3.1. Impact of Rootstocks and Training Systems on ‘IAC 138-22 Maximo’ Grape Variety: Physiological, Biochemical Parameters and Yield
Significant interactions were observed between rootstocks and training systems concerning the electron transport rate (ETR), stomatal conductance (gs), transpiration rate (E), water-use efficiency (WUE), assimilation rate (A), and internal carbon concentration (Ci) in the ‘IAC 138-22 Máximo’ grape variety (
Table 1).
A higher ETR was achieved with the combination of the rootstock 106-8 ‘Mgt’ and the high espalier, as well as with the rootstock ‘IAC 766 Campinas’ and the low espalier. This suggests that these specific combinations are more efficient in capturing and utilizing light energy for electron transport, leading to increased production of ATP and NADPH, which are essential for the synthesis of photoassimilates.
Stomatal conductance (gs) is another vital parameter, referring to the rate of CO2 influx and water vapor efflux through the stomata. An increase in gs, as observed in vines grafted onto the rootstock 106-8 ‘Mgt’ with the low espalier training system, allows for greater CO2 intake, thereby increasing the assimilation rate (A). It was also noted that the transpiration rate (E), which measures water loss through the stomata, increased as well, potentially aiding in maintaining leaf temperature and ensuring continuous water and nutrient flow.
The increased gs and E in vines grafted onto the rootstock 106-8 ‘Mgt’ may have contributed to the lower water-use efficiency (WUE) in this combination of rootstock and training system, indicating that, despite the increased photosynthesis, water was not used as efficiently, which may result in lower biomass accumulation.
In both training systems, vines grafted onto the rootstock ‘IAC 766 Campinas’ exhibited a higher internal carbon concentration (Ci), suggesting that despite the high stomatal conductance (gs), CO2 fixation may not have been as efficient due to lower Rubisco activity and reduced water-use efficiency (WUE). Another hypothesis is that mesophyll cells consume CO2 during photosynthetic assimilation, consequently resulting in lower CO2 concentration in the intercellular airspace compared to the ambient air outside the leaf. The internal carbon concentration (Ci) reflects the concentration of CO2 within the leaves and indicates the balance between CO2 fixation and its entry through the stomata.
Thus, it was observed that the correct choice of rootstock and training system can optimize photosynthesis and water use in vines, resulting in more efficient and productive plants. These interactions directly influence physiological parameters, such as the ETR, gs, E, WUE, A, and Ci, and consequently, the agronomic performance of the ‘IAC 138-22 Máximo’ vines.
No interaction was observed between the rootstocks and training systems for the SPAD index and total chlorophyll content in the ‘IAC 138-22 Máximo’ vine, with only rootstocks showing a significant isolated effect. Higher SPAD indices and chlorophyll contents were obtained with the use of the rootstock 106-8 ‘Mgt’ (
Table 2). Vines with higher SPAD indices exhibit greater green coloration intensity, as a result of higher concentrations of photosynthetic pigments, particularly chlorophylls. This aspect is particularly important for grapes intended for processing, where a high SPAD index and chlorophyll content enhance light utilization during photosynthesis, increasing carbohydrate accumulation, which will be converted into sugars in the berries and energy for the next production cycle.
Associating these results with photosynthetic parameters, it can be inferred that vines with a higher chlorophyll content and SPAD index, as observed with the rootstock 106-8 ‘Mgt’, exhibit greater photosynthetic efficiency. This is reflected in a higher ETR and CO2 assimilation rate (A), promoting better photosynthetic performance.
No significant interaction was observed between rootstocks and training systems for the productive and physicochemical variables of the ‘IAC 138-22 Máximo’ grape must. Therefore, the isolated effect of the variables was assessed. Significant differences were found for the production, yield, cluster fresh weight, soluble solids, and pH, among the training systems. Except for pH, all the variables showed higher results when the ‘IAC 138-22 Máximo’ vine was trained with a high espalier (
Table 3).
