Abstract
Tetratricopeptide repeat (TPR) proteins play numerous roles in plant growth and development by mediating protein–protein interactions in biological systems by binding to peptide ligands. Although genome-wide analyses of the TPR gene family in other species have been performed, its evolution and function in Cucurbitaceae remain unclear. In this study, 144 TPR genes from 11 genomes of eight Cucurbitaceae species with a heterogeneous distribution on the chromosomes were characterized. Based on the homology between Cucurbitaceae and Arabidopsis, the TPR genes were divided into four groups, and the evolutionary relationships of the Benincaceae and Cucurbitaceae tribes were also represented in a phylogenetic tree. Using the ‘DHL92′ genome as a reference, an integrated chromosome map was obtained containing 34 loci, 4 of which were common to the Cucurbitaceae. Cis-regulatory element analysis showed that these elements are essential for melon development and responses to light, phytohormones, and various stresses. CmTPR tissue- and development-specific expression analysis revealed differential expression patterns under normal growth conditions. Furthermore, the CmTPR genes responded to various abiotic stressors. Overall, this study offers insights into the evolutionary history of the TPR gene family in Cucurbitaceae and provides valuable information for elucidating the potential role of CmTPR genes during development and under different stresses in melon.
1. Introduction
Cucurbitaceae account for 2.6% of the total global cultivated area of vegetable crops and are among the most genetically diverse crops worldwide [1]. Cucurbits are one of the most popular varieties of vegetables and fruits, and their production has increased annually in recent years (http://faostat.fao.org/, accessed on 26 June 2023) [2]. Cucurbit crops can be naturally exposed to various abiotic and biotic stresses during their lifetime, such as salt, high or low temperatures, and powdery mildew [3,4,5]. Many nucleotide-binding leucine-rich repeat (NB-LRR) proteins belong to the TPR (tetratricopeptide repeat) gene family, a class of genes containing conserved TPR motifs that are involved in many important life activities and contribute to plant response to various environmental stresses [6,7,8].
During the long evolutionary process, higher plants have gradually developed defense mechanisms to cope with these adversities, adjusting their gene expression through signaling responses, morphology, structure, and physiological functions to adapt to the complex and changing external environment. The TPR domain has 34 amino acid repeat motifs in its structural domain and is thought to function as a mediator of protein–protein interactions involved in cell cycle regulation, transcriptional repression, protein transport, RNA synthesis, and stress response [9,10]. TPR-containing proteins might be required for the specific recognition of RNA substrates and might also be part of multi-subunit protein complexes that could form a superhelical structure that serves as a scaffold to mediate protein–protein interactions, giving the TPR protein the ability to adapt to and complement regions of interactions with target proteins [11,12].
The TPR gene family is widely distributed in nature, and proteins containing a conserved TPR motif have been found in microorganisms, plants, animals, and other species [7,13,14]. TPR proteins were first discovered in yeast, and over 20,000 TPR proteins have been identified to date [7,13]. Mutations in the TPR protein significantly affect the infectious ability of Borrelia burgdorferi, suggesting that conserved TPR motifs are associated with Borrelia burgdorferi virulence in microorganisms [15]. In animals, mutations in the TPR gene family have been associated with the pathogenesis of various diseases, such as breast cancer [16,17]. In plants, TPR proteins mediate protein–chaperone-protein interactions and participate in various environmental stresses and hormone signaling processes [18,19]. TPR proteins promote transient interactions between proteins and related functional domains, enabling them to perform key roles in different cellular processes, conferring adaptability or tolerance to different environmental stress in plants; for example, TaTPR1 is able to respond to adversities in wheat, such as low-temperature and high-salt stress [19]. Genome-wide characterization of TPR genes has been widely performed in various plants, including Arabidopsis, rice, and tomato [10,20]. Plant are often hampered in their growth and development by various abiotic and biotic stresses [21,22]. AtCHIP contains three TPR repeats that enhance plant temperature sensitivity and modulate membrane channel proteins in response to temperature stress [18]. The NCA1 protein interacts with the CAT2 (Catalase 2) protein via its TPR repeat sequence to maintain intracellular H2O2 homeostasis under abiotic stress conditions [23]. SPY-containing TPR repeats mediate gibberellin and cytokinin signaling pathways, which are negative regulators of the GA (gibberellin) signaling pathway [24]. TT1 positively regulates ABA-regulated (abscisic acid) stress responses and increases plant sensitivity to salt and osmotic stresses [19]. Previous studies have shown that the SlTPR gene family is differentially expressed in different developmental periods and tissues, that members of the SlTPR gene family are capable of responding to a wide range of biotic and abiotic stresses, and that the specific response mechanisms may differ [20]. Silencing SlTPR2 and SlTPR4 using VIGs reduces the ability of silenced plants to respond to biotic and abiotic stresses [20].
The genomes of several economically important species of cucurbit have been published, including five Benincaseae tribes (Cucumis melo L., Cucumis sativus L., Citrullus lanatus subsp., Lagenaria siceraria Standl., and Benincasa hispida Cogn.) and three Cucurbiteae tribes (Cucurbita moschata Duchesne, Cucurbita maxima Duchesne, and Cucurbita pepo Duchesne) [25,26,27,28,29,30,31,32,33,34,35]. Although the genome-wide characterization of TPR genes has been performed in several species, the identification and functional analysis of this gene family is still lacking in cucurbit crops. Based on the genome of cucurbit crops, using bioinformatics to identify the number of TPR gene family members, and via the analysis of gene structure, system evolution, gene collinearity, participation pathways, expression patterns, combination of transcriptome and quantitative real-time PCR (qRT-PCR) assay was used to examine the changes in the expression of each member under various environmental stress. In conclusion, this study provides new insights into the evolutionary history of the TPR gene family and points to a prospective subset of candidate genes for future TPR functional analyses.
2. Materials and Methods
2.1. Plant Materials and Treatment
The plant material used in this study was the melon cultivar ‘Yangjiaomi’, which was grown in an artificial climate chamber in Liaocheng university with a photoperiod of 16 h/8 h and day/night temperatures of 26 °C/23 °C. After the seedlings grew three leaves, melon plants with uniform growth were chosen for salt stress, chilling stress, and high-temperature–high-humidity (HTH) stress, respectively. According to the settings of previous studies, salt stress was performed using 300 mM NaCl for 2, 4, and 6 d (days), low-temperature stress was performed at 4 °C for 6 and 12 h (hours), and HTH stress (day and night temperatures of 45 °C/35 °C, soil humidity of 100%, and humidity of 90%) was performed for 1 and 2 d [36,37,38,39,40,41,42,43,44]. Ten melon plants each of the control and treatment melons were collected to quantify the relative expression of CmTPR gene family members. Three biological replicates were performed for each treatment group.
2.2. Identification of TPRs and Their Biochemical Characterization
Based on the recently published genome-wide characterization of TPR genes in Cucurbitaceae species, 144 TPR proteins were obtained from eight Cucurbitaceae species [20,36,37]. Utilizing the native Blast program with the e-value set to 1.0 × 10−5, these protein sequences were used as queries to obtain predicted protein files for C. melo, C. sativus, C. lanatus, L. siceraria, C. moschata, C. maxima, C. pepo, and B. hispida from the Cucurbit Genome Database (http://cucurbitgenomics.org/, accessed on 30 July 2023), and this method was used in previous research, such as with the CmTCS gene family, ClOMT gene family, and SlTPR gene family [27]. The TPR protein sequences were analyzed using the online domain tool CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 3 August 2023), and a TPR gene was confirm if it contained at least one conserved TPR sequence. Using the ExPASy proteomics server online tool (http://expasy.org, accessed on 13 August 2023) under standard mode, basic information (such as the number of amino acids, molecular weight, instability index, aliphatic index, GRAVY) of the TPR genes was obtained from the cucurbit crops.
