Abstract
The increase in global temperature calls for ambitious action to reduce the release of greenhouse gases into the atmosphere. The transportation sector contributes up to 25% of the total emissions released, mainly from the burning of vehicle fuel. Therefore, scientists from all around the world are focusing on finding a sustainable alternative to conventional vehicle fuel. Biofuel has attracted much attention, as it shows great potential for the replacement of traditional fossil fuels. However, the main bottlenecks of biofuel are the ongoing controversial conflict between food security with biofuel production. Therefore, this study focuses on a sustainable extraction of lipids from microalgae for the production of biofuel using a liquid biphasic flotation system coupled with sugaring-out method. This is the first study to combine the methods of liquid biphasic flotation system with the sugaring-out technique. It represents a holistic study of optimum and effective conditions needed to extract lipids from the system and to understand the reliability of sugar solution as the agent of cell disruption. At the 15-min flotation time, 150 g/L of fructose solution with a 1:2 mass separating agent-acetonitrile ratio successfully extracted up to 74% of lipid from Chlorella sorokiniana CY-1. Two types of fatty acid methyl esters were recovered from the study, with C5:0 being the main component extracted.
1. Introduction
Unsustainable usage and management of fossil fuels can lead to fuel shortage in the future as they are non-renewable resources [1]. The global temperature has increased by 1.09 °C since the industrial revolution due to the emission of greenhouse gases from anthropogenic activities [2]. The transportation sector accounts for more than a quarter of the global greenhouse gas emissions, rooted in the burning of vehicle fuel [3]. It is essential to find an alternative to current conventional fossil fuels in order to ensure a sustainable future. Raw materials from food crops such as corn, wheat, rice and sugarcane are currently being used for the production of biofuel. The use of food crops as a feedstock can trigger the conflict between food security and biofuel production [1]. Microalgae have become an attractive source of biofuel production. This biofuel is known as third-generation biofuel, where the use of microalgae as the feedstock can mitigate the conflict between food security and biofuel production [1].
Microalgae can produce higher biomass per unit area compared to terrestrial plants, and can easily grow in wastewater and seawater-based mediums that make it a sustainable option for biofuel production [1,4]. Apart from tackling the drawbacks of fossil fuel and food crop biofuel, microalgae can also act as a natural carbon sink, whereby about 455 g of microalgae can convert 816.5 g of carbon dioxide (CO2) from the atmosphere into oxygen [5]. The biomass of microalgae is relatively small compared to other plants, but it can produce up to more than 75% of the total oxygen required by the living things [4]. It also has one of the greatest growth rates, with biomass of microalgae increasing by double in just 13 h of cultivation, which can support a continuous supply of raw material for biofuel production [6]. Lipids are one of the major components in microalgae that make up between 5–50% of the microalgae dry weight [7]. It is worthwhile exploring the methods of lipid extraction from microalgae to ensure a sustainable supply and production of biofuel.
The long process of traditional lipid extraction using the Bligh and Dyer method or the Folch method, require extensive usage of hazardous chemicals that can have a negative impact to the environment and society. The mechanisms of the Bligh and Dyer method is to break the hydrogen bonds and electrostatic forces between protein and membrane lipid, allowing the lipid to escape from the microalgae cell [8], while the Folch method applies the partitioning of lipids in a biphasic mixture of chloroform and methanol, which disrupts the hydrogen bonds between lipids and proteins. The Bligh and Dyer and Folch methods are efficient and easy for extracting lipid from microalgae; however, these methods are not recommended to be used at the industrial level because it will require a large usage of toxic chemicals such as chloroform, which can increase the total cost of operation. Other options for more eco-friendly methods include supercritical CO2 extraction, Soxhlet solvent, expeller press, and liquid triphasic systems were introduced to mitigate the limitations of the traditional methods. Although these methods do not involve the usage of hazardous chemicals, the processes are time-consuming, high energy required, complicated processing and needs expensive operating equipment that will lead to a high overall production cost [8].
A recent work investigated the salting-out effect of lipid extraction using liquid biphasic flotation (LBF) systems [9], which was the first study to unveil the potential of LBF system in microalgae lipid recovery. Salting-out is a separation technique that uses salt as the mass separating agent (MSA) to ease the formation of two-phase system. This technique usually requires the use of high salt concentration that can activate unwanted chemical reactions, which may not be suitable for the extraction of biological molecules, and can cause an alteration of the pH value of the solution that can also lead to equipment corrosion and fouling [10].