The yield of the vines trained with a high espalier was 38.52% higher than those trained with a low espalier, demonstrating greater compatibility of the cultivar with this training system, possibly due to its high vigor. The ‘IAC 138-22 Máximo’ vine requires a system that allows for better distribution of the branches and the vegetative canopy, thus providing more efficient leaf distribution and greater solar radiation capture. This benefits the photosynthetic process, increasing photoassimilate production and, consequently, the yield. Additionally, this training system resulted in heavier clusters, which directly contributes to the observed higher yield.
The high espalier system promotes an increase in the soluble solids in the must of the ‘IAC 138-22 Máximo’ grape (15.25 °Brix). Although the training system did not influence the titratable acidity and the SS/TA ratio, the low espalier system produced fruit with an acidity of 0.98% tartaric acid and an SS/TA ratio of 14.91, values below the requirements in Brazilian legislation, which stipulates a maximum acidity of 0.9% tartaric acid and a minimum SS/TA ratio of 15 for grapes intended for processing. This result was also observed when the vines were grafted onto the rootstock 106-8 ‘Mgt’, which had a titratable acidity of 1.02% tartaric acid and an SS/TA ratio of 14.17.
When a grape cultivar exhibits high acidity, low soluble solids content, or any other undesirable chemical characteristic, it becomes crucial to diversify cultivars for blending purposes. This aims to balance the potential limitations of each cultivar, thus meeting the required standards for beverage production and enhancing the quality of juices and wines.
The high espalier system, by providing better canopy distribution, supports a higher ETR and assimilation rate, optimizing light utilization and increasing ATP and NADPH production. This not only improves photosynthesis, but also enhances the efficiency of photoassimilate production, resulting in a higher soluble solids content and better grape must quality. Therefore, choosing an appropriate training system, such as the high espalier, in combination with efficient rootstocks, is crucial for maximizing the productivity and quality of the ‘IAC 138-22 Máximo’ vine.
A well-distributed canopy allows more leaves to receive direct light, increasing the CO2 assimilation rate and light-use efficiency. Increased photosynthetic activity promotes a higher concentration of carbohydrates, which are utilized for the growth and development of the clusters, explaining the greater cluster fresh weight and higher soluble solids concentration. Thus, the high espalier offers optimal conditions to maximize the photosynthetic efficiency and productivity of the ‘IAC 138-22 Máximo’ vine.
The rootstock ‘IAC 766 Campinas’ provided the ‘IAC 138-22 Máximo’ grape must with lower titratable acidity, increasing the SS/TA ratio and improving the grape flavor.
Improvements to the photosynthetic efficiency and carbohydrate production of grapevines are essential for fruit growth and development. Consequently, the increased absorption and availability of nutrients, common to vigorous rootstocks, may lead to reduced titratable acidity, balancing the SS/TA ratio and enhancing the flavor and quality of the must.
Moreover, the greater vigor of the ‘IAC 766 Campinas’ rootstock may be associated with its better adaptation to various environmental and soil conditions, promoting more balanced and healthy vine growth. This results in a more efficient distribution of resources within the plant, contributing to a more uniform development of the aerial parts and, particularly, the clusters, leading to better overall fruit quality.
Significant interactions were observed between rootstocks and training systems for the total phenolic compounds, monomeric anthocyanins, and antioxidant activity (DPPH and FRAP) of the ‘IAC 138-22 Máximo’ grapevine (
Table 4). The combination of the high espalier with the ‘IAC 766 Campinas’ rootstock resulted in higher levels of monomeric anthocyanins and total phenolic compounds in the grapes, increasing the antioxidant activity as expressed by DPPH and FRAP values (
Table 4).
The presence of high levels of phenolic compounds, anthocyanins, and antioxidant activity in the berry skins of ‘IAC 138-22 Máximo’ grapes, especially when combined with the ‘IAC 766 Campinas’ rootstock and the high espalier system, offers numerous health benefits. Anthocyanins and flavonoids are positively related to vine exposure to solar radiation, which justifies the high antioxidant activity observed in grapes cultivated with this combination of training system and rootstock.
The high antioxidant activity observed, as measured by the DPPH and FRAP methods, indicates that these grapes have a significant capacity to combat cellular damage caused by reactive oxygen species.
The higher concentration of these compounds in the combination of the high espalier and the ‘IAC 766 Campinas’ rootstock suggests an increased synthesis of flavonoids.
