1. Introduction
World olive (
Olea europaea L.) oil production was just over 3.3 million tons, and nearly 23.5 million tons of olives were produced on 10.3 million hectares in 2021, which is the last year from which we have final data [
1]. In that year, Spain, the world’s largest producer of olive oil, produced 1.5 million tons of olive oil (44.6% of the total) and 8.3 million tons of olives (35.1%) on 2.6 million hectares [
1]. However, olive tree cultivation is facing a dramatic change worldwide. After centuries of rain-fed, low-density orchards planted with local cultivars, olive growing is rapidly transitioning in the last few decades to large, super high-density irrigated orchards [
2,
3], which are mostly achieved using one single cultivar: Arbequina [
4]. However, Arbequina is self-incompatible [
5], and the high yields needed to control vegetative growth strongly depend on cross-pollination from neighboring cultivars that act as unsuspected pollinizers [
6,
7]. This benefit is possible in traditional olive countries due to the airborne condition and massive production of pollen grains in this wind-pollinated taxon. In fact, several authors have demonstrated through seed paternity analyses that most fruit produced in supposedly monovarietal orchards are sired by cross-pollination from distant sources [
8,
9]. Unfortunately, this free cross-pollination does not exist in new, recent areas of olive cultivation, and the varietal mosaic in traditional olive countries is becoming more and more reduced, compromising the benefits of this free open-pollination.
Olive blooms in panicles developed in 1-year-old shoots. The panicle is the unit of fructification in olive and bears staminate (pistil-aborted) as well as hermaphrodite flowers in a variable number (usually between 12 and more than 20). The hermaphrodite flower is composed of four greenish small sepals, four larger white petals, two opposite stamens producing plenty of pollen grains and a pistil where a large stigma stands out. The style of the pistil is, on the contrary, short. The ovary contains four ovules in two locules, although usually only one ovule is fertilized and becomes the seed of the fruit (drupe). Staminate flowers, produced in a variable proportion in the panicle of the olive, share the basic morphology of the hermaphrodite flowers, but the pistil in them is rudimentary or completely absent. Olive flowers are receptive from anthesis, when anthers dehiscence starts and the stigma receptivity allows pollen adhesion. Olive is considered preferentially allogamous for most authors with a preference for cross-pollen to achieve the fertilization of the ovule becoming seed. The preferential allogamy is based on a self-incompatibility system that is still under dispute, although recent work strongly suggests that, in olive, self-incompatibility corresponds to the sporophytic system [
10,
11].
Given the preferential allogamy of most olive cultivars, Arbequina included, and the increasing interest in very large monovarietal high-density orchards, we have studied in two consecutive years the self-incompatibility behavior of Arbosana, which is a low-vigor cultivar often selected as an alternative to Arbequina in different countries and growing conditions [
12,
13,
14,
15]. Despite its wide use in high-density orchards, Arbosana self-incompatibility response has not yet been determined.
2. Materials and Methods
2.1. Plant Material and Study Site
An experiment to determine Arbosana self-incompatibility response was carried out during the seasons 2022 and 2023 on adult olive trees planted in 2011 in a high-density orchard located on the Rabanales Campus of the University of Córdoba (Córdoba, Spain; 37°56′05″ N, 4°43′00″ W, at 160 m altitude). The trees are drip-irrigated with volumes between 1000 and 2000 m3/ha, and pests and diseases are controlled by IPM programs. Harvest and pruning is performed mechanically, as usual in high-density orchards trained in hedgerow systems. Arbosana is noted for its reduced vigor, good adaptation to super high-density plantations, early production and heavy and regular yields of very good quality olive oil. Its good agronomic performance justifies its increased acreage. Arbosana accounts for around 25% of high-density orchards and represents a clear alternative to the main cultivar Arbequina for warm areas, such as the experimental site, where it produces as much as Arbequina.
