Abstract
Third-generation (3G) biorefineries harnessing industrial off-gases have received significant attention in the transition towards a sustainable circular economy. However, uncertainties surrounding their techno-economic feasibility are hampering widespread commercialization to date. This study investigates the production of single-cell protein (SCP), a sustainable alternative food and feed protein, from steel mill off-gas through an efficient coupled fermentation approach utilizing acetate as an intermediate. A comprehensive model that comprises both the gas-to-acetate and the acetate-to-SCP fermentation processes, as well as gas pretreatment and downstream processing (DSP) operations, was developed and used to perform a techno-economic analysis (TEA). Sensitivity analyses demonstrated that significant cost reductions can be achieved by the process intensification of the gas-to-acetate fermentation. As such, an increase in the acetate concentration to 45 g/L and productivity to 4 g/L/h could lead to a potential cost reduction from 4.15 to 2.78 USD/kg. In addition, the influence of the production scale and other economic considerations towards the commercialization of off-gas-based SCPs are discussed. Conclusively, this research sheds light on the practical viability of a coupled fermentation process for SCP production by identifying key cost-influencing factors and providing targets for further optimization of the acetate platform, fostering sustainable and economically feasible bio-based innovations.
1. Introduction
Over the past decades, waste prevention and reduction have become increasingly important. In this context, the transition to a circular economy and a reduction in greenhouse gas (GHG) emissions are key action points, prompting the need for innovative and sustainable solutions. Biorefineries have gained considerable attention as they redesign industrial processes towards the use of renewable feedstocks and biological conversion technologies, mitigating their environmental impact. However, current first-generation (1G) biorefineries are often constrained by the requirement for high-purity feedstocks, entailing the utilization of arable land and thus leading to competition with food crops [1]. Alternatively, second-generation (2G) biorefineries use non-food biomass such as agricultural residues and organic waste, yet face challenges due to the higher complexity and the cost of processing these feedstocks [2]. Recently, another groundbreaking approach has been put forth in the form of a third-generation (3G) biorefinery harnessing carbon from industrial off-gases, an abundant and sustainable carbon source [3].
A key challenge of 3G biorefineries is energy-efficient carbon fixation. For this reason, gas fermentation using acetogenic bacteria, which can efficiently assimilate CO2 and H2 as well as CO through the native Wood–Ljungdahl pathway, appears as a propitious route [4]. One of today’s most prominent examples of this technology is the ethanol production process of LanzaTech (US), which is able to convert off-gas from the basic oxygen furnaces (BOFs) of steel mills [5]. Considering that the iron and steel industry is responsible for a substantial 25% share of industrial GHG emissions, such a gas fermentation process is regarded as a favorable carbon capture and utilization (CCU) technology [6]. The BOF gas is rich in CO, which can be used as both a carbon and energy source by the acetogens [7]. In addition, thanks to the robustness of the acetogens, BOF gas can be supplied to the gas fermentation process without extensive prior gas purification [5].
Whereas the acetogenic metabolism is known for its metabolic efficiency, its product spectrum is rather limited [7]. To overcome this limitation, a coupled fermentation approach with acetate as an intermediate can be employed. This coupled fermentation consists of (1) an anaerobic gas fermentation producing acetate, the main natural product synthesized by acetogens, and (2) an aerobic acetate fermentation to synthesize higher-value compounds. For the former, Moorella thermoacetica serves as the homoacetogenic model organism, producing acetate at a high yield and capable of reaching an acetate concentration of 30 g/L at a volumetric productivity of 0.57 g/L/h [8,9]. The produced acetate can be assimilated by a wide variety of microbial production strains since it only takes one enzymatic conversion to form the central molecule acetyl-CoA [10].
In fact, this coupled fermentation concept has been employed for the acetate-based production of triacylglycerides, polyhydroxyalkanoates, and single-cell protein (SCP), amongst others [11,12,13]. In particular, the use of SCP has recently been considered highly promising because of its environmental merits. Since the production of microbial protein is highly efficient in land and nutrient utilization, it could alleviate the burden that traditional protein production for food and feed exerts on the environment [14,15]. To produce SCP from acetate, Cupriavidus necator, formerly known as Ralstonia eutropha or Alcaligenes eutrophus, has emerged as an interesting production strain. It stands out as a well-established acetate-utilizing strain, reaching high cell densities exceeding 100 g/L [11]. In addition, SCP from C. necator has been reported to be of superior quality because of its high protein content of around 70%, valuable amino acid profile, and similar or better digestibility than the current commonly used protein sources such as soy or whey [16].
Hence, while offering a carbon-neutral system for sustainable protein production, the production of off-gas-derived SCP perfectly aligns with the need for innovative CCU technologies. Moreover, the proposed coupled fermentation approach with acetate as the intermediate has recently been identified as one of the most energy-efficient metabolic routes compared with alternative intermediates and direct gas fermentation [17]. Nevertheless, to date, the techno-economic feasibility of this intricate process remains unexplored, leaving the untapped potential for widespread implementation yet to be revealed.
To bridge this gap, we developed a comprehensive model that incorporates the coupled fermentation with acetate as the intermediate in a 3G biorefinery, utilizing BOF gas to produce SCP feed. Besides the anaerobic and aerobic fermentations, the model also includes the required gas pretreatment and downstream processing (DSP), as well as additional operations to enhance waste management and circularity. Analyses of the cost breakdown and capital investment, as well as sensitivity analyses, were performed aiming to provide a better understanding of the process and to identify the main factors affecting its techno-economic feasibility. By doing so, this study not only elucidates the vast potential of the investigated 3G biorefinery for off-gas-derived SCP production but also provides concrete insights and practical recommendations that serve as a crucial guideline to empower further research on the coupled fermentation platform.