The combination of the high espalier and the ‘IAC 766 Campinas’ rootstock proved effective in maximizing the levels of these bioactive compounds in the grape skins. This training system and rootstock favor light exposure and canopy distribution, optimizing photosynthesis [
14] and secondary metabolite production.
Consequently, the increase in phenolic compounds and anthocyanins not only improves the functional and sensory quality of the grapes, but also enhances the health benefits, making these grapes highly beneficial for both fresh consumption and for the production of juices and wines. Therefore, the choice of appropriate training systems and rootstocks is crucial for maximizing the beneficial chemical and biochemical attributes of ‘IAC 138-22 Máximo’ grapes for consumers.
3.2. Impact of Rootstock and Training Systems on ‘BRS Violeta’ Grape Variety: Physiological, Biochemical Parameters and Yield
For the variables, namely the electron transport rate (ETR), stomatal conductance (gs), CO
2 assimilation rate (A), internal carbon concentration (Ci), and SPAD index of the ‘BRS Violeta’ vine, a significant interaction between the rootstocks and training systems was observed (
Table 5). The combination of ‘IAC 766 Campinas’ with the low espalier resulted in reduced energy dissipation and a higher ETR, indicating a less stressful condition for the scion cultivar and resulting in less wear on the vines. This combination also increased the CO
2 assimilation rate and reduced the internal carbon concentration, reinforcing the benefits of this combination for vine cultivation (
Table 5).
The lower energy dissipation observed with the combination of ‘IAC 766 Campinas’ and a low espalier suggests that this interaction optimizes the capture of light energy, directing it more efficiently towards photosynthetic processes.
Furthermore, the reduction in the internal carbon concentration (Ci) observed with this interaction suggests that the available CO
2 is rapidly fixed during photosynthesis, preventing CO
2 accumulation in leaf cells and potentially reducing oxidative stress [
27,
28].
For the SPAD index, a significant interaction was observed, with the ‘IAC 766 Campinas’ rootstock providing higher SPAD values in ‘BRS Violeta’ vines, regardless of whether it was combined with a low or high espalier. The SPAD index is an indirect indicator of chlorophyll content, with high SPAD values being associated with greater photosynthetic capacity of the plant.
The presence of higher chlorophyll content suggests that the ‘IAC 766 Campinas’ rootstock is particularly effective in enhancing the photosynthetic efficiency of ‘BRS Violeta’, providing optimal conditions for the production of ATP and NADPH, necessary for CO2 assimilation.
The combination of the ‘IAC 766 Campinas’ rootstock with both training systems (low or high espalier) maximizes the SPAD index, contributing to a higher ETR, gs, and A. The increased ETR facilitates electron transfer between the photosystems, while a higher gs enhances the gas exchange and CO2 uptake. The combination of these physiological and biochemical characteristics optimizes photosynthesis, promoting more vigorous growth of ‘BRS Violeta’ vines.
There were no significant interactions between the training systems and rootstocks in terms of the transpiration rate (E), water-use efficiency (WUE), and chlorophyll content; therefore, these variables were analyzed separately (
Table 6). For the photosynthetic pigments, no significant differences were observed in any of the evaluated variables.
The ‘BRS Violeta’ hybrid grown with a low espalier has higher water-use efficiency (WUE), particularly when combined with the 106-8 ‘Mgt’ rootstock, which also resulted in a lower transpiration rate (E). Since the WUE indicates the plant’s capacity to assimilate a greater amount of carbon dioxide with less water lost through transpiration, a higher water-use efficiency implies a lower E, which can contribute to better carbohydrate synthesis efficiency and reduced vulnerability to water stress.
The increased WUE observed with the low espalier and the 106-8 ‘Mgt’ rootstock suggests an optimization in the plant’s water balance, allowing efficient CO2 assimilation without excessive water loss through transpiration (E). This trait is particularly advantageous in environments with limited water resources, where the ability to maintain efficient photosynthesis and carbohydrate production with reduced water consumption is crucial. Therefore, selecting training systems and rootstocks that maximize the WUE can significantly improve the sustainability and productivity of the ‘BRS Violeta’ vine under water stress conditions.
