Abstract
Fresh-cut fruits, renowned for their convenience and nutritional value, are susceptible to rapid deterioration, compromising their quality and shelf life. In this study, a sustainable and environmentally friendly edible coating was developed based on sodium alginate (SA; 1% w/v), cedar mucilage (CM; 4% w/v), and calcium chloride (2% w/v), applied using a layer-by-layer technique to preserve the quality and prolong the shelf life of fresh-cut melon. Fruits were cut into cubes coated or uncoated by dipping and subsequently packaged in a polyethylene terephthalate container and stored for 15 days at 4 °C. Physicochemical traits and qualitative features such as polyphenol, flavonoid, carotenoid, ascorbic acid content, as well as antioxidant activity, were assessed. Furthermore, the enzymatic antioxidant system and the ascorbate–glutathione cycle were investigated. The coating reduced weight loss and enhanced polyphenol, flavonoid, and ascorbic acid content and antioxidant activity during cold storage. Edible coating (SA + CM) represents a valid tool to extend the postharvest life, improve the storability, and enhance the physicochemical and qualitative traits of fresh-cut melon. Further research is required to optimize coating formulations and application techniques to maximize their effectiveness and commercial viability in the food industry.
1. Introduction
Challenges stemming from time constraints throughout the evolution of society and the prioritization of convenience are frequently cited as primary factors contributing to the underconsumption of fresh fruits and vegetables, resulting in levels that fall below the World Health Organization’s recommended daily intake [1,2,3,4]. In recent years, consumer habits regarding fruit and vegetable consumption have undergone significant changes, with today’s consumers increasingly recognizing the importance of a balanced, nutrient-rich diet in promoting overall health and reducing the risk of chronic disease [5]. This has led to an increased demand for fresh, convenient, and high-quality produce, particularly fresh-cut fruits [6]. Fresh-cut melon provides consumers with a convenient and ready-to-eat option, saving time and effort in preparation. It eliminates the need for washing, peeling, and cutting, making it an attractive choice for busy individuals or those seeking quick and easy snacks. Therefore, marketing melons as convenient, ready-to-eat fresh-cut products in slices or cubes is an attractive option for both consumers and retailers, given that melons are relatively inconvenient to consume due to their large size and the presence of a significant inedible portion [7].
Melons (Cucumis melo L.), belonging to the Cucurbitaceae family, are not only refreshingly delicious fruits but also beneficial for health. Melons contain typical nutrients, such as minerals and vitamins, as well as a wide range of carotenoids, flavonoids, and phenolic acids with biological properties, such as antioxidant, anticancer, antineurodegenerative, and anti-inflammatory activity [8]. The primary drawback associated with fresh-cut fruits is their short shelf life, frequently lasting fewer than two weeks [9]. Cutting or minimally processing fruits causes alterations in physiological properties due to the loss of cellular integrity, which can impact their quality, appearance, texture, and nutritional value [10]. Understanding these physiological changes and implementing appropriate postharvest handling techniques and storage strategies are crucial for maintaining the freshness and marketability of fresh-cut fruits [7]. Different techniques, such as cold storage [11,12], edible coatings [8,13,14,15,16,17,18,19], ozone [20], modified storage atmosphere [7,21,22], ultraviolet radiation [23], and cold plasma [24], have been employed to preserve the quality and safety of fresh-cut melons.
Edible coatings (ECs) are a promising technology used to improve the quality, safety, shelf life, and overall consumer appeal of various ready-to-eat fruits [25]. These coatings, typically made from natural polymers such as proteins, polysaccharides, lipids, or combinations thereof, form a protective layer on the surface of the fruit that helps to prolong the fruit’s shelf life and marketability. One of the main advantages of edible coatings is their ability to reduce the rate of physiological processes, such as respiration and ethylene production, associated with fruit ripening and deterioration during storage [26]. Several studies have tested the effectiveness of several ECs realized with different compounds such as chitosan, alginate, calcium chloride, pectin, gellan, mucilage, hydrocolloid, and Aloe vera gel on fresh-cut melon [15,17,18,21,27,28,29].
The aim of this study was to evaluate the effectiveness of a layer-by-layer edible coating on ready-to-eat melon by analyzing its physicochemical, qualitative, and enzymatic properties during cold storage.
2. Materials and Methods
2.1. Raw Materials and Processing
Cantaloupe melons (Cucumis melo var. cantalupensis) were purchased from a local market (Caserta, Italy) and stored at 4 °C and 95% relative humidity until processing. Fruits were selected for size uniformity, the absence of fungal infection, and soluble solid content between 8 and 10 °Brix, corresponding to commercial ripeness, as suggested by Martiñon et al. [15]. Whole melons were washed with running water, immersed in sodium hypochlorite (110 ppm) for 2 min, rinsed with sterile distilled water, peeled aseptically, and sliced into 2 cm sided cubes.
2.2. Edible Coatings and Experimental Design
Cedar mucilage was extracted from fruit peel according to the method described by Sortino et al. [30] and used to prepare a 4% (w/v) mucilage solution. Two other different coating solutions were prepared in sterile distilled water: sodium alginate (1%, w/v, Sapore puro, Turin, Italy) and calcium chloride (2%, w/v, Sigma-Aldrich, Milan, Italy). The layer-by-layer technique was performed as previously reported by Trevino-Garcia et al. [31]. Melon cubes were randomly divided into two batches, and the first batch was dipped for 2 min in a calcium chloride solution, cedar mucilage solution, sodium alginate, and finally calcium chloride solution (coated sample). Dipping in each solution was interspersed with a drying period of approximately three minutes under a laminar flow hood at room temperature. Fruit from the second lot underwent the same treatment as the treated fruit, but the dipping solutions were replaced with sterile distilled water (uncoated sample). The coated and uncoated samples, containing approximately 150 g of melon cubes, were placed in plastic containers (polyethylene terephthalate) with lids and stored at 4 °C and 95% relative humidity for fifteen days. Three biological replicates were prepared for control and coated samples at each time point. Analyses were performed on days 0, 5, 10, and 15, with daily visual monitoring throughout the storage period (Figure S1).
2.3. Physicochemical Traits
Weight loss was determined by weighing the fresh-cut melon at each sampling and calculating the difference in weight. The initial sample weight was measured after dipping treatment. The results are expressed as a percentage of weight loss according to the following equation: Weight loss (%) = [(initial sample weight) − (sample weight at each sampling)]/(initial sample weight) × 100. The total soluble solid (TSS) content was determined on the extracted juice using a digital refractometer (Sinergica Soluzioni, DBR35, Pescara, Italy) and expressed in °Brix. Titratable acidity (TA) was determined by basic acid titration with 0.1 mol L−1 NaOH and expressed as grams of citric acid equivalent (CAE) per 100 mL of juice (g CAE 100 mL−1), and pH values were measured at 25 °C using a digital pH meter (model 2021, XS PH 50 VIO LAB, Torino, Italy).
Firmness was measured using a digital penetrometer (Turoni, Forlì, Italy) with a 5 mm diameter probe, and the results are expressed in newtons (N).