2. Methodology
2.1. Modeling and Simulation Software
SuperPro Designer v13 (Intelligen, Scotch Plains, NJ, USA) was employed to model and simulate the biorefinery for SCP production from industrial off-gas. The same software tool was used to conduct economic assessments of the process, providing pricing models for the facility-dependent costs.
2.2. Process Design
An integrated process was designed for the continuous production of SCP from BOF gas through coupled fermentation with acetate as the intermediate. The complete process consists of eight sections, including the core production process as well as waste management operations to create circularity within the process. The model input parameters were derived from the literature and based on engineering discernment. The key assumptions for each of the process sections are discussed below. The detailed SuperPro Designer model used in this study is available upon request.
2.2.1. Medium Preparation
The medium contains the nutrients for both the gas-to-acetate and the acetate-to-SCP fermentation processes [13]. First, the water is purified by ion exchange and reverse osmosis. Then, the nutrients are dissolved and sterilized through in-line sterilization, except for the heat-sensitive compounds, namely the trace elements and vitamins, which are sterilized by filtration [18]. The complete sterilized medium is continuously added to the first bioreactor, from which the spent medium is fed to the second bioreactor together with the produced acetate.
2.2.2. Gas Pretreatment
BOF gas with a mass composition of 55.96% CO, 20.48% CO2, 0.27% H2, 23.27% N2, and 0.02% O2 was selected as the feedstock [5,19]. Since anaerobic conditions are required during the gas-to-acetate fermentation, oxygen traces are removed in a catalytic system [20]. In addition, to prevent catalyst poisoning caused by iron oxide dust and other particulates in the steel mill off-gas, a gas filtration step is included [21]. Subsequently, the purified gas is compressed to 9 bar and cooled to 60 °C to match the fermentation conditions.
2.2.3. Anaerobic Gas-to-Acetate Fermentation
The acetogenic species M. thermoacetica was selected to convert the BOF gas. The anaerobic gas fermentation process is operated under its optimal conditions for acetate production, namely a temperature of 60 °C and pH 6.0 [22]. To improve the gas transfer efficiency, the operating pressure was set at 8.5 bar and a bioreactor with an airlift design was selected, using a maximum nominal volume of 600 m3 [23,24]. A 90% overall CO conversion efficiency was assumed, of which 10% is converted into biomass and 90% is used for acetic acid production resulting in the formation of ammonium acetate with NH4OH as the neutralizer, according to the following stoichiometric reactions:
with being the molecular formula for M. thermoacetica biomass for which the reaction was based on the elemental balance [8,25].
A continuous fermentation mode is employed with the implementation of an external filtration unit for cell recycling. The latter consists of a hollow-fiber membrane that retains most of the cells, whereas a filtrate stream consisting of the produced ammonium acetate and the residual nutrients is continuously extracted [13]. A small cell fraction is removed using a bleed stream to keep the biomass concentration constant and assure the culture’s viability. By implementing this continuous fermentation mode, a steady state is attained. The values of the key fermentation parameters for the benchmark process were based on the work of Hu et al. (2016) and the findings obtained during pilot-scale gas fermentation trials conducted at the Bio Base Europe Pilot Plant (Ghent, Belgium) (Table 1) [13]. Finally, the filtered acetate stream is blended and cooled in a buffer tank, with a residence time of 2 h, to serve as the substrate for the second fermentation stage.

Table 1.
Benchmark process values for the gas-to-acetate fermentation.
2.2.4. Aerobic Acetate-to-SCP Fermentation
The dilute acetate stream is fed to the second fermentation, where it is assimilated by C. necator to produce SCP. The temperature is controlled at 30 °C and air is continuously sparged at 0.3 vvm. The process is conducted in a continuously stirred tank reactor (CSTR) using a maximum nominal volume of 350 m3. Three stoichiometric reactions were included for this fermentation, namely the dissociation of ammonium acetate, the production of biomass, and the neutralization of the residual NH3 with H3PO4:
with being the molecular formula for C. necator biomass for which the reaction was based on the elemental balance [25].
A continuous fermentation mode is employed to keep the volume constant while feeding the dilute acetate stream; hence, similar to the gas-to-acetate fermentation, a steady state is reached. The values of key fermentation parameters for the benchmark model listed in Table 2 were based on the work of Vlaeminck et al. [11].

Table 2.
Benchmark process values for the acetate-to-SCP fermentation.
2.2.5. DSP of SCP
The effluent broth of the acetate-to-SCP fermentation is treated in a series of continuous DSP operations to produce SCP suitable for feed applications. First, a two-step microfiltration is performed, including concentrating the cells to 90 g/L and diafiltration to wash the cells and remove water-soluble contaminants [26]. Then, the concentrated broth is pasteurized at a temperature of 90 °C for 1 h. Finally, water is removed from the product stream using a three-effect evaporation system and a spray-drying system, which reduce the water content to 80% and 10%, respectively [26].
2.2.6. NH3 Recovery
Part of the NH3 contained in the ammonium acetate produced in the gas-to-acetate fermentation is consumed by C. necator for biomass formation in the acetate-to-SCP fermentation. Recovery of the unused NH3 is implemented by including an absorption unit that retains 90% of the NH3 contained in the off-gas stream of the acetate-to-SCP fermentation [27]. The use of water for scrubbing enables the generation of NH4OH, which is reused as a neutralizer in the gas-to-acetate fermentation.
2.2.7. Anaerobic Digestion and Gas Combustion
The residual biomass and nutrients present in the bleed stream from the gas-to-acetate fermentation and in the filtrate stream from the two-step microfiltration during DSP are valorized by anaerobic digestion for biogas generation. The streams are collected and blended in a storage tank before entering a series of two anaerobic digestion tanks operating at a temperature of 37 °C with a maximum volume of 15,000 m3 and a residence time of 240 h [28]. The produced biogas is upgraded by removing the H2S with an absorption unit, compressed to 11 bar, and sent to a buffer tank [29]. Besides the biogas, the off-gas from the gas-to-acetate fermentation, containing unconverted CO and H2, is also an energy-containing gas stream. Both the biogas and the fermentation off-gas are combusted for heat generation in the spray-drying system used during DSP, thereby reducing natural gas utilization [30].
