Abstract
Basil is a culinary herb in high demand all year round, either fresh, dried, or frozen. Field basil crops are commercially predominant, while greenhouse crops can cover the demand for fresh basil during the off-season. The leaf aspect of basil has great importance for the food industry, and a great diversity of genotypes has been created. The aim of this research was to conduct a comparative characterization of 12 basil genotypes (green and purple leaf) that present interest for breeding programs from a national germplasm collection. The study examines the accumulation of photosynthetic pigments in basil cultivated in field versus greenhouse conditions as indicators of plant performance and herb quality from the perspective of ensuring fresh plant material all year round. The experimental median indicated that photosynthetic leaf pigments accumulated in higher concentrations in the field relative to the greenhouse conditions, in descending order: β-carotene, lutein, chlorophyll a, and chlorophyll b. The trend was not as consistent for chlorophyll b, since four out of twelve genotypes presented higher levels in the greenhouse than in the field, while the overall values were not much lower in the greenhouse than the field (16.82%). All genotypes accumulated much higher carotenoid contents in field conditions relative to greenhouse conditions (>200%) and could also provide better nutritional advantages given their demonstrated health benefits. The differences in photosynthetic leaf pigments have both nutritional (the carotenoids), shelf-life, and processing relevance (chlorophylls) and serve as quality markers.
1. Introduction
Culinary herbs are in high demand all year round. There is a strong tradition of using herbs such as basil in seasoning food products and fresh dishes, particularly in Europe and Asia [1]. According to Codex Alimentarius, the world market for basil was evaluated at over 3 million USD (thousands) and is increasing [2]. Out of several species of Ocimum distinguished by morphology and flavor, mainly two have numerous cultivars: O. basilicum L. and O. sanctum L. Basil plants are used fresh, frozen, or dried. By drying, some of the top notes are lost, and basil becomes less aromatic compared to when fresh or frozen [3].
The aspect of fresh herb material is important for restauranteurs, and different colors are in demand for decorating foods [3]. The health-promoting properties and leaf aspects of basil plants, in particular, the color, are important driving choices for customers. Basil is used in functional syrup [4], health-promoting drink blends [5], pasta, pesto, sauces, salads, stews, pickles, vinegar, aromatic oil, and “bouquet garni”, while some cultivars are specifically used for emblematic dishes. Medicinal and other uses are also documented [3]. In addition, basil is a melliferous crop [6]. The constant demand for fresh plant material in the food industry raises the question of whether cultivation conditions significantly influence the parameters of fresh basil herbs.
Currently predominant in the commercial segment are field-grown crops, which are more advantageous economically [7]. However, given the demand for fresh basil all year round, in a controlled environment, crops can supply fresh basil outside the field-growing season [8,9]. Together, these cover most of the market’s needs. The pots of basil are a retail commodity in demand [9]. The potted plants can be destined for kitchen settings, for ornamental purposes, or for transplanting in the garden or field. Hence, it represents the starter quality of the sold material. For field crops, herbs are usually harvested for processing at the full flowering growth stage 65 on the BBCH (Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie) scale [10]. This stage is insightful for the culminant assimilation potential that plants can reach. These two technological methods (potted plants and field crops at flowering) are important from a practical point of view. Due to this, it is proposed that they are highly relevant for a comparative study.
The high diversity of basil phenotypes can be distinguished based on descriptive characteristics that are standardized by the UPOV (International Union for the Protection of New Varieties of Plants), which refer mainly to leaf blades in terms of form, size, margins, intensity of coloration, glossiness, and blistering, in addition to flower characteristics and plant habitus [11]. Among the dozens of genotypes of basil used today, there are differences in morphology, genetics, and chemical composition due to centuries of crossing and selection [12,13].
The commercial crops are ensured optimal conditions, which are important for the quality of the harvested vegetal material, which further impacts marketability. The leaf pigments of basil are important parameters tracked in relation to shelf life [14] and processing [15]. Yet, the literature provides surprisingly few studies on such qualitative parameters of crops comparatively under routine, commercial-like conditions, which creates a gap of knowledge that this work attempts to bring to attention.
The aim of this work was to provide a comparative characterization of 12 basil genotypes from the national germplasm collection that present interest for breeding programs. The genotypes chosen for this study include some of the most widely used internationally and a few autochthonous cultivars and lines (both green and purple). The analysis was performed in the same phenophase (full flowering, BBCH 65). The objective of the study was the identification of photosynthetic leaf pigment variation across genotypes in greenhouse versus field conditions.
2. Materials and Methods
2.1. Biologic Material
The seeds were provided by the Plant Genetic Resources Bank for Vegetable, Floriculture, Aromatic, and Medicinal Plants from Buzău, Romania. The voucher specimens were deposited in the Scientific Herbarium of USAMV Cluj-Napoca; voucher codes are provided in Table 1.

Table 1.
The Ocimum genotypes and experimental variants.
The genotypes chosen for this study include some of the most widely used internationally, used as a reference in comparison to valuable autochthonous cultivars and lines (green and purple) of importance for breeding [17]. The existence of both purple and green genotypes makes basil a good model plant for the study of photosynthetic apparatus adaptation under contrasting conditions or factors, which can be conveniently put into evidence through measurements of photosynthetic pigments. The studied genotypes are presented in Figure 1.
