumber seedlings inoculated with gray mold (GM) at 0 h, 6 h, 24 h, and 72 h, and maintaining environmental humidity after inoculation was necessary. Collectively, the qRT-PCR results corresponded well with the RNA-seq data, confirming the accuracy of the datasets (Figure 5D). The expression of CsTRM5 gradually decreased after inoculation. In contrast, the expression of CsTRM18 and CsTRM26 increased after inoculation at 6 h and remained at elevated levels. The expression of CsTRM21 showed a rapid increased after inoculation at 6 h, but the expression decreased at 24 h and 72 h to below that observed at 0 h post inoculation. This indicates that CsTRMs exhibit different response mechanisms to gray mold infection.
4. Discussion
Studies have shown that a single reference genome is insufficient for capturing the full diversity within a species [67]. Therefore, we carried out a thorough analysis to identify and characterize the TRM family in 13 different cucumber varieties. Although, in the previous study, 28 members of the TRM family were identified [54], in this study, a novel member, CsTRM29, which is only present in PI183967, was discovered (Table 1). Moreover, only five CsTRMs show a uniform protein length in all 13 cucumber accessions, and all the identified TRM proteins have amino acid variations including insertions, deletions, single amino acid changes, and frame shifts (Table S1). Some CsTRMs underwent changes not only in gene structure but also in conserved motifs (Figure 1). Therefore, in this study, we found rich variations occurred in CsTRMs from the pan-genomes of 13 cucumber accessions, and these variations will provide a base for discovering TRM genes with novel functions, which will accelerate the breeding of new cucumber varieties [44].
It is widely recognized that there exists a correlation between gene expression and gene function. The cucumber fruit typically have three fused carpels [68]; the carpel number is an important fruit trait that affects the fruit shape, size, and internal quality [57]. In the lines with different carpel numbers, there were no significant differences observed in the expression of CsTRMs (Figure 3A), suggesting that CsTRMs might not play a critical role in regulating the number of carpels in cucumber fruits. However, in the short-fruited lines (409 and CsFUL1A-OX-29), CsTRM5, 6, 10, 21, and 27 were significantly downregulated (Figure 3B,C), indicating that these genes might play crucial roles in regulating cucumber fruit length. Interestingly, the expression of CsTRM26 is lower in the short-fruited line 409 than in the long-fruited line 408, but higher in the short-fruited line CsFUL1A-OX-29 than in the wild type. This could be due to differing genetic backgrounds or the possibility that CsTRM26 does not regulate cucumber fruit length.
Thus far, TRMs have been reported to be functional in plant organ growth, but not in plant response to stresses. But an increasing number of works of research suggested that, apart from their crucial roles in mechanical architecture and cell division, microtubules are also implicated in plants’ adaptation to severe environmental conditions [69]. Since some TRMs are microtubule-binding proteins, they might participate in stress responses. Therefore, we analyzed the expression patterns of CsTRMs under certain stress conditions in this study. Many CsTRM genes showed expression changes at varying degrees under different stress conditions (Figure 4 and Figure 5). Under salt and heat stress conditions, the expression of CsTRM3 and CsTRM8 was significantly upregulated, while CsTRM11 and CsTRM21 were significantly downregulated (Figure 4); however, under inoculation with PM, BC, or DM, the expression of CsTRM14 was significantly increased, while the expression of CsTRM21 showed significant changes after inoculation with PM and BC (Figure 5). These results might indicate that different CsTRMs respond to abiotic or biotic stresses. Remarkably, CsTRM21 plays a crucial role in regulating the fruit shape (Figure 3B,C) and in responding to biotic stresses (Figure 4 and Figure 5). Therefore, this study provided not only a base for the function of CsTRMs in stress tolerance, but also a cross-talk point between organ growth and biotic stresses.
