Abstract
The effect of three levels of salinity on physio-biochemical traits in 10 Alcea rosea (hollyhock) varieties were evaluated. It was observed that salt stress increased both the total phenolic content (TPC) and total flavonoid content (TFC) in some varieties and decreased them in others. The greatest increases in both TPC and TFC were recorded in the Saman variety (104% and 62%, respectively) when cultivated under severe salt stress, indicating that this is the most salt-tolerant variety amongst those tested. The most abundant phenolic compound recorded was ellagic acid, and the phenolic compounds that showed the greatest increases in concentration due to salt stress were p-coumaric acid (87% in the Isfahan variety) and chlorogenic acid (142% in the Mahallat variety). Salt stress was also shown to decrease the production of diphenyl-2-picrylhydrazyl (DPPH) in all varieties. The highest concentration of DPPH (133%) was recorded in the Shiraz 1 variety, grown under conditions of severe salt stress. Salt stress also increased the mucilage content present in the petals, leaves, and seeds of some of the selected varieties. These data suggest that the selection of salt-tolerant varieties of hollyhock for direct cultivation or for use in future breeding programs is feasible.
1. Introduction
Saline soils and groundwater pose a serious environmental threat to global food security [1]. Unfortunately, the area of arable land in which salinity is problematic is expanding rapidly due to increases in evaporation and decreases in precipitation as a result of global climate change [2], the use of saline irrigation, recycled wastewater, lack of proper drainage, excessive fertilizer use, and the global spread of desertification [3,4,5].
High soil salinity leads to ion toxicity, ion imbalance, reduction of osmotic potential, and deterioration of the physical structure of soil [6]. These phenomena have subsequent effects upon the morphology, physiology, and biochemistry of crop species when subjected to salt stress during their cultivation. This results in a reduction in both crop quality and yield [5,7,8].
In response to such stress stimuli, plant cells often produce a range of phenolic compounds, as these low-molecular-weight non-enzymatic antioxidants can aid in the scavenging of reactive oxygen species (ROS) [9]. These phenolic compounds have been shown to be effective in protecting biological systems against various oxidative stresses, playing a crucial role in the maintenance of redox homeostasis [10]. Therefore, the adaptation of plants to salt stress is likely to be influenced by the homeostasis between ROS and the production of phytochemicals such as polyphenols and flavonoids.
Phenolic compounds, including flavonoids, are the most abundant secondary metabolites in the plant kingdom. These compounds play numerous biochemical and molecular roles in plants, including cell signaling, plant defense, mediation of auxin transport, antioxidant activity, and free radical scavenging [11]. Several mechanisms have been proposed to explain the observed increase in secondary metabolites when plants are exposed to abiotic stressors, such as high levels of salinity. According to the growth-defense trade-off hypothesis, the energy and carbon assimilated by photosynthesis are primarily consumed for cell maintenance and plant growth, while the remainder is used for the synthesis of defense chemicals. A second mechanism proposed by Kleinwächter and Selmar (2015) is that under drought and salt stress, stomatal closure limits CO2 influx into leaves; thereby reducing the Calvin cycle and consequently, NADPH consumption. Therefore, the stressed plant redirects its metabolic pathways towards the synthesis of highly reduced compounds, i.e., phenols, isoprenoids, or alkaloids, in order to prevent the formation of ROS and thus avoid an over-reduced physiological cell state [12].
A variety of agricultural management practices, such as flushing and leaching, have been used to reduce the damage caused by salt stress, but these treatments are both costly and slow in terms of implementation [2,5]. Therefore, there is a need to develop and apply new and more economically viable strategies to mitigate the negative effects of high salinity in the salt-stressed regions of the world. One such approach is to select salt-tolerant varieties within the crop species of interest. Using this approach, Grieve et al. (2012) and Ashrafi et al. (2018) screened several plant species under different levels of soil salinity and subsequently, categorized them as very sensitive, sensitive, moderately sensitive, moderately tolerant, tolerant, and very tolerant to salt [6,8]. This approach has also been used in barley to identify salt-tolerant genotypes of this species [2,7].
An alternative approach is to cultivate aromatic and medicinal plant species of high economic value, whose levels of agronomically important secondary metabolites are known to increase under salt stress. Salt stress has been shown to induce an accumulation of proline, total phenolics, and other antioxidants in Rosmarinus officinalis. The phenolic content of leaves of this species was shown to increase significantly at 50 mM NaCl (from 0.91 to 1.59), to 4.22 at 100 mM NaCl, and then to decrease at 150 mM NaCl (to 3.47). The phenolic content in different tissues of Mentha pulegium L. from plants cultivated under different levels of salt stress has also been reported. The phenolic content of the leaves of plants grown under salt-stressed conditions was about 3.5 times higher than that of the control [9]. In addition, the results of a study on basil (Ocimum basilicum L.) showed that the vegetative growth of this species was reduced with increasing salinity, and a concomitant increase in the production of phenolic compounds was observed [13].
The observed increase in the production of secondary metabolites induced by salt stress in some medicinal plant species suggests that the cultivation of salt-tolerant medicinal plants may be an economically viable option for the use of marginal lands with saline soil and/or groundwater. The subject of the current study, Alcea rosea L. (hollyhock), is an aromatic, perennial, horticultural species, whose roots, seeds, shoots, and flowers contain aromatic secondary metabolites that are widely used in developing countries to alleviate symptoms of cough and sore throat, abdominal pain, and flatulence [14].
Hollyhock is physiologically well adapted to the hot and dry conditions prevalent in the majority of Iranian arable land. Bearing in mind the limited ‘clean’ water resources and the widespread problem of salt-contaminated irrigation water, the cultivation of plants that are able to withstand salt stress is very relevant to the current agricultural situation in Iran. Many studies have been published describing the unique medicinal properties of this species [15]. However, very little has been published on the physiological response of hollyhock to salt stress or on the biosynthesis of the secondary metabolites in this species.
Therefore, the main objective of the current study is to investigate the effect of salt stress on the production of mucilage, phenolic acids, and flavonoid acids in a selection of indigenous Iranian hollyhock varieties to provide physiological data on which to base the future selection of salt-tolerant genotypes associated with high levels of agronomically important secondary metabolites.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
A total of 10 Iranian hollyhock varieties were used in the current study (Isfahan, Khafr, Khomeini Shahr, Mahallat, Mashhad, Saman, Shahin Shahr, Shiraz 1, Shiraz 2, and Tabriz). Their seeds were harvested in 2017. The varieties and their main characteristics are listed in Table 1. For cultivation of the plants, the seeds were first soaked in water overnight prior to planting. Here, the objective was to loosen the testa of the seed to facilitate optimal seed germination. The seeds were then disinfected with sodium hypochlorite (5 g L−1) for 5 min and washed with distilled water to remove any residual disinfectant. The treated seeds were then planted.

Table 1.
Geographic origin and floral phenotype of selected hollyhock varieties.
2.2. Field Experiments
The field experiments were conducted over two growing seasons (17 March to 21 November 2019 and 11 March to 5 December 2020) at the Agricultural Research Center of the College of Agriculture, Isfahan University of Technology, located in Lavark, Nejaf-Abad, Iran (latitude: 32°32′ N; and longitude: 51°23′ E; altitude: 1630 m). The annual rainfall and average temperature in this region were 126 mm and 18.7 °C and 133.6 mm and 18.3 °C in 2019 and 2020, respectively. Before planting, an analysis of the characteristics of the farm soil (at the test site) was carried out by sampling at a depth of 60 cm, and the results were as follows. The soil at the research center was shown to be a fine loam with pH = 7.5, EC = 1.8 dS m−1. bulk density = 1.47 g cm−3, soil organic C content = 0.7%, and P and K contents = 7.4 and 45.6 mg kg−1, respectively. After cultivation, an analysis of the characteristics of the field soil (at the test site) was performed by sampling at a depth of 60 cm and 30 cm, and the results were as follows. At a depth of 30 cm, the pH = 7.6, EC = 11.37 dS m−1, soil organic C content = 0.7%, and P and K contents = 32.4 and 41 mg kg−1, respectively, and at a depth of 60 cm, the pH = 7.52, EC = 7.22 dS m−1, soil organic C content = 0.81%, and P and K contents = 25.3 and 32 mg kg−1.
