Abstract
The determination of grape quality parameters is intricately linked to the mineral composition of the fruit; this relationship is increasingly affected by the impacts of climate change. The conventional chemical methodologies employed for the mineral quantification of grape tissues are expensive and impracticable for widespread commercial applications. This paper utilized Laser-Induced Breakdown Spectroscopy (LIBS) to analyze the mineral constituents within the skin, pulp, and seeds of two distinct Vitis vinifera cultivars: a white cultivar (Loureiro) and a red cultivar (Vinhão). The primary objective was to discriminate the potential variations in the calcium (Ca), magnesium (Mg), and nitrogen (N) concentrations and water content among different grape tissues, explaining their consequential impact on the metabolic constitution of the grapes and, by extension, their influence on various quality parameters. Additionally, the study compared the mineral contents of the white and red grape cultivars across three distinct time points post veraison. Significant differences (p < 0.05) were observed between the Loureiro and Vinhão cultivars in Ca concentrations across all the dates and tissues and for Mg in the skin and pulp, N in the pulp and seeds, and water content in the skin and pulp. In the Vinhão cultivar, Ca differences were found in the pulp across the dates, N in the seeds, and water content in the skin, pulp, and seeds. Comparing the cultivars within tissues, Ca exhibited differences in the pulp, Mg in the skin and pulp, N in the pulp and seeds, and water content in the skin, pulp, and seeds. These findings provide insights into the relationship between the grape mineral and water content, climatic factors, and viticulture practices within a changing climate.
1. Introduction
The taste and overall quality of grapes are influenced by their chemical composition [1]. The essential macronutrients, namely calcium (Ca), magnesium (Mg), and nitrogen (N), contribute to the growth, development, and metabolic processes of grapevines [2]. Ca contributes to membrane stability, enhances disease resistance, and supports vital metabolic pathways, such as Ascorbate-Glutathione, contributing to the overall health of grapevines [2,3]. Mg, which is essential for photosynthesis and enzyme activation, promotes the general health of grapevines [1]. Concurrently, the N profiles proteins that optimize the grapevine canopy’s growth, development, and vitality [2]. The allocation of these components within grape tissues impacts the final yield and the overall quality of the harvested fruit [4].
Environmental, genetic, and agricultural factors collectively influence the plant nutrient transport mechanisms, impacting ion and water uptake efficiency and mineral assimilation [5,6]. Beyond the factors shaping grape composition and taste, the dynamic relationship of these external elements is consequential in the context of climate change and viticulture practices. Understanding the elemental composition of grapes is essential for optimizing cultivation practices, encompassing factors like irrigation and fertilization, and ensuring the long-term sustainability of vineyards. Climate change-induced water availability shifts directly affect the grape composition [7,8]. Moreover, pollutants present in the environment [9] and soil degradation attributed to heavy metals [10] can manifest in various grape organs, potentially interfering with the overall composition.
While the existing research has underscored the importance of Ca [11], Mg [6], and N [5], there is a lack of studies exploring the variations in their distribution across different grape tissues like the skin, pulp, and seeds, during maturation. This paper explores the impact of elemental dynamics on grape maturation and quality parameters, which can benefit grape growers and winemakers.
The elemental quantification of grape tissues has relied on traditional methods such as acid digestion coupled with atomic absorption spectrophotometry (AAS) or inductively coupled plasma optical emission spectrometry (ICP-OES) [12]. However, these conventional techniques present inherent constraints that limit their applicability for routine analyses in the viticultural sector. The sample preparation requirements, time-intensive procedures, and dependence on specialized equipment, reagents, and skilled operators pose challenges, particularly in large-scale vineyards [10,13]. By recognizing these limitations, alternative non-destructive methods have been developed to address these challenges. For example, X-ray fluorescence has demonstrated efficacy in characterizing the element composition of biological samples [14]. However, the limitations in quantifying the elements with atomic numbers (Z) higher than 12 and the need to consider radiation effects during analysis necessitate the careful evaluation of these methods. Also, Vis-NIR has been used to quantify the nutrients [15,16].
Laser-Induced Breakdown Spectroscopy (LIBS) has emerged as a promising alternative method for elemental analysis in agriculture and food science [17]. This rapid and destructive analytical approach involves using a neodymium-doped yttrium aluminum garnet laser to generate pulses, ablating and exciting the targeted object, such as the grape tissue, and producing a plasma plume. The optical radiation emitted from this plasma is then captured and examined to interpret the sample’s elemental composition. Despite the weakness of producing limited molecular structure information, LIBS presents distinct advantages over the traditional techniques. In contrast to the costly conventional methods, LIBS has minimal sample preparation requirements, a high spatial resolution, and reduced operational costs [12], making it an attractive choice for the elemental tissue analysis of small samples of grapes [18].
The literature reports portable LIBS that can be used for element quantification [19,20]. This study, building upon the groundwork laid by Tosin, et al. [21], seeks to leverage the advantages of LIBS in characterizing the elemental composition of grapes, explicitly focusing on calcium (Ca), magnesium (Mg), and nitrogen (N) in the skin, pulp, and seeds.
Integrating the relationships systematically, quantitatively, and causally between the soil, climate, and plant physiology is imperative. The utilization of LIBS-based nutrition diagnosis has emerged as a tool, providing reinforced precision in discerning the grapevine nutritional requirements [14], which helps to improve the grape quality and facilitates wise and precise management practices, thereby advocating for more sustainable viticultural methods in response to dynamic climate change scenarios [22]. While the focal point of this research centers on precision viticulture, these discernments and technological developments hold the potential for broader applications encompassing diverse fruit crops.