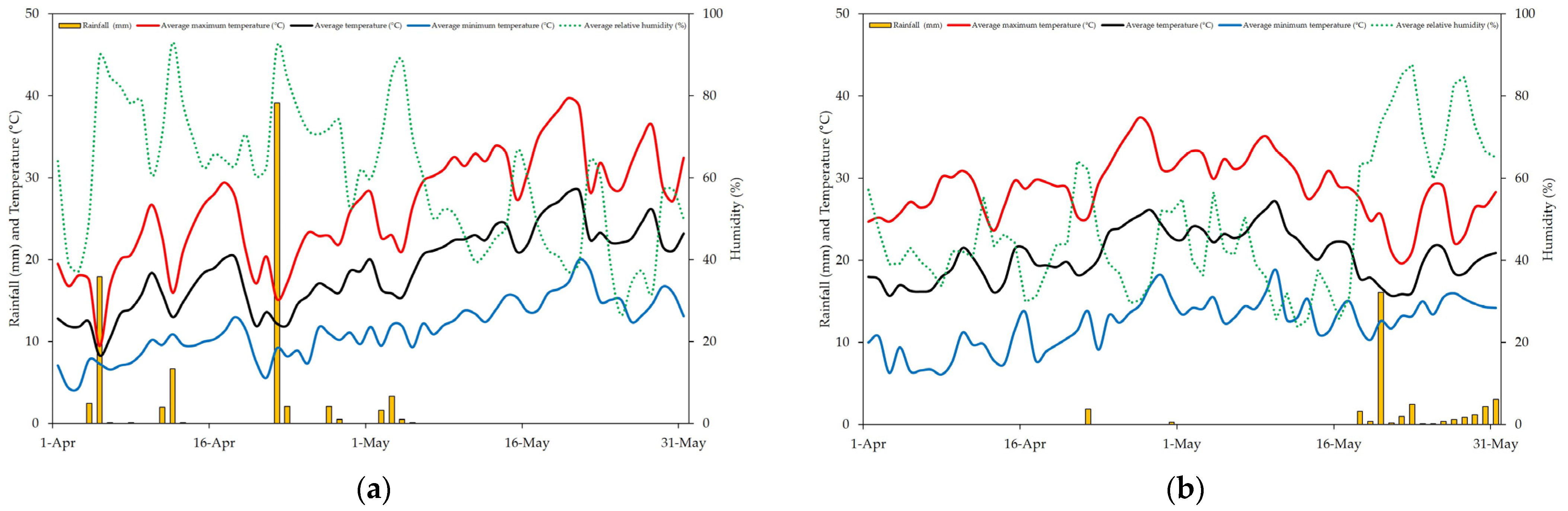
The average temperature at the experimental site during the bloom and fruit set periods (April and May) of 2022 was 18.8 °C with an average relative humidity of 60.3% and total rainfall reaching a significant volume of 78.7 mm in less than two months. The 2023 season was slightly warmer (an average of 20.5 °C) and drier (average relative humidity of 48.1%) with less rainfall totaling 32.6 mm. The weather conditions during the bloom and fruit set periods in 2022 and 2023 are shown in
Figure 1. Climactic data were retrieved from a weather station located nearby at the Campus of the University of Córdoba.