2.2.8. Wastewater Treatment
The digestate effluent stream from the anaerobic digestion still contains undigested organic compounds with a considerable amount of organic duty. This is treated by aerobic oxidation in an activated sludge system comprised of an aeration tank for biodegradation and a settling tank for clarification. The activated sludge is partially recycled, whereas the sludge bleed is concentrated with a belt filter. Since an absence of toxic compounds was considered, additional processing was deemed unnecessary and a sludge disposal cost of 10 USD per metric ton (Mton) was taken into account [31]. Finally, the treated water is passed through a granular media filter to remove solid particles [28]. Most of the purified water is used in medium preparation, in which 20% of the fresh water is added to circumvent the potential accumulation of toxic compounds. A smaller part of the purified water is used in the biogas upgrading absorption unit and in the diafiltration during DSP. In the latter, the condensed water of the evaporation system is also completely reused.
2.3. Economic Assessment
For the economic assessment, a facility with a lifetime of 20 years, an annual SCP production capacity of 20,000 Mton, and an annual operational activity of 49 weeks was modelled. To estimate the operating costs and capital investment, a simulation-based approach was employed using economic calculations integrated into the SuperPro Designer software. The operating costs consist of the following costs: raw materials, facility (including plant maintenance, depreciation, and overhead expenses), labor, consumables, laboratory/quality control/quality assurance (lab/QC/QA), and utilities. The capital investment comprises direct fixed capital (including equipment purchase, installation, piping, instrumentation, buildings, etc.), working capital (estimated as the capital required to cover one month of expenses), and startup and validation costs (estimated as 5% of the direct fixed capital). Sensitivity analyses were conducted through a comprehensive set of simulations in which the sensitivity variables were systematically adjusted. As such, the influence of key fermentation performance parameters, namely the acetate productivity (1–6 g/L/h) and the acetate concentration (30–60 g/L) of the gas-to-acetate fermentation, and the impact of production scale (5000–50,000 Mton/y) on the unit production cost and capital investment were evaluated.
3. Results and Discussion
3.1. Process Simulation and Conversion Yield
The benchmark process was modeled and simulated following the assumptions described in the Section 2. In Figure 1, a simplified block flow diagram indicating the eight process sections is shown. With the assumed productivities of 1.0 g/L/h acetate and 2.0 g/L/h SCP for the coupled fermentations, a total of 11 airlift bioreactors (432 m3) and 5 CSTRs (299 m3) are required to sustain the production of 2.5 Mton/h of dry C. necator biomass. Moreover, since acetate becomes inhibiting for M. thermoacetica at high concentrations, the acetate concentration was limited to 30 g/L [22]. This is achieved through a continuous fermentation mode in the gas-to-acetate fermentation, requiring the addition of 169 Mton/h fresh medium. Moreover, 7 Mton/h NH3 solution (24.5 w%) is added in this first fermentation step to keep the pH constant, thereby forming ammonium acetate. In the acetate-to-SCP fermentation step, all acetate is consumed to produce 14 g/L C. necator dry biomass, leading to a pH increase that is counteracted by the addition of H3PO4 (85 w%). The 158 Mton/h effluent from this second fermentation is concentrated and washed in a two-step microfiltration process. Following this, the C. necator culture is inactivated through pasteurization. Finally, the stream is dried, resulting in a final product composed of 87% SCP, 10% water, and 3% salts.
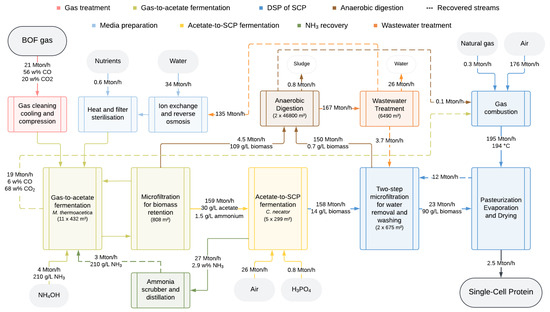
Figure 1.
Simplified block flow diagram indicating the eight process sections. The material flows and dimensions of the main unit operations, including process tank volumes as well as membrane and heating surface areas are given for the benchmark process.
Optimization of resource management through the recovery of materials and energy, as well as wastewater treatment, is crucial for the overall sustainability of the process [15]. Therefore, the NH3 recovery, anaerobic digestion, and wastewater treatment sections were included. By using NH3 recovered from the off-gas of the acetate-to-SCP fermentation, 42% less NH4OH solution is needed. In the anaerobic digestion section, the residues of biomass, salts, and nutrients are treated, resulting in the formation of biogas. This biogas and the gas-to-acetate fermentation off-gas are used for the heat generation required in the spray-drying system, hence 96% less natural gas is needed. The purified water obtained from the wastewater treatment allows an 80% reduction in freshwater usage for medium preparation. Furthermore, the complete production process was designed in a continuous operation mode, enabling steady-state operation and consistent product quality. This enhances the overall space–time yield as it eliminates the need for startup and shutdown procedures, thus minimizing downtime and reducing the operational costs. The technical feasibility of this continuous coupled fermentation process was enhanced by including buffer vessels at several crucial points, such as before each fermentation section.
