Abstract
Vitis vinifera is an important fruit crop which is mainly consumed fresh or used for the production of wine. Genetic improvement programs through New Genomic Techniques (NGTs) aim to develop grapevine varieties resistant to biotic and abiotic stresses or enhancing nutraceutical properties. In order to apply NGTs, maintaining embryogenic calluses from flower tissues is critical. Optimizing culture conditions—pH, gelling agents, temperature, light, growth regulators, and gas composition—is essential for inducing efficient embryogenic responses tailored to each genotype/explant. Ethylene, a pivotal gaseous plant hormone, significantly influences tissue culture by affecting organogenesis and embryogenesis processes in several plants. Modulating ethylene levels shows promise for improving tissue culture vitality. This study evaluates in Vitis vinifera the effects of silver thiosulfate (STS) and salicylic acid (SA) on embryogenic callus growth, specifically investigating their roles in maintaining and inducing embryogenic competence. STS, particularly at 40 µM and 60 µM concentrations, effectively preserved embryogenic competence in Italia and Red Globe calluses, while high SA concentrations showed varied and occasionally adverse effects. At the same time, STS markedly suppressed the non-embryogenic callus growth in recalcitrant variety Italia, potentially increasing the ratio between embryogenic to non-embryogenic calluses development.
1. Introduction
Vitis vinifera is one of the most important species in the world; it is primarily consumed as fresh fruit or employed for wine production [1].
In recent years, as a result of climate changes and modifications in consumer tastes, several genetic improvement programs have been launched to develop vine varieties resistant or tolerant to biotic (such as downy mildew, powdery mildew, and gray mold) and abiotic stresses (such as water stress and increased temperatures), also taking into account the consumers’ preference towards seedless berries with nutraceutical qualities.
The different breeding programs include both traditional techniques, such as crossing, and new ones, such as New Genomic Techniques (NGTs). These techniques are a powerful tool that can greatly enhance breeding programs. In the application of NGTs, obtaining and maintaining embryogenic calluses derived in vitro from flower tissues plays a fundamental role [2,3].
Most grapevine cultivars are considered recalcitrant, and the majority of available genetic transformation protocols rely on embryogenic calluses, which often require continuous production [4].
The long-term multiplication and maintenance of these calluses, as well as the differentiation, maturation, and subsequent conversion of embryos into plantlets, are particularly delicate steps. In addition, the embryonic regeneration potential of most woody plant embryogenic cultures in vitro diminishes or is lost after long-term culture. Moreover, variations related to genotype necessitate protocol improvements for virtually every cultivar [5,6,7].
Several factors can induce embryogenic responses [8]. To obtain an efficient response from plant tissues, it is necessary to establish optimal culture media and environmental conditions for each genotype/explant including pH, gelling agents, temperature, light, growth regulators, and a gaseous environment [9,10,11].
The most frequently studied components of the culture atmosphere are carbon dioxide, oxygen, and ethylene [12]. The latter has been recognized as one of the most important growth factors in plant tissue culture, being the first identified gaseous plant hormone that regulates a wide variety of processes affecting plant growth and development [13]. In fact, all plants produce ethylene in vitro [14] and the requirement for closed containers to prevent contamination of the culture causes its accumulation at high levels [15].
All organogenesis and embryogenesis processes can be affected by ethylene production [13,16,17]. Bai et al. [18] showed that the down-regulation of not only ethylene biosynthesis but also the ethylene response is critical for the induction of somatic embryogenesis. In the cited work, the authors further demonstrated that ethylene interfered with the beginning of somatic embryogenesis by inhibiting YUCCA gene (YUC) expression. YUC may be involved in local auxin biosynthesis and subsequent auxin distribution.
Initial stress conditions resulting from explant cuts and the transition to the new in vitro environment can lead to the release and accumulation of stress-related compounds, such as ethylene [19].
Several studies have focused on the modulation of this hormone and have demonstrated, in different fruit species, its ability to inhibit or improve both organogenesis shoots’ generation and the growth of calluses and its subsequent embryogenic differentiation [20,21,22,23,24,25].