Three salinity levels (control (EC = 0.1 mM NaCl), moderate (100 mM NaCl) and high salinity (180 mM NaCl)) and 10 hollyhock varieties were used in this investigation. Since no previous studies on the effect of salinity stress on the measured traits in hollyhock had been published, we decided to use the levels of salinity used in previous studies of other members of the Malvaceae family–i.e., control, moderate and severe levels of salinity stress.
In our study, the 10 hollyhock varieties were evaluated using a randomized complete block design with three replications. An analysis of variance (ANOVA) was performed using Proc GLM in the SAS 9.2 software to examine the differences among the three levels of salinity, variety, growing season, and their interactions. Treatment means were compared using the least significant difference (LSD) test at p ≤ 0.05.
The total number of experimental units was 90 (3 × 3 × 10), with each unit occupying an area of 3.6 m2. The number of plants in the plots was the same. The plot area was considered to be appropriate for this study because this plot size left enough plants for measurements after removing for the margin effect. Each plot consisted of five rows, which were 150 cm long, with row spacing of 60 cm and 30 cm between plants (Figure 1a,b). For the control treatment, the plants were irrigated with fresh water (EC = 0.1 mM NaCl) throughout the experiment, and the salinity treatments were subsequently applied to the experimental plants at the eight-leaf stage of development. Each experimental plot received the same quantity of irrigation for both the control and experimental conditions. The experimental plots were irrigated using an upstream tank that delivered a stock solution of sodium chloride (1 mM NaCl) via a calibrated flowmeter to maintain the desired salt concentration (100 mM NaCl and 180 mM NaCl). Plots were considered fully watered when the soil moisture in the root zone exceeded 80% of field capacity (ψ = −0.06). Soil EC (electrical conductivity) at 0–40 cm soil depth was measured at harvest for all treatments. The observed mean soil EC values were 2.5, 6.2, and 11.3 dS m−1 for the control and saline field conditions, respectively.

Figure 1.
Growth stages of hollyhock varieties to illustrate different growth stages of plants in the two consecutive years of this study. (a) The first growing season: May, June, July, and September 2019 with budding, flowering, seed ripening, and ready-to-harvest seeds, respectively. (b) The second growing season: regrowth of bushes in March, rapid growth of bushes in April, budding in May, and flowering in June 2020. (c) Photographs illustrating the different floral phenotypes of the selected hollyhock (Alcea rosea L.) varieties.
Sampling was performed after the symptoms of salinity stress appeared on the plants, which occurred about six weeks after the stress was applied. To carry out the sampling, first the plants on the margin were omitted, and plants that were representative of the plot were selected. For example, the semi-dried petals harvested from the field were completely dried in a closed space away from direct sunlight and at room temperature and stored as whole petals in glass containers. One day before the analyses, the petal samples were ground. Five samples were collected from each plot to measure all the traits except petal yield, and in laboratory conditions, the experiments were performed with three repetitions.
2.3. Assessment of Total Phenolic Content (TPC)
The total phenolic content of the petal extracts was determined using Folin–Ciocalteau reagent and external calibration with tannic acid [11]. The results were expressed as tannic acid equivalents per gram dry weight (mg TAE g−1 DW).
For this method, 10 g of petals were ground into a powder and extracted with 200 mL of 80% methanol in an orbital shaker (150 rpm) for 24 h at 25 °C. The extraction process was then repeated three times, and the extracts were filtered. Tenfold diluted Folin–Ciocalteau reagent (2.5 mL), methanolic extract (0.5 mL), and 7.5% sodium carbonate (2 mL) were mixed and then heated for 15 min at 45 °C, after which the absorbance was read at 765 nmm. TPC was expressed as tannic acid equivalents per gram dry weight of the sample.
High Performance Liquid Chromatography (HPLC) of Phenolic Compounds
The petal samples were dried, ground, and extracted with methanol (80% v/v), and the extract was then analyzed using the HPLC (model Agilent 1090, Santa Clara, CA, USA). Briefly, three grams of powdered petal was extracted in methanol (HPLC grade, Merck, Darmstadt, Germany), which was later removed from the combined extracts via evaporation under reduced pressure to dryness. For this purpose, the methanol dried extract was dissolved in 80% (v/v) methanol. The extracts were then filtered through Whatman No.1 filter paper. Finally, the extract was filtered through a 0.22 μm nylon disk filter, and 20 μL of the filtered extract was injected onto an HPLC column and the chromatogram was recorded at 260–330 nm.
Prior to injection into the analytical HPLC system, all the standards (gallic acid, caffeic acid, p-coumaric acid, ferulic acid, ellagic acid, rosmarinic acid, syringic acid, vanillic acid, 4-hydroxybenzoic acid, chlorogenic acid, luteolin, quercetin, rutin, and apigenin) were dissolved in methanol at concentrations ranging from 0.2 to 20 mg/L. HPLC grade methanol was used to dissolve the standards. The stationary phase consisted of a 250 mm × 4.6 mm (5 μm) C18 column (Waters Crop., Milford, MA, USA) (10 mm × 4 mm I.D.), and the mobile phase consisted of formic acid (0.1% v/v) in acetonitrile (99.8% v/v) at a flow rate of 0.8 mL/min with the wavelength adjusted to 200–400 nm. The mobile phase consisted of a combination of A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The performance of the gradient conditions was characterized by the following specifications: a linear step from 10% to 26% solvent B (v/v) for 40 min, followed by 65% solvent B for 70 min, and finally to 100% solvent B for 75 min. The phenolic and flavonoid determinations were reported as μg per g dry weight. The standard curves of the identified compounds are shown in Figure 2.
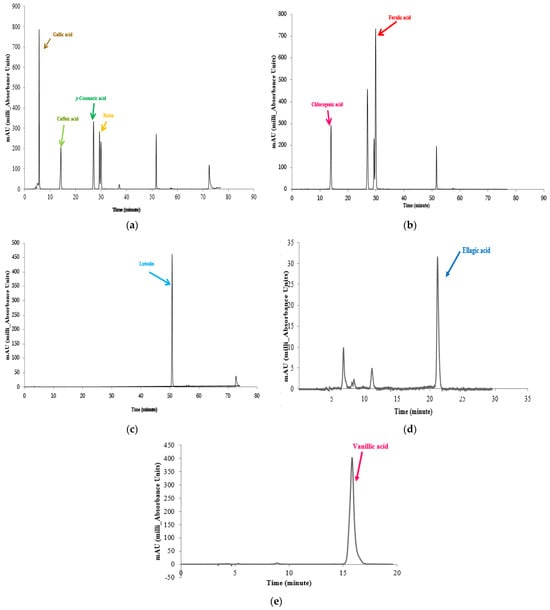
Figure 2.
Standard curves for the composition of gallic acid, caffeic acid, p-coumaric acid and rutin (a). Standard curve for the composition of chlorogenic acid and ferulic acid (b). Standard curve for the composition of luteolin (c). Standard curve for the composition of ellagic acid (d). Standard curve for the composition of vanillic acid (e).
2.4. Assessment of Total Flavonoid Content
The determination of total flavonoid content was based on the formation of a flavonoid-aluminum complex, according to the method by Rahimmalek and colleagues with minor modifications [16].
In this procedure, 0.5 mL of the methanolic extract was mixed with distilled water (2 mL) and NaNO2 solution (5%, 0.15 mL). After 6 min, an AlCl3 solution (10%, 0.15 mL) was also added and allowed to stand for 6 min before an NaOH solution (4%, 2 mL) was added to the solution thus obtained.
Finally, distilled water was added to the mixture (5 mL) and allowed to stand for 15 min, and the absorbance was recorded at a wavelength of 510 nm. The total flavonoid content (TFC) was expressed as quercetin equivalents per gram dry weight (mgQUEg−1 DW).
2.5. Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Assay
The antioxidant activity of the petal tissues was determined using the DPPH assay, as described by Gul et al. (2017) [17] with some minor modifications. In this experiment, the stable purple-colored chemical compound, 2, 2-diphe nylpicrylhydrazyl (DPPH), was used to determine free radical-scavenging ability. In brief, for the antioxidant activity assessments, the extracts were diluted in pure methanol at various concentrations including 50, 100, 300, and 500 mg/L before the DPPH solution (0.1 mM, 5 mL) was mixed with 100 μL of the diluted extracts. Each mixture thus obtained was then shaken vigorously and incubated for 30 min at room temperature in the dark for completion of the reaction. A mixture consisting of methanol plus the DPPH solution was used as a negative control, and BHT was employed as the positive control compound for comparison. Finally, radical scavenging activity was expressed as IC50 (μg mL−1)—the antiradical dose required to cause a 50% inhibition. The absorbance of the samples was measured using a UV-visible spectrophotometer (U-1800, Hitachi, Tokyo, Japan) at 517 nm with methanol (80% v/v) as a blank. The inhibition percentage was plotted against the sample concentration, and 50% of the IC50 (inhibitory concentration) of the DPPH values was defined using a linear regression analysis.