This study aims to address and overcome the limitations identified in the existing research concerning the application of LIBS in the analysis of plant tissues, with a specific focus on vineyards. Constraints, such as the influence of water content on measurements, the necessity to handle sample volumes, and the requirement for sample preparation pellets, have been noted in prior studies, as identified by Senesi, Cabral, Menegatti, Marangoni and Nicolodelli [12]. Furthermore, the challenges have been acknowledged in quantifying the elements within dried tissues, along with considerations regarding the influence of nitrogen in the atmosphere [23,24]. These challenges posed barriers to the implementation of LIBS in viticultural backgrounds.
This work tests and evaluates the possible resolution of these challenges, contributing to the advancement and refinement of LIBS application in plant tissue analysis, particularly within viticulture. Thus, this paper has two main objectives: (i) to extract elemental samples (Ca, Mg, and N) from the grape tissues, specifically the skin, pulp, and seeds; (ii) to conduct comparative analyses between the two grape varieties and across different dates after veraison. The specific goals include utilizing the same volume (0.098 cm3) for the skin, pulp, and seeds to identify the elemental constituents and water content.
2. Materials and Methods
2.1. Test Site
This research was conducted within the Região dos Vinhos Verdes located in the northwestern region of Portugal, Vila do Conde, specifically at the Campus Agrário de Vairão (41°19′31.91″ N; 8°40′27.45″ W). The area is defined by a Mediterranean climate, presenting an average annual temperature of 14.9 °C and an average yearly precipitation of 1149 mm (Climate-Data.org). The soil composition is predominantly from shales of the shale–greywacke complex.
The grapes were randomly sampled in the year 2020 on three dates after the veraison (14 August 2020) of two grape cultivars: Loureiro (Vitis International Variety Catalogue (VIVC) 25085), characterized as a white grape cultivar; and Vinhão (VIVC 13100), classified as a red grape cultivar (Table 1). On each sampling date, the following considerations were made, aiming to minimize the potential sources of variability: (i) prioritizing grape bunches that were representative and uniformly sized and matured within the same cultivar; (ii) considering the position of grape bunches on the vine to mitigate variations stemming from differing sunlight exposure, nutrient availability, and microclimatic conditions; and (iii) incorporating the accumulation of biomass and grape bunch size in the selection process to ensure that the chosen samples were at similar stages of development.

Table 1.
Sampling dates of the Loureiro and Vinhão cultivars.
2.2. Characterization of the Grapes
Using a scalpel, the separation of grape tissues into skin, pulp, and seeds was meticulously executed. After weighing, the tissues dried in an oven set at 45 °C for four days. Following this drying period, re-weighing the dried tissues allowed the water content (%) calculation based on the observed weight differential.
The dried tissues were individually macerated using a grinder, and from these processed tissues, a pellet with an approximate volume of 0.098 cm3 was assembled (Figure 1). This prepared palette served as the sample for subsequent analysis utilizing LIBS.
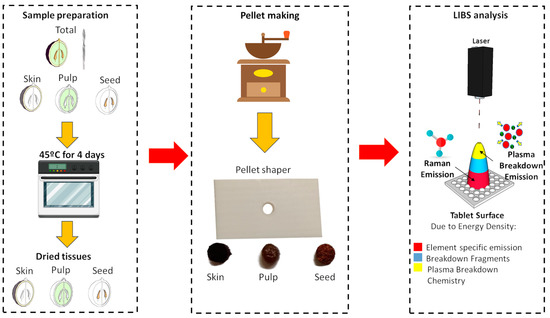
Figure 1.
Sample preparation, pellet making, and LIBS analysis of the skin, pulp, and seeds of the Loureiro and Vinhão cultivars.
2.3. LIBS Instrumentation
The in-house-assembled system utilized a Q-Switched Nd:YAG (neodymium-doped yttrium aluminum garnet) laser operating at a fundamental wavelength of 1064 nm, featuring an 8 ns pulse duration and a maximum adjustable energy of 200 mJ [25]. The arrangement included lenses and mirrors for the laser pulse focusing on the sample surface, complemented by two crossed visible diode laser pointers aligned with the primary laser’s focal point. This alignment facilitated positioning the sample’s region of interest at the optimal height and X–Y coordinates (Figure 2).
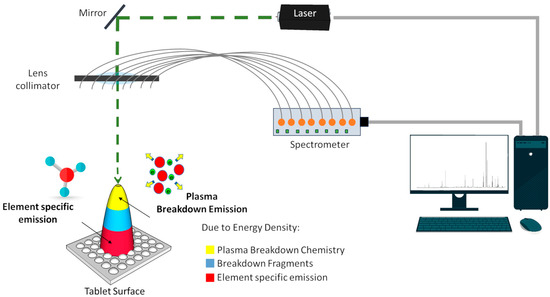
Figure 2.
Experimental setup for Laser-Induced Breakdown Spectroscopy (LIBS), showcasing laser incidence, spectral data acquisition, and manual adjustments for focus. Adapted from Ribeiro, Capela, Ferreira, Martins, Jorge, Guimarães and Lima [24].