Therefore, the regulation of ethylene content or the control of its biosynthesis are promising approaches to increase the efficiency of plant tissue culture protocols. Compounds containing silver ions, such as silver nitrate (AgNO3) or silver thiosulphate (STS), have been identified as inhibitors that block the action of ethylene receptors without affecting ethylene biosynthesis [26,27]. Furthermore, silver ions are known to possess antimicrobial properties, which play a crucial role in safeguarding explants from potential infections [28]. Salicylic acid (SA) has been demonstrated to be a growth regulator and a powerful inhibitor of ethylene biosynthesis via anoxia phenomena [15,29].
Despite ethylene being an important factor for the in vitro culture in several species, little is known about the influence of its levels on grapevine in vitro culture. Therefore, we have evaluated the effect of silver thiosulphate and salicylic acid on embryogenic callus growth in Vitis vinifera. Specifically, we assessed their role in maintaining embryogenic competence over time and in inducing competence in calluses that have lost it. We investigated the effect on embryogenic competence in four different table grape varieties highlighting genotype-dependent behavior. Overall, our data suggest new clues that will help to improve protocols for somatic embryogenesis that are also suitable for recalcitrant varieties.
2. Materials and Methods
2.1. Plant Materials and Sterilization
Immature inflorescences of four Vitis vinifera L. varieties—Glera B., Italia B., Red Globe Rs., and Victoria B.—were harvested from 15-year-old plants grown in the experimental field of the CREA (Azienda Lamarossa, Rutigliano, Southern Italy; 40°57′26″ N; 17°00′26″ E) between April and May 2022.
The inflorescences were collected in the stages of early flower development I to III [30] when the pollen mother cells (PMC) were in the early premeiotic phase and early formation of the tetrad [31] (Figure 1). Inflorescences were collected at this stage, as from previous work, because this is the optimal period for the aforementioned varieties to obtain embryogenic calluses [32]. After staining of crushed anthers with acetocarmine [33], the flower’s development phase was determined by the examination of the microsporogenesis stage with an optical microscope (Nikon Labophot Mod. Y by Nikon Corporation, Tokyo, Japan).
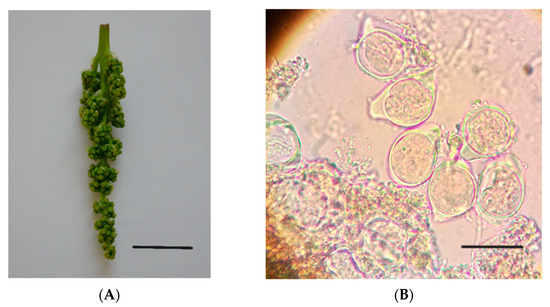
Figure 1.
(A) Inflorescence of the ‘Italia’ variety; (B) PMC with callose wall (stage III). Bars: (A) 1.5 cm; (B) 30 µm.
The freshly collected explants were immersed in ethanol (70% v/v) for 30 s. Then, they were sterilized by immersion with mild stirring for 2 min in sodium hypochlorite (2% active chlorine) solution to which 2 drops of Tween 20 were added. After rinsing in sterile water 3 times, the explants were plated on a cultural substrate.
Whole flowers were used as explants instead of the stamens and ovaries, as already proposed by other authors [32,34,35]. Using whole flowers is not only time-saving but also allows greater ease of cultivation, results in less damage to stamens and ovaries, and enables a greater number of explants to be plated.
2.2. Media and Culture Conditions
Initially, the explants were cultivated in the following media for callus induction (PIV) [2,3,36] which was composed of Nitsch and Nitsch mineral salt [37], Murashige and Skoog vitamins [38], 6% sucrose, 0.3% Gellan Gum, 4.5 µM 2,4-dichlorophenoxyacetic acid (2,4-D), and 8.9 µM 6-benzylaminopurine (BAP),with the addition of the citric acid (CA) as an antioxidant at a dose of 0.15 g L−1 [39].
The pH was adjusted to 5.8 with 1 M potassium hydroxide (KOH) before autoclaving (121 °C for 20 min). Explants were cultured in Petri dishes with a diameter of 90 mm containing 25 mL of medium (70 explants/plate) sealed with PARAFILM®, (Pechiney Plastic Packaging Company, Chicago, IL, USA). The cultures were maintained at 26 °C in the dark.
After the formation of the calluses (approximately 3–4 months), for their long-term maintenance and multiplication, the medium used (C1C) has a composition similar to the medium used for callus induction except for the growth regulator concentration: 1 µM BAP, 5 µM 2,4-D [36,40,41]. This substrate (C1C), as well as the initial medium, also included citric acid. This addition is routinely performed in our laboratory because we found that it improves both the formation of embryogenic callus and contributes to its maintenance over time [32].