For the annual production of 20,000 Mton SCP, 172,000 Mton BOF gas is required, which corresponds to about one-third of the total BOF gas emitted by a conventional steel mill with an annual steel production capacity of 5 million Mton [19,32]. Furthermore, thanks to its microbial robustness, this process can also utilize other industrial off-gases, such as the syngas obtained by the gasification of woody biomass, a common waste stream of current 1G and 2G biorefineries [33]. The overall carbon fixation yield of CO into SCP equals 21%, whereas the rest of the carbon dissipates at different points in the process:
- A total of 55% is emitted through the off-gas of the gas-to-acetate fermentation. More specifically, 10% accounts for the amount of CO that remains unconverted, whereas the remaining 45% is emitted as CO2 during the conversion of CO to M. thermoacetica biomass and acetate (Equations (1) and (2));
- A total of 18% is emitted as CO2 through the off-gas of the acetate-to-SCP fermentation when converting ammonium acetate (Equation (5));
- Totals of 5% and 1% are fixed in the discarded biomass of M. thermoacetica (removed in the bleed stream during the gas-to-acetate fermentation) and C. necator (lost in the two-step microfiltration during the DSP of SCP), respectively, which are treated by anaerobic digestion.
Remarkably, the carbon yield could potentially be improved by introducing CO2 conversion. Both M. thermoacetica and C. necator are capable of consuming CO2 if additional energy in the form of H2 is provided. However, some critical challenges arise when aiming to achieve this complete carbon conversion. For M. thermoacetica, it is known that the simultaneous assimilation of CO and H2 within one fermentation is metabolically conflicting due to hydrogenase inhibition by CO [13]. For C. necator, this would require the establishment of an aerobic gas fermentation process, which is known to be technically challenging since the CO2/H2/O2 gas mixture is potentially explosive [34]. Furthermore, the Calvin cycle natively used by C. necator for aerobic CO2 fixation is far less efficient than the acetogenic Wood–Ljungdahl pathway, hence requiring a larger amount of H2 and thus increasing the costs [17]. In this respect, current advances in metabolic engineering focusing on the co-utilization of CO and H2 for M. thermoacetica, as well as decreasing the H2 requirement for C. necator, are highly valuable [35,36,37].
3.2. Capital Investment and Breakdown of the Unit Production Cost
Economic analysis of the benchmark process model for a 20,000 Mton/y SCP facility resulted in an estimation of the capital investment and unit production cost of USD 320 million and 4.15 USD/kg, respectively. The contribution of each process section and the breakdown of the unit production cost are shown in Figure 2.
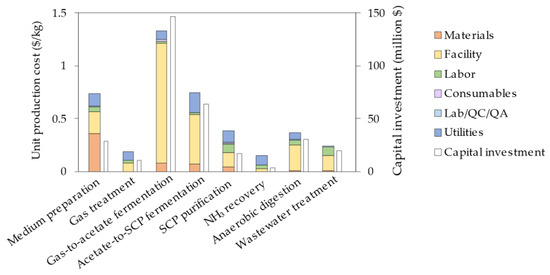
Figure 2.
Breakdown of the unit production cost and capital investment per process section for the benchmark process.
The facility-dependent costs make up 58% of the total production costs, mainly resulting from the large number of bioreactors needed. The utilities, encompassing electricity and heat transfer agents, account for 18% of the total costs. Remarkably, despite the acetate-to-SCP bioreactors (299 m3) being smaller than the gas-to-acetate bioreactors (432 m3), their utilities cost per bioreactor is significantly higher. This difference can be attributed to the fact that a CSTR demands approximately five times more electricity than an airlift bioreactor, primarily due to the energy-intensive motor required for stirring. In addition, the acetate-to-SCP fermentation process demands more cooling as the cultivation temperature is 30 °C for C. necator, whereas it is 60 °C for M. thermoacetica. The material costs are relatively small, since the use of several compounds typically required for the startup, cleaning, and sterilization of the process tanks and other equipment are largely reduced by operating in a continuous mode. In addition, the main material streams, namely water and NH3, are recovered in the process. Finally, it is also crucial to note that whereas the feedstock cost typically encompasses 50% of the production cost in a traditional biorefinery, this cost was decreased to 5% when accounting for the treatment of the BOF gas. In addition, carbon taxation could further reduce this cost and could even lead to additional revenue. As such, a carbon tax of 50 USD/Mton, which is advised as the minimum amount for 2030 (to achieve the Paris Agreement’s temperature targets), would result in an extra gain of 0.03 USD/kg SCP or 593,000 USD/y [38].
3.3. Impact of Process Intensification on the Production Costs
The economic analysis of the benchmark process shows that the coupled fermentation part is responsible for 67% of the capital investment and 50% of the unit production costs. Therefore, the influence of key fermentation performance parameters on these costs was thoroughly investigated via sensitivity analysis. Although the benchmark process values were defined based on current published findings, the aim of this analysis is to determine a new set of target process values for an intensified process that can serve as an ambitious yet achievable and realistic goal for future developments within this field.
More specifically, a sensitivity analysis of the acetate productivity and the acetate concentration was performed while keeping the throughput constant. The input process values used for the sensitivity analysis range from 30 to 60 g/L and from 1 to 6 g/L/h. The lower values represent the benchmark process, whereas the higher values were determined based on the highest values published to date for acetate production from gases. Notably, these upper values have so far only been obtained in distinct fermentation processes (59 g/L at 0.8 g/L/h and 18 g/L at 6 g/L/h) [8]. This can be explained by the aforementioned inhibition of acetate at high concentrations, resulting in a trade-off between acetate productivity and concentration. However, this could be alleviated by metabolic advances increasing the acetate tolerance of acetogens, as well as enhanced process design and control, including specialized industrial equipment [39]. The unit production costs resulting from the simulations of the various process scenarios are presented in Figure 3.