The sample holder relied on three stages, with control over X, Y, and Z via manual adjustment directions using precision knobs. The system comprises eight optical fibers and collimators aligned with the system’s focal point, collecting and directing plasma light into an eight-channel CCD spectrometer (Avantes, Apeldoorn, The Netherlands). This spectrometer enabled spectral detection within the wavelength range of approximately 180 to 920 nm, boasting a resolution between 0.06 nm and 0.18 nm. Three spectra were acquired for each sample, and the resulting averaged spectrum was considered for analysis.
All the tests were conducted in ambient air, and the distance between the sample and the collecting optics varied randomly, aiming to cover the entire longitudinal extent of the sample during scanning. A detailed overview of this specific LIBS setup can be found in the description provided by Ribeiro, Capela, Ferreira, Martins, Jorge, Guimarães and Lima [24].
2.4. Element Identification
This paper adopted a qualitative methodology to discern the presence of calcium (Ca), magnesium (Mg), and nitrogen (N) in grape skin, pulp, and seeds. The primary goal of LIBS lies in deducing the chemical composition of samples through the analysis of their spectra. Each chemical element exhibits distinct wavelengths of light, giving rise to well-defined lines within the spectrum. However, instances may arise where the emission wavelengths of different elements overlap. The resulting LIBS spectra had a high resolution of approximately 0.1 nm, and the number of variables for this configuration was 16,361. As a result of the large amount of data, principal component analysis (PCA) was applied to study the main differences among the elements assessed between the tissues (skin, pulp, and seeds) and the two grape cultivars.
Two reference databases, the OSCAR [26] and NIST databases [27], were evaluated regarding their suitability for interpreting the samples’ spectra and building a database. Both databases were employed for elemental computation from the periodic table, with certain elements exclusively identified in one of the databases. In the whole analysis, a total of 37 elements from the periodic table were computed. However, only Ca, Mg, and N were identified in the assessed samples.
The spectral data obtained from LIBS were compared with the spectra available in the OSCAR [26] and NIST [27] databases for matches or similarities between the peaks or patterns. The elements (Ca, Mg, and N) present in each sample were identified by matching the peaks or patterns from the LIBS spectra with the corresponding entries in the built database.
2.5. Statistical Analysis
Repeated measures analysis of variance (RM-ANOVA), employed to account for non-identical samples, was complemented by a post hoc test using Duncan’s method to assess the variations in water content between the two grape cultivars across the skin, pulp, and seeds on the three evaluation dates. Furthermore, RM-ANOVA, followed by the post hoc test, was conducted to discern significant statistical differences in Ca, Mg, and N characterization within the skin, pulp, and seeds of both grape cultivars across the three assessment dates. This study considered both the non-significant (p > 0.05) and significant (p < 0.05) outcomes for comprehensive examination and interpretation.
3. Results
Figure 3 presents the average signal intensity relating to the skin, pulp, and seeds of the Loureiro and Vinhão cultivars in S1 (47 days after veraison). The diagram incorporates the respective wavelengths corresponding to each element identified according to the generated database. Ca is characterized by five peaks at 317.936 nm, 617.128 nm, 646.273 nm, 649.378 nm, and 866.097 nm. Mg exhibits three peaks at 279.535 nm, 280.24 nm, and 285.18 nm; N shows six peaks at 744.2 nm, 746.753 nm, 821.533 nm, 822.248 nm, 824.184 nm, and 867.938 nm.
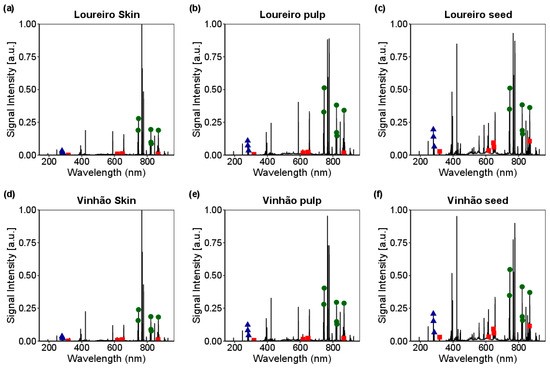
Figure 3.
The average signal intensities of the Ca (■), Mg (▲), and N (●) in the skin, pulp, and seed tissues of the Loureiro cultivar (a–c) and the Vinhão cultivar (d–f) 47 days after veraison (S1). The sample size for each group is specified as n = 20 for Loureiro and n = 16 for Vinhão.
Table 2 presents statistical differences in the Ca, Mg, and N concentrations across the skin, pulp, and seed tissues of the two grape cultivars during the three assessment dates. Regarding the Ca concentrations within these tissues, the Loureiro cultivar displays significant variations across all three assessment dates for all the tissues. The higher Ca levels in Loureiro compared to that of Vinhão at S3 may be attributed to overripening, which is known to elevate the Ca concentration in the tissues [28]. In the white grapes, as they ripen and overripen, cell wall breakdown occurs, releasing or redistributing Ca ions from the cell walls, potentially influencing the measured Ca content [29].

Table 2.
The average LIBS spectra intensity peaks of Ca, Mg, and N and water content (% w/w) for each tissue on the three assessment dates (S1, S2, and S3) for the Loureiro and Vinhão cultivars.
On the contrary, the Vinhão cultivar only diverges in the pulp tissue. The comparative analysis of the two cultivars reveals uniform Ca concentrations in the skin and consistent concentrations in the seeds during S3.