5. Conclusions
In this study, we conducted a pan-genome analysis to identify the TRM gene family in cucumber, identifying a total of 29 members, including a novel member, CsTRM29, which is exclusively present in PI183967. Only five of the CsTRMs have consistent protein lengths among the 13 accessions. All CsTRM proteins showed amino acid variations. Furthermore, the transcriptomic data of fruits with different shapes indicate that CsTRMs play a significant role in regulating the fruit length but not in controlling the carpel number. And transcriptomic data from various stress conditions highlighted the comparative analysis of the CsTRM expression in response to abiotic and biotic stressors, and CsTRM14 was identified as responding to salt stress, powdery mildew, gray mold, and downy mildew. Notably, CsTRM21 plays a role in regulating both the fruit shape and resistance. In summary, this study offers a reference for exploring the potential role of TRMs for fruit shape and stress resistance in cucumber.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10090908/s1. Table S1. The variation of amino acids in different cucumber accessions. Table S2. Orthologous relationships between cucumber and Arabidopsis, tomato, rice, maize. Table S3. RNA-seq data (FPKM values) of CsTRM genes in the fruit. Table S4. RNA-seq data (FPKM values) of CsTRM under abiotic and biotic stresses. Table S5. The primer sequences used for the qRT-PCR.
Author Contributions
Conceptualization, Z.R., L.W. and C.C.; methodology, L.Z. and K.W.; validation, L.Z.; software, L.Z., K.W., Z.W. and S.C.; formal analysis, L.Z. and K.W.; investigation, L.Z. and K.W.; data curation, L.Z. and K.W.; writing—original draft preparation, L.Z.; writing—review and editing, Z.R.; supervision, L.W. and Z.R.; project administration, Z.R., L.W. and C.C.; funding acquisition, Z.R. and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the National Natural Science Foundation of China (32172605 and 31972419) and the ‘Taishan Scholar’ Foundation of the People’s Government of Shandong Province (ts20130932).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We extend our appreciation to the anonymous reviewers for their valuable suggestions to help improve this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Drevensek, S.; Goussot, M.; Duroc, Y.; Christodoulidou, A.; Steyaert, S.; Schaefer, E.; Duvernois, E.; Grandjean, O.; Vantard, M.; Bouchez, D.; et al. The Arabidopsis TRM1-TON1 interaction reveals a recruitment network common to plant cortical microtubule arrays and eukaryotic centrosomes. Plant Cell 2012, 24, 178–191. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, G.T.; Kim, I.J.; Park, J.; Kwak, S.S.; Choi, G.; Chung, W.I. LONGIFOLIA1 and LONGIFOLIA2, two homologous genes, regulate longitudinal cell elongation in Arabidopsis. Development 2006, 133, 4305–4314. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Ng, P.Q.; Shi, S.S.; Fan, D.; Li, J.; Zhao, J.; Wang, H.; David, R.; Mittal, P.; Do, T.; et al. Arabidopsis TRM5 encodes a nuclear-localised bifunctional tRNA guanine and inosine-N1- methyltransferase that is important for growth. PLoS ONE 2019, 14, e0225064. [Google Scholar] [CrossRef]
- Tang, J.; Jia, P.; Xin, P.; Chu, J.; Shi, D.Q.; Yang, W.C. The Arabidopsis TRM61/TRM6 complex is a bona fide tRNA N1-methyladenosine methyltransferase. J. Exp. Bot. 2020, 71, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, C.; Azimzadeh, J.; Pastuglia, M.; Bellini, C.; Grandjean, O.; Bouchez, D. The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell 2002, 14, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, J.; Nacry, P.; Christodoulidou, A.; Drevensek, S.; Camilleri, C.; Amiour, N.; Parcy, F.; Pastuglia, M.; Bouchez, D. Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell 2008, 20, 2146–2159. [Google Scholar] [CrossRef]