The pH was adjusted to 5.8 with KOH 1 M. The cultures were maintained under the aforementioned conditions. The calluses were transferred to fresh substrate monthly for one year. It is possible to determine the embryogenic competence of the callus from its morphology [42]. As a result, the calluses deteriorated, and certain varieties experienced a decrease in their embryogenicity.
2.3. Ethylene Inhibitors
After a year of culture, both the embryogenic calluses and the calluses that had lost this competence were grown on media with different ethylene inhibitors to test their effect. These media had a similar composition to C1C, but without citric acid, and were supplemented with various ethylene inhibitors such as silver thiosulphate (STS at 20, 40, 60 μM) and salicylic acid (SA at 25, 50, 75 μM). As a control, the C1C substrate was used without both citric acid and ethylene inhibitors (control).
STS and SA were added after the media had cooled. Silver thiosulphate was prepared fresh; a 10 mM stock solution was prepared by mixing 1 volume of 100 mM sodium thiosulphate solution with 4 volumes of 100 mM silver nitrate solution [22]. The calluses were transferred on fresh substrate monthly. The number of vital and embryogenic calluses was recorded after 12 weeks.
The Nitsch and Nitsch mineral salt, Murashige and Skoog vitamins, sodium thiosulfate, silver nitrate, and plant growth regulators were purchased from Duchefa, Haarlem, The Netherlands. The Gellan Gum was purchased from Alfa Aesar by Thermo Fisher Scientific, Bond Street Ward Hill, Waltham, MA, USA. Acetocarmine was purchased from Chem Cruz, Santa Cruz Biotechnology, Inc., Finnel, Dallas, TX, USA.
Callus quality was evaluated by differentiating calluses according to their morphology and embryogenic competence in agreement with López-Pérez et al. [42].
2.4. Statistical Analysis
A total of 4 replicates for each treatment were conducted, using 10 explants for each replicate. The data were tested for normality by Shapiro–Wilk normality test, followed by Levene’s test for equality of variances, as the data were not normally distributed. To assess significant differences, we used either the non-parametric Kruskal–Wallis’s test followed by Dunn post hoc or the Kruskal–Wallis’s rank sum test followed by Conover’s Test. All statistical analyses were performed using R Statistical Software (R version 4.2.0 (22 April 2022 ucrt) [43]. The R packages that were utilized are as follows, listed in alphabetical order: car [44], conover.test [45], and dunn.test [46].
3. Results
The four table grape varieties showed different behaviors depending on the medium composition.
Responses to the ethylene modulation were genotype-dependent in agreement with what has previously been reported in the literature [25].
Specifically, for the Italia variety, the tests were carried out on embryogenic calluses to determine their ability to maintain this competence (embryogenic) and on calluses that had lost their competence after a year of in vitro culture, following the aging (not embryogenic). The percentage of total vital calluses of the Italia variety (Table 1) cultured on the substrates STS 40 and C1C was significantly higher than the control with average values of 100.0% and 98.1%, respectively. STS 60 showed the best results regarding the maintenance of embryogenic capacity, with 66.8% of embryogenic calluses (Figure 2). The lowest percentage values are found in SA 50 for both the calluses viability and the maintenance of their embryogenic capacity.

Table 1.
Mean values of the effects of different substrates on the viability of calluses, and embryogenic calluses in ‘Italia’ (embryogenic).
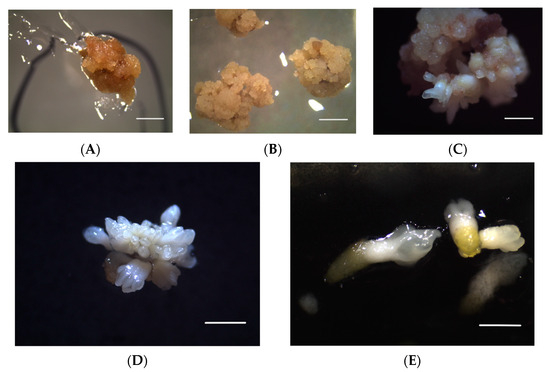
Figure 2.