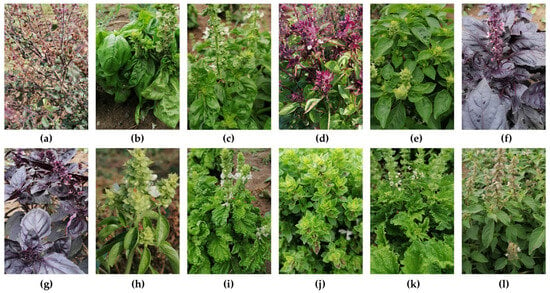
Figure 1.
Basil genotypes: (a) O. basilicum ‘Aromă de cuișoare’; (b) O. basilicum ‘Aromă de trandafir’; (c) O. basilicum ‘Aromat de Buzău’; (d) O. basilicum ‘Ierusalim’; (e) O. basilicum ‘L7’; (f) O. basilicum ‘L10’; (g) O. basilicum ‘L12’; (h) O. basilicum ‘L50’; (i) O. basilicum f. bulatum; (j) O. basilicum f. minimum; (k) O. basilicum var. crispum; and (l) O. citriodorum ‘Macedon’; (original).
2.2. Location and Conditions
The experiment was located in a controlled climate in the greenhouse (46°45′27.3″ N 23°32′38.5″ E) and in the field conditions of the Agro-Botanical Garden (46°45′35.0″ N 23°34′26.8″ E) of the University of Agricultural Sciences and Veterinary Medicine from Cluj-Napoca, Romania. The climate is temperate-continental. In the experimental year (2022), the average temperature of 10.5 °C was within the average values of the previous decade, while the annual sum of precipitation in 2022 (454.07 mm) was 23.65% lower relative to the average of the previous decade [18].
The cultivation technology followed standard methods. Basil is usually propagated via seedlings. Direct sowing is less suitable due to small seeds, which cause poor emergence in field conditions. Hence, the routine technological approach is to obtain seedlings, which are transplanted in the field, resulting in higher crop success [19].
The sowing was conducted on 17 March in the greenhouse in peat substrate. The seeds germinated within 7 days. The seedlings destined to be planted in the field were transferred in plug trays, while those destined for cultivation in the greenhouse were transferred in pots (Ø 15 cm). In the greenhouse, 10 plants from each genotype were maintained. The temperature in the greenhouse was maintained at 25 °C, and from the month of May, the greenhouse was not heated. The greenhouse has an automatic system that ensures proper ventilation and airing.
Some of the seedlings were transferred to the experimental field in May 2022, by planting seedlings at distances between rows of 50 cm and between plants in a row of 25 cm. The surface of the experimental field was 180 m2. Each genotype was planted in three rows. The soil of the experimental field had an alkaline pH, with a good NPK supply and humus content [20].
The harvest was conducted at full flowering (BBCH 65) both from the greenhouse crop and from the field crop for comparative analysis.
2.3. Extraction of Chlorophyll
Fresh basil genotype samples (0.2 g) were homogenized, and 90% acetone was used as an extraction solvent in a round-bottom flask using a magnetic stirrer. After 2 h of extraction, the solution was filtered, and the extraction procedure was repeated until the residue became colorless. Absorbance was read at 645 and 663 nm using the V-530 UV-VIS Spectrophotometer (Jasco, Eason, MD, USA). The formulas for dosing chlorophyll a and chlorophyll b were as follows [21]:
Chlorophyll a (mg/g) = (11.75 × A663 − 2.35 × A645) × V/g
Chlorophyll b (mg/g) = (18.61 × A645 − 3.96 × A663) × V/g
Chlorophyll b (mg/g) = (18.61 × A645 − 3.96 × A663) × V/g
In Formula (1), A645 and A663 represent the optical density at a specific wavelength, V represents the volume of the extract (mL), and g represents the samples’ weights (mg).
2.4. Extraction of Carotenoids
The extraction of carotenoids from 1 g of the fresh basil genotype sample was conducted using a mixture of methanol, ethyl acetate, and petroleum ether (1:1:1, v/v/v) [22]. The extraction was repeated until the residue became colorless. The organic phase was separated and evaporated to dryness using a rotary evaporator. The residue was dissolved in diethyl ether (10 mL) and treated with an equal volume of potassium hydroxide (30% methanolic solution) for saponification (for 2 h). The samples were transferred into a separation funnel and washed with saline solution until the aqueous phase reached a neutral pH. The organic phase was dried over anhydrous sodium sulfate and evaporated under vacuum. For the quantification of individual carotenoids, the extracts were diluted with ethyl acetate, filtered through a membrane filter (PTFE, 0.45 µm pore size, Millipore, Darmstadt, Germany), and analyzed on a Shimadzu HPLC system equipped with an LC-20 AT binary pump (Prominence), a degasser DGU-20 A3 (Prominence), and a photodiode array detector SPD-M20 (HPLC-PDA) (Shimadzu U.S.A. Manufacturing, Inc, Canby, OR, USA). The carotenoids were separated on a YMC C30 column (24 cm × 4.6 mm, 5 µm particle size) using a mixture of two solvents as the mobile phase: solvent A: methanol/tert-butyl methyl ether/water (81:15:4, v/v/v) and solvent B: tert–butyl methyl ether/methanol/water (90:7:3, v/v/v). The flow rate used was 0.8 mL/min. Gradient elution started with 1% B at 0 min and increased to 100% B by 160 min. The identification of individual carotenoids was made based on UV-VIS spectra with those of lutein and β-carotene used as standards.