2.6. Mucilage Content
Petal, leaf, and seed samples of the hollyhock varieties were air dried and ground in a mortar and pestle. The mucilage content of these samples was then estimated according to the technique reported by Kalyanasundaram et al. (1980) [18]. For each sample (petal, leaf, and seed), ten milliliters of 0.1 N HCl was heated to boiling in a l00 mL Coming flask. The flask was then removed from the flame, and 1 g of the dry sample was added. Heating was resumed and when all samples (petal, leaf, and seed) had changed color, the flask was removed from the flame and the samples were filtered through Whatman No. 1 filter paper while still hot. To separate residual traces of mucilage, the samples were washed twice in 5 mL of hot water, and the solution obtained was filtered. The combined filtrate—containing the dissolved mucilage—was mixed with 60 mL of 95% (v/v) ethanol and stirred and allowed to stand for 5 h at room temperature. Finally, the supernatant was decanted, and the precipitate was oven dried at 50 °C for 48 h. The weight of the dry precipitate was taken as the total mucilage content.
2.7. Petal Yield
Because hollyhock is an indeterminate plant, flowers were harvested throughout the growing season during both years of cultivation in order to estimate the petal yield of the plants (Figure 1c). Flowers were harvested from each plant twice a week for approximately 7 months. The harvested flowers were then air dried for a period of 4 d. This procedure was carried out continuously until the end of the flowering stage of development. After drying, the weight of the flowers was measured. At the end of the growing season and at the end of the flowering stage, the petal yield was obtained from the sum of the weights of the petals collected during the growing season.
2.8. Statistical Analysis
The recorded data were subjected to an analysis of variance (ANOVA), and the least significant difference (LSD) test for the comparison of means using SAS software (ver. 9.2). The experiment was conducted as a randomized complete block design with three replications. Data on the biochemical characteristics, mucilage content, and flower yield obtained in the three replications over the two study years were combined, while data on the phenolic acid composition were those obtained in one growing season with two replications. A principal component analysis (PCA) was performed and correlation coefficients were obtained using GraphPad software (Prism version 9.0, San Diego, CA, USA).
3. Results
3.1. Total Phenolic Content
Table 2 shows the analysis of variance (ANOVA) for the biochemical characteristics, mucilage content, and flower yield of the hollyhock varieties studied under control and salt-stressed conditions in both 2019 and 2020. The interaction of year and stress, the interaction of variety and stress, and the interaction of stress and year were all shown to be significant at the 0.1 probability level (Table 2).

Table 2.
Analysis of variance (ANOVA) for the biochemical properties, mucilage content, and flower yield of the hollyhock varieties studied under control and salt-stress conditions in 2019 and 2020.
The total quantity of phenolic compounds was observed to increase by 16% and 21% in 2019 and 2020, respectively, in plants cultivated under conditions of severe salt stress when compared to plants grown under control conditions. As the length of the period of salt stress increased, the level of phenolic compounds was also observed to increase. Hence, the highest increase in phenolic compounds was observed in the second year of cultivation (Table 3).

Table 3.
Mean comparisons of stress and agronomic years (interaction) in relation to traits of hollyhock varieties.
Table 4 shows the interaction between plant variety and level of salinity upon the phenolic acid composition of the plant tissues. As mentioned above, the response to salt stress varied depending on the variety and the salinity level to which the plants were exposed (Table 4). In the majority of varieties, the quantity of phenolic compounds was observed to increase when the plants were grown under conditions of both moderate and high salt stress when compared to the control (Table 4).

Table 4.
Mean comparisons of stress and varieties (interaction) in relation to traits of hollyhock varieties in 2019 and 2020.
With increasing salt stress, an increased total phenolics content was observed in the majority of varieties. The largest increase was observed in the Saman variety, in which there was a 104% increase in the level of phenolics produced under high salt stress compared to the control. The smallest increase was observed in the Shahin Shahr variety, with an increase of 7%. In some varieties, such as Tabriz and Isfahan, no significant difference was observed between severe salt stress and control conditions. It is of interest to note that in some genotypes, a reduction in the amount of total phenolics was observed with increasing salt stress, and the largest decrease was observed in the Shiraz 1 variety with a 41% reduction. The highest quantity of TPC was observed in the Saman variety grown under severe salt stress with 79.12 mg TAE g−1 DW (Table 4). However, in half of the varieties tested, the total amount of phenolic compounds present increased in the second year compared to the first year of cultivation. The largest increase in the total phenolic compound content was observed in the Khomeini Shahr and Khafr varieties, which increased by 42% and 43%, respectively, while in the Mashhad, Saman, Shahin Shahr, Isfahan, and Shiraz 2 varieties, no significant difference was observed between the two growing seasons. In addition, a 25% decrease was observed in the Tabriz variety (Table 5).

Table 5.
Mean comparisons of agronomic years and variety (interaction) in relation to traits of hollyhock varieties.
In this study, gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ellagic acid, ferulic acid, vanillic acid, and ellagic acid were the most abundant phenolic acids detected. For illustration purposes, an example chromatogram of one variety of hollyhock is depicted in Figure 3, in which phenolic and flavonoid determinations are reported as mg per 100 g of the sample’s dry weight. The interaction of variety and salt stress upon the production of gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, vanillic acid, ellagic acid, luteolin, and rutin was shown to be significant at the 1% probability level (Table 6).
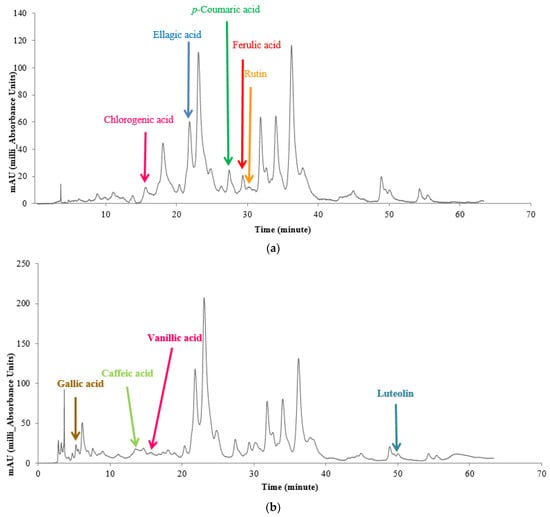
Figure 3.
HPLC chromatographic separation of methanolic extract of Alcea rosea L. in 330 nm (a) and 270 nm (b). The chromatogram of a sample grown under severe salt stress showing the identified peaks of gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, vanillic acid, ellagic Acid, luteolin, and rutin compounds.

Table 6.
Analysis of variance of phenolic acids and flavonoids of the hollyhock varieties studied under control and salt-stress conditions.
The gallic acid levels were observed to range from 7.66 (Mashhad under control conditions) to 30.92 (Mahallat under severe salt stress) mg per 100 gDW (Table 7). The gallic acid content was observed to increase by 119% in Mahallat and decrease by 69% in Tabriz under severe salt stress (Table 7). The chlorogenic acid content ranged from 3.86 (Khomeni Shahr under control conditions) to 27.7 (Shiraz 1 under control conditions) mg per g DW. Chlorogenic acid increased by 1143% in Mahallat but decreased by 63% in Shiraz 1 under high salt stress (Table 7). The caffeic acid content ranged from 15.76 (Mashhad under high salinity stress) to 152.18 (Shiraz 1 under control conditions) mg per 100 g DW under our experimental conditions. In the Tabriz variety, this level increased by 53% and in Khafr and Shiraz 1, it decreased by 74% under the highest salt stress treatment (Table 7). The levels of p-coumaric acid ranged from 3.9 mg per 100 g DW (dry weight) (Shiraz 2 under control) to 26.62 (Isfahan under moderate salt stress). The largest increase (126%) was observed in the Mahallat variety, while the largest decrease (21%) was observed in the Khafr variety under severe salt-stress conditions (Table 7). The ferulic acid levels ranged from 8.28 (Mashhad under severe salt stress) to 51.04 (Saman under control conditions) mg per 100 g DW. The ferulic acid levels increased by 210% in Shiraz 2 under severe salt stress and decreased by 79% in Khafr also under severe salt-stress conditions (Table 7). Vanillic acid ranged from 12.24 (Mashhad under control conditions) to 151.66 (Shiraz 1 under control conditions) mg per 100 g DW (Table 7). The largest increase (183%) was recorded in Shiraz 2 under high salt stress, and the largest decrease (83%) was recorded in Shiraz 1 also under high salinity stress conditions. Under our experimental conditions, the ellagic acid content ranged from 87 (Shahin Shahr under high salinity stress) to 969.6 (Tabriz under high salinity stress) mg per 100 g DW. Its level increased by 237% in Tabriz under the highest salt-stress conditions but was shown to be reduced by 76% in Shahin-Shahr also under severe salt-stressed conditions (Table 7).