2.3. Phylogenetic Relationship, Exon/Intron Structure, and Protein Motif
To investigate the phylogenetic relationships of TPR proteins between Cucurbitaceae and Arabidopsis, TPR protein sequences from Arabidopsis and Cucurbitaceae were aligned and analyzed using the neighbor-joining (NJ) method with MEGA-X and a bootstrap test with 1000 iterations [38]. We further divided the TPR genes into subfamilies based on the homology between the Cucurbitaceae species and Arabidopsis. The TBtools software version 17763.0 was used to extract and visualize the exons and introns of the TPR gene family from the genomes of Cucumis melo L. cv. DHL92. The TPR protein motifs of melon were predicted using the MEME (Multiple EM Motif Elicitation) online tool (https://meme-suite.org/meme/tools/meme, accessed on 3 August 2023), and the maximum number of motifs was set as 5 under the default mode. Gene structures were synthesized using the TBtools software with the ‘Gene Structure View (Advanced)’ module under the default mode for visualization [37].
2.4. Chromosomal Location and Collinearity Analyses
Using the Mapchat software under the standard mode, the genome data for C. melo, C. sativus, C. lanatus, L. siceraria, C. moschata, C. maxima, C. pepo, and B. hispida obtained from the Cucurbitaceae genome website database (http://cucurbitgenomics.org/, accessed on 17 August 2023) and a previous study [27], we visualized the chromosomal distribution of TPR genes. Using TBtools and MCScanX, we further analyzed the tandem duplication events of the TPR gene family between the cucurbit species and C. melo with the e-value set to 1.0 × 10−10 [37,39]. Similarly, using TBtools with the MCScanX method, we investigated the segmental duplication events and covariance of gene pairs in the different cucurbit species with the e-value set to 1.0 × 10−10 [37,39]. The standards for identification of tandem duplicate genes were neighboring homologous genes located on the same chromosome with only 1 gene inserted in the middle, or the length and similarity of two gene sequences being >70% [4].
2.5. Promoter Cis-Regulatory Element and Gene Ontology Analysis
To gain further insight into the TPR gene family, we characterized the cis-promoter regulatory elements of the TPR genes. We examined the sequences within 2000 bp upstream of the ATG promoter and searched for these sequences in the Cucurbitaceae genome. The identification of cis-elements in promoters was performed using the PlantCARE online tool (http://bioinformatics.psb.ugent.be/webtools/plantcare, accessed on 9 November 2023) under the default mode [40], and the statistical analysis and visualization of the identified cis-acting elements in the promoters were performed using a heatmap in the R language. Using the agriGo online tool (http://systemsbiology.cau.edu.cn/agriGOv2/, accessed on 3 August 2023) under the default mode, we used Fisher’s exact test as the statistical test method, Go (Gene Ontology) terms were analyzed for TPR genes in C. melo, C. sativus, and C. lanatus, and Go terms with a Q-value ≤ 0.05 were considered overrepresented.
2.6. Expression Analysis of CmTPR Genes
Using a published RNA-seq dataset, the expression of CmTPR genes was examined in different organs and developmental periods using an online website (https://melonet-db.dna.affrc.go.jp/ap/top, accessed on 15 November 2023) [41]. Heatmaps were generated to illustrate the spatiotemporal expression of callus, dry seeds, root, stem (downside and upside), shoot apex, leaves (young and 6th–12th), tendril, flower (anther male, petal female, and stigma female), ovary (0–4 DAF (days after flowering)), fruit flesh (8–50 DAF), and fruit epicarp (8–50 DAF).
Using RNA-seq datasets published by Wang et al., Weng et al., and Diao et al., CmTPR gene expression was analyzed under abiotic stress [42,43,44]. According to the methodology of Cheng et al., the expression level of the gene transcript was obtained by analyzing the raw transcriptome data uploaded to the NCBI database using the ‘Trimmomatic’, ‘Kallisto’, and ‘Trans Value Sum’ modules of the TBtools software [37]. All published RNA-seq data were shown as heat maps plotted using the R package (Heatmap package). To experimentally explore the expression profiles of the CmTPR genes under abiotic stress, and qRT-PCR was then carried out. Total RNA was extracted with an RNA-extraction Kit (Vazyme, Nanjing, China) and reverse transcribed into cDNA using a Vazyme Reverse Transcription Kit with reference to the instruction manual. The real-time fluorescence quantitative PCR reaction was performed using the Roche SYBR Green Master method. Relative expression was calculated according to the 2−ΔΔCT method [45]. Differences between treatments were tested by ANOVA (one-way analysis of variance), and a p-value ≤ 0.05 was considered statistically significant. Data were analyzed using the SPSS software Version 29.0 and expressed as mean ± SD (standard deviation) of three biological replicates. All primers used for the qRT-PCR are listed in Table S1.
3. Results
3.1. Identification of TPR Genes in Cucurbitaceae
In total, 144 TPR proteins were identified in eight Cucurbitaceae species (C. melo, C. sativus, C. lanatus, L. siceraria, C. moschata, C. maxima, C. pepo, and B. hispida) (Tables S2 and S3). We used the genomes of eleven cucurbit crops to identify TPR genes, two of which were used for melon (Cucumis melo L. cv. DHL92 (10) and Cucumis melo subsp. agrestis (10), as well as two genomes of cucumber, Cucumis sativus L. cv. Gy14 (8) and Cucumis sativus L. var. sativus var. 9930 (12); we also used two watermelon genomes, Citrullus lanatus subsp. vulgaris cv. Charleston Gray (14) and Citrullus lanatus subsp. vulgaris cv. 97103 (18) (Table 1). The remaining five genomes were Lagenaria siceraria var. USVL1VR-Ls, Cucurbita moschata var. Rifu, Cucurbita maxima var. Rimu, Cucurbita pepo subsp. Pepo, and Benincasa hispida var. B227, in which 10, 19, 17, 16, and 10 TPR genes were identified, respectively (Table 1). Compared with similar TPR gene copy numbers in species of the Benincaseae tribe (C. melo, C. sativus, C. lanatus, L. siceraria, and B. hispida), more homologous genes were found in the genomes of the Cucurbitaceae tribe (C. moschata, C. maxima, C. pepo), respectively (Table 1). This may be due to WGD (whole genome duplication), and duplicated sequences are a major factor in genome size differences, which occurred only in the ancestors of Cucurbitaceae [33,34].

Table 1.
Baic information for TPR genes in Cucurbitaceae.
Due to a lack of specific annotations for TPR genes in Cucurbitaceae, we renamed them based on their location on the chromosome (Table S4), which has been commonly adopted in previous studies [46,47]. The distribution of TPR genes was uneven, with three in chr.1, two in chr.11, and one for the other genes in C. melo cv. DHL92 (Figure S1). The physicochemical properties of the TPR genes, such as the number of amino acids, molecular weight, and theoretical PI, were analyzed; it was found that the number of amino acids and the molecular weight showed similar ranges in the Cucurbit crops. The theoretical PI and GRAY characteristics showed similar ranges in C. melo, C. sativus, C. lanatus, L. siceraria, and B. hispida, whereas these characteristics showed greater ranges in the Cucurbitaceae (C. moschata, C. maxima, and C. pepo) (Table 1 and Table S4).