2.5. Statistical Analysis of Leaf Pigments
A Shapiro–Wilk test showed that the normality assumption was not met for the chlorophyll and carotenoid datasets. Further non-parametric tests were applied. A Kruskal–Wallis test was followed by a post hoc test (Dunn), which explored the significance between variants. Results are expressed as median ± median absolute deviation. The Kendall tau correlation was used for studying the relationship between parameters. The non-parametric and correlation tests were performed with PAST 4.0 (NHM, Oslo, Norway). The principal component analysis (PCA) was obtained with Origin software (OriginLab, Northampton, MA, USA).
3. Results
The Kruskal–Wallis test indicated that differences between the medians of the experimental variants were significant (Table 2). The Dunn test further indicated the significance of the differences between variants for each of the leaf pigments analyzed.

Table 2.
Influence of experimental variant on photosynthetic leaf pigments.
3.1. Chlorophylls
The experimental median of chlorophyll a content in greenhouse conditions was 0.52 mg/g, while in field conditions, there was an increase of 110.40% (1.10 mg/g) relative to the greenhouse median. The same trend of higher levels in the field compared to the greenhouse was registered in all 12 studied genotypes, with the exception of the purple leaf, O. basilicum ‘L10’. Among the genotypes in the greenhouse, only one autochthonous cultivar, O. basilicum ‘Aromat de Buzau’, surpassed several others significantly (O. basilicum ‘Aromă de trandafir’, O. basilicum ‘Ierusalim’, and O. basilicum f. bulatum) (Figure 2).
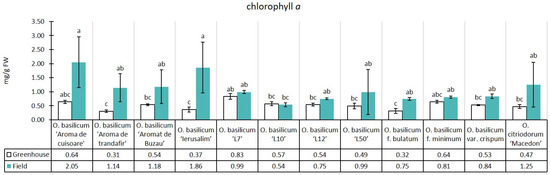
Figure 2.
Chlorophyll a content in greenhouse and open-field basil plants; median ± median absolute deviation (mg/g Fresh Weight). Similar letters indicate that differences between variants are not significant (Dunn test).
The experimental median of chlorophyll b content in greenhouse conditions was 0.28 mg/g, while in the field conditions, there was an increase of 16.82% (0.32 mg/g) relative to the greenhouse median. This trend was not consistent for all genotypes studied. Some genotypes registered a decrease in this pigment in the field conditions compared to the greenhouse, namely: O. basilicum ‘L7’, O. basilicum ‘L10’, and O. basilicum var. crispum; for the genotypes in the greenhouse conditions, the same three had the highest content of these pigments. Among the genotypes in the field conditions, O. basilicum ‘Aromă de cuișoare’ and O. basilicum ‘Ierusalim’ registered the highest median (Figure 3).
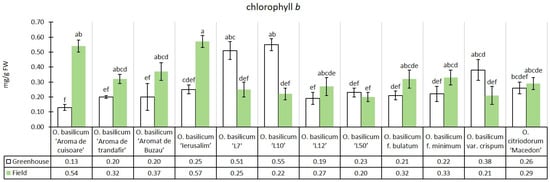
Figure 3.
Chlorophyll b content in greenhouse and open-field basil plants; median ± median absolute deviation (mg/g Fresh Weight). Similar letters indicate that differences between variants are not significant (Dunn test).
The experimental median of total chlorophyll content in the greenhouse conditions was 0.80 mg/g, while in the field conditions, there was an increase of 77.87% (1.42 mg/g) relative to the greenhouse median. With the exception of two genotypes, this trend was found in all genotypes. Only the lines O. basilicum ‘L7’ and O. basilicum ‘L10’ presented lower total chlorophyll content in the field. Among the genotypes in the field conditions, it is notable that the same cultivars registered the highest chlorophyll a and b content, hence also total chlorophyll (Figure 4).
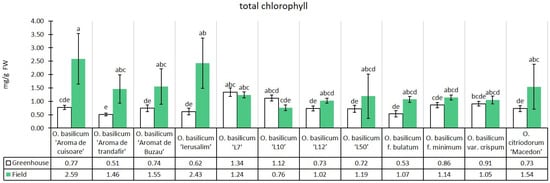
Figure 4.
Total chlorophyll content in greenhouse and open-field basil plants; median ± median absolute deviation (mg/g Fresh Weight). Similar letters indicate that differences between variants are not significant (Dunn test).
3.2. Carotenoids
The experimental median of lutein content in the greenhouse conditions was 0.04 mg/g, while in the field conditions, there was an increase of 259.02% (0.13 mg/g) relative to the greenhouse median. All genotypes had higher lutein content in field conditions than in greenhouse conditions. Both in greenhouse and field conditions, the cultivar O. basilicum ‘Aromă de cuișoare’ had a higher median content (Figure 5).
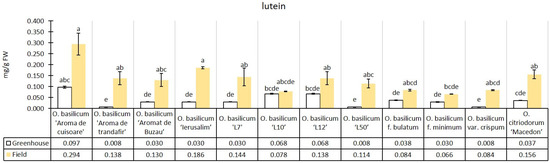
Figure 5.
Lutein content in greenhouse and open-field basil plants; median ± median absolute deviation (mg/g Fresh Weight). Similar letters indicate that differences between variants are not significant (Dunn test).