- Spinner, L.; Gadeyne, A.; Belcram, K.; Goussot, M.; Moison, M.; Duroc, Y.; Eeckhout, D.; De Winne, N.; Schaefer, E.; Van De Slijke, E.; et al. A protein phosphatase 2A complex spatially controls plant cell division. Nat. Commun. 2013, 4, 1863. [Google Scholar] [CrossRef]
- Schaefer, E.; Belcram, K.; Uyttewaal, M.; Duroc, Y.; Goussot, M.; Legland, D.; Laruelle, E.; Tauzia-Moreau, M.D.; Pastuglia, M.; Bouchez, D. The preprophase band of microtubules controls the robustness of division orientation in plants. Science 2017, 356, 186–189. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Chen, B.Q.; Dang, X.; Zhu, L.L.; Rao, J.Q.; Ren, H.B.; Lin, C.T.; Qin, Y.; Lin, D.S. Arabidopsis IPGA1 is a microtubule-associated protein essential for cell expansion during petal morphogenesis. J. Exp. Bot. 2019, 70, 5231–5243. [Google Scholar] [CrossRef] [PubMed]
- Van der Knaap, E.; Chakrabarti, M.; Chu, Y.H.; Clevenger, J.P.; Illa-Berenguer, E.; Huang, Z.J.; Keyhaninejad, N.; Mu, Q.; Sun, L.; Wang, Y.P.; et al. What lies beyond the eye: The molecular mechanisms regulating tomato fruit weight and shape. Front. Plant Sci. 2014, 5, 227. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.Y.; Keyhaninejad, N.; Rodríguez, G.R.; Kim, H.J.; Chakrabarti, M.; Illa-Berenguer, E.; Taitano, N.K.; Gonzalo, M.J.; Díaz, A.; et al. A common genetic mechanism underlies morphological diversity in fruits and other plant organs. Nat. Commun. 2018, 9, 4734. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, M.D.; Wu, S.; Snouffer, A.; Wang, Y.P.; Van der Knaap, E. Plant organ shapes are regulated by protein interactions and associations with microtubules. Front. Plant Sci. 2018, 9, 1766. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Q.; Keyhaninejad, N.; Taitano, N.; Sapkota, M.; Snouffer, A.; van der Knaap, E. A combinatorial TRM-OFP module bilaterally fine-tunes tomato fruit shape. New Phytol. 2023, 238, 2393–2409. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.Q.; Wang, S.S.; Wang, Y.; Chen, X.B.; Zhang, Y.; Gao, C.X.; et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 2015, 47, 949–954. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xiong, G.S.; Hu, J.; Jiang, L.; Yu, H.; Xu, J.; Fang, Y.X.; Zeng, L.J.; Xu, E.; Xu, J.; et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 2015, 47, 944–948. [Google Scholar] [CrossRef]
- Zhou, Y.; Miao, J.; Gu, H.Y.; Peng, X.R.; Leburu, M.; Yuan, F.H.; Gu, H.W.; Gao, Y.; Tao, Y.J.; Zhu, J.Y.; et al. Natural Variations in SLG7 Regulate Grain Shape in Rice. Genetics 2015, 201, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, X.F.; Sun, C.Z.; Song, X.F.; Li, X.L.; Cui, H.N.; Guo, J.Y.; Liu, L.; Ying, A.; Zhang, Z.Q.; et al. CsTRM5 regulates fruit shape via mediating cell division direction and cell expansion in cucumber. Hortic. Res. 2023, 10, uhad007. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.H. On and around microtubules: An overview. Mol. Biotechnol. 2009, 43, 177–191. [Google Scholar] [CrossRef]
- Landrein, B.; Hamant, O. How mechanical stress controls microtubule behavior and morphogenesis in plants: History; experiments and revisited theories. Plant J. 2013, 75, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Nick, P. Microtubules, signalling and abiotic stress. Plant J. 2013, 75, 309–323. [Google Scholar] [CrossRef]