2.4. Bioactive Compounds and Antioxidant Activity
Bioactive compounds and antioxidant activity were determined using a methanolic extract obtained from homogenizing melon cubes in a methanol solution (80% v/v) as described by Ravindranath et al. [32]. The total phenol content (TPC) and flavonoid content (TFC) were determined using the Folin–Ciocalteu method [33] and the aluminum chloride colorimetric method [34], respectively. TPC and TFC results are expressed as milligrams of gallic acid equivalents (GAEs) per 100 g fresh weight (FW) and as milligrams of catechin equivalents (CEs) per 100 g fresh weight (FW), respectively. GAEs and CEs were used as standards and analyzed under the same conditions.
Ascorbic acid content was determined as reported by Petriccione et al. [35] using an assay mixture comprising 300 μL of extract, 0.3% (v/v) metaphosphoric acid, and (5:1, v/v) diluted Folin’s reagent, in a final volume of 2 mL and evaluating the absorbance after an incubation of 10 min at 760 nm. The results are expressed as milligrams of ascorbic acid (AA) per 100 g of fresh weight (FW).
Total carotenoids were extracted from melon cubes using N-N-dimethylformamide (1:2 w/v). The absorbance of the extract was evaluated at three wavelengths (470, 633, and 645 nm) using a UV–VIS spectrophotometer (Model V-630, Jasco, Milan, Italy). Carotenoid content was calculated using Wellburn equations [36], and the results are expressed as milligrams per 100 g fresh weight (FW).
The radical scavenging activity of the methanol extracted samples was evaluated toward 2,2-azinobis-(3-ethylbenzothiazolin-6-sulphonic acid) (ABTS). ABTS assay was performed according to Ravindranath et al. [32], and the results are expressed in µmol of Trolox equivalents (TE) per 100 g fresh weight (FW).
2.5. Hydrogen Peroxide Content
Hydrogen peroxide content was assessed as described by Carvalho et al. [14]. Fresh melon (1:10 w/v) was homogenized in trichloroacetic acid (5% w/v) and centrifuged at 12,000× g for 15 min at 4 °C. The supernatant was mixed with a reaction mixture containing 0.1% trichloroacetic acid and 1 mol L−1 potassium iodide, and the absorbance of the mixture assay was monitored at 390 nm. The results are expressed as mol H2O2 per kg fresh weight (FW).
2.6. Antioxidant Enzymes and Ascorbate–Glutathione Pathway Enzymes
For total protein extraction, melon cubes were ground in liquid nitrogen and homogenized in extraction buffer containing potassium phosphate (pH 7.8, 100 mM), sodium ethylenediaminetetraacetic acid (pH 7.0, 1 mM), dithiothreitol (2 mM), phenylmethanesulfonyl fluoride (1 mM), polyvinylpolypyrrolidone (5% w/v), and glycerol (10% v/v). Ascorbic acid (5 mM) was added only for the extraction of ascorbate peroxidase and ascorbate oxidase enzymes. The homogenate was centrifuged at 18,000× g for 10 min at 4 °C. The resulting supernatant was used to assess enzyme activity, and its protein content was estimated with the Bradford method [37] using bovine serum albumin as standard.
Superoxide dismutase (SOD, EC 1.15.1.1) activity was monitored by spectrophotometric analysis based on the inhibition of the photoreduction of nitro blue tetrazolium chloride (NBT) with riboflavin. The assay mixture contained potassium phosphate buffer pH 7.8 (50 mM), sodium EDTA (0.1 mM), methionine (13 mM), NBT (75 mM), riboflavin (2 mM), and 200 µL of crude enzyme extract. After incubation at room temperature under continuous light, the absorbance of the assay mixture was measured at 560 nm. One SOD unit was defined as the amount of enzyme that inhibited the rate of NBT reduction by 50% under the above assay conditions. SOD activity is expressed as units per g fresh weight (FW).
Catalase (EC 1.11.1.6; CAT) activity was evaluated using the reaction medium described by Carvalho et al. [14].
Ascorbate peroxidase (APX, EC 1.11.1.11) activity was evaluated according to Nakano and Asada [38], with some modifications. The reaction mixture contained potassium phosphate buffer (pH 7, 100 mM), ascorbic acid (0.33 mM), H2O2 (0.35 mM), sodium EDTA (pH 7, 0.66 mM), and 100 µL of the crude enzyme extract. APX activity was evaluated by monitoring the reduction of ascorbic acid at 290 nm.
Ascorbate oxidase (EC 1.10.3.3; AOX) is an enzyme that catalyzes the oxidation of ascorbic acid to dehydroascorbate. AOX activity was determined as suggested by Diallinas et al. [39], with slight modifications. The reaction mixture contained potassium phosphate buffer (pH 7.0 100 mM), H2O2 (88 mM), 100 μL of the crude enzyme extract, and (0.066 mM) ascorbic acid. The reaction was monitored at 265 nm.
Monodehydroascorbate reductase (EC 1.6.5.4; MDHAR) activity was determined spectrophotometrically by monitoring the oxidation of nicotinamide adenine dinucleotide (NADH) at 340 nm [40]. The reaction mixture contained 50 mM potassium phosphate, pH 7.6, 0.25 mM NADH, 2 mM sodium ascorbate, 0.25 U ascorbate oxidase from Cucurbita sp. (Sigma-Aldrich, Milan, Italy), and 50 µL of the crude enzyme extract.
Dehydroascorbate reductase (EC 1.8.5.1; DHAR) activity was evaluated in accordance with Dalton et al. [41], with slight modifications. The reaction mixture contained 100 mM HEPES–KOH (pH 7.0), 0.1 mM EDTA, 2.5 mM reduced glutathione (GSH), 0.2 mM dehydroascorbate (DHA), and 100 µL of the extract. The ascorbate production was monitored at 265 nm.
Glutathione reductase (EC 1.6.4.2; GR) activity was evaluated by monitoring the oxidation of dihydro nicotinamide–adenine dinucleotide phosphate (NADPH) at 340 nm [41]. The reaction mixture contained 50 mM potassium phosphate pH 7.0, 0.5 mM glutathione in the oxidized form (GSSG), 100 µL of the crude enzyme extract, and 0.2 mM NADPH.
The results of the above-reported enzymes are expressed as U per g FW, where 1 U of enzyme activity is the amount of enzyme required for the conversion of 1 μmol of the substrate into product per minute.
2.7. Statistical Analysis
All data are expressed as the mean ± standard deviation. The differences between coated and uncoated fruits were evaluated by one-way ANOVA and Tukey test (p < 0.05) for mean comparisons. Principal component analysis (PCA) and partial least-square discrimination analysis (PLS-DA) were applied to describe the relationship between the physicochemical, nutraceutical, and enzymatic traits to identify the principal components contributing to the majority of the variation within the dataset. Data were mean-centered and scaled to unit variance. These analyses were carried out using SPSS v.20.0 statistical software (IBM Corporation, Armonk, NY, USA) and MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/, accessed on 14 March 2024).