Table 7.
Individual phenolic acid and flavonoid contents of the petals of the hollyhock varieties cultivated under control and salt-stress conditions.
3.2. Total Flavonoid Content (TFC)
The interaction between stress and year, variety and total flavonoid content, and total flavonoid content and year were all shown to be significant at the 1% probability level (Table 2). In the data presented, it was observed that the total flavonoid content increased with increasing salinity in both growing seasons. In 2019 and 2020, increases of 17% and 11% were recorded in plants grown under severe salt stress compared to the control. However, no statistically significant difference was observed between plants grown under severe salt stress during the two-year period (Table 3).
TFC was shown to increase in most varieties and decrease in the remainder, except for Shiraz 1. The largest increase (62%) was obtained in the Saman genotype, while the largest decrease (22%) was observed in Shiraz 1, grown under high salt-stress conditions. No significant difference was observed in the Tabriz variety. The highest amount of TFC was observed in the Mahallat variety with 18.23 mg QE g−1 DW in plants grown under severe salt stress (Table 4).
The total flavonoid content in half of the varieties increased in the second year compared to the first year of cultivation. The highest rate of increase was recorded in the Mahallat (22%) and Shahin Shahr (21%) varieties, and the lowest (4.5%) was observed in the Tabriz variety. The total flavonoid content recorded in the Khomeini Shahr variety was reduced by 29% in the second year compared to the first year (Table 5). In summary, these data indicate that of those tested, the Saman variety is the most salt tolerant.
3.3. Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Assay
IC50 is defined as the concentration of antioxidant required to reduce the initial concentration of DPPH by 50% [19] and is a widely used measure to quantify the antioxidant activity present in biological samples. Thus, the lower the IC50 value, the higher the antioxidant activity. In the present study, it was observed that the IC50 value decreased, which means the antioxidant activity increased in both growing seasons.
The interaction of year and stress was significant at the 5% probability level, and the interaction of stress and variety and the interaction of year and variety were significant at the 1% probability level (Table 2). A decrease of 42% and 35% was observed in plants cultivated under severe salt stress when compared to the control conditions in 2019 and 2020, respectively. The largest reduction was recorded under high salt stress in the second growing season, but the difference between the moderate and severe salt stress levels in the two years was not statistically significant (Table 3).
A consistent decrease in the IC50 value was observed under salt-stressed conditions, and this decrease was shown to be variety specific (Table 4). In most varieties, the amount of DPPH recorded under moderate and severe salt-stress conditions decreased compared to the control (Table 4). The lowest IC50 values (highest antioxidant activity) were recorded in Shiraz 1, Mashhad, and Shiraz 2 under control, moderate, and extreme salt-stress conditions, respectively. The largest decrease in IC50 values (71%) were recorded in the Saman variety grown under severe salt-stress conditions and in the Mahallat variety (58%) when grown under moderate salt-stress conditions. The highest increase (133%) was recorded in Shiraz 1 under severe salt stress. The lowest amount of DPPH was observed in the Shahin Shahr variety grown under severe salt-stress conditions at 270 mg mL−1 (Table 4). In the majority of varieties, a decrease in the amount of DPPH was observed and in the Isfahan, Mashhad, and Shahin Shahr varieties, there was no significant difference between the second year compared to the first year of the experiment (Table 5).
3.4. Mucilage Content
The interaction between stress and year in relation to seed mucilage content was shown to be significant at the 1% probability level. In addition, the interaction of stress and variety in relation to petal, leaf, and seed mucilage content was significant at the 1% probability level (Table 2). The seed mucilage content decreased with increasing stress in the two growing seasons. The content of seed mucilage in plants grown under severe salt stress (compared to the control level) in 2019 and 2020 decreased by 59% and 51%, respectively. However, the difference between these two values was not statistically significant (Table 3). With few exceptions, salt stress was observed to increase mucilage content in the petals, leaves, and seeds of plants grown under severe salt stress compared to the control. Mucilage accumulation was shown to be the highest in petal tissues, followed by leaves and seeds, respectively (Table 4). The highest petal mucilage content was recorded in the Khomeini Shahr, Isfahan, and Shahin Shahr varieties when plants were grown under control, moderate, and severe salt stress, respectively (Table 4). As the salinity level increased, the mucilage content of the plants increased. The highest increase (182%) in petal mucilage was recorded in the Mashhad variety. The smallest increase in petal mucilage was observed in the Khafr variety with a 15% increase. The largest increase in petal mucilage was observed in the Khomeini Shahr variety grown under severe salt stress, for which a 23.1% increase was recorded. The largest increase in leaf mucilage (72%) was observed in the Tabriz variety, while the highest increase in seed mucilage (89%) was measured in the Khafr and Isfahan varieties, which were all grown under severe salt stress. Leaf mucilage increased in all varieties, except the Khomeini Shahr, which decreased by 17% compared to the control. The most leaf mucilage was observed in the Khafr variety under severe salt stress with a 12.33% increase. The lowest rate of seed mucilage increase was observed in the Shahin Shahr variety with an 11% increase. The highest rate of seed mucilage was observed after severe salt-stress treatment in the Khafr variety with 5.4% seed mucilage (Table 4).
3.5. Petal Yield
In terms of petal yield, the interaction of salt stress and variety is significant at the 1% probability level, and the interaction of year and stress is significant at the 5% probability level (Table 2).
It was observed that the petal yield decreased with increasing salinity (Table 4). Under moderate salt stress, the highest reduction (31%) was observed in the Isfahan and Tabriz varieties, while the lowest (11%) was observed in the Khafr variety. However, under high salt stress, the highest (56%) and lowest (45%) reductions were observed in the Isfahan and Khafr varieties, respectively (Table 4). The highest petal yield in the Isfahan variety was observed under control conditions with a yield of 748 kg per ha, and the lowest petal yield was observed in plants grown under severe salt stress with 268 kg per ha (Table 4).
In all varieties, the petal yield increased in the second year compared to the first year. The largest increase in the second year compared to the first year was observed in Shiraz 1 with an increase of 51%, and the lowest increase was recorded in Mahallat with an increase of 18% (Table 4).
3.6. Effect of Salinity Stress Levels on Secondary Metabolites
Figure 4A–C shows the effect of salt stress on the recorded traits including TPC, TFC, gallic acid, chlorogenic acid, p-coumaric acid, ferulic acid, rutin, and luteolin. In general, the results show that moderate salt stress results in an increase in TPC, TFC, chlorogenic acid, p-coumaric acid, and rutin compared to the control levels. Meanwhile, severe salt stress led to decreases in TPC, TFC, rutin, gallic acid, chlorogenic acid, and ferulic acid compared to the control and moderate salt stress. When plants grown under severe salt stress were compared to those grown under moderate stress, the maximum reduction was observed in the levels of ferulic acid (51%), rutin (43%), and chlorogenic acid (28%), respectively. The extent of the reduction in TPC observed in plants exposed to severe salt stress compared to the control was 27%. In fact, although the level of these traits increased under moderate salt stress when compared to the control plants; severe salt stress led to a decrease in the amounts of these compounds.
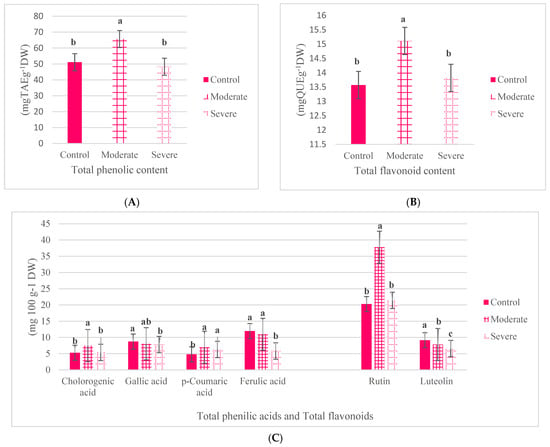
Figure 4.