No significant interactions were observed between rootstocks and training systems for any of the productive or physicochemical variables of the ‘BRS Violeta’ grape must. Therefore, the factors were evaluated individually (
Table 7). Training systems only influenced the soluble solids (SS) content and titratable acidity (TA) of the must.
The highest soluble solids (SS) content (16.32 °Brix) and the lowest titratable acidity (TA) (0.68% tartaric acid) were obtained from grapes of vines trained on a high espalier. The SS content in grapes is primarily composed of sugars (glucose and fructose) and, along with the TA, are direct indicators of fruit quality, closely linked to photosynthetic metabolism and the plant’s resource-use efficiency. The higher SS content achieved with a high espalier suggests better photosynthetic efficiency, as the high espalier configuration allows for improved light distribution over the canopy, enhancing light capture and CO
2 assimilation (A). This efficiency is highlighted by the elevated values of the electron transport rate (ETR), stomatal conductance (gs), and SPAD index, which together contribute to increased sugar production in the fruits (
Table 5).
The lower TA observed in grapes from a high espalier can be explained by the increased CO2 assimilation and greater Rubisco enzyme activity, which channels more carbon into sugar production, reducing the relative concentration of organic acids in the must. This combination is ideal for producing grapes with a more balanced and attractive sensory profile for winemaking.
The ‘IAC 766 Campinas’ rootstock was noted for promoting higher production and productivity in the ‘BRS Violeta’ vine. The increase in these variables can be attributed to several physiological advantages provided by this rootstock; hence, it is recognized for its superior vigor, translating into greater water and nutrient absorption capacity from the soil. This is crucial to meet the high metabolic demands associated with photosynthesis and plant growth.
Thus, the greater photosynthetic efficiency of vines grafted onto ‘IAC 766 Campinas’ is evidenced by the higher values of the ETR, gs, and CO
2 assimilation rate (A) (
Table 5). These parameters indicate that the vines have a greater capacity to capture and utilize solar light for photosynthesis, resulting in higher production of ATP and NADPH, which are essential for carbohydrate synthesis and increased vine production.
Vines grafted onto this rootstock showed higher SS and pH values, indicating an optimal balance between sugar accumulation and must pH. The superior capacity of ‘IAC 766 Campinas’ to support cultivation conditions and maximize carbohydrate production makes it a recommended choice for the ‘BRS Violeta’ vine. The higher CO2 assimilation rate (A) observed with the use of this rootstock indicates that more carbon is being fixed and converted into sugars and other photoassimilates, which are used for fruit growth and development.
Moreover, the improved regulation of must pH with ‘IAC 766 Campinas’ indicates a balance between sugar synthesis and organic acid accumulation, resulting in higher quality fruit for wine and juice production. Specifically concerning pH, studies suggest that the effects of rootstocks may be related to their capacity for potassium extraction from the soil.
In both grapes and beverages, such as grape juice and wine, pH measurement is crucial as it is directly related to anthocyanin stability, which affects the color intensity of these beverages. Therefore, the choice of rootstock not only impacts vine productivity and health, but also the final quality of the grape-derived products, highlighting the importance of ‘IAC 766 Campinas’ in optimizing the production and quality of ‘BRS Violeta’ grapes.
There were interactions among the factors evaluated for the secondary metabolites in the skin of ‘BRS Violeta’ grapes. The combination of using the ‘IAC 766 Campinas’ rootstock with the high espalier resulted in higher levels of total phenolic compounds and monomeric anthocyanins, as well as greater antioxidant activity, using both methods (
Table 8).
The higher concentration of phenolic compounds obtained with the high espalier can be attributed to better sunlight distribution, which promotes increased photosynthetic activity and synthesis of secondary metabolites, such as phenolic compounds.
This same interaction between the rootstock and the training system also resulted in increased anthocyanin content in the grape skins (
Table 8). Biochemically, anthocyanins are pigments responsible for grape coloration and play a significant role in antioxidant activity.
Greater light exposure with a high espalier can induce the expression of genes related to anthocyanin biosynthesis, resulting in higher concentrations of these pigments. Enhanced photosynthetic efficiency, evidenced by the high ETR and increased production of ATP and NADPH (
Table 5), also contributes to the increased synthesis of anthocyanins.