3. Results and Discussion
3.1. Physicochemical Traits
Fresh-cut fruits and vegetables exhibit high respiration rates and produce ethylene in response to injury, which, combined with the expanded surface area per unit volume, improves water loss and the loss of texture, flavor, and nutritional quality [42]. Table 1 shows the changes in the physicochemical traits of coated and uncoated fresh-cut melons during the fifteen days of cold storage.

Table 1.
Total soluble solids (TSSs; °Brix), pH, titratable acidity (TA; g citric acid/100 mL of juice), weight loss (WL; %), and firmness (F; N) in uncoated and coated fresh-cut melons after 0, 5, 10, and 15 days of cold storage at 4 °C.
The WL of samples significantly increased during cold storage. After 15 days of storage, the uncoated samples showed a WL of 4.60 ± 0.54%, while the coated samples exhibited a WL of 2.88 ± 0.25%. The bi-layer coating tested in this study helped to reduce weight loss during cold storage. The EC tested in this study showed better efficacy in reducing WL than the lemon extract and soy protein isolate-based coating tested by Yousuf et al. [18] on fresh-cut melon during cold storage. Furthermore, our results are in agreement with the findings of Trevino-Garza et al. [17] and Chong et al. [27], who tested linseed mucilage–chitosan and chitosan–CaCl2-based ECs in fresh-cut melon, respectively. Weight loss in fresh-cut fruit is mainly due to water loss, but ECs can effectively prevent moisture loss through the fruit surface, resulting in a reduced rate of moisture loss and transpiration and respiration rates, as reported by Chong et al. [27].
Several studies have shown that edible coatings create a protective layer on the fruit surface, which can alter the surrounding atmosphere by serving as a semipermeable barrier, thereby enabling control over the gas exchange between the commodity and its environment, as well as reducing water loss, shriveling, and qualitative decay [8,31,35].
The TSS content is a critical factor that affects the quality and taste of fresh-cut fruits for consumers, contributing significantly to their sweetness and overall sensory appeal [43]. The TSS content decreased over time in both uncoated and coated samples during cold storage. A significant reduction in TSS value was observed after 5 days of storage, with a decrease of 20% and 8.70% in uncoated and coated samples, respectively. At the end of cold storage, the uncoated samples showed a significantly lower value of 7.08 ± 0.21 °Brix compared to the coated samples, with a value of 8.40 ± 0.20 °Brix. Our results are consistent with the studies on fresh-cut melons treated with β-carotene nanocapsules/xanthan gum [19], CaCl2/chitosan [27], and linseed mucilage–chitosan coating [17].
The decrease in TSS content could be linked to the utilization of sugars as a nutrient source by spoilage microorganisms during their growth [44] and the higher rate of juice loss and inherent enzymatic activity [19].
pH values showed significant differences between the uncoated and coated samples throughout cold storage. At the beginning of the experiment (day 0), both uncoated and coated samples exhibited similar pH values of 6.36 ± 0.03 and 6.34 ± 0.05, respectively. The uncoated samples showed a gradual decrease in pH, reaching 5.34 ± 0.03 at the end of cold storage. In contrast, the coated samples displayed more stability in pH, with a slight decrease, and a pH value of 5.87 ± 0.06 after 15 days. pH changes can be due to oxidative and environmental stress during postharvest life [27]. In addition, these stresses also contribute to a reduction in ATPase pumping capacity, thus decreasing the cellular pH [45]. The significant decrease in pH observed in the uncoated samples may be primarily due to these stresses, which had a lower effect on the coated ones.
Similarly, TA levels followed a similar pattern during cold storage in both samples. After 15 days, uncoated samples showed a significant reduction in TA over time (0.07 ± 0.02 g CAE 100 mL−1), compared to the coated samples, which displayed a slower reduction (0.11 ± 0.01 g CAE 100 mL−1). The TA decrease is due to the utilization of organic acids as substrates in metabolic pathways [16]. Several studies have demonstrated that ECs can slow down the TA decrease in fresh-cut fruit during cold storage [46,47].
Firmness is a crucial indicator of the structural integrity and the overall quality of fruits [48]. Our findings revealed significant differences in firmness between coated and uncoated samples over the experiment. Initially, both coated and uncoated samples exhibited similar levels of firmness at day 0 (Table 2). However, as the storage period progressed, a significant decline in firmness was observed in both samples. The coated samples displayed higher firmness compared to the uncoated ones throughout the cold storage period. After 15 days, the firmness of the coated samples (3.23 ± 0.15 N) was significantly higher than that of the uncoated ones (2.50 ± 0.36 N), suggesting that the coating treatment effectively reduced the firmness loss during storage. The firming effect of the coating is due to calcium’s role in binding with complex polysaccharides and proteins that form the cell wall at the cellular level [49]. Several studies on fresh-cut melon have demonstrated that edible coatings can help slow down the softening process and maintain firmness, thus prolonging the marketability of fruits [15,19,27].

Table 2.
Total phenol content (TPC, mg GAE 100 g−1 FW), total flavonoid content (TFC, mg CE 100 g−1 FW), antioxidant activity toward 2,2-azinobis-(3-ethylbenzothiazolin-6-sulphonic acid) (ABTS, µmol TE/100 g FW), ascorbic acid (AA; mg 100 g−1 FW), and carotenoid content (CAR; mg 100 g−1 FW) in uncoated and coated fresh-cut melons after 0, 5, 10, and 15 days of cold storage at 4 °C.
3.2. Bioactive Compounds and Antioxidant Activity
In Table 2, the bioactive compound content and antioxidant activity of the coated and uncoated fresh-cut melon after 0, 5, 10, and 15 days of cold storage are shown.
Phenolic compounds are widely recognized as the primary compounds involved in the overall antioxidant capacity of fruits and vegetables. Cutting or damaging fruit triggers a cellular response that activates phenolic biosynthesis, enhancing the nutritional value and health-promoting properties [50,51,52]. Different studies have demonstrated a significant TPC increase and antioxidant activity following wounding in several fresh-cut fruits and vegetables [53,54,55,56,57,58,59].
Consistent with previous research, our study showed that TPC and TFC increased during cold storage with higher values in the coated samples. In the uncoated samples, TPC ranged from 4.82 ± 0.41 mg GAE 100 g−1 FW on day 0 to 10.53 ± 0.70 mg GAE 100 g−1 FW on day 15, while the coated samples displayed a significant increase in TPC, reaching a value of 15.27 ± 0.30 mg GAE 100 g FW−1 after 15 days of cold storage. This upward trend suggests a gradual accumulation of polyphenols within the samples over time, as demonstrated by Manozzi et al. [8] on fresh-cut melons. Similarly, TFC in the uncoated sample increased from 0.15 ± 0.03 to 0.33 ± 0.01 mg CE 100 g−1 FW. In the coated samples, the values increased from 0.14 ± 0.01 to 0.46 ± 0.01 mg CE 100 g−1 FW.
ECs offer a powerful method for preserving the high levels of polyphenols and flavonoids in fruits by mitigating their interaction with oxidative enzymes. By limiting the availability of oxygen, ECs create an environment where polyphenols and flavonoids are less prone to oxidation [57].