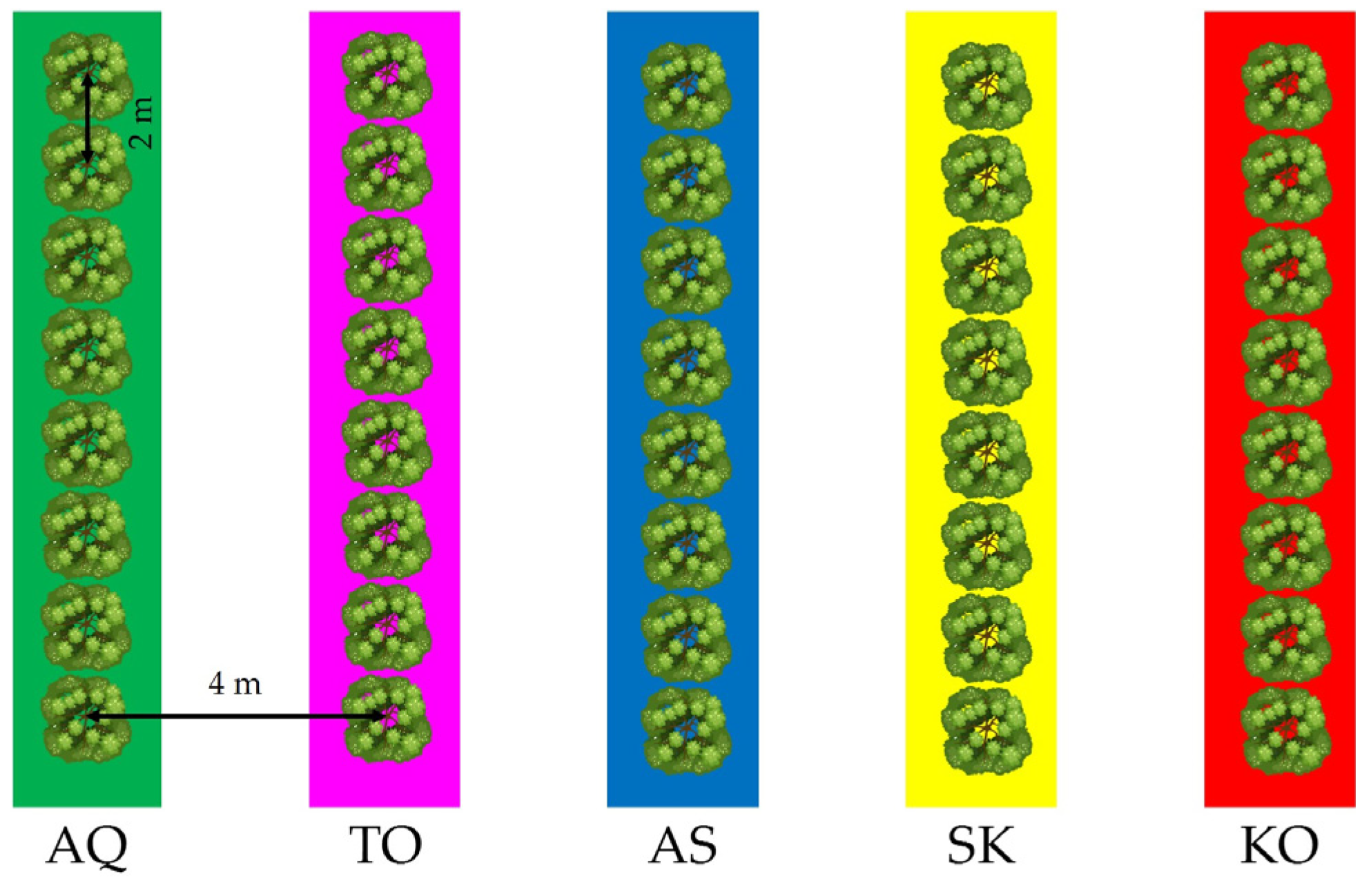
In the experiments, four adult trees of Arbosana were used as replications. The trees were 4 × 2 m spaced and trained as a hedgerow in a multivarietal orchard. Adjacent tree rows of the cultivars Arbequina, Sikitita, Koroneiki and Tosca were included in the orchard. The orchard layout showing the tree rows arrangement of the different cultivars is depicted in
Figure 2.
Arbequina is, so far, the most popular olive cultivar for high-density orchards based on its high and regular productivity, precocious yield and good olive oil quality. Arbequina accounts for around 65% of the area of the high-density plantations carried out all around the world (last estimations are near 100,000 hectares). Sikitita, on the contrary, is a new olive cultivar recently released from the UCO olive-breeding program, suitable for high-density orchards due to its very much reduced plant vigor. It presents the lowest alternate bearing index of the cultivars constituting the experimental orchard. Koroneiki is a Greek cultivar with excellent olive oil quality that is well adapted to semi-arid zones where is very productive, but it shows a higher tendency to alternate bearing. Koroneiki also exhibits higher vigor than the other cultivars tested. Finally, Tosca is the main Italian cultivar used for high-density orchards. It is also a low-vigor cultivar, maturing early in the season, allowing, therefore, harvesting before some other cultivars. In comparative trials carried out from 2013 to 2018 in the same experimental orchard, Tosca has shown lower productivity than Arbequina, Arbosana, Sikitita and Koroneiki [
16].