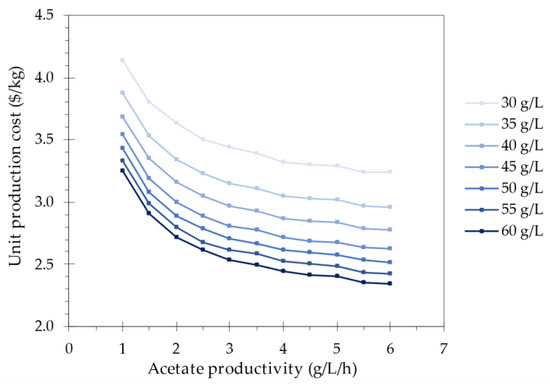
Figure 3.
Impact of increasing acetate productivity and concentration on the unit production cost.
The unit production cost drops significantly when increasing the acetate productivity and acetate concentration, with both having a maximal singular effect of 0.90 USD/kg or a 32% cost reduction. It is important to acknowledge that process changes were performed by adjusting the input values without considering any variations in performance that might arise, such as differences in gas transfer or other transport phenomena. This is especially important for the gas-to-acetate fermentation process, as the rate of acetate production is gas transfer limited at high biological activities [22]. Nevertheless, the bioreactor with an airlift design used for this fermentation is known for its high mass transfer performance. A CO transfer of up to 8 g/L/h has been reported, which would support acetate production up to 4 g/L/h (Equation (2)) [23]. In addition, the increased operating pressure of 8.5 bar also improves the gas transfer [40]. For example, for ethanol production from syngas, the productivity was doubled when increasing the pressure to 5 bar [41].
For both acetate productivity and acetate concentration, the cost decrease stagnates towards the upper values. This cost trend can be attributed to the reduction in the required reactor volume, as presented in Figure 4. Indeed, an increasing acetate productivity and acetate concentration lead to a decrease in the number of required gas-to-acetate and acetate-to-SCP bioreactors, respectively, for the same product output. The latter can be explained by the fact that a higher acetate concentration also leads to a higher C. necator biomass concentration, which in turn results in higher productivity when the specific growth rate remains constant.
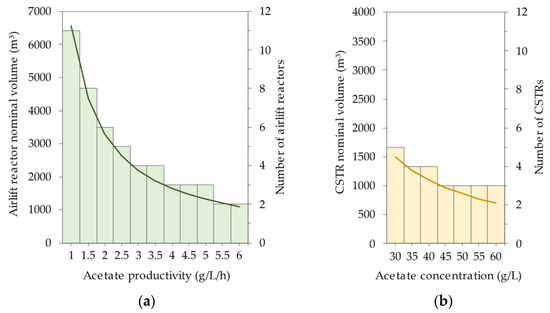
Figure 4.
Nominal reactor volume (lines) and number of reactors (bars) required for (a) the gas-to-acetate fermentation depending on the attained acetate productivity and (b) the acetate-to-SCP fermentation depending on the attained acetate concentration.
The sensitivity analyses revealed that the initial process intensification advances will mainly yield the greatest economic gain, primarily due to the significant reduction in the number of required bioreactors. Since the facility-dependent costs constitute the vast majority of the total unit production cost, this explains the corresponding cost reduction. Based on these insights, the target values for the intensified process model were defined as listed in Table 3. By setting the acetate productivity at 4 g/L/h, only three airlift bioreactors are required. Further process intensification to 5 g/L/h would not lead to a further decrease in this number. Similarly, the acetate concentration was set at 45 g/L, reducing the number of CSTRs to three as well.

Table 3.
Intensified process values for the gas-to-acetate and acetate-to-gas coupled fermentations.
For the intensified process model, the capital investment and unit production costs amount to USD 175 million and 2.78 USD/kg, respectively, resulting in a reduction of 55% and 67%, respectively. The impact of this intensified process model for each process section can be observed by comparing Figure 2 with Figure 5. As expected, a great decrease of 0.83 USD/kg and 0.23 USD/kg is observed for the gas-to-acetate and acetate-to-SCP fermentations, respectively. Furthermore, targeting a higher acetate concentration reduces the amount of water required for medium preparation, resulting in a cost reduction of 0.22 USD/kg. As resources are used more efficiently, energy demand and wastewater generation decrease, resulting in a reduction of the anaerobic digestion and wastewater treatment costs.
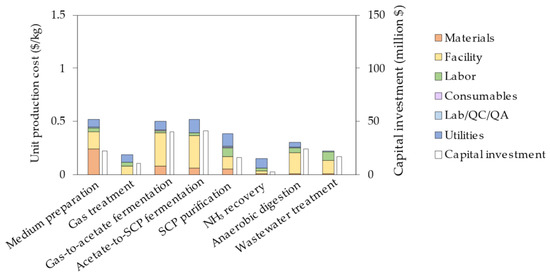
Figure 5.
Breakdown of the unit production cost and capital investment per process section for the intensified process.
3.4. Towards Commercialization
3.4.1. (Dis)Economy of Scale for a 3G Biorefinery
The impact of production scale on the unit production cost and capital investment for both the benchmark and intensified processes are displayed in Figure 6. The economy of scale is visible in the reduction of the unit production cost towards higher production volumes, where fixed costs are spread over a larger number of units. The curve flattens near the end as this benefit becomes relatively smaller. For example, for the intensified process model, a production increase of 5000 Mton/y, corresponding to an additional capital investment of around USD 33 million, leads to a unit cost reduction of 5.2%, from 20,000 to 25,000 Mton/y, whereas this reduction is only 1.6% for 40,000 to 45,000 Mton/y.