In this study, the ascorbic acid content in the uncoated samples ranged from 65.23 ± 0.93 mg 100 g−1 FW on day 0 to 43.91 ± 2.16 mg 100 g−1 FW on day 15, indicating a reduction of 32.7%. Similarly, in the coated samples, the concentration of ascorbic acid decreased from 65.78 ± 0.60 mg 100 g−1 FW on day 0 to 59.47 ± 0.77 mg 100 g−1 FW on day 15, showing a decrease of 9.6%. However, the rate of ascorbic acid decrease appeared significantly slower in the coated samples compared to the uncoated ones, in agreement with Mannozzi et al. [8]. ECs delay the AA loss due to low oxygen permeability, which leads to a reduction in the enzyme activity responsible for AA oxidation [35].
In fresh-cut melon, the presence of ascorbic acid positively influenced the levels of phenols, as demonstrated by Altunkaya et al. [60] in minimally processed lettuce. The synergism observed between these compounds underscores the complex interplay of antioxidants in fresh commodities.
The ABTS radical scavenging capacity increased throughout cold storage, with higher values in the coated samples. The uncoated samples showed values ranging from 1.86 ± 0.09 µmol TE 100 g−1 FW and 5.67 ± 0.15 µmol TE 100 g−1 FW from 0 to 15 days of cold storage, while in the coated samples, it reached 8.78 ± 0.17 µmol TE 100 g−1 FW at the end of the experiment.
Few literature data on ABTS assays performed on coated fresh-cut melon have been reported. Chikhala et al. [61] reported that the inclusion of Lactobacillus plantarum 75 in xanthan coatings significantly retained the radical scavenging capacity during cold storage in cantaloupe melon fresh cuts.
Melon is a good source of carotenoids characterized by high antioxidant activity [62]. During cold storage, we observed a decrease in the rate of carotenoid content from 69.7% to 38.7% in the uncoated and coated samples, respectively. ECs with their oxygen barrier properties contributed to higher levels of carotenoids in the coated samples compared to the uncoated samples, potentially minimizing their oxidation [8,11]. The reduction in the carotenoid content observed over the shelf-life period of fruits and vegetables can be attributed to various factors, including the degradation induced by exposure to oxygen and cellular damage caused by wounds. Carotenoids, due to being sensitive to oxidation, are particularly vulnerable to degradation when exposed to oxygen present in the surrounding environment. This oxidative degradation process can lead to a decline in carotenoid levels, affecting fruits’ nutritional quality [11].
3.3. Enzymatic Antioxidant System
In fresh-cut fruit, antioxidant defense systems mitigate ROS-induced damage and preserve quality [63]. One of the main mechanisms of ROS detoxification involves enzymatic antioxidants. Enzymes such as superoxide dismutase (SOD), catalase (CAT), and enzymes involved in the ascorbate–glutathione cycle, such as ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR), play a key role in the scavenging and neutralization of ROS, thus maintaining cellular redox homeostasis and protecting against oxidative stress [64]. In addition, wounding accelerates the ripening and senescence of postharvest fruits and vegetables, and ROS in injured fruits may act as signaling molecules in response to cut-wounding stress [65,66]. Furthermore, ROS plays an important role in the wound-induced activation of both the primary and secondary metabolism [67].
SOD activity (Figure 1A) exhibited different trends between the uncoated and coated samples during the cold storage period. In the uncoated samples, a significant decrease was observed over time. This decrease was 15.8% after 10 days and 17.4% after 15 days compared to the initial value. In the coated samples, we observed a significant SOD activity increase of 50.3% and 55.4% after 10 and 15 days, respectively, compared to the beginning of the experiment. These results suggest that this coating has a significant effect on SOD during cold storage, inducing an increase in the activity of this enzyme and enhancing the ROS scavenging potential in fresh-cut melon. SOD catalyzes the dismutation of superoxide radicals (O2•−) into oxygen (O2) and hydrogen peroxide (H2O2) This enzymatic reaction is essential to neutralizing superoxide radicals, which are highly reactive and can cause damage to cellular components such as proteins, lipids, and DNA [68].
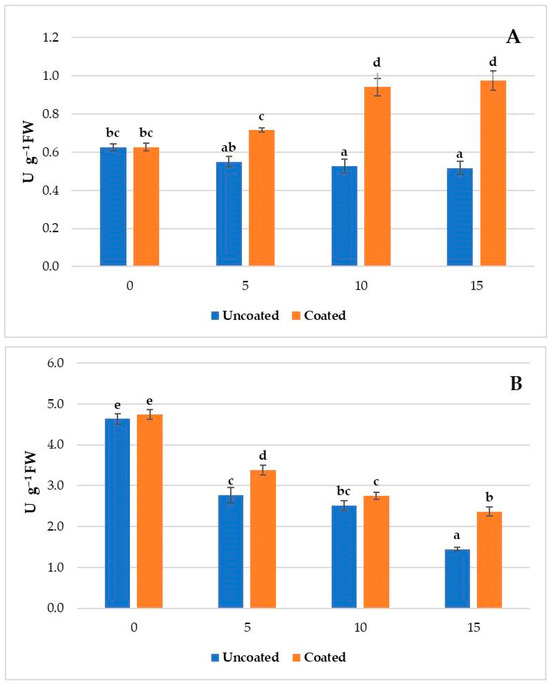
Figure 1.
Superoxide dismutase (A) and catalase (B) activity in coated and uncoated fresh-cut melons after 0, 5, 10, and 15 days of cold storage at 4 °C. Different letters indicate significant differences between different groups (p < 0.05; Tukey test).
CAT activity (Figure 1B) decreased in the coated and uncoated samples during cold storage, with higher values in coated ones. CAT showed lower values in the coated (2.36 µmol g−1 FW) than the uncoated samples (1.45 µmol g−1 FW) after 15 days of cold storage. CAT catalyzes the decomposition of hydrogen peroxide without reducing power, with a high turnover rate but low substrate affinity [69]. Our results are in agreement with those reported in a previous study indicating that a coating comprising chitosan and trans-cinnamaldehyde in fresh-cut melons induced a significant increase (p < 0.05) in CAT activity compared to uncoated samples [14].
In fresh-cut melon, APX and AOX activity displayed different trends during cold storage (Figure 2). APX significantly increased with a higher value in the coated samples, while a significant decrease in AOX was registered. Compared to the uncoated samples, APX activity was 53.3%, 44.6%, and 20.2% higher in the coated fruit after 5, 10, and 15 days, respectively, while the AOX activity in the coated fruit was 5.7%, 27.9%, and 18.8% higher at 5, 10, and 15 days, respectively. The lower APX activity in fresh-cut melon at the beginning of the experiment indicates that CAT plays a predominant role in the H2O2 neutralization, as suggested by Carvalho et al. [14].