Four experimental trees of ‘Arbosana’ were chosen for their uniform and high level of flowering. In 2022, each of these trees received four pollination treatments: self-pollination, open pollination, cross-pollination with Arbequina pollen, and cross-pollination with Sikitita pollen. In the 2023 season, we added a new cross-pollination treatment using Koroneiki pollen.
The self-pollination treatment was achieved by bagging, before bloom, 1-year-old flowering shoots with hand-made tissue paper bags. These hand-made paper bags, although fragile in the event of rain, do not substantially modify the temperature and humidity conditions inside them. Cross-pollination was performed by applying fresh pollen collected from the selected pollen donor cultivars to the stigmas of the open flowers of Arbosana using a fine camel paintbrush. Cross-pollen was repeatedly applied to the open flowers every other day. After performing hand pollination, cross-pollinated shoots were re-bagged to avoid pollen entrance from other neighboring cultivars. Pollen grains used for pollination were repeatedly harvested from the neighboring trees on the same day of pollination. Pollinations were performed between 25 April and 1 May in 2022 and from 13 April to 22 April in 2023. Emasculation of the flowers was not performed, so these flowers received their own pollen plus the pollen from the selected pollinizer. Open-pollinated flowers were left unbagged. Open pollination in a multivarietal orchard represents the optimal pollination treatment due to the unrestricted and continuous pollen arrival from different sources and different pollen donor trees.
2.2. Initial and Final Fruit Measurements
To determine Arbosana response to these pollination treatments, we measured initial and final fruit set for each pollination treatment in four trees as replications. Fruit set was calculated as the number of fruit per inflorescence measured in ten shoots per tree in 2022 and in eight shoots per tree in 2023. The selected shoots bore ten inflorescences each. That uniform flowering level was achieved by thinning the excess inflorescences when necessary. The shoots were located all around the trees at observer height. The shoots were bagged (except open-pollination treatment) before bloom and received the corresponding pollination treatment every other day and at least three times as bloom progressed. After performing hand cross-pollination, the shoots were bagged again. Initial fruit set was calculated as the number of enlarged ovaries (fruitlets) per inflorescence two weeks after bloom. Final fruit set was estimated as the number of fruitlets per inflorescence seven weeks after bloom. With final fruit set data, we calculated the Index of Self-Incompatibility (ISI) by dividing self-pollination fruit set by open- (and cross-) pollination fruit set [
17].
2.3. Pollen–Pistil Interaction
For the study of the pollen–pistil interaction, samples of 20 flowers (occasionally less) of each pollination treatment were collected 1, 2, 4 and 8 days after pollination (dap) in 2022, and 2, 4 and 8 dap in 2023. Pollination of these flowers was carried out the day of anthesis and one day after. Anthesis date was ensured by removing the open flowers on a given day and all closed flowers the next day, so all remaining flowers opened between one given day and the next. Pollination treatments were applied as explained before for fruit set measurements. The sampled flowers were immediately fixed in a FAE solution made with formalin, glacial acetic acid and 70% ethanol in a 1:2:17 ratio by volume and sent to the lab. These samples were kept in vials in the fridge at 4 °C until the evaluation of pollen–pistil interaction under fluorescence microscopy. The parameters analyzed were pollen adhesion, pollen germination, pollen tube growth and fertilization. For processing the flowers, they were softened during 8 h with a solution of NaOH 1N, washed in running water, stained with aniline blue, and gently squashed before observation under fluorescence microscopy using a Labophot microscope (Nikon, Tokyo, Japan).