3.2. Phylogenetic Divergence of TPR Genes in Cucurbitaceae
The evolution of the Benincaseae tribe over millions of years has led to the differentiation of several species, including melons, watermelons, cucumbers, and bottle gourds. To analyze the phylogenetic relationships of the TPR gene family in the Benincaseae tribe using the TPR protein sequences of melon, cucumber, watermelon, bottle gourd, and wax gourd, a phylogenetic tree was constructed following the NJ algorithm, in which the bootstrap replicate value was set to 1000 (Figure S2). The results indicated that the 92 TPR genes from the Benincaseae tribe were split into four groups. Group I comprised most TPR members, with 47 TPR genes. Group II and III included 19 and 23 TPR genes, respectively. Group IV contained the lowest number of three TPR genes (Figure S2). Interestingly, most branches contained homologs from only a single species. Compared to homologs from melon and cucumber, those from watermelon, bottle gourd, and wax gourd were preferentially clustered together, concurring with previous studies [36].
WGD is an intrinsic factor that provides a rich base of genetic material for morphological trait variation and synergistically contributes to the diversification of cucurbit species, with multiple WGD events occurring in the ancestors of Cucurbitaceae [25]. Cucurbitaceae evolved a branch containing nearly 80% of extant species after the WGD event, which coincided with a brief climatic optimum in the middle Eocene, providing suitable environmental conditions for species diversification [25]. A phylogenetic tree was constructed using 19, 17, and 16 TPR protein sequences from C. moschata, C. maxima, and C. pepo, respectively, and the results were divided into four groups (Figure S2). The four groups contained 26, 13, 9, and 4 TPR proteins (Figure S2). Consistent with the Benincaseae tribe, Group I had the most TPR gene members, and Group IV had the fewest TPR gene members. Notably, most TPR proteins in the branches were from different genomes.
To further analyze the phylogenetic relationship of the TPR gene family in cucurbit crops, we used the identified 144 TPR in cucurbit crops and 36 TPR proteins in Arabidopsis to construct an unrooted phylogenetic tree, which showed that all the TPR proteins were divided into four groups, consistent with those in the Benincaseae and Cucurbitaceae tribes (Figure 1). Group I included 99 TPR genes, group II contained 35 TPR genes, and group IV contained the least number of TPR genes, with 5. Most members of the Benincaseae tribe were clustered together, and the TPR proteins from watermelon, bottle gourd, and wax gourd were clustered together on most branches, which is consistent with the evolutionary relationship of the modern cucurbit genome [25,48]. In addition, most TPR members from the tribe Cucurbitaceae gathered preferentially, suggesting that they originated from a common ancestor [33].
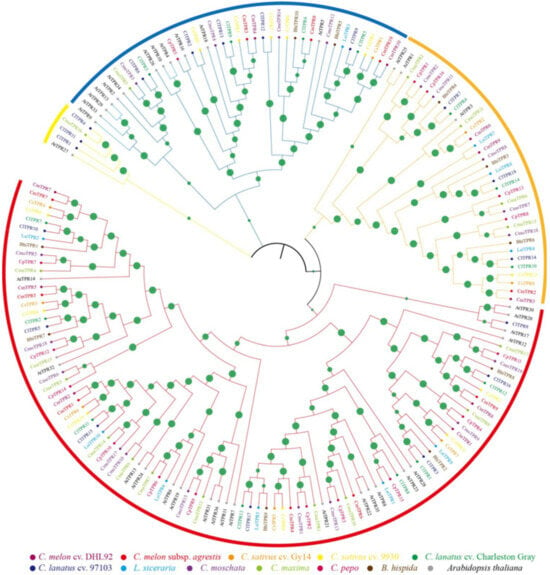
Figure 1.
Phylogenetic tree of TPR genes in Arabidopsis and Cucurbitaceae. An evolutionary tree was constructed using the NJ method to further categorize TPR genes into four groups. Green dots indicate bootstrap values, different colored lines represent different subfamilies, and different colored letters indicate different species.
3.3. Collinearity Analysis of TPR Genes among Different Species
Plant genomes differ in the location and order of genes on their corresponding chromosomes, and comparative analysis between species genomes can illustrate genome evolution [49]. Relationships between species can be investigated by identifying conserved genes in pairs that exist between them [50]. To further understand the evolutionary relationships of TPR genes across species, collinearity analysis was performed using melons from seven other species. Seventy CmTPR genes in melon were linked to seven CsTPR genes and seven ClTPR genes in cucumber and watermelon, respectively (Figure 2 and Table S5). Sixty percent of the CmTPR genes had collinear connections with six LsiTPR genes and six BhiTPR genes in bottle gourd and wax gourd, respectively (Figure 2 and Table S5). More TPR homologous genes were identified in the Cucurbitaceae tribes (C. moschata, C. maxima, and C. pepo), and covariance analysis of melons with these species revealed that 80% of the CmTPR genes were linked to 10 CmoTPR genes (Figure 2 and Table S5). In total, 60% of the CmTPR genes were collinearly linked to seven CmaTPR genes, and 50% CmTPR genes were collinearly connected with six CpTPR genes (Figure 2 and Table S5). The results of the collinear analysis were consistent with those of the phylogenetic tree analysis, where the TPR genes with collinear relationships tended to be on similar branches in the phylogenetic tree. Our results suggest that TPR genes are similarly characterized in different species, and CmTPR genes are reliably identified in melon.
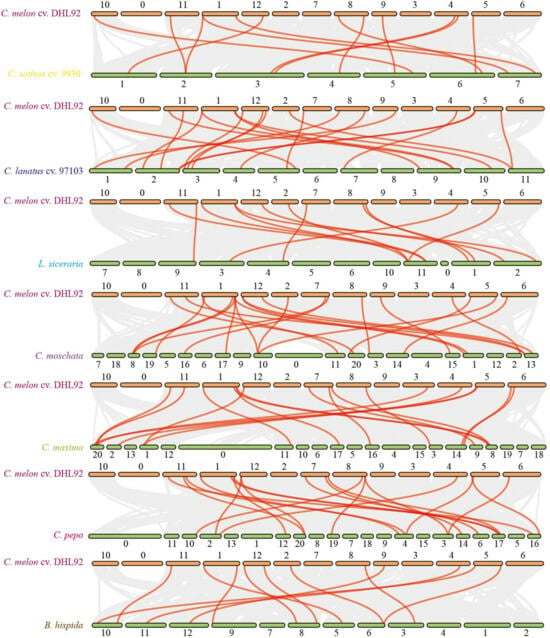
Figure 2.
Interspecific covariance analysis of melon ‘DHL92′and other Cucurbitaceae. From top to bottom: C. sativus cv. 9930, C. lanatus cv. 97103, L. siceraria, C. moschata, C. maxima, C. pepo, and B. hispida.
3.4. Construction of Integration Gene Map for TPR Genes
Similar to other gene families distributed on chromosomes, the distribution of TPR genes in the present study was heterogeneous across chromosomes in the Cucurbitaceae species, and some chromosomes did not contain TPR genes. The ancestral Cucurbitaceae karyotype consists of 12 chromosomes that have evolved into the extant Cucurbitaceae species genome through frequent hybridization and lineage-/species-specific genomic recombination. To investigate the loci of TPR genes on chromosomes, we constructed an integrated gene map containing 34 TPR loci using the melon chromosome as a reference (Figure 3). Of these, only four loci were shared by all species, and the remaining loci were present or absent in the genome (Figure 3). For instance, the first locus was shared by all species on chr.1, and the second locus was shared by only three Cucurbitaceae species on chr.1.