The effect of salt stress on secondary metabolite production. The effect of salt stress on TPC (A), on TFC (B) and chlorogenic acid, gallic acid, p-coumaric acid, ferulic acid, rutin, and luteolin (C). Significance (p < 0.05) is indicated by different letters.
In Figure 4A,B, the observed increase in the levels of TPC, TFC, chlorogenic acid, and rutin in plants exposed to severe salt stress compared to the control is well known. However, there are exceptions for other traits. For example, the amount of ferulic acid was higher in the control than in plants grown under conditions of moderate (but not significant) and severe salt stress, respectively. The level of gallic acid produced in plants grown under control conditions was higher than those under severe salt stress. However, the levels observed at moderate salt stress were not significantly different from the control and those recorded in plants grown under high salt stress. Salt stress does not seem to have had a statistically significant effect on gallic acid synthesis. The level of p-coumaric acid also increased under moderate and severe salt stress compared to the control. However, no significant difference was observed between the amounts of p-coumaric acid produced under conditions of moderate and severe salt stress (Figure 4C). Given the effect of salt stress on TPC and TFC and the phenolic and flavonoid compounds included in our study, it can be concluded that moderate salt stress led to an increase in the amounts of secondary compounds but that severe salt stress led to a decrease in the levels of these compounds.
3.7. Correlation Analysis
The correlations between TPC, TFC, gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, luteolin, vanillic acid, ellagic acid, and rutin traits at all three salinity levels was investigated (and Table 8, Table 9 and Table 10). The evaluation of the correlation coefficients of the total phenolic compounds with phenolic components, including gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, vanillic acid, and ellagic acid, showed that there was no significant correlation between the mentioned traits in the control (Table 8). However, with increasing levels of salt stress, significant and positive correlations, such as the correlation of TPC with gallic acid (r = 0.51 *) and with chlorogenic acid (r = 0.57 *) that were positive and significant, were observed (Table 9).

Table 8.
Correlation between traits of hollyhock varieties at control level in 2020.

Table 9.
Correlation between traits of hollyhock varieties at moderate salt stress levels in 2020.

Table 10.
Correlation between traits of hollyhock varieties at severe salt stress levels in 2020.
No significant correlation was observed between TPC and the majority of phenolic acid compounds at high levels of salt stress (Table 10). This indicates that moderate salt stress led to an increase in phenolic compounds, whilst severe salt stress led to a decrease in the levels of these compounds. In the control, no significant correlation was observed between TFC and luteolin, but a significant positive correlation was observed between TFC and rutin (r = 0.44 *). The correlation between TFC and luteolin was significant and negative and equal to r = 0.569 * (Table 9).
No significant correlation was observed between the recorded traits in the severe salt-stressed plants (Table 10). In the control, positive and significant correlations between caffeic acid and chlorogenic acid (r = 0.9 **), vanillic acid and chlorogenic acid (r = 0.9 **), vanillic acid and caffeic acid (r = 0.99 **), and ferulic acid were observed. Under severe salt stress, no statistically significant correlations were observed Table 10).
3.8. Principle Component Analysis
The results of the principal component analysis (PCA) between the measured traits and the hollyhock plants cultivated under both control and salt-stress conditions in the second year of cultivation (2020) are shown in Figure 5. The cosine of the angles between vectors indicates the extent of the correlation between traits. The acute angles (<90°) represent positive correlations, whereas the wide angles (90°<) indicate negative correlations. The intensity of correlation increases for the angles near 0° and 180°, and the length of the vectors connecting traits to the origin shows the extent of variability and contribution of each trait in the PCA. Under control conditions, TPC and TFC were shown to be negatively correlated with flower yield. The Mahallat and Khomeini Shahr varieties had a positive correlation with flower yield under control conditions, and the Mahallat variety had a strong correlation with TPC and TFC at this level. Shiraz 1 had the highest TPC and TFC values as well as the lowest antioxidant activity (highest IC50 value), while the highest flower yield is attributed to the Mahallat variety.
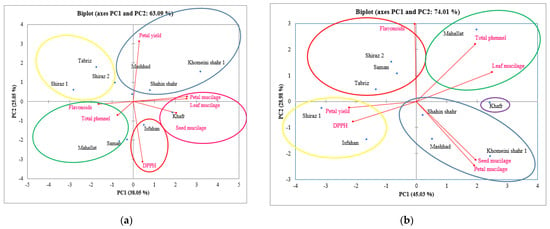
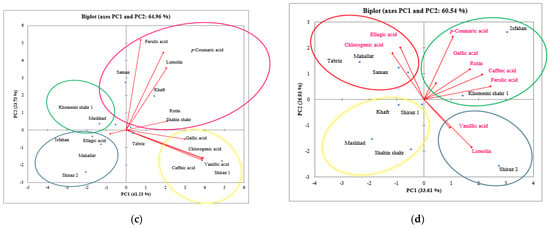
Figure 5.
Projections (axis 1 and 2 of a principal component analysis) of antioxidant activity (DPPH), total phenolic acid content (TPC), total flavonoid acid content (TFC), mucilage (petal, leaf, and seed) content, and petal yield of 10 hollyhock varieties (Isfahan, Khafr, Khomeini Shahr, Mahallat, Mashhad, Saman, Shahin Shahr, Shiraz 1, Shiraz 2, and Tabriz) under control (a) and severe salinity stress; (b) condition in the year 2020. Projections (axis 1 and 2 of a principal component analysis) of gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ellagic acid, ferulic acid, vanillic acid, and ellagic acid of 10 hollyhock varieties (Isfahan, Khafr, Khomeini Shahr, Mahallat, Mashhad, Saman, Shahin Shahr, Shiraz 1, Shiraz 2, and Tabriz) under control (c) and severe salinity stress; (d) condition in the year 2020.
In order to assess the relationship between the traits of TPC, TFC, DPPH, mucilage (petal, leaf, and seed), and yield and the grouping of the populations, analyses were performed on these traits at the control and high stress levels and the biplot of the two components. The PCA results indicate that 63% and 73% of the total variability could be explained by the first two principal components under normal and salt-stress conditions, respectively (Figure 5a,b).
The graphical results of the PCA indicate that 70% and 60% of the total variability could be explained by the first two principal components in the control and stress conditions, respectively (Figure 5a,b).
The PCA plot shows that all the hollyhock varieties studied could be divided into five groups (Figure 5). These multivariate analyses make it possible to make conclusions about the underlying causes of the antioxidant activity of different varieties on the basis of their chemical compositions. The population of Khafr alone was placed in a high mucilage group. The populations of Mashhad, Shahin Shahr, and Khomeini Shahr were placed in a group with high values of flower yield. The populations of Saman, Mahallat, and Isfahan were also in the same group and possessed a high antioxidant capacity.
By evaluating the biplot of TPC, TFC, DPPH, petal mucilage, leaf mucilage, seed mucilage, and petal yield under both control and stress conditions (Figure 5a,b), it was found that the Khafr variety had a high yield in terms of mucilage and that the Saman and Mahallat varieties under control conditions had a high yield in terms of DPPH (in terms of IC50), which means that under salt-stressed conditions, their antioxidant capacity was weak. Also under stress conditions, the Mahallat and Saman varieties were shown to be the most productive in terms of TPC and TFC, respectively. The Shahin Shahr and Khomeini Shahr varieties were shown to be more salt-tolerant varieties and were significant in terms of the petal yield and mucilage yield traits. It seems that the Shiraz 1 and Shiraz 2 varieties are salt sensitive in terms of the aforementioned traits.
According to Figure 5c,d, the populations are divided into four groups. Shiraz 2, Mahallat, and Isfahan were placed in the same group and had strong correlations with regards to their ellagic acid content. The Khafr and Saman varieties were also in the same group and had a strong correlation with respect to their ferulic acid, p-coumaric acid, luteolin, and rutin content. However, Shiraz 1 was placed in a category by itself and contained high levels of vanillic acid, caffeic acid, and gallic acid. The Tabriz and Shahin Shahr varieties were also placed in a separate category. According to Figure 5d, Shiraz 2 was placed in separate category and displayed a high correlation with the luteolin and vanillic acid compounds. The Isfahan and Khomeini Shahr varieties displayed a high correlation with regards to the ferulic acid, gallic acid, rutin, p-coumaric acid, and gallic acid compounds. Saman, Mahallat, and Tabriz also performed strongly in terms of their chlorogenic acid and ellagic acid content.