Regarding the Mg content, the Loureiro cultivar displays fluctuations in the skin and pulp tissues across the three assessment dates. Conversely, the Vinhão cultivar maintains consistent Mg concentrations in internal tissues throughout the three assessment dates. Cross-cultivar analysis reveals variations in the Mg content in the skin tissue of the Loureiro cultivar during S3, as well as in the pulp and seed tissues during S1 and S2.
Concerning N content analysis, the Loureiro cultivar lacks significant differences in the skin tissue across the three assessment dates. In contrast, the Vinhão cultivar demonstrates statistical distinctions only in the seed tissue. The assessment of N concentrations between the two cultivars indicates disparities in the pulp tissue for both the grape cultivars across the three assessment dates and in the seed tissue during S3.
Regarding the water content, there are alterations in the skin and pulp tissues of the Loureiro cultivar, while the seed tissue remains unaffected. On the contrary, the Vinhão cultivar experiences fluctuations in water content across all the three tissues. The variations in water content for both the cultivars were observed in the skin tissue during S3 and in the pulp and seed tissues during S1 and S3. Additionally, the impact of water content on the studied macro elements appears to be more pronounced in the Loureiro cultivar than that in the Vinhão cultivar.
Figure 4 shows the Ca, Mg, and N alterations across the Loureiro cultivar’s skin, pulp, and seeds during the three post-veraison dates. These dynamic changes were observed throughout the assessment period. The representations of principal component 1 (PC1) and PC2 in Figure 4 capture more than 90% of the dataset’s variance.
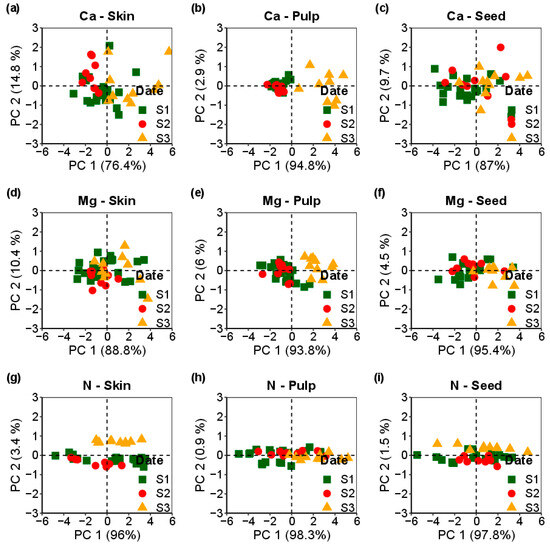
Figure 4.
Principal component analysis of Loureiro cultivar across three assessment dates (S1(■), S2 (●), S3(▲)); (a–c) represent calcium (Ca) concentrations in skin, pulp, and seeds, while (d–f) show magnesium (Mg) concentrations, and (g–i) illustrate nitrogen (N) concentrations in the corresponding tissues.
The rationale behind employing PCA in this study is to demonstrate multivariate information from the elemental concentrations in the grape tissues and across the dates into a concise set of principal components (PC1 and PC2). These two components explain most of the variance within the data, offering a more streamlined representation.
By transforming the data into a lower-dimensional space, PCA aids in identifying the variations and similarities among the samples. The integration of PCA with the results presented in Table 2 enriches the understanding of elemental dynamics during grape maturation. This approach allows to visualize how different grape tissues evolve regarding elemental composition across the assessed sampling dates and improves our insights into the clustering or dispersion of samples in a reduced dimensional space, facilitating the identification of underlying patterns or trends that are not immediately apparent in the tabulated data.
Moreover, the alignment of the PCA results with Table 2 demonstrates the differences in Ca concentrations across the skin, pulp, and seeds for the three assessment dates, the variations in Mg content within the skin and pulp, and the distinctions in the pulp and seeds concerning N. PCA validates the results presented in Table 2, providing a robust analytical framework for interpreting the results.
Figure 5 shows the variations in Ca, Mg, and N concentrations within the Vinhão cultivar’s skin, pulp, and seeds over the three post-veraison dates. Compared to the Loureiro cultivar (Figure 4), these results highlight the differences in macronutrient dynamics during grape maturation between the two cultivars.
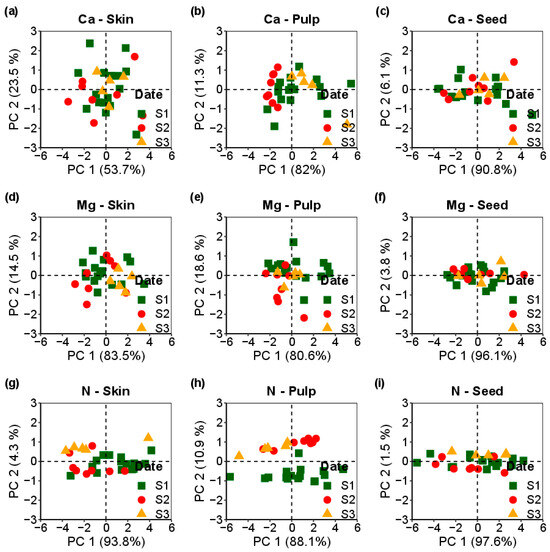
Figure 5.
Principal component analysis of Vinhão cultivar across three assessment dates (S1(■), S2 (●), S3(▲)); (a–c) represent calcium (Ca) concentrations in skin, pulp, and seeds, while (d–f) show magnesium (Mg) concentrations, and (g–i) illustrate nitrogen (N) concentrations in the corresponding tissues.