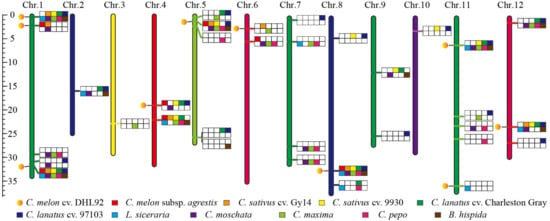
Figure 3.
Comprehensive map of TPR gene loci in Cucurbitaceae. All TPR genes were designated on the 12 chromosomes of melon ‘DHL92′, represented by yellow circle. Other species were represented using different colors in the squares.
3.5. Phylogenetic Relationship, Gene Structure, and Motif Analysis in Melon
To better understand the structural diversity of TPR genes, we investigated the conserved motifs and exon–intron structures of TPR genes in Cucurbitaceae. Five motifs were identified, named motifs 1–5 (Figure 4). Of these, motif 4 was present in three CmTPR genes (CmTPR3/6/9), and motifs 1 and 2 were found in the remaining CmTPR genes (Figure 4). Motif 3 was identified in only two CmTPR genes (CmTPR1/8) (Figure 4). In addition, the exon–intron structure analysis provided an important basis for the evolution of gene family members (Figure 4). The exon–intron structure of the genes suggested that the CmTPR gene family has 3–15 exons as well as different numbers of introns and that the CmTPR genes showed very close similarity in terms of the number of exons and the length of introns on the same branch (Figure 4). The analyses of the other Cucurbitaceae species revealed similar results, with members of the TPR gene family on the same branch showing very close similarities in terms of the number of motifs and structure of exon–intron numbers.
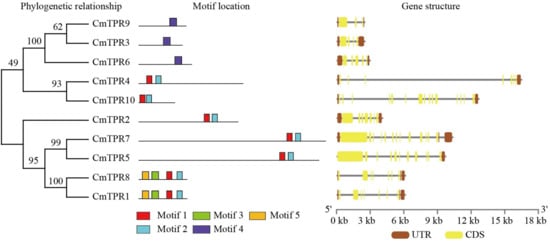
Figure 4.
Phylogenetic tree, motif, and gene structure analysis of CmTPR genes in melon. Motifs 1–5 were conservation motifs in the MEME structure diagram. Brown and yellow columns and black lines represent UTR, CDS, and introns in the exon–intron structure diagram, respectively.
3.6. Analysis of Promoter Cis-Regulator Elements and GO Terms in Melon
Cis-regulator elements in promoters related to phytohormones and other factors can elucidate the role of genes in plant development and environmental stress. To further explore whether CmTPR genes play a key role in multiple stress responses, we extracted 2 kb of the sequence upstream of the initiation codon (ATG) for the cis-regulator element analysis. We identified twenty-eight cis-regulator elements from 10 CmTPR genes in the promoter, of which nine were hormone-related cis-regulator elements, two were Auxin-responsive elements (TGA-element and AuxRR-core), one was an ABA-responsive element (ABRE), three were gibberellin-responsive elements (P-box, TATC-box, and GARE-motif), two were MeJA (Methyl Jasmonate)-responsive elements (CGTCA-motif and TGACG-motif), and one was an SA (salicylic acid)-responsive element (TCA-element). A further 18 environmental response cis-regulator elements were identified, of which the TC-rich repeat was a defense- and stress-responsive element, MBS was a drought-responsive element, LTR (low-temperature-responsive) was a low-temperature-responsive element, and the others were light-responsive elements (Figure 5 and Figure S3). In addition, anaerobic induction, circadian control, and meristem expression elements were detected (Figure 5 and Table S6). To gain a full understanding of the TPR genes, overrepresented gene ontology (GO) terms for the TPR genes were analyzed. A total of five GO terms were found (Q-value ≤ 0.05), including ‘Protein binding,’ ‘Binding,’ ‘Cellular protein modification process,’ ‘Protein modification process’, and ‘Macromolecule modification’ (Table S7). Our results suggest that the CmTPR gene family plays essential roles in plant development and response to various environmental stresses.
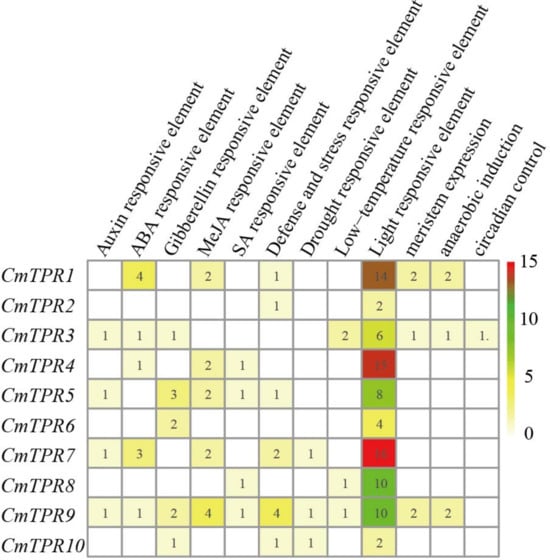
Figure 5.
Cis-regulator element analysis of CmTPR genes in melon. Cis-regulator elements were identified in the promoter region of CmTPR gene. The gradient color from white to red and the numbers in the grid indicate the number of different cis-regulator elements in CmTPR.
3.7. Tissue-Specific Expression of CmTPR Genes in Melon
To investigate the expression of CmTPR genes in different tissues and at different developmental periods in melons, the expression profile of the CmTPR genes was constructed using the transcriptomic data published by Yano et al. [41]. We analyzed 10 CmTPR genes in different tissues and at different developmental stages in melons, including seeds, roots, leaves (young/6th/9th/12th leaves), flowers (anther male and petal/anther/stigma female flowers), and fruit (fruit flesh and epicarp) (Figure 6). Three CmTPR genes (CmTPR1/4/8) were highly expressed in calli, CmTPR3 and CmTPR9 were highly expressed in dry seeds, and five CmTPR genes (CmTPR1/2/3/4/9) were highly expressed in the stem (bottom and top) (Figure 6). Three CmTPR genes (CmTPR1/2/4) were highly expressed in young leaves, whereas CmTPR3 and CmTPR9 were primarily expressed in fully expanded leaves (Figure 6). In addition, CmTPR1/2/3/4/9 were highly abundant during the different periods of fruit ripening (Figure 6). Similar phenomena were observed in flowers, revealing that the development of different tissues may be related to the selective expression of CmTPR genes.
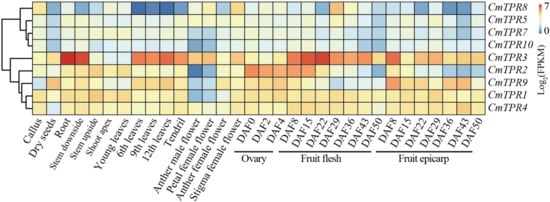
Figure 6.
Tissue and development expression profiles of CmTPR genes under normal growth conditions. The expression abundance of the CmTPR gene was normalized to FPKM and shown as the average of three biological repeat sequences.
3.8. CmTPR Genes Expression in Response to Multiple Stresses
To investigate the expression patterns of the CmTPR gene family under conditions of multiple environmental stresses, we analyzed the expression levels of the CmTPR genes in response to salt, HTH, and cold stress using RNA-seq data [42,43,44]. The transcriptome data showed that CmTPR1/3/4/6 were highly expressed after salt stress, and CmTPR3/4 had significant changes (Figure 7). Transcript abundance analysis of CmTPR1/3/4/6 in melon seedling leaves was performed under salt stress using qRT-PCR, and the different response profiles are shown in Figure 7. CmTPR3/4/6 expression progressively increased and remained at relatively high levels after salt stress (Figure 7). Analysis of the RNA-seq data revealed that CmTPR1/3/4/6 had equally high expression and significant changes in tolerant and susceptible melons after HTH stress (Figure 7). Additionally, the qRT-PCR results indicated that CmTPR3/4/6 expression was significantly increased under HTH stress. In addition, the transcriptome analysis revealed a high transcript abundance of CmTPR3/6/9 under chilling stress (Figure 7). An increasing trend was detected for CmTPR3/6/9 by qRT-PCR under chilling stress conditions (Figure 7).