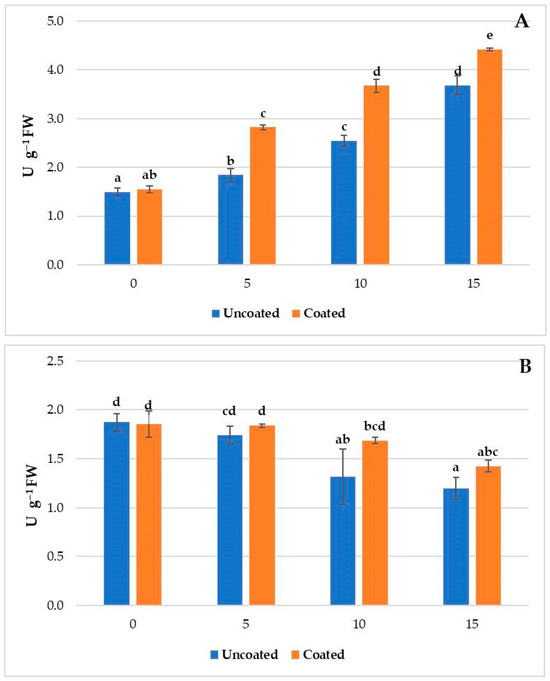
Figure 2.
Ascorbate peroxidase (A) and ascorbate oxidase (B) activity in fresh-cut melon after 0, 5, 10, and 15 days of cold storage at 4 °C. Different letters indicate significant differences between different groups (p < 0.05; Tukey test).
H2O2 is a non-radical reactive oxygen species generated during many particular conditions and is currently universally recognized as an important mediator of redox-regulated processes [68]. H2O2 is a product of oxidative metabolism and can cause cell damage if not neutralized. In our study, a decrease in H2O2 levels was observed in the coated samples over time, showing greater effectiveness in neutralizing H2O2. The coated samples showed significantly lower H2O2 content of 5.88, 12.24, and 17.52 at 5, 10, and 15 days, respectively compared to the uncoated samples (Figure 3A). This could be attributed to the higher activity of antioxidant enzymes, in particular APX and CAT in the coated samples, which work together to neutralize H2O2 and maintain cellular redox homeostasis.
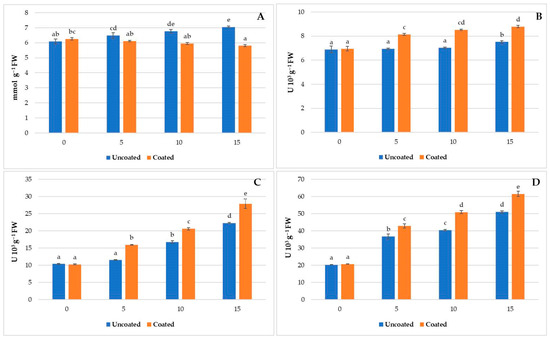
Figure 3.
Hydrogen peroxide content (A), as well as monodehydroascorbate reductase (B), dehydroascorbate reductase (C), and glutathione reductase (D) activity, in fresh-cut melon after 0, 5, 10, and 15 days of cold storage at 4 °C. Different letters indicate significant differences between different groups (p < 0.05; Tukey test).
MDHAR is involved in the conversion of monodehydroascorbate (MDHA) to its reduced form, ascorbic acid (AsA). In our study, we observed an increase in MDHAR activity (Figure 3B) over time in both the coated and uncoated samples. Compared to the uncoated samples, the MDHAR activity in the coated fruit was 17.11, 20.70, and 17.09% higher at 5, 10, and 15 days, respectively. This suggests that this coating may positively influence the reducing capacity of MDHA, leading to an increase in MDHAR activity. Elevated MDHAR activity, in turn, may contribute to the enhanced availability of ascorbic acid, a crucial substrate for the subsequent phase of the AsA–GSH cycle. This cascade of events potentially underscores the beneficial impact of the coating on the overall efficiency of the cycle and cellular antioxidant defense mechanisms [70].
DHAR catalyzes the conversion of dehydroascorbate (DHA) to its reduced form, ascorbic acid (AsA), utilizing reduced glutathione (GSH) as an electron donor [68]. Coating affected DHAR activity in the same way as MDHAR, increasing its activity by 38.28, 23.11, and 25.05% at 5, 10, and 15 days, respectively, compared to uncoated (Figure 3C). This suggests an improvement in AsA recycling efficiency, as increased DHAR activity implies a faster conversion of DHA to AsA. This process is important for maintaining high levels of AsA in the cycle, providing essential protection against oxidative stress.
GR is involved in the recycling of GSSG into GSH, using NADPH as a cofactor. In our study, coating caused an increase in GR activity compared to the uncoated fresh-cut melon throughout cold storage (Figure 3D) with significantly higher values in coated samples. The GR activity in coated fruit was 16.40%, 25.97%, and 20.79% higher at 5, 10, and 15 days, respectively, compared to the uncoated samples. High GR activity promotes the rapid recycling of GSSG into GSH, maintaining a high GSH/GSSG ratio, which is crucial for the cell’s antioxidant defense [68].
ROS are byproducts of cellular metabolism and are produced in small amounts under normal physiological conditions acting as signaling molecules [66]. In fresh-cut fruit, ROS levels become elevated due to cut-wounding processes causing membrane damage and reducing the shelf life [65]. In fresh-cut melons, higher ROS accumulation was found to be associated with higher respiration rates and ATP and ADP levels [71]. Hydrogen peroxide (H2O2) is one of the most common and stable ROS found in biological systems. This study showed that in the uncoated fresh-cut samples, there was a significant increase in H2O2 levels of about 17.6% compared to the coated samples at the end of cold storage. Exogenous coating treatments enhance the efficiency of the ascorbate–glutathione cycle, a crucial antioxidant pathway involved in the detoxification of ROS, as demonstrated in different fresh-cut fruits such as apple [72,73], pear [74], melon [14], peach [75], and avocado [70].
During cold storage, the enzymes involved in the AsA–GSH cycle exhibited different responses between coated and uncoated fresh-cut melon. Coating during storage enhanced the activities of SOD, APX, MDHAR, DHAR, and GR, resulting in reduced H2O2 content and increased AA content (Figure 4).
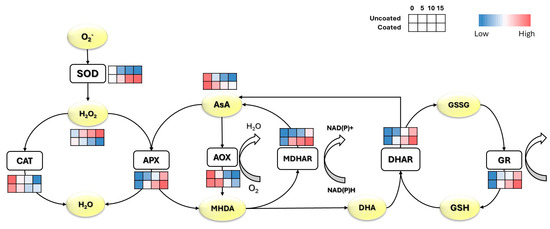
Figure 4.
Response of the fresh-cut melons AsA−GSH cycle under coating treatment during 15 days of cold storage at 4 °C. Each row in the heat map corresponds to one sample (first: uncoated; second: coated).
3.4. Multivariate Data Analysis
Two multivariate analysis techniques, namely PCA and PLS-DA, were employed to analyze traits in the coated and uncoated samples in fresh-cut melon during cold storage.
PCA revealed significant insights considering the dataset. The first two PCs explained a substantial portion of the variance, totaling 98.1%. This high cumulative variance elucidates that much of the variability within the data could be effectively summarized using these two PCs. Analyzing the contribution of each PC, PC1 explained 78.8% of the total variance in the dataset while PC2 accounted for an additional 19.3% of the variance (Figure 5A).