Calluses of the ‘Italia’ variety on a substrate with STS 60: (A) viable but non-embryogenic callus; (B) embryogenic callus; (C,D) differentiation of embryos; (E) embryos. Bars: 5 mm.
Moreover, experiments on the Italia calluses that had lost embryogenic capacity showed that the substrates that better maintained the non-embryogenic calluses proliferation were the control and SA 25 and 50 (Figure 3), while there was no significant difference in inducing embryogenic capacity (Table 2).
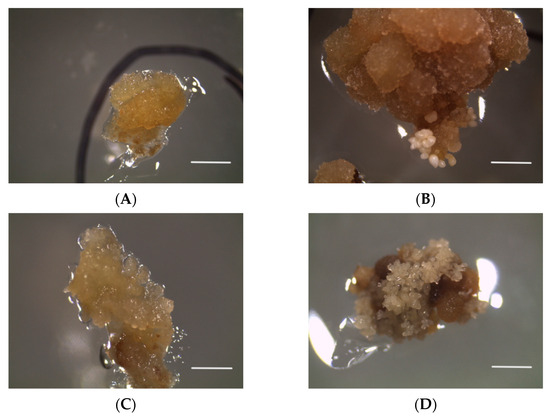
Figure 3.
Calluses of the ‘Italia’ variety (not embryogenic): (A) callus not embryogenic—control; (B) conversion to embryogenic callus on SA 25; (C) callus not embryogenic on SA 50; (D) conversion to embryogenic callus on STS 20. Bars: 5 mm.

Table 2.
Mean values of the effects of different substrates on the viability of calluses, and embryogenic calluses in ‘Italia’ (not embryogenic).
On the other hand, a peculiar selective activity of STS in suppressing the non-embryogenic calluses growth in any tested concentration was observed. At the same time, although not statistically significant, the sporadic conversion back to embryogenic competence was still observed with STS.
In Red Globe (Table 3), the best results for the vitality tests were recorded in the control, STS 20, SA 25, and SA 50, with 100% of vital calluses. Focusing on the percentage of the embryogenic calluses, the highest values were observed in C1C, STS 60, and SA 75 with all vital calluses confirmed to be still embryogenic.

Table 3.
Mean values of the effects of different substrates on the viability of calluses, embryogenic calluses, and embryo development in ‘Red Globe’.
There were no significant differences between the various media and control in the Glera variety, either in the number of viable or embryogenic calluses, as can be seen from Table 4.

Table 4.
Mean values of the effects of different substrates on the viability of calluses, and embryogenic calluses in ‘Glera’.
In Victoria, all the treatments maintained 100% of vital calluses, except for STS 60. For the remaining treatment, no significantly different values were recorded, even though only STS 20 regenerated embryos (Table 5).

Table 5.
Mean values of the effects of different substrates on the viability of calluses, embryogenic calluses, and embryo development ‘Victoria’.
4. Discussion
In this study, we investigated the influence of some ethylene inhibitors, specifically STS and SA, on the maintenance and induction of embryogenic competence in Vitis vinifera calluses. The choice of STS as the source of Ag+ ions for ethylene action inhibition has been made because it has been found to have greater efficacy compared with AgNO3 in previous studies [47]. In addition, in the presence of S2O32− as the anion, the absorption of silver is enhanced [48]. Our findings demonstrated significant differences in the behavior of the four grapevine varieties (Italia, Red Globe, Glera, and Victoria) in response to the media composition, highlighting how ethylene’s influence is genotype-dependent on in vitro culture processes.
We observed that STS at various concentrations often improved callus viability across the studied varieties. For instance, in the Italia embryogenic calluses, STS 40 and STS 60 showed the highest callus viability rates. This is in agreement with previous reports suggesting that silver ions can effectively inhibit ethylene action, promoting better growth conditions for in vitro cultures [15,18]. In addition, the antimicrobial properties of silver ions likely contributed to these results by preventing potential infections [14]. In fact, although good laboratory practices are of great importance in reducing infections in plants tissue culture, it is still an event that happens and can quickly erase many months of work. Although the numbers from our experiments did not allow us to clarify whether there is a benefit to reduced frequencies of infections in tissue cultures, this aspect must certainly be taken into consideration as already exploited in other cultures, in particular with the use of nanoparticles [49].