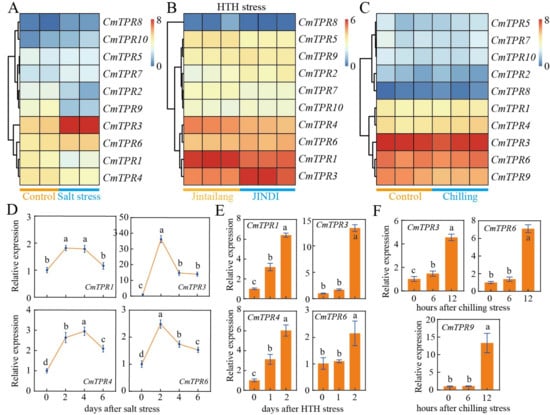
Figure 7.
Expression profiles of CmTPR gene in melon under different abiotic stresses. (A) Heatmap of CmTPR expression under normal growth conditions and salt (NaCl) stress. (B) Heatmap displaying CmTPR expression in tolerant (‘JINDI’) and sensitive (‘Jintailang’) varieties under HTH (high temperature and humidity) stress. (C) Heatmap of CmTPR expression under normal growth conditions and chilling stress. (D) Expression profiles of representative CmTPR genes at 0, 2, 4, and 6 d (days) after salt stress. (E) Expression profiles of representative CmTPR genes at 0, 1, and 2 d (days) after HTH stress. (F) Expression profiles of representative CmTPR genes at 0, 3, and 6 h (hours) after chilling stress. Different letters in the bar graph indicate significant differences in the expression of representative CmTPR between control and other time points at a significance level of 0.05.
4. Discussion
The TPR gene family containing conserved TPR structural motifs is widely involved in biological processes such as the cell cycle, gene expression, protein translocation, RNA shearing, transcriptional repression, and response to stress. TPR genes are widespread in plants; for instance, 26 SlTPR genes have been identified in tomatoes [20]. However, TPR gene family members have not yet been identified in Cucurbitaceae. With the sequencing of cucurbit crop genomes completed sequentially, we used bioinformatics to analyze the genome of cucurbit crops. A total of 144 TPR genes were identified in eight cucurbit species (C. melo, C. sativus, C. lanatus, L. siceraria, C. moschata, C. maxima, C. pepo, and B. hispida), and they were widely and irregularly distributed on chromosomes [27,28,29,30,32,33,34,35,51]. A total of 10 CmTPR genes were identified in melon, and 8 and 12 CsTPR genes were found in Cucumis sativus L. cv. Gy14 and Cucumis sativus L. var. sativus var. 9930, respectively. Totals of 14 and 18 ClTPR genes were identified in Citrullus lanatus subsp. vulgaris cv. Charleston Gray and Citrullus lanatus subsp. vulgaris cv. 97103, respectively. Because the occurrence of WGD, the number of TPR genes were different between cultivars of the same species [33,52]. Additionally, ten LsiTPR and ten BhiTPR genes were identified in L. siceraria and B. hispida, respectively. In total, 19 CmoTPR, 17 CmaTPR, and 16 CpTPR genes were identified in the Cucurbiteae tribe (C. moschata, C. maxima, and C. pepo). Taken together, the numbers of TPR genes in the Cucurbiteae tribe were, on average, higher than those in the Benincaseae tribe, which might be due to a WGD event during the origin of the genus Cucurbiteae tribe in Cucurbitaceae [33,52].
Tandem duplication events play an important role in the expansion of gene family members and in the conservation of the topology of the gene family [36]. The TPR genes were divided into four groups in the phylogenetic tree, consistent with the evolutionary analyses of the Cucurbiteae and Benincaseae tribes, with Group I consisting of the most members, followed by Group II and Group IV with the fewest members. Based on the scenario analysis of cucurbit species, melon diverged from cucumber at ~6.51 Mya (million years ago), from watermelon at ~19.06 Mya, from cucurbits at ~36.13 Mya, from the progenitor A of Cucurbita at ~30.75 Mya, and from the progenitor B of Cucurbita at ~26.28 Mya [33,52]. It is worth noting that among the members of the Cucurbiteae tribe, the majority of the members of subgenome B, as opposed to subgenome A, usually congregate with members of the Benincaseae tribe. Consistent with this evolutionary scenario, TPR genes are more likely to cluster together in melons and cucumbers than their homologs in other cucurbit species, which is consistent with the analysis of other gene families identified in cucurbit species [36].
The number of genes in a species depends on the gene duplication events that occur at irregular frequencies across subspecies [47]. Using melon chromosomes as a reference, integrated gene mapping was performed based on gene covariance among cucurbit species. Similar to previous studies, an integrated gene map with 34 loci was obtained, of which 4 were shared, and polymorphisms (present/absent) were observed at 30 loci [36]. In the comprehensive gene map, members of Cucurbiteae tribe always appeared at the same locus, and there were 10 loci with only TPR gene members from the Cucurbitaceae species. This reflects the evolutionary relationships between TPR genes in Cucurbitaceae species.
Exon–intron structures can reflect evolutionary relationships within a gene family [53]. Introns are self-splicing reverse transcription elements that play key roles in shaping the genomes of organisms [54]. Changes in gene structure and motifs are relatively reliable parameters for assessing the evolution of a gene family [55]. CmTPR4/10 have longer introns compared to the other CmTPR gene members, while CmTPR3/6/9 have shorter introns. The same motif was described on a similar branch in the phylogenetic tree; for example, motif 4 was observed in CmTPR3/6/9, which might be a motif unique to Group I. And CmTPR1/8 contained motifs 1, 2, 3, and 5, whereas the motifs unique to group IV might be motifs 3/5 (Figure 4). Phylogenetic analysis of the gene structure revealed that CmTPR genes were structurally similar in the same branch, which is consistent with the results of the analysis of other gene families [20,47,56].
GO term enrichment analysis can characterize the properties of genes and gene products; cis-regulatory elements are involved in the regulation of gene expression and play an important role in the regulation of gene transcription initiation [40,57]. In this study, five GO terms associated with processes such as transcriptional repression and protein transport were identified in the cucurbit species. Chen et al. classified cis-regulatory elements into eight categories related to plant development and hormones in melons and used this as a basis for hypothesizing that CmCH3 genes perform their biological functions through different signaling pathways [58]. A total of 28 cis-regulatory elements were identified in the CmTPR gene promoter and were categorized into 12 different response groups (Auxin-, ABA-, Gibberellin-, MeJA-, SA-, Defense and stress, drought, low temperature, light-responsive element, meristem expression, anaerobic induction, and circadian control) (Figure 5). The expression pattern of the genes was mediated by cis-regulator elements that have sites recognized and bound by transcription factors, and the cis-regulator elements in CmTPR genes might have an important role in plant growth and response to stress. Light-responsive elements were commonly shared by all the CmTPR genes, while other cis-regulatory elements were also widely distributed in the promoters of the CmTPR gene, which again supported our phylogenetic view that CmTPR genes might be functionally conserved/diversified.