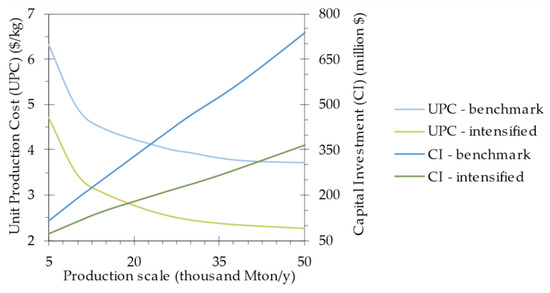
Figure 6.
Effect of the production scale for the benchmark and intensified process models on the unit production cost and capital investment.
Potential disadvantages of increasing production scale, known as diseconomies of scale, should also be considered. For a biorefinery, these disadvantages are often related to feedstock availability, which is especially important when establishing 3G biorefineries using gaseous feedstocks. In this case, a smaller facility offers the benefit of seamless integration at the manufacturing site of another plant emitting industrial off-gas. A notable example is the commercial Steelanol plant, which uses Lanzatech’s technology to produce ethanol from BOF gas and comprises four airlift bioreactors directly connected to the steel mill of Arcelor Mittal in Belgium [5]. In a hypothetical scenario where these four 500 m3 bioreactors would be repurposed for the production of acetate and coupled with four 300 m3 CSTRs, the SCP production scale would amount to 25,000 Mton/y for the intensified process. In this case, the unit production cost and capital investment would amount to 2.58 USD/kg and USD 208 million, respectively. Moreover, in addition to steel mill off-gas, other off-gases can also be used, including fermentation off-gas. As such, this coupled fermentation facility could be integrated into an existing biorefinery, thereby increasing its overall feedstock conversion yield [42].
3.4.2. Introducing Off-Gas-Derived SCP Feed to the Market
By applying the intensified process model, the SCP production cost could be reduced from 4.15 to 2.78 USD/kg. Despite this 33% cost reduction, this remains higher than the current selling prices of competing products, such as fish meal (1.79 USD/kg). However, to make a fair comparison, several other aspects of off-gas-derived SCP should be considered in relation to traditional protein sources. Firstly, SCP produced via the proposed coupled fermentation process offers a more sustainable route. The environmental impact of the current plant and animal protein production industries, involving deforestation, biodiversity loss, and GHG emissions, renders them inadequate to meet the escalating needs of the growing global population [43]. In contrast, the production of SCP in bioreactors is highly efficient in its use of resources such as energy, water, and land [15]. Secondly, CCU of industrial off-gas is not only environmentally favorable but is also economically beneficial, as carbon taxes and emissions trading systems (ETS) are spreading and rising [44]. Thirdly, recent studies focusing on the nutritional quality of C. necator SCP for feed purposes appear highly promising, even showing positive effects for growth using SCP in the diets of fish and shellfish, amongst others [45].
Taking into account the merits of off-gas-derived SCP compared with traditional protein sources, a higher selling price could be justified, thereby offering an economically attractive path toward commercialization. For example, assuming a 20,000 Mton/y production facility that implements the intensified process model and sets the selling price at 3.00 USD/kg, the estimated return on investment is 8.4%, corresponding to a payback time of 12 years. Finally, the market is rapidly expanding as the protein demand keeps on growing and attention is increasingly drawn to SCP. According to current projections, the market value of SCP is expected to triple by 2030, reaching USD 18.5 billion [46,47]. Hence, there is an urgent need for innovations that can fill this gap, and the proposed route for off-gas-derived SCP via acetate shows great potential in this context.
4. Conclusions
A detailed analysis of the techno-economic aspects associated with the implementation of a coupled fermentation approach using acetate as the intermediate for SCP feed production from industrial off-gas was presented. The cost analysis of the benchmark model disclosed a unit production cost of 4.15 USD/kg, primarily attributed to facility-dependent costs. Sensitivity analyses demonstrated that significant cost reductions can be achieved by optimizing the gas-to-acetate fermentation process, as this particularly decreases the required number of bioreactors. Elevating the acetate concentration and acetate productivity to 45 g/L and 4 g/L/h, respectively, could reduce the unit production cost to 2.78 USD/kg.
In conclusion, this research contributes to a better understanding of the techno-economic viability of a large-scale 3G biorefinery utilizing a coupled fermentation process with acetate as the intermediate for SCP production. The results highlight the key factors influencing production costs and lay the foundation for further research and optimization of the acetate platform. By providing concrete targets and identifying related research needs, this study paves the way for future advancements and explorations within this field, fostering sustainable and techno-economically feasible bio-based innovations.
Author Contributions
Conceptualization, E.U., K.Q., S.-S.K., N.M. and R.G.F.; methodology, E.V., S.-S.K., N.M. and R.G.F.; formal analysis, E.V.; writing—original draft, E.V.; writing—review and editing, E.U., K.Q., T.D., S.-S.K., N.M., R.G.F., D.P. and K.D.W.; visualization, E.V.; supervision, D.P., K.D.W. and W.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FWO (Research Foundation Flanders, Belgium) through the doctoral fellowship of E.V. (1SE0421N) and by VLAIO (Flanders Innovation & Entrepreneurship, Belgium) through the AC2GEN project (Flanders Industry Innovation Moonshot Strategic Basic Research for clusters).
Data Availability Statement
All data used, generated, and analyzed during this study are included in the SuperPro Designer process model (available upon request) and this published article.
Acknowledgments
All authors want to thank Ellen Verhoeven for her invaluable contribution to this research and dedication to providing essential expertise while preparing the paper.
Conflicts of Interest
N.M., R.G.F. and D.P. work for Intelligen (Scotch Plains, NJ, USA), the company that markets the SuperPro Designer software tool.