The ability to maintain or induce embryogenic competence was also influenced by the media composition. In the Italia variety, STS 60 was particularly effective in maintaining embryogenic competence. The selective activity of STS in suppressing the non-embryogenic calluses growth is very interesting since it could increase the overall ratio of embryogenic to non-embryogenic calluses. This is even more crucial considering that this activity was found in a recalcitrant variety such as Italia. This outcome supports the hypothesis that reducing ethylene levels can enhance somatic embryogenesis by stabilizing auxin distribution, as indicated by earlier studies [5,17].
On the other hand, SA showed contrasting results. In our study, SA has been considered mainly as an ethylene biosynthesis inhibitor; however, it is clear that this is a simplification. SA is a hormone in all respects with multiple potential effects on many gene expression pathways. It is well known for mediating host responses upon pathogen infection with viruses, fungi, insects, and bacteria, to the point of being used for exogenous SA treatments that boost the defense system of the host [50]. Moreover, it was shown that SA in several, but not all, plant systems can enhance somatic embryogenesis, through different proposed mechanisms and, especially, at different but generally very low concentrations [51,52,53,54]. How SA might promote somatic embryogenesis in several plant systems is still unclear. According to our experiments, which include both recalcitrant and non-recalcitrant varieties, even at the lowest SA tested concentration, no clear nor significant effect in enhancing embryogenesis was detected for Vitis vinifera.
The effects of citric acid inclusion in the culture media induce differences that we have previously reported [32] and these were confirmed by the data presented in this study, albeit always with varietal differences. In the Citrus genus, the citric acid seems to have a more pronounced effect than other acids of the Krebs’ cycle in promoting the growth of Citrus calluses [15]; therefore, we hypothesize that the improvement of the calluses viability with citric acid could be attributed to its involvement in the Krebs’ cycle for energy and coenzyme reduced production.
Vitis vinifera is considered a recalcitrant species, with some exceptions, where recalcitrance means the inability of plant cells, tissues, and organs to respond to tissue culture manipulations. In fact, the response to in vitro regeneration varies from the quite simple, although still requiring the long times typical of tree species, to the very difficult or non-existent. Unfortunately, this is a big issue for grapevine, because there are few and not very interesting varieties that respond well to in vitro culture conditions, while most grapevine varieties have a medium-low to very low response to regeneration [32]. The only countermeasure available so far to counter the deleterious effects of recalcitrance has been to tailor the individual varieties of interest to the best cultivation conditions. This could increase the possibility of success towards in vitro regeneration, a point emphasized by Preece and Read [12]. In our study, significant genotype-dependent responses to ethylene inhibitors were highlighted. While the Italia and Red Globe varieties exhibited notable improvements in callus viability and embryogenic competence with STS treatments, the Glera variety showed no significant differences across treatments. Taking into account that the first two varieties mentioned are moderately or very recalcitrant, while the third responds better to in vitro culture conditions, these results go in the direction of a useful tailoring of tissue culture protocols to specific genotypes.
5. Conclusions
In this article, we addressed several key points related to the effects of ethylene inhibitors on the long-term maintenance of the embryogenic callus of Vitis vinifera L. First, the response to ethylene inhibitors varies markedly among grapevine genotypes, highlighting the necessity for genotype-specific optimization in tissue culture protocols.
The STS emerges as a potent inhibitor of ethylene action and enhances the viability and embryogenic competence of Vitis vinifera calluses, albeit with varying efficacy across different grapevine varieties. STS’s ability to maintain and induce embryogenic competence in selected grapevine varieties could be further exploited, considering the introduction of this compound in the routine procedures applied to grapevine tissue culture.
The addition of citric acid improved callus viability, likely through its involvement in some metabolic pathways, underlying the importance of optimizing basal medium constituents.
SA demonstrated limited efficacy and occasionally adverse effects on callus viability and embryogenic competence compared with STS, indicating its lesser suitability for the callus vitality of grapevine varieties studied.
Future research should focus on further elucidating the molecular mechanisms underlying ethylene’s production in somatic embryogenesis and the interaction between ethylene and other phytohormones. Additionally, exploring the combined effects of ethylene inhibitors with other culture conditions and growth regulators may yield more refined and effective protocols for the in vitro culture of diverse Vitis vinifera genotypes.