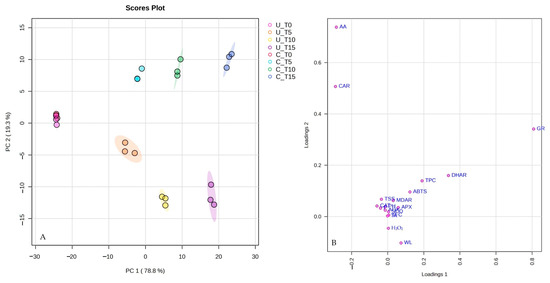
Figure 5.
PCA scores (A) and loading plots (B) of analyzed features on fresh-cut melon after 0 (T0), 5 (T5), 10 (T10), and 15 (T15) days of cold storage at 4 °C (U: uncoated and C: coated).
TPC, TFC, ABTS, APX, DHAR, and GR activities were positively correlated with PC1, whereas firmness, TSS, pH, TA, AA, CAR, CAT, SOD, AOX, and MDHAR activity were positively correlated with PC2. WL and H2O2 content were negatively correlated with PC2 (Figure 5B). Overall, PCA provided valuable insights into the behavior of the analyzed samples throughout the cold storage period, highlighting how their characteristics change and diverge over time in the coated and uncoated samples.
PLS-DA was employed to assess the relationship between the analyzed traits and two key factors such as edible coating and the duration of cold storage. The first two latent components that accounted for 98.1% of the total variance were integrated to realize a bi-plot (Figure 6A). Overall, the PLS-DA model demonstrated both good goodness of fit (R2Y = 0.76) and reliable predictive performance (Q2 (cum) = 0.71). These results suggest that the model is robust and capable of effectively capturing the relationships between predictor variables (traits) and response variables (presence or absence of an edible coating and duration of cold storage), thereby providing valuable insights and predictive capabilities for the analyzed dataset.
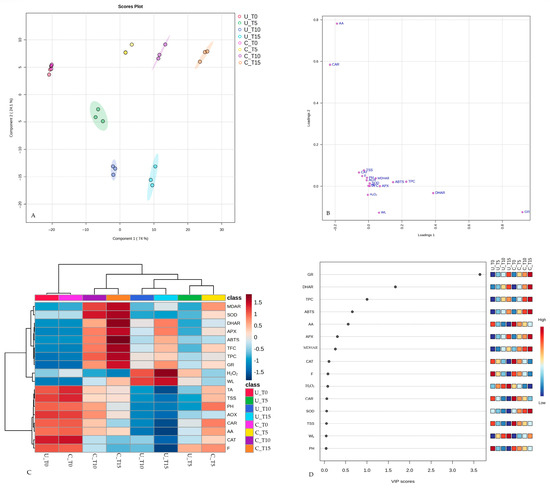
Figure 6.
PLS-DA score plot (A); PLS-DA score plot loaded with different analyzed traits (B); normalized heatmap (dark red hues indicate high levels and light blue hues indicate low levels) and dendrogram based on hierarchical clustering analysis of PLS-DA data (colored boxes on the right show the relative concentration of each analyzed trait) (C); VIP scores (D).
The GR and DHAR activity were more closely associated with edible coating, while CAR and AA were associated with storage time (Figure 6B). Hierarchical cluster analysis (HCA) was performed on the dataset of analyzed traits to visualize clusters of samples sharing similar features linked to the edible coating (Figure 6C). Heatmaps highlighted the levels of low and high values of the analyzed traits, resulting in a matrix for each sample. At the top of the heatmap, a cladogram revealed two primary clusters observed in the PLS-DA plot. The first cluster consisted of coated and uncoated samples at the beginning of the experiment, while the second cluster was composed of two subgroups: The first consisted of coated samples after 10 and 15 days of cold storage, while the other consisted of uncoated samples after 5, 10 and 15 days and coated sample after 5 days of cold storage. The outcomes of this analysis indicated that the dendrogram generated by HCA effectively discriminated between postharvest treatments in a storage-time-dependent manner.
The values of variable importance (VIP) scores allow for the identification of which variables (VIP > 1.0) contribute most significantly to the classification or discrimination achieved by the PLS-DA model. The top three analyzed traits with VIP scores greater than 1 were TPC, GR, and DHAR (Figure 6D). PCA and PLS-DA provided a clear differentiation between the coated and uncoated samples, highlighting the efficacy of coating in influencing analyzed attributes during cold storage. Multivariate analysis represents a valuable approach to elucidate the complex interactions between postharvest treatments and storage conditions in fruits. Furthermore, these techniques have been used to identify patterns and correlations within large datasets, elucidating the impact of postharvest treatments on fruit metabolism and quality attributes [72,74,76].
4. Conclusions
A layer-by-layer edible coating comprising sodium alginate, cedar mucilage, and calcium chloride is effective in extending the postharvest life in fresh-cut melon during cold storage. This innovative coating preserved the physicochemical traits, maintained the quality and nutraceutical attributes, and improved the antioxidant enzymatic system that regulates oxidative stress and the ascorbate–glutathione cycle.
Edible coatings are an economically feasible and eco-friendly tool for developing more effective postharvest management strategies to ensure the delivery of high-quality fresh-cut fruits to consumers. Coating treatments for the postharvest preservation of fresh-cut fruit offer a promising way to address both economic and environmental challenges in the food supply chain. By harnessing the benefits of coatings, stakeholders in the agriculture and food industry can contribute to global efforts to achieve sustainable food production and reduce food waste.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10050465/s1, Figure S1: Images of uncoated (U) and coated (C) fresh-cut melons during 15 days of storage at 4 °C.
Author Contributions
Conceptualization, M.P.; methodology, D.C., M.T.P. and E.F.; software, D.C., M.T.P. and E.F.; validation, D.C. and E.F.; formal analysis, D.C., G.C., M.T.P. and E.F.; investigation, D.C., G.C., M.T.P. and E.F.; resources, M.P.; data curation, D.C., M.T.P. and E.F.; writing—original draft preparation, D.C., M.T.P. and E.F.; writing—review and editing, M.P.; visualization, M.P.; supervision, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Decreto Direttoriale n. 1211 del 30 luglio 2020, Project n. ARS01_00640 “POFACS—Conservabilità, qualità sicurezza dei prodotti ortofrutticoli ad alto contenuto di servizio”, CUP B84E20000250005.