- Sampathkumar, A.; Yan, A.; Krupinski, P.; Meyerowitz, E.M. Physical forces regulate plant development and morphogenesis. Curr. Biol. 2014, 24, R475–R483. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W. Regulation of developmental and environmental signaling by interaction between microtubules and membranes in plant cells. Protein Cell 2016, 7, 81–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, S.; Chen, Q.; Sun, Y.; Li, Y. Histone H2B monoubiquitination regulates salt stress-induced microtubule depolymerization in Arabidopsis. Plant Cell Environ. 2017, 40, 1512–1530. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Z.; Jin, J.W.; Zhang, J.R.; Wang, D.; Bai, X.C.; Xie, W.F.; Hu, T.M.; Zhao, X.; Mao, T.L.; Qin, T. MDP25 mediates the fine-tuning of microtubule organization in response to salt stress. J. Integr. Plant Biol. 2022, 64, 1181–1195. [Google Scholar] [CrossRef]
- McNally, F.J.; Roll-Mecak, A. Microtubule-severing enzymes: From cellular functions to molecular mechanism. J. Cell Biol. 2018, 217, 4057–4069. [Google Scholar] [CrossRef]
- Bao, Z.R.; Xu, Z.J.; Zang, J.Z.; Bürstenbinder, K.; Wang, P.W. The morphological diversity of plant organs: Manipulating the organization of microtubules may do the trick. Front. Cell Dev. Biol. 2021, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.R.; Guo, Y.; Deng, Y.L.; Zang, J.Z.; Zhang, J.H.; Deng, Y.T.; Ouyang, B.; Qu, X.L.; Bürstenbinder, K.; Wang, P.W. Microtubule-associated protein SlMAP70 interacts with IQ67-domain protein SlIQD21a to regulate fruit shape in tomato. Plant Cell 2023, 35, 4266–4283. [Google Scholar] [CrossRef] [PubMed]
- Gantet, P.; Masson, F.; Domergue, O.; Marquis-Mention, M.; Bauw, G.; Inze, D.; Rossignol, M.; de la Serve, B.T. Cloning of a cDNA encoding a developmentally regulated 22 kDa polypeptide from tobacco leaf plasma membrane. Biochem. Mol. Biol. Int. 1996, 40, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki-Takeuchi, N.; Miyano, M.; Maeshima, M. A plasma membrane-associated protein of Arabidopsis thaliana AtPCaP1 binds copper ions and changes its higher order structure. J. Biochem. 2008, 144, 487–497. [Google Scholar] [CrossRef]
- Tanaka-Takada, N.; Kobayashi, A.; Takahashi, H.; Kamiya, T.; Kinoshita, T.; Maeshima, M. Plasma Membrane-Associated Ca2+-Binding Protein PCaP1 is Involved in Root Hydrotropism of Arabidopsis thaliana. Plant Cell Physiol. 2019, 60, 1331–1341. [Google Scholar] [CrossRef]
- Giovannoni, M.; Marti, L.; Ferrari, S.; Tanaka-Takada, N.; Maeshima, M.; Ott, T.; De Lorenzo, G.; Mattei, B. The plasma membrane-associated Ca2+ -binding protein, PCaP1, is required for oligogalacturonide and flagellin-induced priming and immunity. Plant Cell Environ. 2021, 44, 3078–3093. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Theerawitaya, C.; Kageyama, H.; Cha-Um, S.; Takabe, T. Expression of developmentally regulated plasma membrane polypeptide (DREPP2) in rice root tip and interaction with Ca(2+)/CaM complex and microtubule. Protoplasma 2015, 252, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Klein, M.L.; Hernández-Reyes, C.; Batzenschlager, M.; Ditengou, F.A.; Lace, B.; Keller, J.; Delaux, P.M.; Ott, T. The Medicago truncatula DREPP Protein Triggers Microtubule Fragmentation in Membrane Nanodomains during Symbiotic Infections. Plant Cell 2020, 32, 1689–1702. [Google Scholar] [CrossRef]
- Dou, L.; He, K.; Higaki, T.; Wang, X.; Mao, T. Ethylene Signaling Modulates Cortical Microtubule Reassembly in Response to Salt Stress. Plant Physiol. 2018, 176, 2071–2081. [Google Scholar] [CrossRef]
- Yang, J.; An, B.; Luo, H.; He, C.; Wang, Q. AtKATANIN1 Modulates Microtubule Depolymerization and Reorganization in Response to Salt Stress in Arabidopsis. Int. J. Mol. Sci. 2019, 21, 138. [Google Scholar] [CrossRef]