Anthocyanins, along with other phenolic components present in ‘BRS Violeta’ grapes, are of extreme importance in the production of red wines and, particularly, juices. Therefore, the correct choice of training system and rootstock, which have greater affinity with the ‘BRS Violeta’ cultivar, is essential to maximize the accumulation potential of antioxidant compounds in grapes.
Vines trained with a high espalier and grafted onto the ‘IAC 766 Campinas’ rootstock exhibited higher antioxidant activity (DPPH: 639.87 µmol g−1 of grape skin; and FRAP: 43.84 µmol Fe kg−1 of grape skin). These results indicate that the increased synthesis of phenolic compounds and anthocyanins as a result of a high espalier not only enhances the nutritional quality of the grapes, but also boosts their antioxidant capacity.
Therefore, the choice of training system and rootstock has a significant impact on the phytochemical composition and antioxidant activity of ‘BRS Violeta’ grapes. The high espalier, combined with the ‘IAC 766 Campinas’ rootstock, proved to be the most effective combination for maximizing the concentration of phenolic compounds, anthocyanins, and antioxidant activity. This combination not only improves the nutritional and sensory quality of the grapes, but also enhances the photosynthetic efficiency and resistance to environmental stresses, promoting more sustainable and high-quality production.
3.3. Principal Component Analysis (PCA)
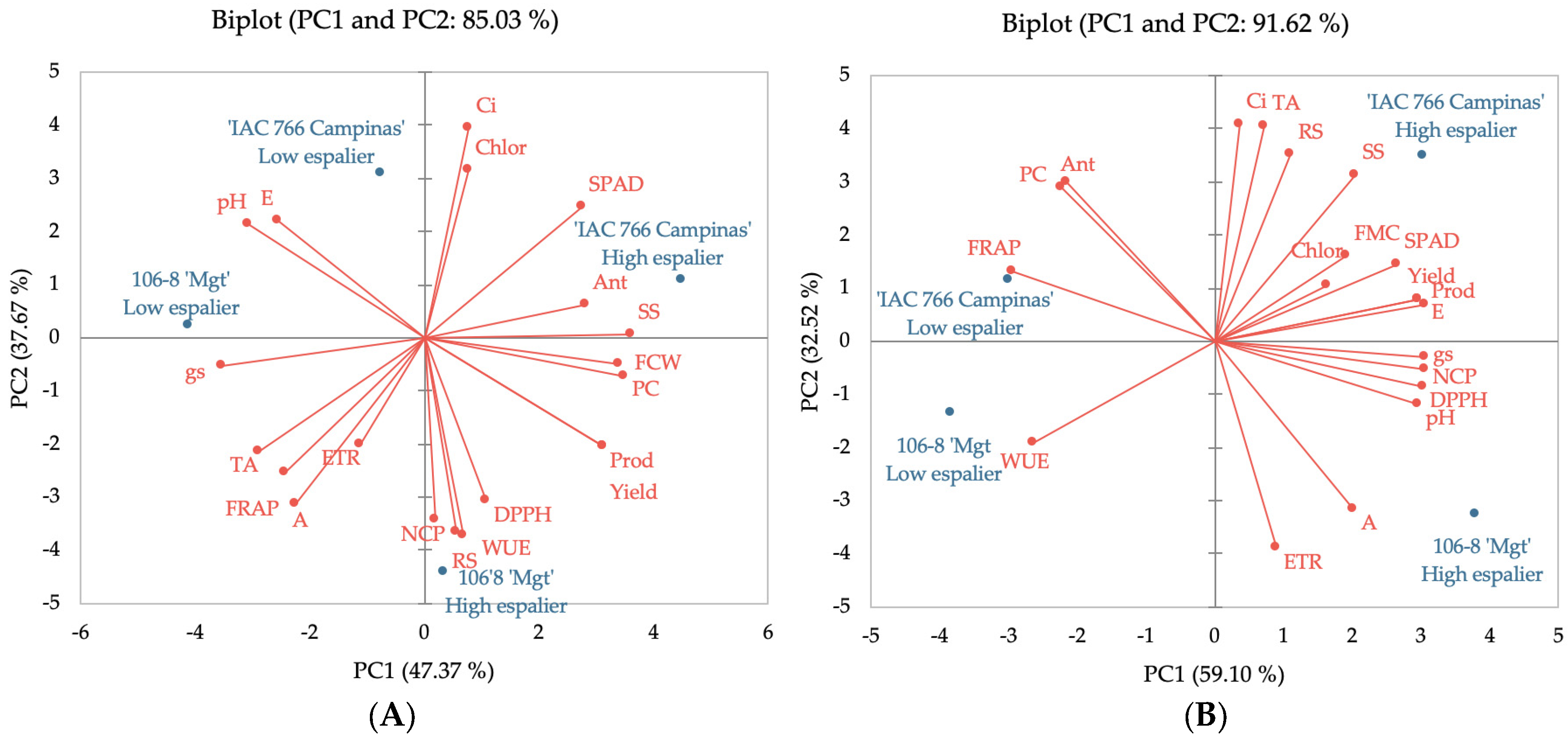
Principal Component Analysis (PCA) of the ‘IAC 138-22 Máximo’ vine reveals that the first two principal components (PCA 1 and PCA 2,
Figure 2A) account for 85.03% of the total variance in the data. It is observed that parameters related to photosynthetic efficiency, such as the SPAD index, internal carbon concentration (Ci), assimilation rate (A), and electron transport rate (ETR), are positively correlated and associated with the ‘IAC 766 Campinas’ rootstock and the high espalier. This indicates that this combination promotes better conditions for photosynthesis, resulting in higher production of photoassimilates and higher levels of phenolic compounds and anthocyanins, which are crucial for the nutritional and sensory quality of the grapes and wines.
Parameters, such as the pH, yield, productivity, and soluble solids, are also positively correlated with a high espalier, highlighting the importance of this training system in maximizing the quality of grape must. The 106-8 ‘Mgt’ rootstock, associated with the low espalier, showed lower efficiency in regard to all these parameters, suggesting reduced physiological and biochemical performance of the ‘IAC 138-22 Máximo’ vines under these conditions.
In the PCA of the ‘BRS Violeta’ vine (
Figure 2B), the first two principal components explain 91.62% of the total variance in the data. Similar to ‘IAC 138-22 Máximo’, the photosynthetic efficiency parameters (SPAD, Ci, A, ETR) are strongly associated with the ‘IAC 766 Campinas’ rootstock combined with the high espalier. This rootstock demonstrated notable superiority in maximizing the photosynthetic efficiency and, consequently, the production of phenolic compounds, anthocyanins, and antioxidant activity.
Additionally, must quality parameters, such as the soluble solids (SS) content, yield, and titratable acidity (TA) are positively correlated with the high espalier and the ‘IAC 766 Campinas’ rootstock. This indicates that this combination not only improves the photosynthetic efficiency, but also optimizes the physical and chemical qualities of the grapes. In contrast, the low espalier and the 106-8 ‘Mgt’ rootstock are associated with lower levels of phenolic compounds, anthocyanins, and antioxidant activity (DPPH and FRAP), reflecting the reduced physiological and biochemical performance of the grapes from this combination.