The experimental median of β-carotene content in the greenhouse conditions was 0.01 mg/g, while in the field conditions, there was an increase of 619.19% (0.09 mg/g) relative to the greenhouse median. This was the highest relative content increase in the field compared to the greenhouse out of the pigments analyzed. All genotypes had higher β-carotene content in field conditions than in greenhouse conditions. Both in greenhouse and field conditions, the cultivar O. basilicum ‘Aromă de cuișoare’ had higher content compared to the other 11 genotypes (Figure 6).
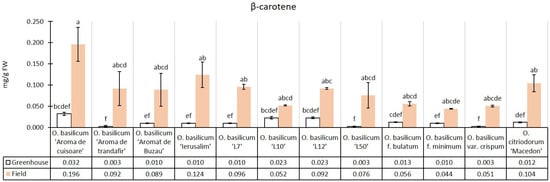
Figure 6.
The β-carotene content in greenhouse and open-field basil plants; median ± median absolute deviation (mg/g Fresh Weight). Similar letters indicate that differences between variants are not significant (Dunn test).
The experimental median of the total carotenoids content in the greenhouse conditions was 0.05 mg/g, while in the field conditions, there was an increase of 348.76% (0.22 mg/g), relative to the greenhouse median. All genotypes had higher total carotenoids content in the field conditions than in the greenhouse conditions. Both in greenhouse and field conditions, the cultivar O. basilicum ‘Aromă de cuișoare’ had a higher total carotenoids median. The two lines with purple leaves presented similar carotenoids medians (both lutein and β-carotene) in greenhouse conditions but not in the field, where O. basilicum ‘L12’ surpassed O. basilicum ‘L10’, although not significantly. By comparison, in terms of chlorophylls, the two purple lines were more similar in the field than the greenhouse, regarding medians (Figure 7).
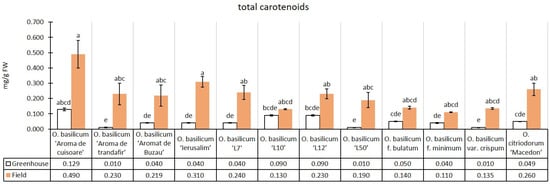
Figure 7.
Total carotenoids content in greenhouse and open-field basil plants; median ± median absolute deviation (mg/g Fresh Weight). Similar letters indicate that differences between variants are not significant (Dunn test).
3.3. Relationship between Photosynthetic Leaf Pigments
The correlation analysis revealed a significant positive monotonic relationship between both chlorophyll a, chlorophyll b, and total chlorophyll content for plants in the greenhouse conditions (Figure 8a) and field conditions (Figure 8b).
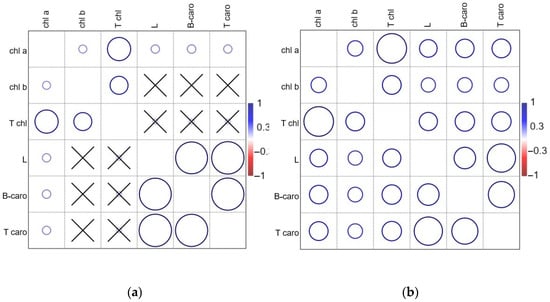
Figure 8.
Kendall tau coefficients (τ) for the plants in the greenhouse (a) and plants in the field (b). The size of the bubble is proportional to the coefficient value; crossed “×” is not significant at α 0.05; chl a—chlorophyll a; chl b—chlorophyll b; L—lutein; B-caro—β-carotene; T chl—total chlorophyll; T caro—total carotenoids.
There was a significant positive monotonic relationship between lutein, β-carotene and total carotenoids in both the greenhouse and the field (Figure 8a,b). By comparison, in field conditions, plants displayed significant positive monotonic relationships between total chlorophylls and carotenoids: lutein (τ = 0.55), β-carotene (τ = 0.59), and total carotenoids (τ = 0.59), which were not significant for plants in the greenhouse (Figure 8a,b).
3.4. Principal Component Analysis of Photosynthetic Pigments
The principal components 1 and 2 from the biplot (Figure 9) together explain 85.80% of the variance registered by the photosynthetic leaf pigment parameters studied. The extracted eigenvectors showed that total carotenoids had the highest coefficient for PC1 (0.58), while the ratio of total chlorophyll to total carotenoids had the highest coefficient for PC2 (0.66), suggesting a stronger response or greater sensitivity of these parameters to the experimental variables. The negative correlation between the ratio of chlorophyll a to chlorophyll b and the ratio of total chlorophyll to total carotenoids is also evident by the angle between these vectors on the PCA projection (Figure 9). The experimental variants cluster distinctively in two groups. The greenhouse variants cluster on the vector of the total chlorophyll to total carotenoid ratio, while the field variants cluster on the total carotenoids vector.
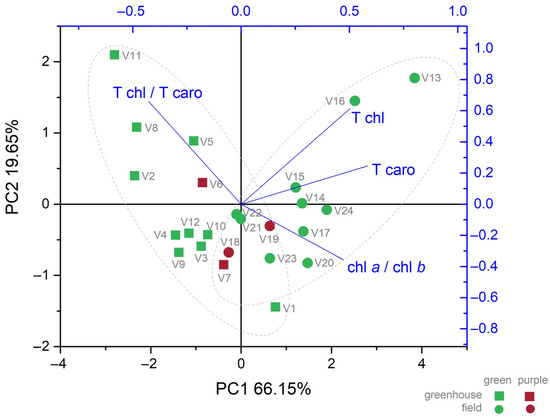
Figure 9.