References
- Azapagic, A. Sustainability Considerations for Integrated Biorefineries. Trends Biotechnol. 2014, 32, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, B.F.; Takors, R. Recent Progress in the Synthesis of Advanced Biofuel and Bioproducts. Curr. Opin. Biotechnol. 2023, 80, 102913. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-Generation Biorefineries as the Means to Produce Fuels and Chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef]
- Molitor, B.; Richter, H.; Martin, M.E.; Jensen, R.O.; Juminaga, A.; Mihalcea, C.; Angenent, L.T. Carbon Recovery by Fermentation of CO-Rich off Gases—Turning Steel Mills into Biorefineries. Bioresour. Technol. 2016, 215, 386–396. [Google Scholar] [CrossRef]
- Collis, J.; Strunge, T.; Steubing, B.; Zimmermann, A.; Schomäcker, R. Deriving Economic Potential and GHG Emissions of Steel Mill Gas for Chemical Industry. Front. Energy Res. 2021, 9, 642162. [Google Scholar] [CrossRef]
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old Acetogens, New Light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef]
- Kantzow, C.; Mayer, A.; Weuster-Botz, D. Continuous Gas Fermentation by Acetobacterium Woodii in a Submerged Membrane Reactor with Full Cell Retention. J. Biotechnol. 2015, 212, 11–18. [Google Scholar] [CrossRef]
- Rosenbaum, F.P.; Müller, V. Moorella Thermoacetica: A Promising Cytochrome- and Quinone-Containing Acetogenic Bacterium as Platform for a CO2-Based Bioeconomy. Green Carbon 2023. [Google Scholar] [CrossRef]
- Kiefer, D.; Merkel, M.; Lilge, L.; Henkel, M.; Hausmann, R. From Acetate to Bio-Based Products: Underexploited Potential for Industrial Biotechnology. Trends Biotechnol. 2020, 39, 397–411. [Google Scholar] [CrossRef]
- Vlaeminck, E.; Quataert, K.; Uitterhaegen, E.; De Winter, K.; Soetaert, W.K. Advanced PHB Fermentation Strategies with CO2-Derived Organic Acids. J. Biotechnol. 2022, 343, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Molitor, B.; Mishra, A.; Angenent, L.T. Power-to-Protein: Converting Renewable Electric Power and Carbon Dioxide into Single Cell Protein with a Two-Stage Bioprocess. Energy Environ. Sci. 2019, 12, 3515–3521. [Google Scholar] [CrossRef]
- Hu, P.; Chakraborty, S.; Kumar, A.; Woolston, B.; Liu, H.; Emerson, D.; Stephanopoulos, G. Integrated Bioprocess for Conversion of Gaseous Substrates to Liquids. Proc. Natl. Acad. Sci. USA 2016, 113, 3773–3778. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liang, H.; Panda, S.; Fung, V.K.Y.; Zhou, J.F.J.; Zhou, K. C2 Feedstock-Based Biomanufacturing of Value-Added Chemicals. Curr. Opin. Biotechnol. 2022, 73, 240–245. [Google Scholar] [CrossRef]
- Van Peteghem, L.; Sakarika, M.; Matassa, S.; Pikaar, I.; Ganigué, R.; Rabaey, K. Towards New Carbon–Neutral Food Systems: Combining Carbon Capture and Utilization with Microbial Protein Production. Bioresour. Technol. 2022, 349, 126853. [Google Scholar] [CrossRef]
- Angenent, S.C.; Schuttinga, J.H.; van Efferen, M.F.H.; Kuizenga, B.; van Bree, B.; van der Krieken, R.O.; Verhoeven, T.J.; Wijffels, R.H. Hydrogen Oxidizing Bacteria as Novel Protein Source for Human Consumption: An Overview. Open Microbiol. J. 2022, 16, e187428582207270. [Google Scholar] [CrossRef]
- Claassens, N.J.; Cotton, C.A.R.; Kopljar, D.; Bar-Even, A. Making Quantitative Sense of Electromicrobial Production. Nat. Catal. 2019, 2, 437–447. [Google Scholar] [CrossRef]
- Vaghari, H.; Anarjan, N.; Najian, Y.; Jafarizadeh-Malmiri, H. Sterilization Process. In Essentials in Fermentation Technology; Berenjian, A., Ed.; Springer: Cham, Switzerland, 2019; pp. 85–103. ISBN 9783030162306. [Google Scholar]
- Uribe-Soto, W.; Portha, J.F.; Commenge, J.M.; Falk, L. A Review of Thermochemical Processes and Technologies to Use Steelworks Off-Gases. Renew. Sustain. Energy Rev. 2017, 74, 809–823. [Google Scholar] [CrossRef]
- Yan, Q.; Wan, C.; Street, J.; Yan, D.W.; Han, J.; Yu, F. Catalytic Removal of Oxygen from Biomass-Derived Syngas. Bioresour. Technol. 2013, 147, 117–123. [Google Scholar] [CrossRef]
- Sandlöbes, S.; Senk, D.; Sancho, L.; Diaz, A. In-Situ Measurement of CO- and CO2-Concentrations in BOF Off-Gas. Steel Res. Int. 2011, 82, 632–637. [Google Scholar] [CrossRef]
- Hu, P.; Rismani-Yazdi, H.; Stephanopoulos, G. Anaerobic CO2 Fixation by the Acetogenic Bacterium Moorella thermoacetica. AIChE J. 2013, 59, 3176–3183. [Google Scholar] [CrossRef]
- Puiman, L.; Abrahamson, B.; Van Der Lans, R.G.J.M.; Haringa, C.; Noorman, H.J.; Picioreanu, C. Alleviating Mass Transfer Limitations in Industrial External-Loop Syngas-to-Ethanol Fermentation. Chem. Eng. Sci. 2022, 259, 117770. [Google Scholar] [CrossRef]
- Simpson, S.D.; Collet, C.; Forster, R.L.S.; Cockrem, M.C.M.; Oakley, S.D.; Kopke, M. Carbon Capture in Fermentation. U.S. Patent US20100323417A1, 23 December 2010. [Google Scholar]