Pollen adhesion and germination was measured soon after anthesis (1, 2 and 4 dap in 2022, and 2 and 4 dap in 2023). Pollen adhesion was calculated by counting the number of pollen grains adhered to the stigma of the flowers. Pollen germination was estimated as the number of them showing a pollen tube of at least the diameter of the pollen grain. Pollen tube growth and fertilization were evaluated 2, 4 and 8 dap. Pollen tube growth was estimated on a scale from 0 to 3 as follows: 0 for no pollen tubes growing in the style, a value of 1 when 1 to 5 pollen tubes were present in the upper part of the style, a value of 2 when 5 to 25 pollen tubes were present in the style, and a value of 3 for more than 25 pollen tubes per flower growing toward the ovary. For the evaluation of fertilization, the four ovules of each flower were dissected from the ovary and observed under fluorescence microscopy. The presence of a pollen tube in the micropyle of the ovules, usually in only one, was considered proof of fertilization. This procedure was followed the same way in both seasons.
2.4. Seed-Paternity Analyses
Finally, to determine the pollen genotype achieving ovule fertilization, samples of fruit from the shoots used for fruit set analyses were randomly selected (when in excess) and collected soon before harvest to determine the paternity of their seeds. Based on the 2022 results, we decided to limit the analyses of seed paternity in 2023 samples to open-, self- and to the new cross-pollination treatment using Koroneiki pollen. Seed-paternity analyses were carried out following the procedure described by Seifi et al. [
18], slightly modified. In addition to the genotyping of the seed embryos, fresh olive leaves were sampled from the five different genotypes (Tosca, Sikitita, Koroneiki, Arbequina and Arbosana) present in the experimental orchard and acting as potential pollen donors. Genomic DNA was extracted from the embryos and leaves using a NucleoSpin™ Plant II kit (Macherey-Nagel, Hoerdt, France) according to the manufacturer’s instructions. Eight polymorphic SSR markers were used for genotyping: ssrOeUA-DCA-(3, 5, 15, 18) [
19], GAPU-(71B, 101) [
20], and EMO-(3, 90) [
21]. PCR reaction was performed in a final volume of 12.5 µL containing 1x supplied AllTaq PCR buffer, 1x supplied Q-Solution, 2 mM MgCl2, dNTP mix (0.2 mM of each dNTP), 1.25 U of AllTaq DNA polymerase (Qiagen, Hilden, Germany), 0.2 mM reverse locus specific primer, M13(-21) tail labeled with fluorescent dye 6-FAM, VIC, NED or PET (Applied Biosystems, Foster City, CA, USA), 0.1 mM elongated forward primer, and 4 µL of undiluted template DNA for olive embryos or 40 ng of template DNA for potential pollen donors. The amplification was performed on a SimpliAmp™ Thermal Cycler (Thermo Fisher Scientific, Waltham, MA, USA) according to the two-step touch-down amplification profile [
22]. One µL of PCR product mix, including amplicons with four different fluorescent dyes, was added to 10.7 µL Hi-Di™ Formamide (Thermo Fisher Scientific, Waltham, MA, USA) and GeneScan™ 500 LIZ size standard (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed on a SeqStudio™ Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). Electropherograms were scored with GeneMapper version 5 software (Thermo Fisher Scientific, Waltham, MA, USA).
In the first step of the analysis, the five potential pollen donor cultivars cited above were genotyped to obtain their reference profiles and then all embryos were genotyped. In a second step, paternity analysis was performed for all embryos produced from open pollination and for those from self-pollination and cross-pollination treatments in which the presumed father alleles were not detected. Then, the potential pollen donors were identified using paternity analyses using CERVUS 3.0 software (
http://www.fieldgenetics.com; accessed on 20 June 2024) [
23]. Each CERVUS run consisted of an allele frequency analysis followed by a simulation in which the number of potential fathers was set to five (the cultivars equally represented in the orchard), while the proportion of candidate fathers sampled was set to 80%. The proportion of typed loci was set to the percentage estimated by the allele frequency analysis. A minimum of four typed loci were required for the progeny to be analyzed for paternity, and the number of simulated progenies was set to 100,000. The genotyping error in the simulation and in the assignment of the most likely pollen donor was set at 1%, as possible errors can occur during the allele-calling phase. Finally, the genotypes of the embryos and the available genotypes for the potential pollen donor cultivars were used to calculate the natural logarithm of the likelihood ratio (LOD) scores. These ratios compare the probability that an individual is the parent of a given progeny divided by the probability that these two individuals are unrelated. The genotype with the highest LOD score above the threshold (determined based on the simulation) for a given parent–offspring pair is considered the most likely pollen donor. If the pollen parent was not among the cultivars present in the experimental orchard, it was assumed that the pollen originated from olive cultivars grown outside the orchard.