Biplot of principal components for photosynthetic leaf pigments of twelve basil genotypes in greenhouse (square points) and field (round points); T chl—total chlorophyll; T caro—total carotenoids; chl a/chl b—chlorophyll a to chlorophyll b ratio; T chl/T caro—total chlorophyll to total carotenoids ratio.
4. Discussion
This work addresses a practical aspect by comparatively assessing photosynthetic leaf pigments, which are frequently associated with plant health, the marketability, attractiveness, and health-promoting properties of basil in the field and greenhouse. The purpose was to clarify some aspects regarding the differences in the accumulation of these pigments resulting from greenhouse and field cultivation conditions. The large differences between sites (greenhouse and field) could depend partially on the site’s microclimatic conditions and the water relations in plants.
Leaf pigments have basic functions for assimilation and photoprotection, acting as indicators of adaptability [23]. Due to the fundamental importance of these pigments in plant performance, understanding their variation in crop settings could help optimize their production systems. Because diversity and genotypic variability in basil germplasm collections are important in supporting breeding efforts [24,25], comparative screening of relevant genotypes is of great interest. Findings from this study suggest that, overall, chlorophylls and carotenoids accumulated in higher concentrations in basil leaves in the field compared to the greenhouse conditions, with few exceptions. These findings are in consensus with a previous study that compared these parameters in eight basil genotypes in field versus greenhouse conditions [26]. The cited study showed that average chlorophyll a content was higher in field conditions (1.495 mg/g FW) and lower in the greenhouse (0.938 mg/g FW); the chlorophyll b content also reached higher levels in the field (0.294 mg/g FW) than in the greenhouse (0.191 mg/g FW). The average β-carotene was 0.0601 mg/g FW in the field and 0.0442 mg/g FW in the greenhouse; the lutein content was 0.0664 mg/g FW in field conditions and 0.0499 mg/g FW in the greenhouse. However, there was one exception to this trend; ‘Osmin Purple’ presented higher levels in greenhouse conditions than in field conditions [26]. Compared to these average experimental values, the experimental medians reported in this study are in close range, even though they were conducted in different geographical regions. The median chlorophyll b in the field and β-carotene in the field and greenhouse were higher than the average values reported under similar conditions by Kopsell et al. [26]. Contrary to our findings and the study cited above, another comparative study indicated that total carotenoids had higher values in the greenhouse conditions compared to the field conditions for two cultivars: purple (Ocimum basilicum ‘Red Rubin’) and green basil (Ocimum basilicum var. minimumum ‘Greek’) [27].
The accumulation pattern of photosynthetic pigments in green versus purple genotypes is also relevant. In this sense, a comparison of the adaptation of green and purple basil genotypes (Ocimum basilicum, O. basilicum × O. americanum, and O. basilicum ‘Rubin’), showed that the carotenoid content between these did not differ significantly, yet a different dynamic was noticed with exposure to intense light: carotenoids increased in the green genotypes and decreased in the purple one [28].
Germplasm collections are important genetic repositories that provide the basis for plant breeding work, and comparative screening is insightful for defining breeding objectives. A comparison of 21 microgreen basil genotypes from an Italian and Iranian collection showed variability in chlorophyll a content between 0.27 and 0.93 mg/g FW, chlorophyll b content between 0.08 and 0.24 mg/g FW, total chlorophyll content between 0.35 and 1.18 mg/g FW, and carotenoid content between 0.05 and 0.28 mg/g FW. The carotenoid and total chlorophyll content were positively significantly correlated with the highest antioxidant potential composite index [29], which is further associated with desired health-promoting properties. Compared to these values, the greenhouse basil values from this study were in relatable ranges regarding the accumulation of photosynthetic pigments in greenhouse conditions. However, considering the different phenophases of sampling, it could be explained that some values were higher in the study reported here.
A study of the relationship between the photosynthetic pigments of basil plants showed a stronger relationship between photosynthetic pigments in greenhouse conditions than in the field [26]. Compared to these results, in our study, the relationship between photosynthetic chlorophyll pigments was stronger in the field conditions. However, the relationship between carotenoids was stronger in the greenhouse, as indicated by the coefficient values.
Chlorophyll a and b are the fundamental pigments of the photosynthetic apparatus that gives green plants their color. Chlorophyll b is synthesized from chlorophyll a and can be recovered back to chlorophyll a through a process called the chlorophyll cycle [30]. Chlorophyll a has the fundamental role of light harvesting and converting the energy of photons into chemical energy [31]. Chlorophyll b plays a role in light harvesting under low light conditions and photoprotection under intense light while also acting as a key factor in ontogenetic signaling [32]. Chlorophyll a is about 2–3 times more abundant than b. The chlorophyll levels are measured to track plant responses to environmental conditions and have been reliably used to understand the mechanisms of plant stress [33].