As indicated in Table 2, the Loureiro cultivar exhibits statistically significant differences in the Ca, Mg, and N concentrations across the assessed tissues. In contrast, the Vinhão cultivar shows significant deviations only in Ca within the pulp and N within the seeds. These distinctions suggest that the macronutrient dynamics vary during the maturation process, further accentuating the unique responses of the two grape cultivars.
4. Discussion
Incorporating Laser-Induced Breakdown Spectroscopy (LIBS) into viticulture marks a departure from the traditional, resource-intensive wet laboratory techniques such as ICP-OES for the elemental quantification of grape tissues. In this study, the potential utilization of LIBS across various grape tissues and cultivars signifies a progressive step toward exploring its applicability and efficacy, particularly in the context of shifting climate patterns. By employing a qualitative framework, this paper enhances the discernment of essential elements, such as calcium (Ca), magnesium (Mg), and nitrogen (N), within the internal tissues of grapes, specifically the skin, pulp, and seeds, across two distinct grape cultivars.
The relationship between water availability and macronutrient dynamics, particularly in the white cultivars, is a complex aspect of plant physiology and nutrient management [1,30]. Water, serving as a conduit for nutrient uptake, facilitates the absorption of essential macronutrients, like Ca, Mg, and N, by the plant roots [30]. The variations in water content influence the elemental constitution of the tissues of grape cultivars, especially in the white cultivars [31], as demonstrated in this study (Table 2). The physical and chemical characteristics of the soil, influenced by the water content, directly impact the solubility and mobility of these macronutrients, influencing their availability to the plant [32]. For example, the Ca levels increase in the grapes under well-hydrated conditions [31]. The dynamic nature of the rhizosphere, where the root and microbial activities intersect, is susceptible to changes in the water content, further influencing nutrient cycling [33].
Additionally, the physiological responses of plants to water availability, particularly stress-induced reactions, can affect nutrient uptake and assimilation [30,31]. The white cultivars may exhibit specific sensitivities and responses to the variations in water availability, emphasizing the importance of adapted irrigation strategies [7]. Therefore, the relationship between the water and macronutrients necessitates a better understanding of the optimized agricultural practices (e.g., irrigation), enhanced nutrient management (e.g., fertilization), and the robust growth and quality of grape cultivars (e.g., pruning and harvest time).
The differences in Ca, Mg, and N concentrations between the Loureiro and Vinhão cultivars (Table 2) reflect the complex interaction of the genetic, physiological, and environmental factors governing grapevine nutrient dynamics [30,31]. The differences across the assessed grape cultivars at the three distinct assessment dates emphasize the unique nutrient profiles inherent to each variety. While the Loureiro cultivar is more susceptible to macronutrient changes [31] during the maturation of all the tissues, the Vinhão cultivar tends to equilibrate the concentration (Table 2). During grape maturation, Ca tends to accumulate in the grapes, especially contributing to the flavonoid pathways [34], and Mg is present in the enzyme activator [6]. The differences in N content observed in both the cultivars from 47 days after veraison (DAV) until 61 DAV, especially in the seeds (Table 2 and Figure 4 and Figure 5), explain the need for this element during seed development [35]. Also, N is fundamental in metabolic processes, emphasizing the dynamic relationship between grape maturation, nutrient accumulation, and the metabolic intricacies specific to each grape cultivar [2]. However, the Loureiro cultivar in S3 exhibits signs of overripeness due to the higher concentration of Ca [28]. This observation aligns with the phenomenon observed in the white grapes, where the process of ripening and overripening triggers cell wall breakdown [29]. During this process, Ca ions are released or redistributed from the cell walls, introducing a potential influence on the measured Ca content [29,31].
The application of LIBS in the element analysis of grapes presents distinct advantages. Firstly, LIBS offers a novel and exploratory approach, providing a potential opportunity for refining and validating the technique for element analysis [10,17]. The promise lies in its ability to offer cost-effective and expeditious alternatives to the traditional methods employed in evaluating the elemental contents of grape tissues [20]. Also, it acknowledges the challenges associated with wet lab procedures, particularly the limitations imposed by the availability of biological material and emphasizes the potential of LIBS to overcome such constraints. In this study, characterizing the elements (Ca, Mg, and N) in the skin, pulp, and seeds with the same low sample volume (0.098 cm3) was possible. This may be a solution to the need for a low-cost method to assess a small sample of biological tissues [18,36]. The adaptability of LIBS technology can mitigate the cost and time commitments typically associated with wet lab procedures, positioning it as a pragmatic solution for on-field elemental composition characterization [12]. However, LIBS still needs a sample preparation for analysis.
In this study, the Ca, Mg, and N analyses of grape tissues relied on the data inferred from the online databases OSCAR [26] and NIST [27]. This approach was chosen because the standardized methods (e.g., ICP-OES) are non-optimized for small tissue aliquots (e.g., 0.098 cm3), non-expedited, expensive, and time-consuming [14,37]. These standardized methods were deemed impractical for this study’s low technology-readiness level (TRL) due to the associated costs of the standardized methods for small tissue samples, highlighting the need for an early-stage, cost-effective technology. This paper’s results serve as proof of the use of LIBIS in demonstrating the feasibility of internal tissue quantification.
The application of LIBS faces more limitations in comparison to those of the standardized methodologies like ICP-OES. The qualitative nature of LIBS, influenced by the uncertainties in plasma formation and chemistry [38], coupled with dynamic complexities introduced by factors, like the humidity, carbon content, and other elements [39], poses challenges to accurate quantitative measurements. Despite its potential for studying a broader spectrum of elements [12], it is fundamental to recognize and acknowledge the current limitations of LIBS in achieving precise quantitative analyses.