- Kumar, S.; Jeevaraj, T.; Yunus, M.H.; Chakraborty, S.; Chakraborty, N. The plant cytoskeleton takes center stage in abiotic stress responses and resilience. Plant Cell Environ. 2023, 46, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.A.; Bajer, A.S. Microtubule converging centers and reorganization of the interphase cytoskeleton and the mitotic spindle in higher plant Haemanthus. Cell Motil. Cytoskelet. 1994, 27, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Faleri, C.; Cresti, M.; Cai, G. Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta 2016, 243, 43–63. [Google Scholar] [CrossRef]
- Parveen, S.; Rahman, A. Actin isovariant ACT7 modulates root thermomor-phogenesis by altering intracellular auxin homeostasis. Int. J. Mol. Sci. 2021, 22, 7749. [Google Scholar] [CrossRef]
- Pressman, E.; Harel, D.; Zamski, E.; Shaked, R.; Althan, L.; Rosenfeld, K.; Firon, N. The effect of high temperatures on the expression and activity of sucrose-cleaving enzymes during tomato (Lycopersicon esculentum) anther development. J. Hortic. Sci. Biotechnol. 2006, 81, 341–348. [Google Scholar] [CrossRef]
- Zheng, Y.; Anderson, S.; Zhang, Y.; Garavito, R.M. The structure of sucrose synthase-1 from Arabidopsis thaliana and its functional implications. J. Biol. Chem. 2011, 286, 36108–36118. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Jayakodi, M.; Stein, N.; Mascher, M. Plant pangenomes for crop improvement; biodiversity and evolution. Nat. Rev. Genet. 2024, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, J.; Wang, T.; Chen, C.; Liu, J.; Xu, Z.; Zhang, Q.; Wang, L.; Ren, Z. Pan-Genome-Wide Identification and Transcriptome-Wide Analysis of DREB Genes That Respond to Biotic and Abiotic Stresses in Cucumber. Agriculture 2022, 12, 1879. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, Q.; Lu, H.; Li, Y.; Wang, A.; Tian, Q.; Zhan, Q.; Lu, Y.; Zhang, L.; Huang, T.; et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018, 50, 278–284. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Peng, H.; Liu, S.; Zhou, G.; Zhang, H.; Liu, Z.; Shi, M.; et al. Pan-Genome of Wild and Cultivated Soybeans. Cell 2020, 182, 162–176. [Google Scholar] [CrossRef]
- Hufford, M.B.; Seetharam, A.S.; Woodhouse, M.R.; Chougule, K.M.; Ou, S.; Liu, J.; Ricci, W.A.; Guo, T.; Olson, A.; Qiu, Y.; et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science 2021, 373, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Lu, H.; Du, H.; Wang, H.; Chen, W.; Chen, Z.; He, Q.; Ou, S.; Zhang, H.; Li, X.; et al. Pan-genome analysis of 33 genetically diverse rice accessions reveals hidden genomic variations. Cell 2021, 184, 3542–3558. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhang, H.; Liu, Z.; Wang, Y.; Xing, L.; He, Q.; Du, H. Plant pan-genomics: Recent advances, new challenges, and roads ahead. J. Genet. Genom. 2022, 49, 833–846. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Ma, J.; Jiang, W.; Jin, L.; Zhang, Z.; Guo, Y.; Zhang, J.; Sui, Y.; Zheng, L.; et al. De novo assembly of soybean wild relatives for pan-genome analysis of diversity and agronomic traits. Nat. Biotechnol. 2014, 32, 1045–1052. [Google Scholar] [CrossRef]
- Golicz, A.A.; Bayer, P.E.; Barker, G.C.; Edger, P.P.; Kim, H.; Martinez, P.A.; Chan, C.K.; Severn-Ellis, A.; McCombie, W.R.; Parkin, I.A.; et al. The pangenome of an agronomically important crop plant Brassica oleracea. Nat. Commun. 2016, 7, 13390. [Google Scholar] [CrossRef]
- Gao, L.; Gonda, I.; Sun, H.; Ma, Q.; Bao, K.; Tieman, D.M.; Burzynski-Chang, E.A.; Fish, T.L.; Stromberg, K.A.; Sacks, G.L.; et al. The tomato pan-genome uncovers new genes and a rare allele regulating fruit flavor. Nat. Genet. 2019, 51, 1044–1051. [Google Scholar] [CrossRef]