Carotenoids are essential for the life of plants, and there are over 800 known carotenoids [34]. In plant tissues, they absorb light in the blue region (280–550 nm), which explains their yellow/orange/red color and act as an accessory photosynthetic pigment that widens the spectrum of light absorbed by plants. Under excess illumination, they play a photoprotective and antioxidant role. At high altitudes, carotenoids accumulate in plants in order to absorb the excessive near-ultraviolet and blue light that could inhibit photosynthesis. There is mounting evidence that these pigments play a role in plant adaptation to growing conditions [35]. Both carotenoids and apocarotenoids derived from them by oxidation have roles in environmental sensing and acclimation. Moreover, light and temperature are major factors driving carotenoids synthesis and accumulation. The general trend suggests an increased carotenoid content with light intensity, while heat and cold can have opposite effects on different carotenoids [36]. Because carotenoids in basil plants play a role in the plant’s adaptation to light conditions, their concentration in leaves may increase for green-leaf basil genotypes exposed to intense light. Since the purple basil genotypes have, in addition to carotenoids, anthocyanins for photoprotection [28], their photosynthetic pigment dynamics may differ.
Besides these roles in plants, dietary intake of carotenoids is also important for human health, such as β-carotene, a precursor of vitamin A with beneficial roles for eye health, the immune system, and lutein, a macular pigment that can decrease eye diseases related to aging [37]. Diversified diets can ensure intake of over 50 types of carotenoids and have been associated with a decreased risk of cancer, cardiovascular disease, and cognitive disorders. About 90% of carotenoids in dietary intake consist of α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin [38]. Hence, carotenoid biofortification of edible leaf crops is an objective in agriculture [36]. A study showed that an addition of 15% basil leaf puree in biscuits was favorable and can potentially become a strategy to counteract β-carotene and calcium deficiency in breastfeeding mothers [39]. Based on the current study, for the biofortification of food products with carotenoids, the field-grown plants of cultivars O. basilicum ‘Aromă de cuișoare’, O. basilicum ‘Ierusalim’ and O. citriodorum ‘Macedon’ are recommended. Due to the lower carotenoid content of greenhouse plants, they are less recommended for food biofortification and more for food seasoning. The great advantage of potted plants is that they can ensure fresh leaves during the off-season to cover a critical period of the year when fresh basil cannot be procured from the field in a temperate climate.
Evidence for the interest in basil crops is reflected in the number of studies from recent years. These studies often investigated the reaction of plants to different types of stress [40,41] or the plant’s behavior in non-conventional growth conditions, such as hydroponics [42]. Particularly, stress studies under controlled experimental conditions could be insightful at determining the optimal parameters and physiological limits of plants that might further help breeding programs or more accurately determine the productivity-limiting factors. This trend in specialty literature can be explained by the prioritization of the middle- and long-term aims and the potential challenges of basil crops, while at the same time leaving some current aspects understudied. Future water shortages or soil salinity have justifiably enjoyed particular attention, yet today, commercial basil is still overwhelmingly grown under classic conditions. From these commercial crops, basil is delivered in different forms to the food industry, reaching millions of customers; hence, we argue that key parameters such as the ones studied here should continue to receive scrutiny due to their immediate implications in such settings.
5. Conclusions
Basil is a culinary plant important for the food industry, and the market demand is covered by field crops and greenhouse crops. This research studied the genotypic variation of photosynthetic leaf pigments for twelve accessions (of green and purple leaf) from the national germplasm collection, with the purpose of providing comparative evidence for differences arising from contrasting growing conditions.
All the studied species and varieties of basil can be successfully cultivated both in the field and in protected spaces (such as greenhouses). Cultivation in protected spaces provides fresh plant material (staggered sowing can be considered) to complement field crops with minimal maintenance work (sowing, potting, watering, and suppressing the inflorescence of the main stem to stimulate branching of the stem).
The experimental median indicated that chlorophylls and carotenoids accumulated in higher concentrations in basil leaves under field conditions compared to greenhouse conditions, with some exceptions for chlorophyll b. The carotenoid content (lutein, β-carotene, and total carotenoids) was also higher in all genotypes under the field conditions compared to the greenhouse conditions.
Among these genotypes, noticeably, O. basilicum ‘Aromă de cuișoare’ and O. basilicum ‘Ierusalim’ accumulated chlorophyll and carotenoids in higher amounts compared to the other genotypes, and for this reason, they are recommended to be considered in breeding programs for biofortified basil. The two lines with purple leaves from this study, in terms of carotenoids, were more similar in the greenhouse than in the field conditions, but in terms of chlorophyll, they were more similar in the field than the greenhouse. This suggests that each genotype has its own strategy to respond to environmental conditions.
The objectives of the plant-breeding programs could include identifying and creating suitable genotypes for controlled environment crops and field crops in order to optimize the production systems of this valuable aromatic plant and maximize their health-promoting properties.
Author Contributions
Conceptualization, I.C., A.B., D.V., R.V., A.S., M.I.C., V.H. and C.V.; methodology, A.B.; software, I.C.; formal analysis, A.B.; investigation, I.C.; resources, A.B., D.V., M.I.C. and C.V.; data curation, I.C. and A.B.; writing—original draft preparation, I.C.; writing—review and editing, I.C., A.B., D.V., R.V., A.S., M.I.C., V.H. and C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data used for this research are available in the content of this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stefanaki, A.; van Andel, T. Chapter 3—Mediterranean Aromatic Herbs and Their Culinary Use. In Aromatic Herbs in Food; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 93–121. ISBN 978-0-12-822716-9. [Google Scholar]
- Codex Alimentarius Commission 2017. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/tr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-736-03%252Fsc03_CRD16x.pdf (accessed on 12 May 2024).