Despite its advantages, LIBS is not without challenges related to atomization and ionization, particularly after the material undergoes the drying process [40]. The emission peaks generated by LIBS often exhibit a low intensity, creating difficulties in determining the elements at low concentrations without potential recourse to pyrolysis [23], which emphasizes the necessity of studying the LIBS technique’s efficacy in analyzing dried plant materials, recognizing the inherent limitations in emission intensity.
In an agricultural context, a consideration for LIBS is its capability to detect light elements [24], such as Boron, which are important for plant nutrition [1]. However, the carbon present in plant tissues absorbs a part of the laser energy, restricting the energy for atomizing the elements in lower concentrations [23]. This interference raises doubts about LIBS’s accuracy in detecting and quantifying trace elements and may generate uncertainty about its use for agricultural applications.
In addition, atmospheric N introduces complexities in LIBS analysis. Although analysis did not occur under vacuum conditions, the detected N during analysis is likely to be atmospheric N, rather than an accurate representation of the N content in the plant material [23]. This atmospheric interference poses potential challenges in result interpretation, emphasizing the need for consideration when analyzing the nitrogen-related data acquired through LIBS from plant samples.
Despite the limitations, using LIBS in on-field elemental composition [19] characterization introduces new perspectives in metabolomics research. Nevertheless, validating LIBS could pave the way for broader applications [41,42], potentially inspiring and facilitating future metabolomics research endeavors. The advantages of LIBS (in situ), including its non-destructive nature and rapid analysis capabilities, make it an attractive tool for exploring the intricate elemental composition of grapes in situ. This technology addresses the current challenges and stimulates a forward-looking perspective by encouraging a more extensive and nuanced exploration of the metabolomic landscape. It raises an understanding of the complex interplay between the elements in grapes and their potential implications for viticulture and oenology, which are particularly relevant for climate change adaptation and sustainable agricultural practices.
5. Conclusions
Incorporating Laser-Induced Breakdown Spectroscopy (LIBS) into viticulture represents a departure from the traditional, resource-intensive wet laboratory techniques, such as ICP-OES, for the elemental quantification of grape tissues. This study explores the potential utility of LIBS across various grape tissues and cultivars, marking a progressive step in understanding its applicability.
The complex relationship between water availability and the macronutrient dynamics, especially in the white cultivars, emerges as a critical aspect. The differences in Ca, Mg, and N concentrations between the Loureiro and Vinhão cultivars reflect genetic, physiological, and environmental factors governing the grapevine nutrient dynamics. Despite its advantages, LIBS has more constraints compared to those of the standardized methodologies like ICP-OES, focusing on a limited set of elements due to the small sample size limitations. The challenges related to atomization, ionization, and interference with atmospheric N require careful consideration. This study′s limitations underscore the need for future research to enhance LIBS’s capabilities and explore its potential in broader agricultural applications.
Further wet lab validation of LIBS results suggests broader applicability, extending beyond the studied elements (Ca, Mg, and N), thereby facilitating its potential integration for determining the various macro and micro elements within grapes and, by extension, other fruits. This multifaceted utility positions LIBS to introduce new avenues for precision viticulture and metabolomic investigations in the context of climate change and sustainable agriculture.
Author Contributions
Conceptualization, R.T., R.M. and M.C.; methodology, F.M.-S. and R.M.; validation, R.M. and M.C.; formal analysis, R.T.; investigation, R.T., R.M. and M.C.; resources, R.M.; data curation, R.T.; writing—original draft preparation, R.T.; writing—review and editing, R.T., F.M.-S., R.M. and M.C.; supervision, R.M. and M.C.; project administration, M.C.; funding acquisition, R.M. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Renan Tosin and Filipe Monteiro-Silva acknowledge Fundação para a Ciência e Tecnologia (FCT) PhD research grant refs. SFRH/BD/145182/2019 and SFRD/BD/09136/2020. Rui Martins acknowledges the Fundação para a Ciência e Tecnologia (FCT) research contract grant (CEEIND/017801/2018). This work is financed by National Funds through the FCT, Fundação para a Ciência e a Tecnologia, I.P. (Portuguese Foundation for Science and Technology) within the project OmicBots, High-Throughput Integrative Omic-Robots Platform for a Next-Generation Physiology-based Precision Viticulture, with the reference PTDC/ASP-HOR/1338/2021.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.M.; Orchard, B.A.; Keller, M. Mineral sinks within ripening grape berries (Vitis vinifera L.). Vitis—J. Grapevine Res. 2006, 45, 115. [Google Scholar]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative Stress Under Macronutrient Deficiency in Plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Gülcü, M.; Uslu, N.; Geçgel, Ü. Determination of Bioactive Compounds and Mineral Contents of Seedless Parts and Seeds of Grapes. S. Afr. J. Enol. Vitic. 2017, 38, 212–220. [Google Scholar] [CrossRef]