Sugaring-out is a promising method for the recovery of biomolecules, as it can overcome the limitations of the salting-out method. The major advantages of the sugaring-out method include its eco-friendly and straightforward process, its cost-effectiveness, its low energy requirement, as the sugar acts as the agent of cell pre-treatment, and the fact that it is able to preserve the pH of the solution, the properties of the extractants and avoid equipment corrosion [10,11]. A study carried out by Abdallah et al. [11] investigated four modes of liquid–liquid extraction: dispersive liquid–liquid extraction, binary and ternary salting-out extraction, and sugaring-out liquid–liquid extraction. Of these four modes, sugaring-out liquid–liquid extraction showed the highest efficiency. Microalgae protein was successfully recovered using the sugaring-out method [10], sulfonamides from honey [12], pepsin, trypsin, and bovine serum albumin [13], metal ions such as platinum (IV), palladium (II) and rhodium (III) [14], and HIV drugs [15]. A comprehensive review of the success of sugaring-out liquid–liquid extraction has been performed by Fu et al. [16].
Based on past works and the literature review, there have been no studies on sugaring-out liquid–liquid extraction for lipid recovery from microalgae. Therefore, this study intends to fill in this knowledge gap, focusing on the concept of sugaring-out for the extraction using the LBF system. It represents a thorough study on the suitability of sugaring-out-LBF integrated system for lipid extraction with the objectives of investigating the optimum and effective conditions of the system, and understanding on the effectiveness of sugar as the agent of cell-disruption. This study could potentially open new opportunities for advanced technologies in microalgae biorefinery.
2. Materials and Methodology
2.1. Materials
The following chemicals and solvents used in this study were of analytical grade and purchased from R&M chemicals (Selangor, Malaysia): glucose, sucrose, fructose, maltose, acetonitrile, methanol, chloroform, buffer solution, sodium hydroxide, sodium nitrate, dipotassium phosphate, epsomite, citric acid, sodium carbonate, calcium chloride dihydrate, ammonium iron (III) citrate, ethylenediamine tetraacetic acid, boric acid, manganese (II) chloride tetrahydrate, zinc sulfate heptahydrate, sodium molybdate dihydrate, copper sulfate pentahydrate, cobalt (II) nitrate hexahydrate, n-hexane, and methyl nonadecanoate.
2.2. Cultivation and Harvesting of Microalgae Biomass
Previous literature reported high lipid productivity from Chlorella sorokiniana CY-1 [17]. Thus, this species of microalgae (Chlorella sorokiniana CY-1) was used in this study, supplied from the National Cheng Kung University, Taiwan. The Chlorella sorokiniana CY-1 was grown in a BG-11 medium. To reduce the risk of contamination on the cultivation, the transfer of the microalgae strain/broth into the BG-11 medium was performed in a laminar flow chamber. The first stage of the cultivation, also known as the pre-cultivation, was performed in a 250 mL photobioreactor (Semenyih, Malaysia). The growth of microalgae was monitored until constant biomass was achieved, by measuring the optical density of the sample at wavelength of 680 nm using UV–vis Spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) [9]. The microalgae from the pre-cultivation took 7 days to obtain constant biomass. The second stage of the cultivation is known as the batch cultivation, where larger volume of photobioreactor (1000 mL) was used for microalgae cultivation. BG-11 medium was prepared in the 1000 mL photobioreactor and 36 mL of microalgae broth from the pre-cultivation was transferred into the 1000 mL photobioreactor. The growth of the microalgae was monitored daily until it reached a constant concentration of microalgae, measured by UV–vis Spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) at 680 nm [9]. It took 10 to 14 days for the microalgae from the batch culture to achieve its maximum growth. The cultivation parameters were set at 450 rpm agitation, 200 µmol/m2/s and 400 mL/min continuous supply of artificial lights and CO2, respectively, in room temperature ranging from 23 °C and 25 °C. The microalgae biomass was separated from the broth using a centrifuge (Eppendorf, 5430, Selangor, Malaysia) set at 6500 rpm for 5 min. The supernatant was discarded. To allow a consistent ratio of biomass to water through the experiment, the pellet was placed in freeze dryer (Alpha 1–2 Ldplus, Christ, Osterode, Germany) for 24 h before dissolving into deionized water. The dried microalgae biomass was kept and preserved at −20 °C for later use.
2.3. Microalgae Preparation and Cell Disruption
Dry microalgae biomass was dissolved in 2 mL deionized water, then mixed directly with the sugar solution containing 250 g/L of glucose for lipid extraction. To understand the effectiveness of sugar as the agent of cell-disruption, the total lipid yield was compared with the total lipid recovery from a pre-treated microalgae cell.
Dry microalgae biomass was dissolved in 2 mL deionized water, instead of mixing it directly with sugar solution, the microalgae mixture was beforehand placed in the microwave oven (MC455THRCSR/SM, Samsung, Selangor, Malaysia) at the optimum parameters (90 s microwave radiation exposure, 450 W microwave power at 80% duty cycle) [9]. The pre-treated microalgae biomass was then transferred into the LBF system for lipid extraction.