Figure 5c,d show that the Shiraz 2 variety cultivated under both the control and salt-stressed conditions had a markedly different yield compared to the other varieties and was placed in a different group with its vanillic acid and luteolin content as the indicator compounds. Under control conditions, the Mahallat and Saman varieties were particularly productive, and they are indicators in terms of ellagic acid composition, which is the most abundant compound measured in the petal extracts of the hollyhock varieties. Although for the Shiraz 1 and Shiraz 2 varieties under control conditions, the vanillic acid and ellagic acid content were at a high level, under salt-stress conditions, they showed a sharp decrease in the synthesis of these compounds. The Mashhad, Khafr and Shiraz 1 varieties were all greatly affected by exposure to salt stress. They are the most salt sensitive varieties of those tested. In contrast, the Mahallat and Saman varieties had the highest yield and in terms of TPC, TFC, chlorogenic acid and ellagic acid, they are the most productive variety. In general, the Saman variety was found to contain significant amounts of polyphenols such as luteolin, p-coumaric acid and ferulic acid. Perhaps, these polyphenols played a larger contribution to the antioxidant activity than the other phenolic compounds.
4. Discussion
In the current study, exposure to moderate and severe salt stress was shown to significantly increase the total phenolic content in some of the varieties tested compared to the control plants (0 mM NaCl), such as Kahfr and Shiraz 1, while in others, such as Mahallat and Shriraz 2, a decrease in the amount of these compounds was observed when exposed to salt stress. Previous studies have reported similar results in salt-stressed plant species. For example, Valifard et al. (2014) reported an increase in phenolic content in response to salt stress in Salvia mirzayani [20]. In another study, Lim et al. (2012) reported an increase in phenolic content under the influence of salt stress in Fagopyrum esculentum M., and the highest increase was associated with isoorientin, orientin, rutin, and vitexin compounds [21]. Bistgani et al. (2019), who studied Thymus vulgaris L. and Thymus daenensis reported that the total phenolic content in these species increased by about 20% after the application of 60 mM NaCl compared to the plants grown under control conditions [22].
Soil salinity is known to be a major environmental constraint on plant growth and productivity and is a serious problem especially in agricultural systems that rely heavily on irrigation. Salt stress causes a shortening of the photosynthetic electron transport chain and promotes the production of reactive oxygen species (ROS), resulting in oxidative stress. Higher plants have developed a variety of adaptive mechanisms to reduce the oxidative damage resulting from salt stress through the biosynthesis of a cascade of antioxidants. Among them, phenolic compounds, such as phenolic acids, flavonoids, and proanthocyanidins, play an important role in scavenging free radicals. Phenolic acids are secondary metabolites widely distributed throughout the plant kingdom that are critical for plant growth and development and are produced in response to adverse environmental factors (light, cold, salinity, pesticide, heavy metal, etc.) and in response to injuries [11,20,21,22]. Therefore, environmental stresses that induce oxidative damage often result in the increased biosynthesis of phenolic compounds [23]. Thus, the study of phenolic acids present in salt-stressed plants can help provide a better understanding of the role of these compounds in plant stress resistance [24,25].
In our study, the most abundant phenolic compound was ellagic acid, and the greatest increase in a phenolic compound induced by salt stress was recorded for p-coumaric acid and chlorogenic acid. A previous study reported the accumulation of phenolic acids in the leaves of Amaranthus tricolor L. (a leafy vegetable) in response to salt stress, including caffeic acid, chlorogenic acid, ferulic acid, gallic acid, 4-hydroxybenzoic acid, p-coumaric acid, salicylic acid, sinapic acid, and vanillic acid [26]. A significant increase in the accumulation of p-coumaric acid can reduce oxidative pressure because p-coumaric acid has high radical scavenging activity due to its hydroxyl nature. The presence of ferulic acid under osmotic stress may be related to the strengthening of the plant cell wall and overall reduction in cell elongation. In addition, ferulic acid is known to play a role in drought tolerance by increasing lipid peroxidation through the activation of antioxidant enzymes and by increasing proline and soluble sugar content in cucumber leaves [20]. These data, together with the differential accumulation of TPC and TFC, provide further evidence that phenolic compounds play important physiological and biochemical roles in plant cells, particularly in ameliorating abiotic stress [27].
The observed levels of both chlorogenic acid and p-coumaric acid were shown to increase under salt-stressed conditions. An increase in the amount of ferulic acid and p-coumaric acid was observed in salt-tolerant varieties, such as Saman and Mahallat (Table 7). The presence of ferulic acid under osmotic stress may be related to the strengthening of the plant cell wall and a reduction in cell elongation. Salt-tolerant plants have higher levels of certain phenolic acids and suffer less from metabolic stress disorders when exposed to salt stress. The scavenging capacity of hydroxyl radicals in seedlings grown under high levels of salt stress increased to the maximum after caffeic and sinapic acid treatments. The proven sequence of efficacy was as follows: caffeic acid > chlorogenic acid > ferulic acid > p-coumaric acid [28].
In addition, it was found that salt stress led to an increase in flavonoids. As mentioned above, salt stress is known to significantly affect the accumulation of secondary metabolites in plant tissues, and flavonoids are effective scavengers of ROS in plants exposed to salt stress [29]. Salt has been shown to increase phenolic and flavonoid content as observed in Leucojum aestivum [30], yam plants, and grapevine [31]. A significant increase in flavonoid content was observed in the leaves of Carthamus tinctorius L. under different NaCl concentrations (50, 100, and 150 mM) [32]. Martinez et al. (2016) also reported that the increased levels of flavonoids in tomato plants exposed to abiotic stress correlated with the protective effect of these substances against stress-induced biochemical damage [10]. Furthermore, in a study of Stevia rebaudiana under conditions of low salt stress (30 mM NaCl), the content of diterpene glycosides was increased (8.25%), and conversely, exposure to higher salt levels (90 mM NaCl) resulted in a decrease (4.2%) [33].
Our results indicate that the Saman hollyhock variety, which was observed to produce increased levels of TPC and TFC when grown under both severe and moderate salt-stress conditions, could achieve the highest levels of DPPH radical scavenging activity and is therefore a highly salt-tolerant variety. DPPH free radicals are neutralized by the interaction of antioxidants and DPPH through electron transfer. In this regard, other studies have shown an increase in DPPH antioxidant activity when phenolic and total flavonoid concentrations increase [34]. For example, Lim et al. (2012) reported that DPPH levels increased in wheat under moderate salt stress [21]. Another study reported that higher levels of DPPH radical scavenging activity were correlated with improved stress tolerance in rice, cucumber, and wheat seedlings [35].
The results of our study show that salt stress in hollyhock caused an increase in mucilage content in the petals, leaves, and seeds in some varieties and a decrease in others. Mucilage is a polysaccharide mixture with a highly variable chemical composition that may play an important role in salt tolerance by modulating water retention and regulating water uptake, ion homeostasis, and ion transport [36]. For example, Ghanem et al. (2010) reported an increase in mucilage content in shoots, stems, and roots in response to salt stress in Kosteletzkya virginica [36]. In another more recent study, Golkar et al. (2017) cultivated Plantago ovata genotypes under salt stress, and the expressed mucilage content increased in all genotypes as salt increased from the control level (0 mM) to 100 mM NaCl and then decreased at 200 mM NaCl [36,37].
Moderate abiotic stress often causes the stomata in mesophile angiosperms to close and thereby limit gas exchange, which results in a decrease in the absorption of carbon dioxide [38,39]. The reduction of stomatal conductance thus reduces the amount of carbon dioxide available for carboxylation, which leads to a reduction in photosynthetic uptake and as a result, a reduction in the production of assimilates sufficient for growth and yield [40]. Severe levels of stress also cause a disturbance of plant metabolism, disturbance in the enzyme production and structural activities of cells, and finally, a reduction in yield [41].
In line with this observation, Razmjoo et al. (2008) found that the fresh and dry flower weights of Matricaria chamomila were reduced by salinity [42], which is in agreement with our data. Elevated salt levels are known to cause a wide variety of detrimental effects on plant growth and development.