- Bertoldi, D.; Larcher, R.; Bertamini, M.; Otto, S.; Concheri, G.; Nicolini, G. Accumulation and distribution pattern of macro- and microelements and trace elements in Vitis vinifera L. cv. Chardonnay berries. J. Agric. Food Chem. 2011, 59, 7224–7236. [Google Scholar] [CrossRef] [PubMed]
- Verdenal, T.; Dienes-Nagy, Á.; Spangenberg, J.E.; Zufferey, V.; Spring, J.-L.; Viret, O.; Marin-Carbonne, J.; Van Leeuwen, C. Understanding and managing nitrogen nutrition in grapevine: A review. OENO One 2021, 55, 1–43. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Buesa, I.; Pérez, D.; Castel, J.; Intrigliolo, D.S.; Castel, J.R. Effect of Deficit Irrigation on Vine Performance and Grape Composition Ofvitis Viniferal. Cv. Muscat of Alexandria. Aust. J. Grape Wine Res. 2017, 23, 251–259. [Google Scholar] [CrossRef]
- Candar, S.; Açıkbaş, B.; Ekiz, M.; Zobar, D.; Korkutal, I.; Bahar, E. Influence of Water Scarcity on Macronutrients Contents in Young Leaves of Wine Grape Cultivars. Ciência Técnica Vitivinícola 2021, 36, 104–115. [Google Scholar] [CrossRef]
- Bora, F.D.; Bunea, C.I.; Chira, R.; Bunea, A. Assessment of the Quality of Polluted Areas in Northwest Romania Based on the Content of Elements in Different Organs of Grapevine (Vitis vinifera L.). Molecules 2020, 25, 750. [Google Scholar] [CrossRef]
- Singh, V.K. Review: Application of LIBS to Elemental Analysis and Mapping of Plant Samples. At. Spectrosc. 2021, 42, 99–113. [Google Scholar] [CrossRef]
- Potortiota, A.G.; Lo Turco, V.; Saitta, M.; Bua, G.D.; Tropea, A.; Dugo, G.; Di Bella, G. Chemometric analysis of minerals and trace elements in Sicilian wines from two different grape cultivars. Nat. Prod. Res. 2017, 31, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Senesi, G.S.; Cabral, J.; Menegatti, C.R.; Marangoni, B.; Nicolodelli, G. Recent advances and future trends in LIBS applications to agricultural materials and their food derivatives: An overview of developments in the last decade (2010–2019). Part II. Crop plants and their food derivatives. TrAC Trends Anal. Chem. 2019, 118, 453–469. [Google Scholar] [CrossRef]
- Silva, F.M.; Queirós, C.; Pinho, T.; Boaventura, J.; Santos, F.; Barroso, T.G.; Pereira, M.R.; Cunha, M.; Martins, R.C. Reagent-less spectroscopy towards NPK sensing for hydroponics nutrient solutions. Sens. Actuators B Chem. 2023, 395, 134442. [Google Scholar] [CrossRef]
- Andrade, D.F.; Pereira-Filho, E.R. Direct Determination of Contaminants and Major and Minor Nutrients in Solid Fertilizers Using Laser-Induced Breakdown Spectroscopy (LIBS). J. Agric. Food Chem. 2016, 64, 7890–7898. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.A.A.C.; Fiorio, P.R.; Rizzo, R.; Rossetto, R.; Vitti, A.C.; Dias, F.L.F.; Oliveira, K.A.d.; Bárbara Neto, M. Detection of nutritional stress in sugarcane by VIS-NIR-SWIR reflectance spectroscopy. Ciência Rural 2023, 53, e20220543. [Google Scholar] [CrossRef]
- Monteiro-Silva, F.; Jorge, P.A.; Martins, R.C. Optical Sensing of Nitrogen, Phosphorus and Potassium: A Spectrophotometrical Approach Toward Smart Nutrient Deployment. Chemosensors 2019, 7, 51. [Google Scholar] [CrossRef]
- Andrews, H.B.; Martin, M.Z.; Wymore, A.M.; Kalluri, U.C. Rapid in situ nutrient element distribution in plants and soils using laser-induced breakdown spectroscopy (LIBS). Plant Soil 2023, 1–10. [Google Scholar] [CrossRef]
- Tosin, R.; Monteiro-Silva, F.; Martins, R.; Cunha, M. Precision maturation assessment of grape tissues: Hyperspectral bi-directional reconstruction using tomography-like based on multi-block hierarchical principal component analysis. Biosyst. Eng. 2023, 236, 147–159. [Google Scholar] [CrossRef]
- Rakovský, J.; Čermák, P.; Musset, O.; Veis, P. A review of the development of portable laser induced breakdown spectroscopy and its applications. Spectrochim. Acta Part B At. Spectrosc. 2014, 101, 269–287. [Google Scholar] [CrossRef]
- Arantes de Carvalho, G.G.; Bueno Guerra, M.B.; Adame, A.; Nomura, C.S.; Oliveira, P.V.; Pereira de Carvalho, H.W.; Santos, D.; Nunes, L.C.; Krug, F.J. Recent advances in LIBS and XRF for the analysis of plants. J. Anal. At. Spectrom. 2018, 33, 919–944. [Google Scholar] [CrossRef]
- Tosin, R.; Monteiro-Silva, F.; Martins, R.; Cunha, M. LIBS-Based Analysis of Elemental Composition in Skin, Pulp, and Seeds of White and Red Grape Cultivars. Eng. Proc. 2023, 48, 43. [Google Scholar]
- Miras-Avalos, J.M.; Intrigliolo, D.S. Grape Composition under Abiotic Constrains: Water Stress and Salinity. Front. Plant Sci. 2017, 8, 851. [Google Scholar] [CrossRef]
- Moros, J.; Laserna, J. Laser-Induced Breakdown Spectroscopy (LIBS) of Organic Compounds: A Review. Appl. Spectrosc. 2019, 73, 963–1011. [Google Scholar] [CrossRef]
- Ribeiro, R.; Capela, D.; Ferreira, M.; Martins, R.; Jorge, P.; Guimarães, D.; Lima, A. X-ray Fluorescence and Laser-Induced Breakdown Spectroscopy Analysis of Li-Rich Minerals in Veins from Argemela Tin Mine, Central Portugal. Minerals 2021, 11, 1169. [Google Scholar] [CrossRef]
- Martins, R.C.; Jorge, P.; Silva, E.; De Almeida, J.; Martins, A. A Method and Apparatus for Characterisation of Constituents in a Physical Sample from Electromagnetic Spectral Information. U.S. US20210270744A1, 31 July 2018. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020026165 (accessed on 26 December 2023).