- Spence, C. Sweet Basil: An Increasingly Popular Culinary Herb. Int. J. Gastron. Food Sci. 2024, 36, 100927. [Google Scholar] [CrossRef]
- Ervina, E.; Bryant, K.; Dwi, L.N.F.; Wahyudi, D. Sensory Characteristics and Consumer Liking of Basil Syrups (Ocimum basilicum L.) in Different Sensory Settings. Pol. J. Food Nutr. Sci. 2023, 73, 233–241. [Google Scholar] [CrossRef]
- Abidoye, A.O.; Ojedokun, F.O.; Fasogbon, B.M.; Bamidele, O.P. Effects of Sweet Basil Leaves (Ocimum Basilicum L) Addition on the Chemical, Antioxidant, and Storage Stability of Roselle Calyces (Hibiscus Sabdariffa) Drink. Food Chem. 2022, 371, 131170. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.A.; Crișan, I.; Vârban, D.; Stoie, A.; Vârban, R. Melliferous Aromatic Plants. Hop. Med. Plants 2023, 31, 67–81. [Google Scholar]
- Bączek, K.; Kosakowska, O.; Gniewosz, M.; Gientka, I.; Węglarz, Z. Sweet Basil (Ocimum basilicum L.) Productivity and Raw Material Quality from Organic Cultivation. Agronomy 2019, 9, 279. [Google Scholar] [CrossRef]
- Majkowska-Gadomska, J.; Kulczycka, A.; Dobrowolski, A.; Mikulewicz, E. Yield and Nutritional Value of Basil Grown in a Greenhouse. Acta Agroph. 2017, 24, 455–464. [Google Scholar]
- Teliban, G.-C.; Popa, L.D.; Buducea, M.; Precupeanu, C.; Stan, T.; Agapie, A.; Naie, M.; Dumitru, I.; Stoleru, V. Research on the Obtaining Basil in Pots, in Greenhouse Conditions. In Proceedings of the ISB-INMA THE’ 2021 Agricultural and Mechanical Engineering, Bucharest, Romania, 29 October 2021. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph. 2018. Available online: https://www.openagrar.de/receive/openagrar_mods_00042351 (accessed on 12 May 2024).
- UPOV Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability Ocimum basilicum L. 2016. Available online: https://www.upov.int/edocs/tgdocs/en/tg200.pdf (accessed on 12 May 2024).
- Ćavar Zeljković, S.; Komzáková, K.; Šišková, J.; Karalija, E.; Smékalová, K.; Tarkowski, P. Phytochemical Variability of Selected Basil Genotypes. Ind. Crops Prod. 2020, 157, 112910. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M. Essential Oils Content, Composition and Antioxidant Activity of Selected Basil (Ocimum basilicum L.) Genotypes. S. Afr. J. Bot. 2022, 151, 675–694. [Google Scholar] [CrossRef]
- Sharma, R.; Bhatia, S.; Kaur, P. Influence of Packaging and Storage Conditions on Biochemical Quality and Enzymatic Activity in Relation to Shelf Life Enhancement of Fresh Basil Leaf. J. Food Sci. Technol. 2018, 55, 3199–3211. [Google Scholar] [CrossRef]
- Chaves, R.P.F.; de Araújo, A.L.; Lopes, A.S.; Pena, R.d.S. Convective Drying of Purple Basil (Ocimum basilicum L.) Leaves and Stability of Chlorophyll and Phenolic Compounds during the Process. Plants 2023, 12, 127. [Google Scholar] [CrossRef]
- Index Seminum Hortus Agro-Botanicus Napocensis; AcademicPres: Cluj-Napoca, Romania, 2023; ISSN 1223-6055.
- Vînătoru, C.; Mușat Zamfir, B.; Bratu, C.; Peticila, A. Results and Perspectives in Ocimum Basilicum (Basil) Breeding at Vegetable Research and Development Station Buzău. Sci. Pap. Ser. B Hortic. 2019, 58, 161–168. [Google Scholar]
- Climate Data of 2022, Cluj-Napoca, Romania. Available online: https://en.tutiempo.net/climate/romania.html (accessed on 9 July 2024).