Author Contributions
Conceptualization, L.R.F. and C.B.; Data curation, T.B.; Formal analysis, T.B.; Funding acquisition, R.V.; Investigation, L.R.F., B.S. and M.D.; Methodology, L.R.F.; Resources, M.F.C. and C.B.; Writing—original draft, L.R.F. and T.B.; Writing—review and editing, L.R.F., T.B., M.F.C., F.A.M.M., M.D. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Union–Next Generation EU-MUR PNRR “AGRITECH National Research Centre for Agriculture Technologies”–Spoke 1–CUP: C23C22000450006.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Roma, Italy, 2021; ISBN 978-92-5-134332-6. [Google Scholar]
- Iocco, P.; Franks, T.; Thomas, M. Genetic Transformation of Major Wine Grape Cultivars of Vitis vinifera L. Transgenic Res. 2001, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Franks, T.; Gang He, D.; Thomas, M. Regeneration of Transgenic Shape Vitis Vinifera L. Sultana Plants: Genotypic and Phenotypic Analysis. Mol. Breed 1998, 4, 321–333. [Google Scholar] [CrossRef]
- Capriotti, L.; Ricci, A.; Molesini, B.; Mezzetti, B.; Pandolfini, T.; Piunti, I.; Sabbadini, S. Efficient Protocol of de Novo Shoot Organogenesis from Somatic Embryos for Grapevine Genetic Transformation. Front. Plant Sci. 2023, 14, 1172758. [Google Scholar] [CrossRef] [PubMed]
- Ya, R.; Li, J.; Zhang, N.; Yu, Q.; Xu, W. Phenotypically Abnormal Cotyledonary Vitis Vinifera Embryos Differ in Anatomy, Endogenous Hormone Levels and Transcriptome Profiles. Tree Physiol. 2023, 43, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-N.; Lu, M.-J.; Zhou, M.; Wang, H.-Y.; Feng, J.-Y.; Wen, Y.-Q. Reduction of Pectin May Decrease the Embryogenicity of Grapevine (Vitis Vinifera) pro-Embryonic Masses after 10 Years of in Vitro Culture. Sci. Hortic. 2023, 309, 111690. [Google Scholar] [CrossRef]
- Domínguez, C.; Martínez, Ó.; Nieto, Ó.; Ferradás, Y.; González, M.V.; Rey, M. Involvement of Polyamines in the Maturation of Grapevine (Vitis vinifera L.‘Mencía’) Somatic Embryos over a Semipermeable Membrane. Sci. Hortic. 2023, 308, 111537. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Reddy, M. In Vitro Plant Propagation: A Review. J. For. Environ. Sci. 2011, 27, 61–72. [Google Scholar] [CrossRef]
- Pěnčík, A.; Turečková, V.; Paulišić, S.; Rolčík, J.; Strnad, M.; Mihaljević, S. Ammonium Regulates Embryogenic Potential in Cucurbita Pepo through pH-Mediated Changes in Endogenous Auxin and Abscisic Acid. Plant Cell Tissue Organ Cult. PCTOC 2015, 122, 89–100. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. Somatic Embryogenesis. An Overview. In Somatic Embryogenesis. Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–8. [Google Scholar]
- Preece, J.E.; Read, P.E. Environmental Management for Optimizing Micropropagation. In Proceedings of the I International Symposium on Acclimatization and Establishment of Micropropagated Plants 616, Sani-Halkidiki, Macedonia, Greece, 19–22 September 2001; pp. 49–58. [Google Scholar]
- Zhang, W.; Hu, W.; Wen, C.-K. Ethylene Preparation and Its Application to Physiological Experiments. Plant Signal. Behav. 2010, 5, 453–457. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 235913. [Google Scholar] [CrossRef] [PubMed]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant Propagation by Tissue Culture: Volume 1. the Background; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 1, ISBN 1-4020-5005-4. [Google Scholar]
- Biddington, N.L. The Influence of Ethylene in Plant Tissue Culture. Plant Growth Regul. 1992, 11, 173–187. [Google Scholar] [CrossRef]