Gene expression patterns are usually closely related to function, and tissue-specific expression profiles of TPR genes may indicate functional diversity to a large extent. The expression of SlTPR2/4/12/14 showed a more pronounced change in different tissues, with SlTPR2 showing the highest expression in fruit flesh and the lowest expression in leaves [20]. We found that CmTPR1/2/4/9 were highly expressed in most tissues, CmTPR1/4 were highly expressed in the callus, and the expression level of CmTPR1/2/3/4 was higher than that of other genes in the root and stem (Figure 6). During leaf development, the expression of CmTPR3 increased gradually and then leveled off, and CmTPR3/4 were highly expressed in the flowers (Figure 6). During fruit ripening, CmTPR3 had a tendency to increase and then decrease, and both CmTPR1/4 had a high expression (Figure 6). Cis-regulatory elements associated with hormones such as auxin, gibberellin, and ethylene were identified in the promoter. TPR genes have been demonstrated to play key roles in response to various stress signaling pathways [59]. In Arabidopsis, AtSGT1b is required for the degradation of the Aux/IAA protein, which is a TPR protein [60]. AtSPY (SPINDLY) is both a repressor of GA responses and a positive regulator of cytokinin signaling, which may modulate GA/cytokinin crosstalk during development [61]. GmTPR interacts with GmETR1-1 (an important ethylene receptor in the soybean ethylene signaling pathway), suggesting that GmTPR is a novel downstream component of the ethylene signaling pathway [62]. AtNCA1, a TPR protein, mediates catalase activity and participates in multiple abiotic stress responses [23]. The SlTPR gene family responds to various abiotic stresses, and SlTPR2/4 expression changes significantly under different abiotic stresses [20]. AtTPR15, which is more closely related to CmTPR1, may be necessary to respond to NaCl stress [63]. In the present study, we found that CmTPR genes might be differentially expressed in a stress-dependent manner, with the transcript levels of CmTPR1/3/4/6 having high levels under salt stress conditions, whereas only one gene (CmTPR3) showed sustained upregulation after salt treatment. CmTPR1/3/4/6 showed high transcript levels after HTH stress, and CmTPR3/6 were significantly upregulated under HTH stress. AtTPR15, on the same branch as CmTPR3, plays a key role in plant stress tolerance by targeting stress-induced ubiquitinated protein aggregates for autophagic degradation [64]. Interestingly, CmTPR1/3/4/6 also showed high transcript levels under cold stress conditions; CmTPR1/3/4 were significantly upregulated, while CmTPR6/9 were significantly downregulated after cold stress. We hypothesized that the CmTPR gene might play an important role in the response to abiotic stress in melons, and further transgenic studies are needed to elucidate its biological function. We identified and summarized the members of the TPR gene family in cucurbit crops and analyzed the evolutionary relationships among them. Our results provide new insights into the characterization of the TPR family and can also contribute to functional genomics exploration for subsequent studies of TPR genes in Cucurbitaceae.
5. Conclusions
In this study, we comprehensively characterized the TPR gene family in Cucurbitaceae and identified 144 TPR genes in eight species of Cucurbitaceae. Based on gene structural and functional attributes, we further categorized the TPR genes into four distinct subfamilies, which helped to elucidate the evolutionary history of the TPR gene family. Using the melon genome as a reference, an integrated gene map containing thirty-four loci was obtained, four of which were common to eight Cucurbitaceae species. The CmTPR gene family has a highly similar exon–intron structure and motif composition within the same branch in the evolutionary tree, and the regulatory functions of different branches are specific to the evolutionary tree. More importantly, CmTPR genes may also be involved in the regulation of abiotic stresses, with CmTPR1/3/4/6/9 cross-responding to salt, HTH, and cold stress. These results provide a valuable resource for a better understanding of the biological role of TPR genes in melons as well as a theoretical basis for the study of TPR genes in Cucurbitaceae.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10010083/s1. Supplemental Table S1. List of qRT-PCR primers used for expression analysis of CmTPR genes. Supplemental Table S2. Identification of conserved domains in TPR proteins on local blast. Supplemental Table S3. Identification of conserved domains in TPR proteins using the CDD tool. Supplemental Table S4. Numbers and characteristic properties of TPR genes in Cucurbitaceae. Supplemental Table S5. Synteny analysis of TPR genes between melon and other Cucurbitaceae. Supplemental Table S6. Identification of cis-regulatory elements in the promoters of CmTPR genes. Supplemental Table S7. Complete list of overrepresented gene ontology (GO) terms for TPR genes. Supplemental Figure S1. Distribution of TPR genes on the chromosomes of various species of Cucurbitaceae. The scale indicates megabases (Mb). Supplemental Figure S2. Phylogenetic tree of TPR genes in Benincaseae and Cucurbitaceae. Supplemental Figure S3. The distribution of cis-regulatory elements in the promoter regions of the CmTPR genes.
Author Contributions
Conceptualization, S.W. and K.Y.; methodology, Y.M.; validation, Y.M. and S.W.; resources, C.W.; data curation, F.D.; writing—original draft preparation, S.W.; writing—review and editing, H.J. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2023QC248), Liaocheng University, China (grant numbers 318052244, 318052290, and 31946221226), and the Key Research and Development Program of Liaocheng (grant number 2022YDNY11). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xin, T.; Tian, H.; Ma, Y.; Wang, S.; Yang, L.; Li, X.; Zhang, M.; Chen, C.; Wang, H.; Li, H.; et al. Targeted creating new mutants with compact plant architecture using CRISPR/Cas9 genome editing by an optimized genetic transformation procedure in cucurbit plants. Hortic. Res. 2022, 9, uhab086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lian, Q.; Zhang, Z.; Fu, Q.; He, Y.; Ma, S.; Ruggieri, V.; Monforte, A.J.; Wang, P.; Julca, I.; et al. A comprehensive genome variation map of melon identifies multiple domestication events and loci influencing agronomic traits. Nat. Genet. 2019, 51, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, M.; Song, M.; Zhang, M.; Chen, J.; Lou, Q. Long-distance control of pumpkin rootstock over cucumber scion under drought stress as revealed by transcriptome sequencing and mobile mRNAs identifications. Hortic. Res. 2022, 9, uhab033. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Guo, Y.; Yan, J.; Zhang, Z.; Yuan, L.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X.; et al. The role of watermelon caffeic acid O-methyltransferase (ClCOMT1) in melatonin biosynthesis and abiotic stress tolerance. Hortic. Res. 2021, 8, 210. [Google Scholar] [CrossRef]