- Srivastava, R.K.; Kumar, S.; Sharma, R.S. Ocimum as a Promising Commercial Crop. In The Ocimum Genome; Shasany, A.K., Kole, C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–7. ISBN 978-3-319-97430-9. [Google Scholar]
- Vârban, D.; Zăhan, M.; Pop, C.R.; Socaci, S.; Ștefan, R.; Crișan, I.; Bota, L.E.; Miclea, I.; Muscă, A.S.; Deac, A.M.; et al. Physicochemical Characterization and Prospecting Biological Activity of Some Authentic Transylvanian Essential Oils: Lavender, Sage and Basil. Metabolites 2022, 12, 962. [Google Scholar] [CrossRef]
- Clapa, D.; Bunea, C.; Borsai, O.; Pintea, A.; Hârța, M.; Ştefan, R.; Fira, A. The Role of Sequestrene 138 in Highbush Blueberry (Vaccinium corymbosum L.) Micropropagation. HortScience 2018, 53, 1487–1493. [Google Scholar] [CrossRef]
- Bunea, C.-I.; Pop, N.; Babeş, A.C.; Matea, C.; Dulf, F.V.; Bunea, A. Carotenoids, Total Polyphenols and Antioxidant Activity of Grapes (Vitis vinifera) Cultivated in Organic and Conventional Systems. Chem. Cent. J. 2012, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The Role of Photosynthesis Related Pigments in Light Harvesting, Photoprotection and Enhancement of Photosynthetic Yield in Planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Gossa, A.G.; Asfaw, B.T. Diversity of Ethiopian Sweet Basil (Ocimum basilicum L.) Germplasm for Quantitative Morphological Traits. Flora 2023, 304, 152313. [Google Scholar] [CrossRef]
- Teodorescu, E.; Berevoianu, R.L.; Burnichi, F.; Petre, C. Phenotypic Evaluation and Economic Efficiency Concerning a Basil Germplasm Collection from S-E Romania. In Proceedings of the XXX International Horticultural Congress IHC2018: International Symposium on Medicinal and Aromatic Plants, Culinary Herbs and Edible Fungi, IV International Jujube Symposium and VI International Symposium on Saffron Biology and Technology, Istanbul, Turkey, 12–16 August 2018; pp. 135–144. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid and Chlorophyll Pigments in Sweet Basil Grown in the Field and Greenhouse. HortScience 2005, 40, 1230–1233. [Google Scholar] [CrossRef]
- Proz, M.d.l.Á.; da Silva, M.A.S.; Rodrigues, E.; Bender, R.J.; Rios, A. de O. Effects of Indoor, Greenhouse, and Field Cultivation on Bioactive Compounds from Parsley and Basil. J. Sci. Food Agric. 2021, 101, 6320–6330. [Google Scholar] [CrossRef]
- Stetsenko, L.A.; Pashkovsky, P.P.; Voloshin, R.A.; Kreslavski, V.D.; Kuznetsov, V.V.; Allakhverdiev, S.I. Role of Anthocyanin and Carotenoids in the Adaptation of the Photosynthetic Apparatus of Purple- and Green-Leaved Cultivars of Sweet Basil (Ocimum basilicum) to High-Intensity Light. Photosynthetica 2020, 58, 890–901. [Google Scholar] [CrossRef]
- Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Biochemical Compounds, Antioxidant Capacity, Leaf Color Profile and Yield of Basil (Ocimum sp.) Microgreens in Floating System. Plants 2023, 12, 2652. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Chlorophyll Cycle Regulates the Construction and Destruction of the Light-Harvesting Complexes. Biochim. Biophys. Acta BBA Bioenerg. 2011, 1807, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Björn, L.O.; Papageorgiou, G.C.; Blankenship, R.E. Govindjee A Viewpoint: Why Chlorophyll a? Photosynth. Res. 2009, 99, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Voitsekhovskaja, O.V.; Tyutereva, E.V. Chlorophyll b in Angiosperms: Functions in Photosynthesis, Signaling and Ontogenetic Regulation. J. Plant Physiol. 2015, 189, 51–64. [Google Scholar] [CrossRef]
- Crișan, I.; Balestrini, R.; Pagliarani, C. The Current View on Heavy Metal Remediation: The Relevance of the Plant Interaction with Arbuscular Mycorrhizal Fungi. Plant Stress 2024, 12, 100439. [Google Scholar] [CrossRef]
- Quian-Ulloa, R.; Stange, C. Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light. Int. J. Mol. Sci. 2021, 22, 1184. [Google Scholar] [CrossRef]
- Maslova, T.G.; Markovskaya, E.F.; Slemnev, N.N. Functions of Carotenoids in Leaves of Higher Plants (Review). Biol. Bull. Rev. 2021, 11, 476–487. [Google Scholar] [CrossRef]
- Dhami, N.; Cazzonelli, C.I. Environmental Impacts on Carotenoid Metabolism in Leaves. Plant Growth Regul. 2020, 92, 455–477. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Grainger, E.M.; Webb, M.Z.; Simpson, C.M.; Chitchumroonchokchai, C.; Riedl, K.; Moran, N.E.; Clinton, S.K. Chapter Eight—Assessment of Dietary Carotenoid Intake and Biologic Measurement of Exposure in Humans. In Methods in Enzymology; Wurtzel, E.T., Ed.; Carotenoids: Biological Functions of Carotenoids and Apocarotenoids in Natural and Artificial Systems; Academic Press: Cambridge, MA, USA, 2022; Volume 674, pp. 255–295. [Google Scholar]
- Ningsih, Y.W.; Devi, M.; Kiranawati, T.M. The Effect of Basil Leaves (Ocimum basilicum) Addition to Contents of β-Carotene, Calcium Levels, and Hedonic Test of Basil Leaves Biscuits; Atlantis Press: Amsterdam, The Netherlands, 2020; pp. 248–254. [Google Scholar]
- Ciriello, M.; Fusco, G.M.; Colla, G.; Kyriacou, M.C.; Sabatino, L.; De Pascale, S.; Rouphael, Y.; Carillo, P. Adaptation of Basil to Salt Stress: Molecular Mechanism and Physiological Regulation. Plant Stress 2024, 11, 100431. [Google Scholar] [CrossRef]
- Copolovici, L.; Lupitu, A.; Moisa, C.; Taschina, M.; Copolovici, D.M. The Effect of Antagonist Abiotic Stress on Bioactive Compounds from Basil (Ocimum basilicum). Appl. Sci. 2021, 11, 9282. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Abass, W.; Morsy, O.; El-Ghobashy, H.; Shaban, Y.; Egela, M. Production of Basil (Ocimum basilicum L.) under Different Soilless Cultures. Sci. Rep. 2021, 11, 12754. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).