- Bashir, M.A.; Silvestri, C.; Salimonti, A.; Rugini, E.; Cristofori, V.; Zelasco, S. Can Ethylene Inhibitors Enhance the Success of Olive Somatic Embryogenesis? Plants 2022, 11, 168. [Google Scholar] [CrossRef]
- Bai, B.; Su, Y.H.; Yuan, J.; Zhang, X.S. Induction of Somatic Embryos in Arabidopsis Requires Local YUCCA Expression Mediated by the Down-Regulation of Ethylene Biosynthesis. Mol. Plant 2013, 6, 1247–1260. [Google Scholar] [CrossRef]
- Feher, A.; Pasternak, T.P.; Dudits, D. Transition of Somatic Plant Cells to an Embryogenic State. Plant Cell Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Dimasi-Theriou, K.; Economou, A.S. Ethylene Enhances Shoot Formation in Cultures of the Peach Rootstock GF-677 (Prunus Persica x P. Amygdalus). Plant Cell Rep. 1995, 15, 87–90. [Google Scholar] [CrossRef]
- Mohiuddin, A.K.M.; Chowdhury, M.K.U.; Abdullah, Z.C.; Napis, S. Influence of Silver Nitrate (Ethylene Inhibitor) on Cucumber in Vitro Shoot Regeneration. Plant Cell Tissue Organ Cult. 1997, 51, 75–78. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Grosser, J.W.; Dutt, M. Silver Compounds Regulate Leaf Drop and Improve in Vitro Regeneration from Mature Tissues of Australian Finger Lime (Citrus australasica). Plant Cell Tissue Organ Cult. PCTOC 2020, 141, 455–464. [Google Scholar] [CrossRef]
- Ptak, A.; Tahchy, A.E.; Wyżgolik, G.; Henry, M.; Laurain-Mattar, D. Effects of Ethylene on Somatic Embryogenesis and Galanthamine Content in Leucojum aestivum L. Cultures. Plant Cell Tissue Organ Cult. PCTOC 2010, 102, 61–67. [Google Scholar] [CrossRef]
- Navarro-García, N.; Martínez-Romero, D.; Pérez-Tornero, O. Assessment of the Impact of Ethylene and Ethylene Modulators in Citrus Limon Organogenesis. Plant Cell Tissue Organ Cult. PCTOC 2016, 127, 405–415. [Google Scholar] [CrossRef]
- Kumar, V.; Ramakrishna, A.; Ravishankar, G.A. Influence of Different Ethylene Inhibitors on Somatic Embryogenesis and Secondary Embryogenesis from Coffea Canephora P Ex Fr. Vitro Cell. Dev. Biol.-Plant 2007, 43, 602–607. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology; Academic Press: Cambridge, MA, USA, 2012; ISBN 0-08-091628-7. [Google Scholar]
- Beyer, E. Silver Ion: A Potent Antiethylene Agent in Cucumber and Tomato1. HortScience 1976, 11, 195–196. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and Hormetic Effects of Silver Nanoparticles on in Vitro Regeneration of Vanilla (Vanilla Planifolia Jacks. Ex Andrews) Using a Temporary Immersion System. Plant Cell Tissue Organ Cult. PCTOC 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Leslie, C.A.; Romani, R.J. Inhibition of Ethylene Biosynthesis by Salicylic Acid. Plant Physiol. 1988, 88, 833–837. [Google Scholar] [CrossRef]
- Gribaudo, I.; Gambino, G.; Vallania, R. Somatic Embryogenesis from Grapevine Anthers: The Optimal Developmental Stage for Collecting Explants. Am. J. Enol. Vitic. 2004, 55, 427–430. [Google Scholar] [CrossRef]
- Yim, B.; Mun, J.-H.; Jeong, Y.-M.; Hur, Y.Y.; Yu, H.-J. Flower and Microspore Development in’Campbell Early’(Vitis Labruscana) and’Tamnara’(V. Spp.) Grapes. Hortic. Sci. Technol. 2015, 33, 420–428. [Google Scholar] [CrossRef]
- Forleo, L.R.; D’Amico, M.; Basile, T.; Marsico, A.D.; Cardone, M.F.; Maggiolini, F.A.M.; Velasco, R.; Bergamini, C. Somatic Embryogenesis in Vitis for Genome Editing: Optimization of Protocols for Recalcitrant Genotypes. Horticulturae 2021, 7, 511. [Google Scholar] [CrossRef]
- Kansas State University. Available online: https://www.k-state.edu/wgrc/electronic_lab/aceto_stain.html (accessed on 29 April 2024).