- Zhao, Z.; Dong, Y.; Wang, J.; Zhang, G.; Zhang, Z.; Zhang, A.; Wang, Z.; Ma, P.; Li, Y.; Zhang, X.; et al. Comparative transcriptome analysis of melon (Cucumis melo L.) reveals candidate genes and pathways involved in powdery mildew resistance. Sci. Rep. 2022, 12, 4936. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, R.S.; Boguski, M.S.; Goebl, M.; Hieter, P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell 1990, 60, 307–317. [Google Scholar] [CrossRef]
- Wei, K.; Han, P. Comparative functional genomics of the TPR gene family in Arabidopsis, rice and maize. Mol. Breed. 2017, 37, 152. [Google Scholar] [CrossRef]
- Goebl, M.; Yanagida, M. The TPR snap helix: A novel protein repeat motif from mitosis to transcription. Trends Biochem. Sci. 1991, 16, 173–177. [Google Scholar] [CrossRef]
- Haucke, V.; Horst, M.; Schatz, G.; Lithgow, T. The Mas20p and Mas70p subunits of the protein import receptor of yeast mitochondria interact via the tetratricopeptide repeat motif in Mas20p: Evidence for a single hetero-oligomeric receptor. EMBO J. 1996, 15, 1231–1237. [Google Scholar] [CrossRef]
- Tsukahara, F.; Urakawa, I.; Hattori, M.; Hirai, M.; Ohba, K.; Yoshioka, T.; Sakaki, Y.; Muraki, T. Molecular characterization of the mouse mtprd gene, a homologue of human TPRD: Unique gene expression suggesting its critical role in the pathophysiology of Down syndrome. J. Biochem. 1998, 123, 1055–1063. [Google Scholar] [CrossRef]
- Cerveny, L.; Straskova, A.; Dankova, V.; Hartlova, A.; Ceckova, M.; Staud, F.; Stulik, J. Tetratricopeptide repeat motifs in the world of bacterial pathogens: Role in virulence mechanisms. Infect. Immun. 2013, 81, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Groshong, A.M.; Fortune, D.E.; Moore, B.P.; Spencer, H.J.; Skinner, R.A.; Bellamy, W.T.; Blevins, J.S. BB0238, a presumed tetratricopeptide repeat-containing protein, is required during Borrelia burgdorferi mammalian infection. Infect. Immun. 2014, 82, 4292–4306. [Google Scholar] [CrossRef]
- Hirano, T.; Kinoshita, N.; Morikawa, K.; Yanagida, M. Snap helix with knob and hole: Essential repeats in S. pombe nuclear protein nuclear. Cell 1990, 60, 319–328. [Google Scholar] [CrossRef]
- D’Andrea, L.D.; Regan, L. TPR proteins: The versatile helix. Trends Biochem. Sci. 2003, 28, 655–662. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Xu, N.; Cui, J.; Guo, X.; Chen, Y.I.; Azziz, R. Small glutamine-rich tetratricopeptide repeat-containing protein alpha (SGTA), a candidate gene for polycystic ovary syndrome. Hum. Reprod. 2008, 23, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Grizot, S.; Fieschi, F.; Dagher, M.C.; Pebay-Peyroula, E. The active N-terminal region of p67phox. Structure at 1.8 A resolution and biochemical characterizations of the A128V mutant implicated in chronic granulomatous disease. J. Biol. Chem. 2001, 276, 21627–21631. [Google Scholar] [CrossRef] [PubMed]
- Connarn, J.N.; Assimon, V.A.; Reed, R.A.; Tse, E.; Southworth, D.R.; Zuiderweg, E.R.; Gestwicki, J.E.; Sun, D. The molecular chaperone Hsp70 activates protein phosphatase 5 (PP5) by binding the tetratricopeptide repeat (TPR) domain. J. Biol. Chem. 2014, 289, 2908–2917. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Li, Q.; Hwang, J.R.; Patterson, C.; Zhang, H. AtCHIP, a U-box-containing E3 ubiquitin ligase, plays a critical role in temperature stress tolerance in Arabidopsis. Plant Physiol. 2003, 132, 861–869. [Google Scholar] [CrossRef]
- Rosado, A.; Schapire, A.L.; Bressan, R.A.; Harfouche, A.L.; Hasegawa, P.M.; Valpuesta, V.; Botella, M.A. The Arabidopsis tetratricopeptide repeat-containing protein TTL1 is required for osmotic stress responses and abscisic acid sensitivity. Plant Physiol. 2006, 142, 1113–1126. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Y.; Cai, Z.; Wang, X.; Liu, Y.; Yu, A.; Chen, X.; Liu, J.; Zhang, Y.; Wang, A. Identification and Functional Analysis of Tomato TPR Gene Family. Int. J. Mol. Sci. 2021, 22, 758. [Google Scholar] [CrossRef]
- Shan, Q.; Liu, M.; Li, R.; Shi, Q.; Li, Y.; Gong, B. γ-Aminobutyric acid (GABA) improves pesticide detoxification in plants. Sci. Total Environ. 2022, 835, 155404. [Google Scholar] [CrossRef]
- Gao, H.; Suo, X.; Zhao, L.; Ma, X.; Cheng, R.; Wang, G.; Zhang, H. Molecular evolution, diversification, and expression assessment of MADS gene family in Setaria italica, Setaria viridis, and Panicum virgatum. Plant Cell Rep. 2023, 42, 1003–1024. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Wang, G.; Cha, J.Y.; Li, G.; Chen, S.; Li, Z.; Guo, J.; Zhang, C.; Yang, Y.; et al. A chaperone function of NO CATALASE ACTIVITY1 is required to maintain catalase activity and for multiple stress responses in Arabidopsis. Plant Cell 2015, 27, 908–925. [Google Scholar] [CrossRef]
- Greenboim-Wainberg, Y.; Maymon, I.; Borochov, R.; Alvarez, J.; Olszewski, N.; Ori, N.; Eshed, Y.; Weiss, D. Cross talk between gibberellin and cytokinin: The Arabidopsis GA response inhibitor SPINDLY plays a positive role in cytokinin signaling. Plant Cell 2005, 17, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, W.; Hu, Y.; Huang, J.; Zhao, Y.; Zhang, L.; Huang, C.H.; Ma, H. Phylotranscriptomics in Cucurbitaceae Reveal Multiple Whole-Genome Duplications and Key Morphological and Molecular Innovations. Mol. Plant 2020, 13, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; González, V.M.; Hénaff, E.; Câmara, F.; Cozzuto, L.; Lowy, E.; et al. The genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA 2012, 109, 11872–11877. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, G.; Lian, J.; Garraway, J.; Niu, Y.; Hu, Z.; Yu, J.; Zhang, M. The Chromosome-Scale Genome of Melon Dissects Genetic Architecture of Important Agronomic Traits. iScience 2020, 23, 101422. [Google Scholar] [CrossRef]
- Yang, L.; Koo, D.H.; Li, Y.; Zhang, X.; Luan, F.; Havey, M.J.; Jiang, J.; Weng, Y. Chromosome rearrangements during domestication of cucumber as revealed by high-density genetic mapping and draft genome assembly. Plant J. 2012, 71, 895–906. [Google Scholar] [CrossRef]