Data Availability Statement
Data is unavailable due to project privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Contini, C.; Boncinelli, F.; Gerini, F.; Scozzafava, G.; Casini, L. Investigating the role of personal and context-related factors in convenience foods consumption. Appetite 2018, 126, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Frewer, L.J.; Risvik, E.; Schifferstein, H. Food, People and Society: A European Perspective of Consumers’ Food Choices; Springer: Berlin/Heidelberg, Germany, 2001; p. 462. [Google Scholar]
- Testa, R.; Schifani, G.; Migliore, G. Understanding consumers’ convenience orientation. an exploratory study of fresh-cut fruit in Italy. Sustainability 2021, 13, 1027. [Google Scholar] [CrossRef]
- Dinnella, C.; Torri, L.; Caporale, G.; Monteleone, E. An exploratory study of sensory attributes and consumer traits underlying liking for and perceptions of freshness for ready to eat mixed salad leaves in Italy. Food Res. Int. 2014, 59, 108–116. [Google Scholar] [CrossRef]
- Goryńska-Goldmann, E.; Murawska, A.; Balcerowska-Czerniak, G. Consumer profiles of sustainable fruit and vegetable consumption in the European Union. Sustainability 2023, 15, 15512. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, M.; Yu, Q.; Mujumdar, A.S.; Yang, C. Fresh food quality deterioration detection and labeling: A review of recent research and application in Supply Chain. Food Bioprocess Technol. 2023. [Google Scholar] [CrossRef]
- Shinde, R.; Vinokur, Y.; Fallik, E.; Rodov, V. Effects of genotype and modified atmosphere packaging on the quality of fresh-cut melons. Foods 2024, 13, 256. [Google Scholar] [CrossRef]
- Mannozzi, C.; Glicerina, V.; Tylewicz, U.; Castagnini, J.M.; Canali, G.; Dalla Rosa, M.; Santina, R. Influence of two different coating application methods on the maintenance of the nutritional quality of fresh-cut melon during storage. Appl. Sci. 2021, 11, 8510. [Google Scholar] [CrossRef]
- Putnik, P.; Roohinejad, S.; Greiner, R.; Granato, D.; Bekhit, A.E.-D.; Bursać Kovačević, D. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A Review. Food Control 2017, 80, 411–419. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Tsironi, T.N. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Müller, C.T.; Rogers, H.J.; Pintado, M. Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef]
- Bett-Garber, K.L.; Greene, J.; Lamikanra, O.; Ingram, D.; Watson, M. Effect of storage temperature variations on sensory quality of fresh-cut cantaloupe melon. J. Food Qual. 2011, 34, 19–29. [Google Scholar] [CrossRef]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate–Chitosan combination. Food Bioprocess Technol. 2013, 7, 1424–1432. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Cabral, M.F.; Germano, T.A.; de Carvalho, W.M.; Brasil, I.M.; Gallão, M.I.; Moura, C.F.; Lopes, M.M.A.; de Miranda, M.R.A. Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Martiñon, M.E.; Moreira, R.G.; Castell-Perez, M.E.; Gomes, C. Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 °C. LWT Food Sci. Technol. 2014, 56, 341–350. [Google Scholar] [CrossRef]
- Moreira, S.P.; de Carvalho, W.M.; Alexandrino, A.C.; de Paula, H.C.; Rodrigues, M.; de Figueiredo, R.W.; Maia, A.G.; de Figueiredo, M.A.T.; Brasil, I.M. Freshness retention of minimally processed melon using different packages and multilayered edible coating containing microencapsulated essential oil. Int. J. Food Sci. Technol. 2014, 49, 2192–2203. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; Correa-Cerón, R.C.; Ortiz-Lechuga, E.G.; Solís-Arévalo, K.K.; Castillo-Hernández, S.L.; Gallardo-Rivera, C.T.; Arévalo Niño, K. Effect of Linseed (Linum usitatissimum) Mucilage and Chitosan Edible Coatings on Quality and Shelf-Life of Fresh-Cut Cantaloupe (Cucumis melo). Coatings 2019, 9, 368. [Google Scholar] [CrossRef]
- Yousuf, B.; Srivastava, A.K.; Ahmad, S. Application of natural fruit extract and hydrocolloid-based coating to retain quality of fresh-cut melon. J. Food Sci. Technol. 2020, 57, 3647–3658. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, J.F. The release kinetics of β-carotene nanocapsules/xanthan gum coating and quality changes in fresh-cut melon (cantaloupe). Carbohydr. Polym. 2017, 157, 1874–1882. [Google Scholar] [CrossRef]
- Selma, M.V.; Ibáñez, A.M.; Allende, A.; Cantwell, M.; Suslow, T. Effect of gaseous ozone and hot water on microbial and sensory quality of cantaloupe and potential transference of escherichia coli O157:H7 during cutting. Food Microbiol. 2008, 25, 162–168. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Raybaudi-Massilia Martínez, R.M.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of superatmospheric and low oxygen modified atmospheres on shelf-life extension of fresh-cut melon. Food Control 2008, 19, 191–199. [Google Scholar] [CrossRef]
- Botondi, R.; Moscetti, R.; Massantini, R. A comparative study on the effectiveness of ozonated water and peracetic acid in the storability of packaged fresh-cut melon. J. Food Sci. Technol. 2016, 53, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Lamikanra, O.; Kueneman, D.; Ukuku, D.; Bett-Garber, K.L. Effect of processing under ultraviolet light on the shelf life of fresh-cut Cantaloupe Melon. J. Food Sci. 2006, 70, C534–C539. [Google Scholar] [CrossRef]
- Tappi, S.; Gozzi, G.; Vannini, L.; Berardinelli, A.; Romani, S.; Ragni, L.; Rocculli, P. Cold plasma treatment for fresh-cut Melon Stabilization. Innov. Food Sci. Emerg. Technol. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent developments in edible films and coatings for fruits and vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Peerzada, G.J.; Sinclair, B.J.; Perinbarajan, G.K.; Dutta, R.; Shekhawat, R.; Saikia, N.; Chidambaram, R.; Mossa, A.-T. An overview on smart and active edible coatings: Safety and Regulations. Eur. Food Res. Technol. 2023, 249, 1935–1952. [Google Scholar] [CrossRef]
- Chong, J.X.; Lai, S.; Yang, H. Chitosan combined with calcium chloride impacts fresh-cut honeydew melon by stabilising nanostructures of sodium-carbonate-soluble pectin. Food Control 2015, 53, 195–205. [Google Scholar] [CrossRef]
- Waqas, A.; Butt, M.S. Application of biodegradable coatings to improve quality and shelf life of minimally processed melon dices. Pak. J. Food Sci. 2014, 24, 82–90. [Google Scholar]
- Riaie, S.; Saadatian, M.; Aghaie, M.; Alizadeh, M.; Hajitaghiloo, R. Application salicylic acid and aloevera gel as edible coating layer to preserving fresh-cut melon slices in cold storage. Int. Food Res. J. 2017, 24, 2456–2459. [Google Scholar]
- Sortino, G.; Inglese, P.; Farina, V.; Passafiume, R.; Allegra, A. The use of opuntia ficus-indica mucilage and aloe arborescens as edible coatings to improve the physical, chemical, and microbiological properties of ‘hayward’ kiwifruit slices. Horticulturae 2022, 8, 219. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; García, S.; Heredia, N.; Alanís-Guzmán, M.G.; Arévalo-Niño, K. Layer-by-layer edible coatings based on mucilages, Pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biol. Technol. 2017, 128, 63–75. [Google Scholar] [CrossRef]