- Alonge, M.; Wang, X.; Benoit, M.; Soyk, S.; Pereira, L.; Zhang, L.; Suresh, H.; Ramakrishnan, S.; Maumus, F.; Ciren, D.; et al. Major Impacts of Widespread Structural Variation on Gene Expression and Crop Improvement in Tomato. Cell 2020, 182, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Guan, Z.; Hu, J.; Guo, C.; Yang, Z.; Wang, S.; Liu, D.; Wang, B.; Lu, S.; Zhou, R.; et al. Eight high-quality genomes reveal pan-genome architecture and ecotype differentiation of Brassica napus. Nat. Plants 2020, 6, 34–45. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Chai, S.; Yang, Z.; Zhang, Q.; Xin, H.; Xu, Y.; Lin, S.; Chen, X.; Yao, Z.; et al. Graph-based pan-genome reveals structural and sequence variations related to agronomic traits and domestication in cucumber. Nat. Commun. 2022, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Che, G.; Gu, R.; Zhao, J.; Liu, X.; Song, X.; Zi, H.; Cheng, Z.; Shen, J.; Wang, Z.; Liu, R.; et al. Gene regulatory network controlling carpel number variation in cucumber. Development 2020, 147, dev184788. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Yan, S.; Yang, W.; Li, Y.; Xia, M.; Chen, Z.; Wang, Q.; Yan, L.; Song, X.; Liu, R.; et al. Transcriptomic analysis reveals the roles of microtubule-related genes and transcription factors in fruit length regulation in cucumber (Cucumis sativus L.). Sci. Rep. 2015, 5, 8031. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, L.; Che, G.; Pan, Y.; Li, Y.; Hou, Y.; Zhao, W.; Zhong, Y.; Ding, L.; Yan, S.; et al. A Functional Allele of CsFUL1 Regulates Fruit Length through Repressing CsSUP and Inhibiting Auxin Transport in Cucumber. Plant Cell 2019, 31, 1289–1307. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, J.; Liang, Y.; Liu, J.; Jia, J. Transcriptomic dynamics provide an insight into the mechanism for silicon-mediated alleviation of salt stress in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 174, 245–254. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Tang, R.; Wang, L.; Chen, C.; Ren, Z. Genome-Wide identification and expression analysis of Hsf and Hsp gene families in cucumber (Cucumis sativus L.). Plant Growth Regul. 2021, 95, 223–239. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Shi, Y.; Qi, X.; Chen, X. Elucidation of the molecular responses of a cucumber segment substitution line carrying Pm5.1 and its recurrent parent triggered by powdery mildew by comparative transcriptome profiling. BMC Genom. 2017, 18, 21. [Google Scholar] [CrossRef]
- Kong, W.; Chen, N.; Liu, T.; Zhu, J.; Wang, J.; He, X.; Jin, Y. Large-Scale Transcriptome Analysis of Cucumber and Botrytis cinerea during Infection. PLoS ONE 2015, 10, e0142221. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.N.; Savory, E.A.; Vaillancourt, B.; Childs, K.L.; Hamilton, J.P.; Day, B.; Buell, C.R. Expression Profiling of Cucumis sativus in Response to Infection by Pseudoperonospora cubensis. PLoS ONE 2012, 7, e34954. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Gao, Z.; Wang, L.; Ren, Z. Localization of quantitative trait loci for cucumber fruit shape by a population of chromosome segment substitution lines. Sci. Rep. 2020, 10, 11030. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.J.; Zhang, Q.X.; Wang, L.N.; Ren, Z.H. Identification and Analysis on TRM Family in Cucumber. J. Shandong Agric. Univ. 2021, 52, 358–363. [Google Scholar]
- Yin, S.; Zhao, L.; Liu, J.; Sun, Y.; Li, B.; Wang, L.; Ren, Z.; Chen, C. Pan-genome Analysis of WOX Gene Family and Function Exploration of CsWOX9 in Cucumber. Int. J. Mol. Sci. 2023, 24, 17568. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.L.; Peng, Y.-B.; Cui, J.X.; Gu, H.T.; Xu, L.Y.; Li, Y.Q.; Xu, Z.H.; Bai, S.N. Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 2004, 220, 230–240. [Google Scholar] [CrossRef]
- Ma, H.; Liu, M. The microtubule cytoskeleton acts as a sensor for stress response signaling in plants. Mol. Biol. Rep. 2019, 46, 5603–5608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).