2.5. Statistical Analyses
Fruit set data were analyzed by analysis of variance and means were compared by Tukey’s test (p < 0.05) using Statistix 8.0 (Analytical Software, Tallahassee, FL, USA). Fertilization levels in the different treatments were compared by Chi-square tests using the same software.
4. Discussion
Arbosana olive exhibited a clear self-incompatible response, with ISI values ranging from 0.15, compared with optimum open pollination, to 0.30 compared to cross-pollination with Arbequina pollen. This means that Arbosana had a substantial reduction in its final fruit set (between three and six times) under self-pollination compared to cross- and/or open-pollination treatments. The differences among treatments in the initial fruit set, a parameter that measures pollinizer efficiency, were in fact substantially more pronounced than the differences found in the final fruit set across both years. This change is due to the markedly more intense fruitlet competition and subsequent abscission (“June drop”) when the initial fruit set is heavier. Therefore, the differences among treatments were attenuated when the fruit population was definitively established seven weeks after bloom. The same reason applies to the significant differences in the initial fruit set between open- and cross-pollination treatments in 2022 that were not statistically maintained at the final fruit set counting. Therefore, although open pollination may increase the initial fruit set with respect to cross-pollination treatments, small differences might not reach statistical significance at the final fruit set and harvest. Some differences emerged also between years with a better, higher, fruit set the second season, despite the weather being warmer and drier. Pollen adhesion and fertilization levels were also generally higher in the second year. This circumstance was likely related to the frequent rainfall during pollination in 2022; rain removes airborne pollen from the atmosphere, as the lower pollen adhesion measured in 2022 suggests. Despite the seasonal differences in fruit set under self-pollination, the self-incompatibility response was still very strong in 2023, as the ISI values show.
The self-incompatibility reaction in Arbosana is characterized by a reduced pollen tube growth in the stigma and style, rejection that leads to lower levels of ovule fertilization. While pollen adhesion on the stigma was also diminished under self-pollination, we do not interpret these differences as due to differences in stigma receptivity among treatments or due to an early rejection of self-pollen grains. The observed differences in pollen adhesion primarily stem from the procedure we used for self-pollination, specifically the constrains imposed by bagging, where the wind agitation of shoots was the sole factor facilitating pollen adhesion in self-pollinated flowers. In contrast, no bagging was performed in open pollination, while in cross-pollination treatments, a copious amount of fresh pollen was applied using a paintbrush. In any case, we do not consider the process of pollen adhesion as critical for the reduced fertilization observed under self-pollination. The value of pollen adhesion data lies in detecting that pollen adhesion was not limiting under self-pollination. Instead, the most significant parameter was the growth of the pollen tube beyond the first layers of stigmatic cells and in the style of the flowers, as previously reported by several authors [
11,
24,
25,
26].