- Onay, A.; Pirinç, V.; Tilkat, E.; Aktürk, Z.; Yildirim, H. Somatic Embryogenesis of Pistachio from Female Flowers. J. Hortic. Sci. Biotechnol. 2004, 79, 960–964. [Google Scholar] [CrossRef]
- Capriotti, L.; Limera, C.; Mezzetti, B.; Ricci, A.; Sabbadini, S. From Induction to Embryo Proliferation: Improved Somatic Embryogenesis Protocol in Grapevine for Italian Cultivars and Hybrid Vitis Rootstocks. Plant Cell Tissue Organ Cult. PCTOC 2022, 151, 221–233. [Google Scholar] [CrossRef]
- Torregrosa, L.; Vialet, S.; Adivèze, A.; Iocco-Corena, P.; Thomas, M.R. Grapevine (Vitis vinifera L.). Agrobacterium Protoc. 2015, 2, 177–194. [Google Scholar] [CrossRef]
- Nitsch, J.; Nitsch, C. Haploid Plants from Pollen Grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- De Paoli, G.; Rossi, V.; Scorzoli, A. Micropropagazione Delle Piante Ortoflorofrutticole; Edagricole: Bologna, Italy, 1994. [Google Scholar]
- Torregrosa, L. A Simple and Efficient Method to Obtain Stable Embryogenic Cultures from Anthers of Vitis vinifera L. VITIS-GEILWEILERHOF 1998, 37, 91–92. [Google Scholar] [CrossRef]
- Martinelli, L.; Gribaudo, I.; Bertoldi, D.; Candioli, E.; Poletti, V. High Efficiency Somatic Embryogenesis and Plant Germination in Grapevine Cultivars Chardonnay and Brachetto a Grappolo Lungo. Vitis 2001, 40, 111–115. [Google Scholar] [CrossRef]
- López-Pérez, A.J.; Carreño, J.; Martínez-Cutillas, A.; Dabauza, M. High Embryogenic Ability and Plant Regeneration of Table Grapevine Cultivars (Vitis vinifera L.) Induced by Activated Charcoal. Vitis 2005, 44, 79–85. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-Project.Org/ (accessed on 1 March 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Dinno, A. Conover.Test: Conover-Iman Test of Multiple Comparisons Using Rank Sums. R Package Version 1.1.6. 2024. Available online: https://cran.r-project.org/web/packages/conover.test/conover.test.pdf (accessed on 1 April 2024).
- Dinno, A.; Rank Sums. Dunn.Test: Dunn’s Test of Multiple Comparisons Using Rank Sums. R Package Version 1.3.5. 2017. Available online: https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf (accessed on 1 April 2024).
- Steinitz, B.; Barr, N.; Tabib, Y.; Vaknin, Y.; Bernstein, N. Control of in Vitro Rooting and Plant Development in Corymbia Maculata by Silver Nitrate, Silver Thiosulfate and Thiosulfate Ion. Plant Cell Rep. 2010, 29, 1315–1323. [Google Scholar] [CrossRef]
- Fortin, C.; Campbell, P.G. Thiosulfate Enhances Silver Uptake by a Green Alga: Role of Anion Transporters in Metal Uptake. Environ. Sci. Technol. 2001, 35, 2214–2218. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in Plant Tissue Culture: The Disclosed and Undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Luo, J.-P.; Jiang, S.-T.; Pan, L.-J. Enhanced Somatic Embryogenesis by Salicylic Acid of Astragalus Adsurgens Pall.: Relationship with H2O2 Production and H2O2-Metabolizing Enzyme Activities. Plant Sci. 2001, 161, 125–132. [Google Scholar] [CrossRef]
- Luo, J.-P.; Jia, J.-F.; Gu, Y.-H.; Liu, J. High Frequency Somatic Embryogenesis and Plant Regeneration in Callus Cultures of Astragalus Adsurgens Pall. Plant Sci. 1999, 143, 93–99. [Google Scholar] [CrossRef]
- Hatanaka, T.; Sawabe, E.; Azuma, T.; Uchida, N.; Yasuda, T. The Role of Ethylene in Somatic Embryogenesis from Leaf Discs of Coffea Canephora. Plant Sci. 1995, 107, 199–204. [Google Scholar] [CrossRef]
- Hutchinson, M.J.; Saxena, P.K. Acetylsalicylic Acid Enhances and Synchronizes Thidiazuron-Induced Somatic Embryogenesis in Geranium (Pelargonium x hortorum Bailey) Tissue Cultures. Plant Cell Rep. 1996, 15, 512–515. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).