Elevated levels of salt reduce the growth of seedlings and flowers, the formation of spikelets, pollen germination, and successful fertilization [43]. Increasing salt concentrations have been shown to negatively affect productivity in a wide variety of medicinal plants including Foeniculum vulgare (fennel), Cuminum cyminum (cumin), Trachyspermum ammi (ajwain), and Silybum marianum (milk thistle) [44]. Growth parameters were also found to be repressed under salt stress in Withania somnifera, Catharanthus roseus, Achillea fragratissima, Salvia officinalis, and Chamomilla recutita [44,45,46].
The results of our study, show that salinity stress in hollyhock caused an increase in TPC and TFC in the petals of plants grown under moderate salt stress (Figure 4A,B), while there were decreases in the petal yield in the control and plants cultivated under conditions of severe salt stress (Table 4). The reduction in growth and yield induced by salt stress may have resulted in a new pattern of resource partitioning providing additional carbon skeletons for phenolic biosynthesis. The highest content of TPC was recorded in moderately salt-stressed plants relative to the control. Similarly in pepper and Cakile maritimie plants, Navarro et al. (2006) and Ksouri et al. (2007) found that salinity induced significant increases in TPC [47]. In fact, plants divert the synthesis of carbohydrates to produce secondary metabolites under saline conditions. It is thought that moderate salinity induces the salt tolerance biochemical pathway by increasing a plant’s content of total phenolic compounds. Environmental signals, such as excess salt concentration, trigger a plant to respond to its growing conditions by controlling the ion partitioning between different sink tissues. In this regard, a study was conducted on safflower, which showed that flavonoids were increased under moderate stress levels compared to control and severe stress conditions [46]. In a study on Salvia mirzayanii, an increase in TPC was reported under salt-stressed conditions. The increase was most pronounced under moderate salt stress [17,23]. In our study, at the moderate stress level, an increase in TFC was observed. In a study on rice, rice varieties showed an increase in TPC and TFC at a salinity level of 5 dS/m, while at a higher salinity level of 10 dS/m, the TPC and TFC levels of all varieties, except the tolerant varieties to salinity, decreased [23].
The observed positive relationship between TPC and TFC is most likely explained by the fact that they originate from the same functional biosynthetic pathway [16], as a consequence of the need to suppress H2O2 production. The varieties differ in their total phenolics and flavonoids and antioxidant activities as measured by DPPH as well as in their individual polyphenolic compounds. Taxonomically related plants seem to show a tendency to produce quite similar phytochemicals, including flavonoids and phenolic compounds [47]. In contrast, negative relationships were observed among DPPH and TPC and leaf mucilage, petal mucilage, and seed mucilage.
Plants grown under salt stress display different growth and metabolomic responses, the extent of which depends on their genetic variation and their content of primary and secondary metabolites [38]. The profiling of secondary metabolites in stressed plants may provide useful information regarding the tolerance or sensitivity of plants to stress [48]. Some sensitive plants respond to stress by accumulating some primary and secondary metabolites as a mechanism to tolerate different types of stresses [49].
From an examination of the total amount of precipitation, the average monthly temperature, the minimum temperature, and the maximum temperature in the years 2019 and 2020, it was observed that there was no significant difference in terms of monthly average temperature, minimum temperature, and maximum temperature between the two growing seasons. However, with regards the total monthly precipitation, a significant difference was observed in the months of February (before planting in the first year and before the active growth of plants in the second year) and September and December. As such, a possible explanation for some of the observed differences in plant performance during the two growing seasons may be related to the total amount of rainfall. However, since the total amount of precipitation is quite low, it seems more likely that the issues of plant establishment and stress memory had a more significant impact on the growth of the plants in 2020 as compared to 2019.
5. Conclusions
We have shown that the ornamental, aromatic/medicinal hollyhock species produces a variety of phytochemicals and that the levels of these metabolites depend on the respective variety in combination with the salinity regime and the interaction between these two factors. Increasing the amount of phenolic and flavonoid compounds in plant tissues can play a vital role in protecting plants against salt stress. As a result, better plant growth is enabled under salt-stressed conditions. The total content of phenolic and flavonoid compounds was shown to increase in some varieties and decrease in others with increases in salt stress. The largest observed increase was observed in the Saman variety grown under severe salt stress, while the largest decrease was observed in Shiraz 1 cultivated under severe salt stress. The Shiraz 1, Shahin Shahr, and Mahallat varieties produced their highest TPC levels under control, moderate, and severe salt stress, respectively. The wide variation among varieties in their response to increasing salinity indicates that the selection of more salt-tolerant varieties for direct use or use in future breeding programs is possible. Among the varieties tested, we recommend the Saman variety for commercial production due to its phenolic and flavonoid content in high salinity soils. Based on these results, it can be concluded that Alcea rosea can be cultivated in salt-affected areas, which could increase its production of secondary metabolites.
Author Contributions
Conceptualization, A.S., J.R. and H.K.; formal analysis, A.S., J.R, T.C.B. and H.K.; investigation, A.S., J.R. and H.K.; data curation, H.K., T.C.B. and A.M.; writing—original draft preparation, A.S., J.R. and H.K.; writing—review and editing, J.R., T.C.B. and A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, and the APC was funded by University of Brescia.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors greatly appreciate the personnel at the research field and lab facility of Agriculture College of Isfahan University of Technology for their assistance and University of Brescia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ashraf, M. Some Important Physiological Selection Criteria for Salt Tolerance in Plants. Flora 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Tavakoli, A.R.; Liaghat, A.; Oweis, T. The Role of Limited Irrigation and Advanced Management on Improving Water Productivity of Rainfed Wheat at Semi-Cold Region of Upper Karkheh River Basin, Iran. Int. J. Agric. Crop Sci. 2012, 4, 939–948. [Google Scholar]
- Yang, Y.; Guo, Y. Elucidating the Molecular Mechanisms Mediating Plant Salt-Stress Responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling Salt Stress Signaling in Plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Said-Al Ahl, H.A.H.; Omer, E.A. Medicinal and Aromatic Plants Production under Salt Stress. A Review. Herba Pol. 2011, 57, 72–87. [Google Scholar]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant Salt Tolerance: Chapter 13. In Agricultural Salinity. Assessment and Management, 2nd ed.; ASCE: Reston, VI, USA, 2012; pp. 405–459. [Google Scholar]
- Mohammadi-Nejad, G.; Nikbakht, E.; Yousefi, K.; Farahbakhsh, H. Evaluation Salinity Tolerance of Safflower (Carthamus tinctorius L.) Genotypes at Different Vegetative Growth Stages. Int. J. Plant Prod. 2010, 1, 105–111. [Google Scholar]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M. Effect of Salt Stress on Growth and Ion Accumulation of Alfalfa (Medicago sativa L.) Cultivars. J. Plant Nutr. 2018, 41, 818–831. [Google Scholar] [CrossRef]