- Ravindranath, V.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Optimization of extraction solvent and fast blue BB assay for comparative analysis of antioxidant phenolics from Cucumis melo L. Plants 2021, 10, 1379. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in Mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Petriccione, M.; De Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of Sweet Cherry during postharvest life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and B, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Diallinas, G.; Pateraki, I.; Sanmartin, M.; Scossa, A.; Stilianou, E.; Panopoulos, N.J.; Kanellis, A.K. Melon ascorbate oxidase: Cloning of a multigene family, induction during fruit development and repression by wounding. Plant Mol. Biol. 1997, 34, 759–770. [Google Scholar] [CrossRef]
- Hossain, M.A.; Asada, K. Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J. Biol. Chem. 1985, 260, 12920–12926. [Google Scholar] [CrossRef]
- Dalton, D.A.; Langeberg, L.; Treneman, N.C. Correlations between the ascorbate-glutathione pathway and effectiveness in legume root nodules. Physiol. Plant. 1993, 87, 365–370. [Google Scholar] [CrossRef]
- Hodges, D.M.; Toivonen, P.M.A. Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biol. Technol. 2008, 48, 155–162. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Lamikanra, O.; Chen, J.C.; Banks, D.; Hunter, P.A. Biochemical and microbial changes during the storage of minimally processed cantaloupe. J. Agric. Food Chem. 2000, 48, 5955–5961. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M. Postharvest Oxidative Stress in Horticultural Crops; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.; Muda, M.M.T. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Soo, K.L.; Wan Zunairah, W.I.; Radhiah, S. Prolonging the shelf life of fresh-cut guava (Psidium guajaya L.) by coating with chitosan and cinnamon essential oil. Heliyon 2023, 9, e22419. [Google Scholar] [CrossRef]
- Qi, H.; Hu, W.; Jiang, A.; Tian, M.; Li, Y. Extending shelf-life of fresh-cut ‘Fuji’ apples with Chitosan-coatings. Innov. Food Sci. Emerg. Technol. 2011, 12, 62–66. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, W.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT 2019, 115, 108447. [Google Scholar] [CrossRef]
- Guan, J.; Lacombe, A.; Rane, B.; Tang, J.; Wu, V.C. A review: Gaseous interventions for Listeria monocytogenes control in fresh apple cold storage. Front. Microbiol. 2021, 12, 782934. [Google Scholar] [CrossRef]
- Hu, W.; Guan, Y.; Feng, K. Biosynthesis of phenolic compounds and antioxidant activity in fresh-cut fruits and vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Rojas-Graü, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.H.; Martín-Belloso, O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Postharvest Biol. Technol. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Li, Y.; Hu, J.; Pang, L.; Fan, H. Browning inhibition and quality preservation of fresh-cut romaine lettuce exposed to high intensity light. Innov. Food Sci. Emerg. Technol. 2012, 14, 70–76. [Google Scholar] [CrossRef]
- Martinez-Hernandez, B.G.; Artes-Hernandez, F.; Gomez, P.A.; Formica, A.C.; Artes, F. Combination of electrolysed water: UV-C and superatmospheric O2 packaging for improving fresh-cut broccoli quality. Postharvest Biol. Technol. 2013, 76, 125–134. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent Mangoes. LWT Food Sci. Technol. 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Berno, N.D.; Tezotto-Uliana, J.V.; Santos Dias, C.T.; Kluge, R.A. Storage temperature and type of cut affect the biochemical and physiological characteristics of fresh-cut purple onions. Postharvest Biol. Technol. 2014, 93, 91–96. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velaìzquez, D.A. Plants as biofactories: Stress-induced production of chlorogenic acid isomers in potato tubers as affected by wounding intensity and storage time. Ind. Crops Prod. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gokmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 107, 1173–1179. [Google Scholar] [CrossRef]
- Chikhala, T.; Seke, F.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Utilizing Xanthan Gum Coatings as Probiotic Bacteria Carriers to Enhance Postharvest Quality and Antioxidants in Fresh-Cut Cantaloupe and Honeydew (Cucumis melo L.) Melons. Foods 2024, 13, 940. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Tremarin, A.; Garcia, E.; Brandão, T.R.S.; Silva, C.L.M. Quality Assessment of Cantaloupe Melon Juice under Ozone Processing. Innov. Food Sci. Emerg. Technol. 2018, 47, 461–466. [Google Scholar] [CrossRef]
- Modesti, M.; Zampella, L.; Petriccione, M. Chitosan mono- and bilayer edible coatings for preserving postharvest quality of fresh fruit. In Polymers for Agri-Food Applications, 1st ed.; Gutierrez, T.J., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 465–486. [Google Scholar]
- Zhang, H.Y.; Ma, Z.M.; Wang, J.J.; Wang, P.; Lu, D.Y.; Deng, S.F.; Lei, H.L.; Gao, Y.F.; Tao, Y.Y. Treatment with exogenous salicylic acid maintains quality, increases bioactive compounds, and enhances the antioxidant capacity of fresh goji (Lycium barbarum L.) fruit during storage. LWT Food Sci. Technol. 2021, 140, 110837. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B.; Rodríguez, S.; Cao, C.-M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Long, Q.; Gao, F.; Han, C.; Jin, P.; Zheng, Y. Effect of cutting styles on quality and antioxidant activity in fresh-cut Pitaya Fruit. Postharvest Biol. Technol. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M.L. Antioxidant defenses in plants with attention to Prunus and Citrus spp. Antioxidants 2013, 2, 340–369. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Magri, A.; Cice, D.; Capriolo, G.; Petriccione, M. Effects of ascorbic acid and melatonin treatments on antioxidant system in fresh-cut avocado fruits during cold storage. Food Bioprocess Technol. 2022, 15, 2468–2482. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, M.; Yang, X.; Xu, J.; Yu, Z. Effect of cutting on the reactive oxygen species accumulation and energy change in postharvest melon fruit during storage. Sci. Hortic. 2019, 257, 108752. [Google Scholar] [CrossRef]
- Magri, A.; Rega, P.; Capriolo, G.; Petriccione, M. Impact of novel active layer-by-layer edible coating on the qualitative and biochemical traits of minimally processed ‘Annurca Rossa del Sud’ apple fruit. Int. J. Mol. Sci. 2023, 24, 8315. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, Z.; Wu, J.; Zhu, S. Effects of pre-treatment with S-nitrosoglutathione-chitosan nanoparticles on quality and antioxidant systems of fresh-cut apple slices. LWT 2021, 139, 110565. [Google Scholar] [CrossRef]
- Magri, A.; Landi, N.; Capriolo, G.; Di Maro, A.; Petriccione, M. Effect of active layer-by-layer edible coating on quality, biochemicals, and the antioxidant system in ready-to-eat ‘Williams’ pear fruit during Cold storage. Postharvest Biol. Technol. 2024, 212, 112873. [Google Scholar] [CrossRef]
- Aboryia, M.S.; El-Gioushy, S.F.; Sami, R.; Aljumayi, H.; Alyamani, A.; Almasoudi, A.; Gawish, M.S. Synergistic Effect of Dipping in Aloe Vera Gel and Mixing with Chitosan or Calcium Chloride on the Activities of Antioxidant Enzymes and Cold Storage Potential of Peach (Prunus persica L.) Fruits. Coatings 2022, 12, 498. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Di Matteo, M. Effects of Passive Modified Atmosphere Packaging on Physico-Chemical Traits and Antioxidant Systems of ‘Dottato’ Fresh Fig. Horticulturae 2022, 8, 709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