- Tracy, B.P.; Jones, S.W.; Fast, A.G.; Indurthi, D.C.; Papoutsakis, E.T. Clostridia: The Importance of Their Exceptional Substrate and Metabolite Diversity for Biofuel and Biorefinery Applications. Curr. Opin. Biotechnol. 2012, 23, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Byeon, S.H.; Lee, B.K.; Raj Mohan, B. Removal of Ammonia and Particulate Matter Using a Modified Turbulent Wet Scrubbing System. Sep. Purif. Technol. 2012, 98, 221–229. [Google Scholar] [CrossRef]
- Gallert, C.; Winter, J. Bacterial Metabolism in Wastewater Treatment Systems. In Environmental Biotechnology: Concepts and Applications; Jördening, H.-J., Winter, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 1–48. ISBN 3527305858. [Google Scholar]
- Misailidis, N.; Petrides, D. Food Industry Wastewater Treatment Plant—Process Modeling and Techno-Economic Assessment (TEA) Using SuperPro Designer; Intelligen, Inc.: Scotch Plains, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and Perspectives in Europe. Renew Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Amann, A.; Weber, N.; Krampe, J.; Rechberger, H.; Zoboli, O.; Zessner, M. Operation and Performance of Austrian Wastewater and Sewage Sludge Treatment as a Basis for Resource Optimization. Water 2021, 13, 2998. [Google Scholar] [CrossRef]
- Ho, M.T.; Bustamante, A.; Wiley, D.E. Comparison of CO2 Capture Economics for Iron and Steel Mills. Int. J. Greenh. Gas Control 2013, 19, 145–159. [Google Scholar] [CrossRef]
- Ciliberti, C.; Biundo, A.; Albergo, R.; Agrimi, G.; Braccio, G.; de Bari, I.; Pisano, I. Syngas Derived from Lignocellulosic Biomass Gasification as an Alternative Resource for Innovative Bioprocesses. Processes 2020, 8, 1567. [Google Scholar] [CrossRef]
- Lambauer, V.; Kratzer, R. Lab-Scale Cultivation of Cupriavidus necator on Explosive Gas Mixtures: Carbon Dioxide Fixation into Polyhydroxybutyrate. Bioengineering 2022, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Valgepea, K.; De Souza Pinto Lemgruber, R.; Abdalla, T.; Binos, S.; Takemori, N.; Takemori, A.; Tanaka, Y.; Tappel, R.; Köpke, M.; Simpson, S.D.; et al. H2 Drives Metabolic Rearrangements in Gas-Fermenting Clostridium autoethanogenum. Biotechnol. Biofuels 2018, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Claassens, N.J.; Bordanaba-Florit, G.; Cotton, C.A.R.; De Maria, A.; Finger-Bou, M.; Friedeheim, L.; Giner-Laguarda, N.; Munar-Palmer, M.; Newell, W.; Scarinci, G.; et al. Replacing the Calvin Cycle with the Reductive Glycine Pathway in Cupriavidus necator. Metab. Eng. 2020, 62, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, J.K.; Valgepea, K.; de Souza Pinto Lemgruber, R.; Casini, I.; Plan, M.; Tappel, R.; Simpson, S.D.; Köpke, M.; Nielsen, L.K.; Marcellin, E. Enhancing CO2-Valorization Using Clostridium autoethanogenum for Sustainable Fuel and Chemicals Production. Front. Bioeng. Biotechnol. 2020, 8, 204. [Google Scholar] [CrossRef]
- Stiglitz, J.E.; Stern, N.; Duan, M.; Edenhofer, O.; Giraud, G.; Heal, G.; Lèbre la Rovere, E.; Moyer, E.; Pangestu, M.; Shukla, P.R.; et al. Report of the High-Level Commission on Carbon Prices; World Bank: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Trček, J.; Mira, N.P.; Jarboe, L.R. Adaptation and Tolerance of Bacteria against Acetic Acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, W.; Bockrath, R.; De Wever, H. Effects of Moderately Elevated Pressure on Gas Fermentation Processes. Bioresour. Technol. 2019, 293, 122129. [Google Scholar] [CrossRef]
- Gaddy, J.L.; Arora, D.K.; Ko, C.W.; Phillips, J.R.; Basu, R.; Wikstrom, C.V.; Clausen, E.C. Methods for Increasing the Production of Ethanol from Microbial Fermentation. U.S. Patent US20030211585A1, 23 October 2007. [Google Scholar]
- Lee, U.; R Hawkins, T.; Yoo, E.; Wang, M.; Huang, Z.; Tao, L. Using Waste CO2 from Corn Ethanol Biorefineries for Additional Ethanol Production: Life-Cycle Analysis. Biofuels Bioprod. Biorefining 2021, 15, 468–480. [Google Scholar] [CrossRef]
- Linder, T. Making the Case for Edible Microorganisms as an Integral Part of a More Sustainable and Resilient Food Production System. ACS Food Sci. Technol. 2023, 11, 1144–1152. [Google Scholar] [CrossRef]
- Thielges, S.; Olfe-Kräutlein, B.; Rees, A.; Jahn, J.; Sick, V.; Quitzow, R. Committed to Implementing CCU? A Comparison of the Policy Mix in the US and the EU. Front. Clim. 2022, 4, 943387. [Google Scholar] [CrossRef]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent Advances in Single Cell Protein Use as a Feed Ingredient in Aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- Single Cell Protein Market Share|Industry Statistics 2030. Available online: https://www.gminsights.com/industry-analysis/single-cell-protein-market (accessed on 12 July 2023).
- Aidoo, R.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. Overview of Single Cell Protein: Production Pathway, Sustainability Outlook, and Digital Twin Potentials. Trends Food Sci. Technol. 2023, 138, 577–598. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).