- Oueslati, S.; Karray-Bouraoui, N.; Attia, H.; Rabhi, M.; Ksouri, R.; Lachaal, M. Physiological and Antioxidant Responses of Mentha pulegium (Pennyroyal) to Salt Stress. Acta Physiol. Plant. 2010, 32, 289–296. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential Oil Composition, Total Phenolic and Flavonoid Contents, and Antioxidant Activity of Thymus Species Collected from Different Regions of Iran. Food Chem. 2016, 220, 153–161. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New Insights Explain That Drought Stress Enhances the Quality of Spice and Medicinal Plants: Potential Applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kadioglu, A.; Turgut, R. Water Stress Effects on the Content of Low Molecular Weight Carbohydrates and Phenolic Acids in Ctenanthe setosa (Rosc.) Eichler. Can. J. Plant Sci. 2000, 80, 373–378. [Google Scholar] [CrossRef]
- Oraee, A.; Shoor, M.; Oraee, T.; Tehranifar, A.; Nema, H. Organic Amendments Role in Reducing Drought Stress in Alcea rosea L. Adv. Hortic. Sci. 2022, 36, 201–214. [Google Scholar] [CrossRef]
- Ardestani, A.; Yazdanparast, R. Antioxidant and Free Radical Scavenging Potential of Achillea santolina Extracts. Food Chem. 2001, 9, 35–46. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Afshari, M.; Sarfaraz, D.; Miroliaei, M. Using HPLC and Multivariate Analyses to Investigate Variations in the Polyphenolic Compounds as Well as Antioxidant and Antiglycative Activities of Some Lamiaceae Species Native to Iran. Ind. Crops Prod. 2020, 154, 112640. [Google Scholar] [CrossRef]
- Gul, R.; Jan, S.U.; Faridullah, S.; Sherani, S.; Jahan, N. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. Sci. World J. 2017, 3, 5873648. [Google Scholar] [CrossRef]
- Sharma, P.K.; Koul, A.K. Mucilage in Seeds of Plantago ovata and Its Wild Allies. J. Ethnopharmacol. 1986, 17, 289–295. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1999, 76, 270–276. [Google Scholar]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of Salt Stress on Volatile Compounds, Total Phenolic Content and Antioxidant Activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, K.J.; Kim, B.K.; Jeong, J.W.; Kim, H.J. Effect of Salinity Stress on Phenolic Compounds and Carotenoids in Buckwheat (Fagopyrum esculentum M.) Sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of Salinity Stress on the Physiological Characteristics, Phenolic Compounds and Antioxidant Activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Minh, L.T.; Khang, D.T.; Thu Ha, P.T.; Tuyen, P.T.; Minh, T.N.; Van Quan, N.; Xuan, T.D. Effects of Salinity Stress on Growth and Phenolics of Rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Kaczyński, P.; Pietkun, M.; Łozowicka, B. Evaluation of Titanium and Silicon Role in Mitigation of Fungicides Toxicity in Wheat Expressed at the Level of Biochemical and Antioxidant Profile. Chemosphere 2022, 308, 136284. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic Acids Composition, Total Polyphenols Content and Antioxidant Activity of Triticum monococcum, Triticum turgidum and Triticum aestivum: A Two-Years Evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of Leaf Color Parameters, Pigments, Vitamins, Phenolic Acids, Flavonoids and Antioxidant Activity in Selected Amaranthus tricolor under Salinity Stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chhabra, M.; Singh, S.K.; Parmar, R.; Kapila, R.K. Genetic Diversity and Population Structure of Critically Endangered Dactylorhiza hatagirea (D. Don) Soo from North-Western Himalayas and Implications for Conservation. Sci. Rep. 2022, 12, 11699. [Google Scholar] [CrossRef] [PubMed]
- Linić, I.; Šamec, D.; Grúz, J.; Vujčić Bok, V.; Strnad, M.; Salopek-Sondi, B. Involvement of Phenolic Acids in Short-Term Adaptation to Salinity Stress Is Species-Specific among Brassicaceae. Plants 2019, 8, 155. [Google Scholar] [CrossRef]
- Ahmad, F.; Kamal, A.; Singh, A.; Ashfaque, F.; Alamri, S.; Siddiqui, M.H. Salicylic Acid Modulates Antioxidant System, Defense Metabolites, and Expression of Salt Transporter Genes in Pisum sativum Under Salinity Stress. J. Plant Growth Regul. 2022, 41, 1905–1918. [Google Scholar] [CrossRef]
- Ates, M.T.; Yildirim, A.B.; Turker, A.U. Enhancement of Alkaloid Content (Galanthamine and Lycorine) and Antioxidant Activities (Enzymatic and Non-Enzymatic) Unders Salt Stress in Summer Snowflake (Leucojum aestivum L.). S. Afr. J. Bot. 2021, 140, 182–188. [Google Scholar] [CrossRef]
- Gohari, G.; Panahirad, S.; Sepehri, N.; Akbari, A.; Zahedi, S.M.; Jafari, H.; Dadpour, M.R.; Fotopoulos, V. Enhanced Tolerance to Salinity Stress in Grapevine Plants through Application of Carbon Quantum Dots Functionalized by Proline. Environ. Sci. Pollut. Res. 2021, 28, 42877–42890. [Google Scholar] [CrossRef]
- Gengmao, Z.; Yu, H.; Xing, S.; Shihui, L.; Quanmei, S.; Changhai, W. Salinity Stress Increases Secondary Metabolites and Enzyme Activity in Safflower. Ind. Crops Prod. 2015, 64, 175–181. [Google Scholar] [CrossRef]
- Shahverdi, M.A.; Omidi, H.; Tabatabaei, S.J. Stevia (Stevia rebaudiana Bertoni) Responses to NaCl Stress: Growth, Photosynthetic Pigments, Diterpene Glycosides and Ion Content in Root and Shoot. J. Saudi Soc. Agric. Sci. 2019, 18, 355–360. [Google Scholar] [CrossRef]
- Santander, C.; Vidal, G.; Ruiz, A.; Vidal, C.; Cornejo, P. Salinity Eustress Increases the Biosynthesis and Accumulation of Phenolic Compounds That Improve the Functional and Antioxidant Quality of Red Lettuce. Agronomy 2022, 12, 598. [Google Scholar] [CrossRef]
- Ahmadi, J.; Pour-Aboughadareh, A.; Ourang, S.F.; Mehrabi, A.A. Screening Wild Progenitors of Wheat for Salinity Stress at Early Stages of Plant Growth: Insight into Potential Sources of Variability for Salinity Adaptation in Wheat. Crop Pasture Sci. 2018, 69, 649–658. [Google Scholar] [CrossRef]
- Edmond Ghanem, M.; Han, R.M.; Classen, B.; Quetin-Leclerq, J.; Mahy, G.; Ruan, C.J.; Qin, P.; Pérez-Alfocea, F.; Lutts, S. Mucilage and Polysaccharides in the Halophyte Plant Species Kosteletzkya virginica: Localization and Composition in Relation to Salt Stress. J. Plant Physiol. 2010, 167, 382–392. [Google Scholar] [CrossRef]
- Golkar, P.; Amooshahi, F.; Arzani, A. The Effects of Salt Stress on Physio-Biochemical Traits, Total Phenolic and Mucilage Content of Plantago ovata Forsk under in Vitro Conditions. J. Appl. Bot. Food Qual. 2017, 90, 224–231. [Google Scholar] [CrossRef]
- Kiani, R.; Arzani, A.; Maibody, S.A.M.M. Polyphenols, Flavonoids, and Antioxidant Activity Involved in Salt Tolerance in Wheat, Aegilops cylindrica and Their Amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.R.B.; Hamid, A.; Islam, M.S. Drought Stress Effects on Water Relations of Wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Ball, R.A.; Purcell, L.C.; Vories, E.D. Short-Season Soybean Yield Compensation in Response to Population and Water Regime. Crop Sci. 2000, 40, 1070–1078. [Google Scholar] [CrossRef]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of Salinity and Drought Stresses on Growth Parameters and Essential Oil Content of Matricaria chamomila. Int. J. Agric. Biol. 2008, 10, 95–107. [Google Scholar]
- Sodani, R.; Mundiyara, R. Salinity Stress: Its Impact on Plant Growth and Development. Agric. Food E-Newsl. 2021, 3, 535–537. [Google Scholar]
- Ashraf, M.; Orooj, A. Salt Stress Effects on Growth, Ion Accumulation and Seed Oil Concentration in an Arid Zone Traditional Medicinal Plant Ajwain (Trachyspermum ammi [L.] Sprague). J. Arid Environ. 2006, 64, 209–220. [Google Scholar] [CrossRef]
- Lakshmanan, G.M.A.; Gomathinayagam, M.; Panneerselvam, R.; Jaleel, C.A. Triadimefon Induced Salt Stress Tolerance in Withania Somnifera and Its Relationship to Antioxidant Defense System. S. Afr. J. Bot. 2008, 74, 126–132. [Google Scholar]
- Ghanavati, M.; Sengul, S. Salinity Effect on the Germination and Some Chemical Components of Matricaria Spp. Asian J. Chem. 2010, 22, 859–866. [Google Scholar]
- Hejazi Mehrizi, M.; Shariatmadari, H.; Khoshgoftarmanesh, A.H.; Dehghani, F. Copper Effects on Growth, Lipid Peroxidation, and Total Phenolic Content of Rosemary Leaves under Salinity Stress. J. Agric. Sci. Technol. 2012, 14, 205–212. [Google Scholar]
- Martucci, M.E.P.; De Vos, R.C.H.; Carollo, C.A.; Gobbo-Neto, L. Metabolomics as a Potential Chemotaxonomical Tool: Application in the Genus Vernonia schreb. PLoS ONE 2014, 9, e93149. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and Metabolomic Profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).