- Gallarati, S.; van Gerwen, P.; Laplaza, R.; Vela, S.; Fabrizio, A.; Corminboeuf, C. OSCAR: An extensive repository of chemically and functionally diverse organocatalysts. Chem. Sci. 2022, 13, 13782–13794. Available online: https://pubs.rsc.org/en/content/articlelanding/2022/SC/D2SC04251G (accessed on 20 May 2023). [CrossRef]
- NIST. NIST Atomic Spectra Database Lines Form. Available online: https://www.physics.nist.gov/PhysRefData/ASD/lines_form.html (accessed on 20 May 2020).
- Kurt, A.; Torun, H.; Colak, N.; Seiler, G.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Nutrient profiles of the hybrid grape cultivar ‘Isabel’ during berry maturation and ripening. J. Sci. Food Agric. 2017, 97, 2468–2479. [Google Scholar] [CrossRef]
- Hocking, B.J. The Role of Calcium in the Cell Wall of Grape Berries. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2015. [Google Scholar]
- Yuan, F.; Schreiner, R.P.; Osborne, J.; Qian, M.C. Effects of Soil NPK Supply on Pinot noir Wine Phenolics and Aroma Composition. Am. J. Enol. Vitic. 2018, 69, 371–385. [Google Scholar] [CrossRef]
- Martins, V.; Unlubayir, M.; Teixeira, A.; Lanoue, A.; Geros, H. Exogenous Calcium Delays Grape Berry Maturation in the White cv. Loureiro While Increasing Fruit Firmness and Flavonol Content. Front. Plant Sci. 2021, 12, 742887. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Juhaimi, F.A.; Gulcu, M.; Uslu, N.; Gecgel, U.; Ghafoor, K.; Dursun, N. Effect of harvest time on physico-chemical properties and bioactive compounds of pulp and seeds of grape varieties. J. Food Sci. Technol. 2017, 54, 2230–2240. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom. 2007, 8, 429. [Google Scholar] [CrossRef]
- Walker, R.P.; Chen, Z.-H.; Técsi, L.I.; Famiani, F.; Lea, P.J.; Leegood, R.C. Phosphoenolpyruvate carboxykinase plays a role in interactions of carbon and nitrogen metabolism during grape seed development. Planta 1999, 210, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.C.; Santos, F.; Cunha, M.; Monteiro-Silva, F.; Tosin, R.; Magalhães, S.; Reis Pereira, M. Method and Device for Non-Invasive Tomographic Characterisation of a Sample Comprising a Plurality of Differentiated Tissues. Patent WO/2023/126532, 6 July 2023. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2023126532&_cid=P12-LK2EKH-19952-1 (accessed on 26 December 2023).
- Mitić, S.S.; Obradović, M.V.; Mitić, M.N.; Kostić, D.A.; Pavlović, A.N.; Tošić, S.B.; Stojković, M.D. Elemental Composition of Various Sour Cherry and Table Grape Cultivars Using Inductively Coupled Plasma Atomic Emission Spectrometry Method (ICP-OES). Food Anal. Methods 2011, 5, 279–286. [Google Scholar] [CrossRef]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part II: Review of instrumental and methodological approaches to material analysis and applications to different fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef]
- Peng, J.; Liu, F.; Zhou, F.; Song, K.; Zhang, C.; Ye, L.; He, Y. Challenging applications for multi-element analysis by laser-induced breakdown spectroscopy in agriculture: A review. TrAC Trends Anal. Chem. 2016, 85, 260–272. [Google Scholar] [CrossRef]
- Ferreira, M.F.S.; Capela, D.; Silva, N.A.; Gonçalves, F.; Lima, A.; Guimarães, D.; Jorge, P.A.S. Comprehensive comparison of linear and non-linear methodologies for lithium quantification in geological samples using LIBS. Spectrochim. Acta Part B At. Spectrosc. 2022, 195, 106504. [Google Scholar] [CrossRef]
- Jull, H.; Künnemeyer, R.; Schaare, P. Nutrient quantification in fresh and dried mixtures of ryegrass and clover leaves using laser-induced breakdown spectroscopy. Precis. Agric. 2018, 19, 823–839. [Google Scholar] [CrossRef]
- Nunes, L.C.; Batista Braga, J.W.; Trevizan, L.C.; Florêncio de Souza, P.; Arantes de Carvalho, G.G.; Júnior, D.S.; Poppi, R.J.; Krug, F.J. Optimization and validation of a LIBS method for the determination of macro and micronutrients in sugar cane leaves. J. Anal. At. Spectrom. 2010, 25, 1453–1460. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).