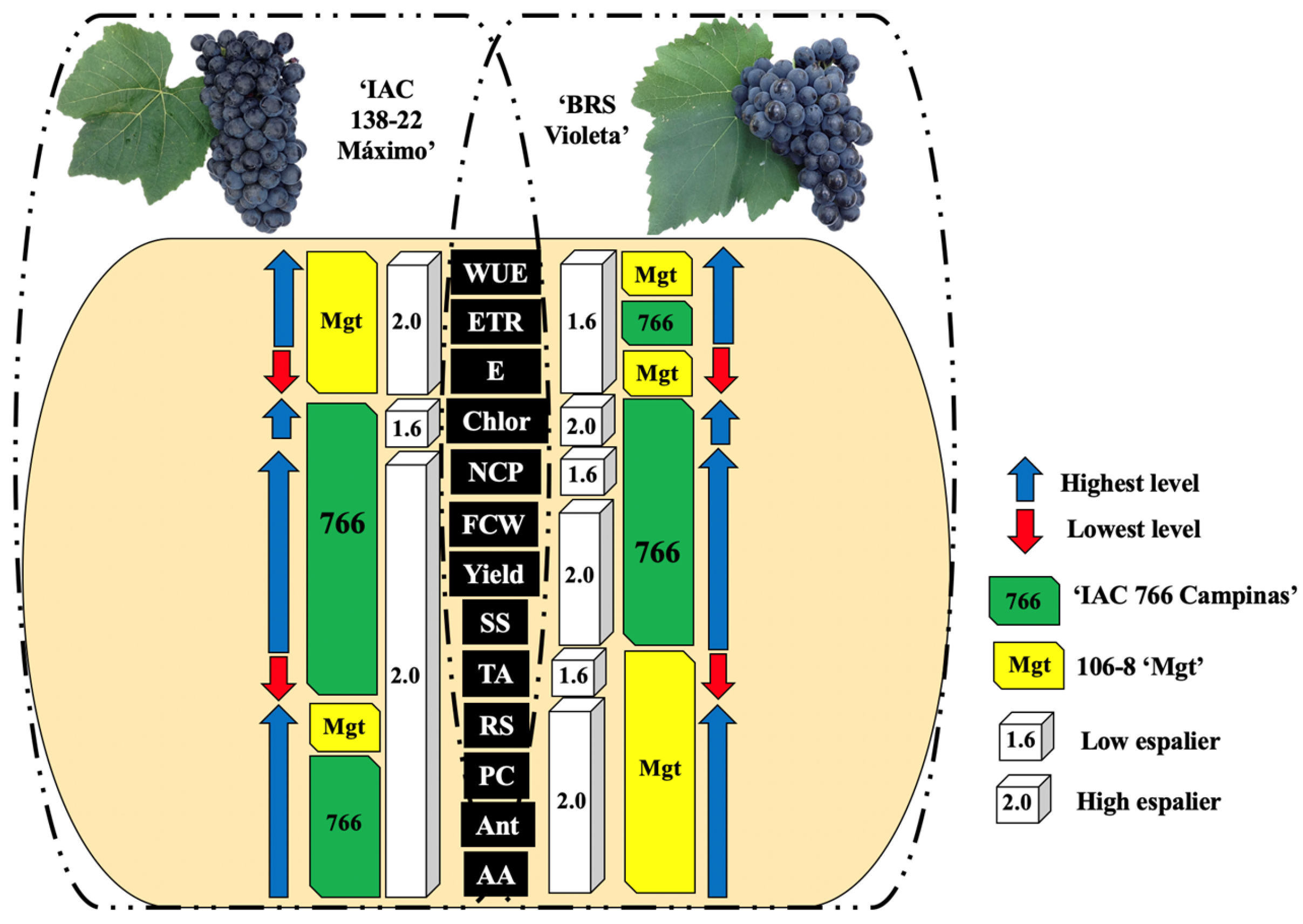
Figure 3 summarizes how different combinations of rootstocks and training systems affect the production and quality of the grapes from the ‘IAC 138-22 Máximo’ and ‘BRS Violeta’ cultivars. The use of the ‘IAC 766 Campinas’ rootstock and the high espalier resulted in increased productivity and improved physiological, physicochemical, and biochemical attributes of the grapes. These results are explained by the higher capacity for water and nutrient absorption, the vigor provided by the rootstocks, and the better light distribution afforded by the high espalier. The integration of these practices can help balance the limitations of each variety, meeting the standards required for juices and wines, and resulting in higher quality products.
In summary, the results indicate that the choice of training system and rootstock has a significant impact on the physiology, biochemistry, and final quality of the grapes from the hybrid vines ‘IAC 138-22 Máximo’ and ‘BRS Violeta’. The combination of the high espalier with the ‘IAC 766 Campinas’ rootstock provides the best conditions for maximizing the photosynthetic efficiency and the synthesis of essential biochemical compounds, resulting in grapes of high nutritional and sensory quality. Therefore, this combination is recommended for the optimized production of these hybrids.