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Reddy, U.; Sun, H.; Bao, K.; Gao, L.; Mao, L.; Patel, T.; Ortiz, C.; Abburi, V.L.; et al. Genome of ‘Charleston Gray’, the principal American watermelon cultivar, and genetic characterization of 1365 accessions in the U.S. National Plant Germplasm System watermelon collection. Plant Biotechnol. J. 2019, 17, 2246–2258. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shamimuzzaman, M.; Sun, H.; Salse, J.; Sui, X.; Wilder, A.; Wu, Z.; Levi, A.; Xu, Y.; Ling, K.S.; et al. The bottle gourd genome provides insights into Cucurbitaceae evolution and facilitates mapping of a Papaya ring-spot virus resistance locus. Plant J. 2017, 92, 963–975. [Google Scholar] [CrossRef]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z.; et al. Karyotype Stability and Unbiased Fractionation in the Paleo-Allotetraploid Cucurbita Genomes. Mol. Plant 2017, 10, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Montero-Pau, J.; Blanca, J.; Bombarely, A.; Ziarsolo, P.; Esteras, C.; Martí-Gómez, C.; Ferriol, M.; Gómez, P.; Jamilena, M.; Mueller, L.; et al. De novo assembly of the zucchini genome reveals a whole-genome duplication associated with the origin of the Cucurbita genus. Plant Biotechnol. J. 2018, 16, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Xu, Y.; Wang, J.; Liu, W.; Zhou, Q.; Luo, S.; Huang, W.; He, X.; Li, Q.; Peng, Q.; et al. The wax gourd genomes offer insights into the genetic diversity and ancestral cucurbit karyotype. Nat. Commun. 2019, 10, 5158. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhang, R.; Yang, X.; Zhu, C.; Li, H.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Comparative Analysis of Calcium-Dependent Protein Kinase in Cucurbitaceae and Expression Studies in Watermelon. Int. J. Mol. Sci. 2019, 20, 2527. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Yano, R.; Ariizumi, T.; Nonaka, S.; Kawazu, Y.; Zhong, S.; Mueller, L.; Giovannoni, J.J.; Rose, J.K.C.; Ezura, H. Comparative genomics of muskmelon reveals a potential role for retrotransposons in the modification of gene expression. Commun. Biol. 2020, 3, 432. [Google Scholar] [CrossRef]
- Wang, L.M.; Zhang, L.D.; Chen, J.B.; Huang, D.F.; Zhang, Y.D. Physiological analysis and transcriptome comparison of two muskmelon (Cucumis melo L.) cultivars in response to salt stress. Genet. Mol. Res. 2016, 15, gmr.15038738. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Rehman, A.; Li, P.; Chang, L.; Zhang, Y.; Niu, Q. Physiological and Transcriptomic Analysis Reveals the Responses and Difference to High Temperature and Humidity Stress in Two Melon Genotypes. Int. J. Mol. Sci. 2022, 23, 734. [Google Scholar] [CrossRef]
- Diao, Q.; Cao, Y.; Fan, H.; Zhang, Y. Transcriptome analysis deciphers the mechanisms of exogenous nitric oxide action on the response of melon leaves to chilling stress. Biol. Plant. 2020, 64, 465–472. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Wang, X.; Yang, X.; Li, Q.; Wang, C.; Chen, C.; Shi, Q.; Ren, Z.; Wang, L. Genome-wide characterization of two-component system (TCS) genes in melon (Cucumis melo L.). Plant Physiol. Biochem. 2020, 151, 197–213. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Kherawat, B.S.; Singh, A.; Dey, P.; Routray, S.; Mohapatra, C.; Saha, D.; Ram, C.; Siddique, K.H.M.; Kumar, A.; et al. Genome-Wide Analysis and Characterization of the Proline-Rich Extensin-like Receptor Kinases (PERKs) Gene Family Reveals Their Role in Different Developmental Stages and Stress Conditions in Wheat (Triticum aestivum L.). Plants 2022, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guan, Y.; Weng, Y.; Liao, B.; Tong, L.; Hao, Z.; Chen, J.; Shi, J.; Cheng, T. Genome-wide identification of the NAC gene family and its functional analysis in Liriodendron. BMC Plant Biol. 2023, 23, 415. [Google Scholar] [CrossRef]
- Li, G.L.; Tang, L.L.; He, Y.H.; Xu, Y.Y.; Bendahmane, A.; Garcia-Mas, J.; Lin, T.; Zhao, G.G. The haplotype-resolved T2T reference genome highlights structural variation underlying agronomic traits of melon. Hortic. Res. 2023, 10, uhad182. [Google Scholar] [CrossRef]
- Tang, H.; Bowers, J.E.; Wang, X.; Ming, R.; Alam, M.; Paterson, A.H. Synteny and collinearity in plant genomes. Science 2008, 320, 486–488. [Google Scholar] [CrossRef]
- Li, H.; Wen, X.; Huang, X.; Wei, M.; Chen, H.; Yu, Y.; Dai, S. Genome-Wide Identification and Characterization of TCP Gene Family Members in Melastoma candidum. Molecules 2022, 27, 9036. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Sun, F.; Wu, R.; Du, H.; Wang, Y.; Jiang, L.; Wu, X.; Wu, X.; Yang, L.; et al. Long-read genome assembly and genetic architecture of fruit shape in the bottle gourd. Plant J. 2021, 107, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Q.; Zheng, Y.; Guo, J.; Yuan, S.; Fu, A.; Bai, C.; Zhao, X.; Zheng, S.; Wen, C.; et al. Cucurbitaceae genome evolution, gene function, and molecular breeding. Hortic. Res. 2022, 9, uhab057. [Google Scholar] [CrossRef] [PubMed]
- Eickbush, T.H. Mobile introns: Retrohoming by complete reverse splicing. Curr. Biol. 1999, 9, R11–R14. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Xu, L.; Chai, P.; Peng, J.; Devarkar, S.C.; Pyle, A.M. Structures of a mobile intron retroelement poised to attack its structured DNA target. Science 2022, 378, 627–634. [Google Scholar] [CrossRef]
- Wen, Z.; Li, M.; Meng, J.; Miao, R.; Liu, X.; Fan, D.; Lv, W.; Cheng, T.; Zhang, Q.; Sun, L. Genome-Wide Identification of the MAPK and MAPKK Gene Families in Response to Cold Stress in Prunus mume. Int. J. Mol. Sci. 2023, 24, 8829. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jia, X.; Yang, Z.; Fu, Q.; Yang, H.; Xu, X. Genome-Wide Identification of PEBP Gene Family in Solanum lycopersicum. Int. J. Mol. Sci. 2023, 24, 9185. [Google Scholar] [CrossRef]
- Lu, P.; Magwanga, R.O.; Guo, X.; Kirungu, J.N.; Lu, H.; Cai, X.; Zhou, Z.; Wei, Y.; Wang, X.; Zhang, Z.; et al. Genome-Wide Analysis of Multidrug and Toxic Compound Extrusion (MATE) Family in Gossypium raimondii and Gossypium arboreum and Its Expression Analysis Under Salt, Cadmium, and Drought Stress. G3-Genes Genomes Genet. 2018, 8, 2483–2500. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, K.; Li, Y.; Bai, C.; Xue, Z.; Wu, Y. Evolutionary Analysis of the Melon (Cucumis melo L.) GH3 Gene Family and Identification of GH3 Genes Related to Fruit Growth and Development. Plants 2023, 12, 1382. [Google Scholar] [CrossRef]
- Schapire, A.L.; Valpuesta, V.; Botella, M.A. TPR Proteins in Plant Hormone Signaling. Plant Signal Behav. 2006, 1, 229–230. [Google Scholar] [CrossRef]
- Gray, W.M.; Muskett, P.R.; Chuang, H.W.; Parker, J.E. Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell 2003, 15, 1310–1319. [Google Scholar] [CrossRef]
- Qin, F.; Kodaira, K.S.; Maruyama, K.; Mizoi, J.; Tran, L.S.; Fujita, Y.; Morimoto, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. SPINDLY, a negative regulator of gibberellic acid signaling, is involved in the plant abiotic stress response. Plant Physiol. 2011, 157, 1900–1913. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, M.; Chen, X.; Ma, Y.; Xu, Z.; Li, L. Characterization of Ethylene Receptors and Their Interactions with GmTPR—A Novel Tetratricopeptide Repeat Protein (TPR) in Soybean (Glycine max L.). J. Integr. Agric. 2013, 12, 571–581. [Google Scholar] [CrossRef]
- Lakhssassi, N.; Doblas, V.G.; Rosado, A.; Esteban Del Valle, A.; Posé, D.; Jimenez, A.J.; Castillo, A.G.; Valpuesta, V.; Borsani, O.; Botella, M.A. The Arabidopsis thaliana TETRATRICO PEPTIDE THIOREDOXIN-LIKE gene family is required for osmotic stress tolerance and male sporogenesis. Plant Physiol. 2012, 158, 1252–1266. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Qi, J.; Chi, Y.; Fan, B.; Yu, J.Q.; Chen, Z. E3 ubiquitin ligase CHIP and NBR1-mediated selective autophagy protect additively against proteotoxicity in plant stress responses. PLOS Genet. 2014, 10, e1004478. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).