Seed-paternity analyses underscore the strong rejection that Arbosana expresses toward its own pollen. Indeed, the uncontroversial paternity analyses indicate that some fruit obtained from bagged shoots were due to cross-fertilization resulting from pollen contamination. Thus, the self-incompatibility response in Arbosana was, in fact, stronger than the values suggested by the ISI values alone. This raises concerns about the procedures used to isolate the flowers and underscores the pervasive nature of olive pollen. High levels of pollen contamination have been previously reported in bagged flowers of olive and other wind-pollinated crops. For instance, Díaz et al. [
8] did not find any seeds from self-fertilization in bagged branches of Picual and Arbequina nor in monovarietal orchards, leading them to conclude the strong self-incompatible condition of these cultivars. Similarly, De la Rosa et al. [
27] found high levels of pollen contamination in bagged flowers intended for self-pollination and recommended installing the pollination bags in advance of bloom to prevent cross-pollen contamination in olive-breeding programs. This precaution was also advocated by Marchese et al. [
7], who found no embryos fathered by Arbequina pollen in self-pollinated flowers due to unexpected contamination with foreign airborne pollen, which was likely present on the branches before bagging. Likewise, Shemer et al. [
25] cautioned that viable pollen of earlier blooming cultivars might be present on the leaves of the maternal olive genotype and can be a possible source of cross-pollen contamination that can be mitigated by bagging shoots well in advance of bloom. We stress that early removal of the pollination bags is also risky and should be delayed as long as reasonably possible due to the prolonged stigma receptivity in olive [
28], where even one single cross-pollen arriving, although late, to the stigma can achieve cross-fertilization if self-incompatibility response is, as shown, robust. In contrast to the high level of pollen contamination observed under self-pollination, low levels of pollen contamination occurred in cross-pollination treatments; this is contamination that we interpret as possible when we re-opened the bags to perform hand pollinations.
Thus, our results conclusively establish Arbosana olive as self-incompatible, and therefore, it cannot be planted alone in large high-density monovarietal orchards. On the contrary, we recommend Arbosana to be inter-planted with inter-compatible cultivars to achieve optimal production in high-density orchards. Thus, identifying suitable pollinizers for Arbosana becomes imperative. In this regard, all three cultivars tested as pollinizers gave good fruit set results, confirmed by the high levels of flower fertilization, and this suggests that all can be chosen as pollen donors’ trees for Arbosana. Particularly noteworthy is the effectiveness of Sikitita as the most successful pollen donor under open-pollination conditions, closely followed by Arbequina in both seasons. It is worth noting, however, that one Sikitita tree row was next to the experimental trees of Arbosana (as Tosca too, however), although in opposite wind direction of the common SW wind at the experimental site in those dates (
Figure 2), while Arbequina (and Koroneiki) trees were slightly more distant but in the prevalent wind direction during bloom. Although long-distance transport of olive pollen has been documented [
29,
30,
31], our study indicates that all embryos were fathered by the cultivars present in the experimental orchard at a distance between 4 and 8 m.
Both Sikitita and Arbequina emerge as highly effective pollen donors for Arbosana, suggesting their suitability as pollinizers of this cultivar. The same approach was taken for Shemer et al. [
25] to recommend Picual as the best pollinizer for the cultivar Barnea. Similarly, our previous results found that the cultivars Drobnica and Lastovka were major pollen donors for the self-incompatible cultivar Oblica [
24]. Seed-paternity analyses have also been instrumental in determining the best pollinizers for Kalamata, as demonstrated by Seifi et al. [
18], who later extended their research to include suitable pollinizers for Barnea, Corregiola, Koroneiki and Mission [
31]. However, when selecting the best pollinizers, additional criteria must be considered. Beyond the demonstrated compatibility between Arbosana and the three pollen donors under study, ideal pollinizers must bloom synchronously with the main cultivar. In this regard, in an adjacent block where the same cultivars were present, bloom phenology was determined for both seasons [
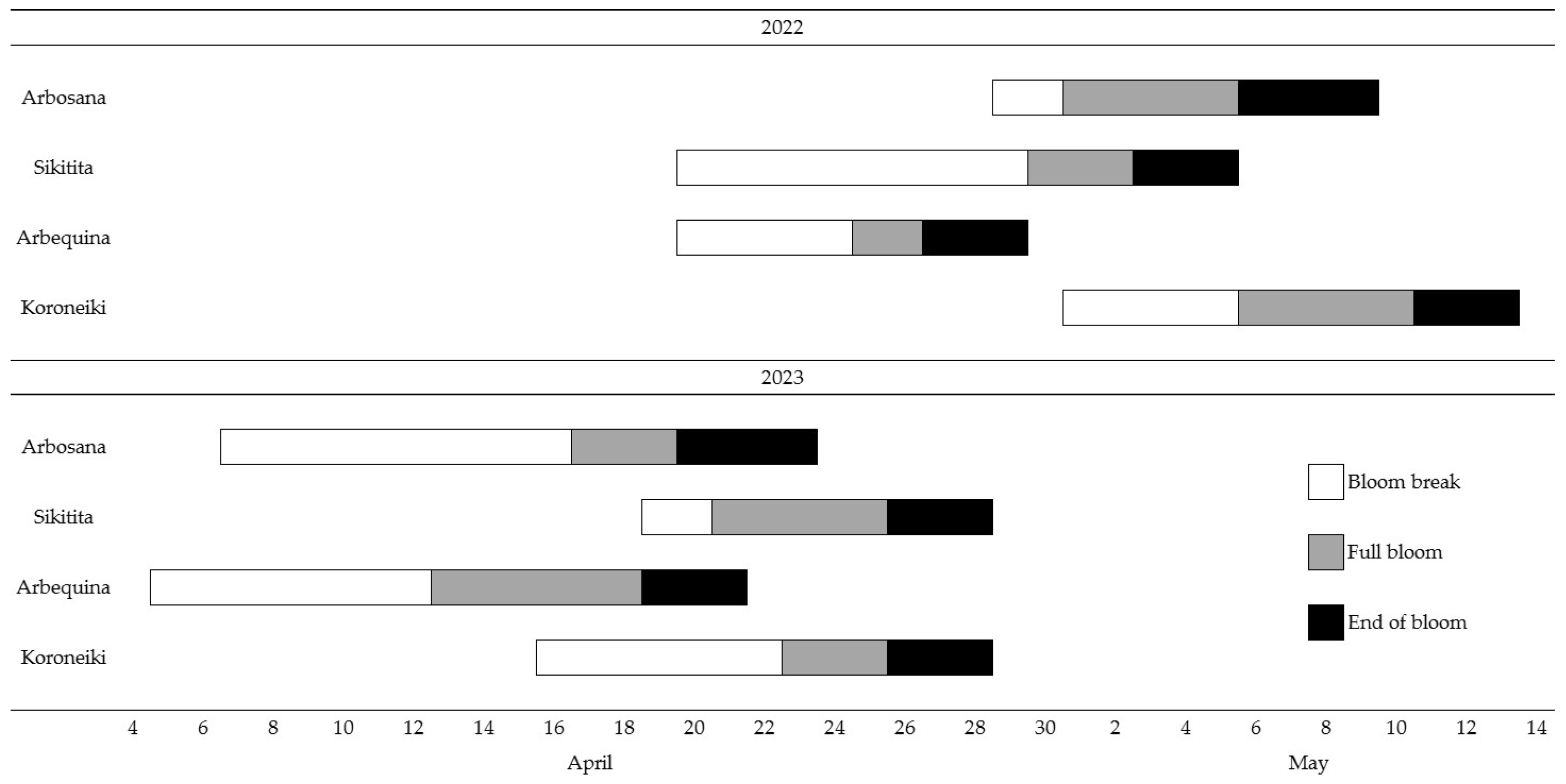
32].
Figure 4 shows that some bloom overlap occurred for all cultivars, although in 2022, Sikitita showed greater overlap with Arbosana full bloom, while in 2023, Arbequina exhibited a slightly greater overlap. However, on-site assessment for extended periods is crucial to establish bloom phenology overlaps accurately. Additional criteria for selecting pollinizers for Arbosana are (i) selecting low-vigor cultivars, which makes chosen pollinizers suitable for high-density orchards, as all tested cultivars are, (ii) producing fruits having the same purpose, in this case oil production, and (iii) being regular producers, i.e., not exhibiting strong alternate bearing, in order to ensure a reliable supply of cross-pollen.