2.4. Optimization of Operating Parameters
The optimum operating parameter was determined using the One-Variable-at-a-Time (OVAT) method whereby, one variable was tested at one time, keeping the other variables at constant (Table 1). The study was performed in a small-scale LBF system of 50 mL. The initial parameters were set to 25 mL of 250 g/L glucose, 25 mL of 100% acetonitrile, 1:1 bottom phase and top phase ratio, 5 min flotation time and 50% microalgae biomass concentration determined from the previous work [10,18,19].

Table 1.
The operating parameters of the system. All experiments were performed in triplicates.
2.5. Total Lipid Content
Formation of two-phase system achieved from the LBF system. The bottom phase consisted of MSA (sugar solution) with microalgae biomass, while the top phase consisted of acetonitrile with the extracted lipid. The top phase formed from the extraction was vaporized using a rotary evaporator (EYELA, Shanghai, China) at 80 °C with a vacuum pressure of 90 mbar. This was done to separate the acetonitrile solution from the extractants. The remaining contents consisting of the extracted lipid were added with 4 mL of 25% methanolic sodium hydroxide solution and left at 100 °C for 0.5 h [9]. 3 mL of chloroform: methanol mixture with 2:1 ratio was added after the sample cooled down at room temperature [20]. The sample was vigorously mixed for 2 min using a vortex and centrifuged for 10 min at 7400 rpm. The lipid layer was extracted and dried in ambient temperature for 24 h. The weight of dried lipid was noted.
2.6. Lipid Profile
The extracted lipid was transesterified into Fatty Acid Methyl Esters (FAMEs) as described in the previous study [7]. Then, 3 mL of methanol with 5% sulfuric acid was added into the sample and vortexed for 5 s before it was placed into a water bath for 3 h at 70 °C. The sample was cooled down to room temperature, n-hexane and milliQ water with 1:1 ratio was added into the sample and vortexed for 5 s with a continuous mix in a test tube rotator (Evergreen, Semenyih, Malaysia) for 15 min then centrifuged for 5 min at 7400 rpm. Then, 2 mL of the hexane layer was extracted and washed with deionized water and placed in a centrifuge (Eppendorf Asia Pacific, Selangor, Malaysia) for 5 min at 7400 rpm. The top layer was extracted to determine the lipid profile. The lipid profile was then analyzed using a gas chromatography (Perkin Elmer Clarus 690 GC, Perki-nElmer, Inc., Waltham, MA, USA) with a flame ionization detector (GC-FID) [9]. Then, 5 mg of methyl nonadecanoate was added to the sample as a control. The presence of methyl nonadecanoate acted as a reference to measure the detected FAME.
The samples were injected into Agilent Technologies analytical laboratory instrument column (InertCap Pure-WAX, GL Sciences, Tokyo, Japan) (30 m × 0.25 mm × id 0.25 µm) with helium at 3.0 mL/min for 3 min, at 35 °C. The temperature setting was gradually increased by 4 °C/min until it reached 220 °C and kept for 35 min. The temperature of the injector and detector were set at 250 °C and 260 °C respectively. Continuous flow of helium gas was set at 103.4 kPa [9].
2.7. Statistical Analysis
IBM SPSS Statistic version 26 analytical software was used to understand the relationship between the variables using the Pearson’s correlation coefficient analysis.
3. Results and Discussion
3.1. Optimum Conditions of Sugaring-Out Assisted LBF System
3.1.1. Effect of Types of Mass Separating Agent
The types of MSA, such as glucose, sucrose, fructose and maltose, play a major role in the sugaring-out liquid–liquid extraction method. The process of extraction occurs when there is an interaction between acetonitrile-water and sugar molecules, allowing the formation of the two-phase system. The formation of the two-phase system is driven by the hydrogen bonding and dipole–dipole interaction by the water molecule that supports the formation of a three dimensional cluster structure that enclosed the sugar and water molecules together [10]. From this study, all four types of the MSA used were able to form a two-phase system with acetonitrile. Sankaran et al. [10] noted a similar observation on the effect of the sugaring-out method for microalgae protein extraction. The discovery of the sugaring-out extraction method was made by Wang et al. [21], who observed instant formation of two-phase system when acetonitrile was added into sugar solution. The results showed that 95% (w/w) acetonitrile remained in the top phase, while the water molecules immersed to the lower phase, to support the formation of hydrogen bonding with the glucose molecule, accomplishing the two-phase system. Thus, from the observation of this study, the formation of the two-phase system suggests that all types of the MSA (glucose, sucrose, fructose and maltose) used can form hydrogen boding with the water molecule.
Sucrose and maltose are disaccharides that comprise of two monosaccharides bonded together, while fructose and glucose are monosaccharides, made of a single simple sugar unit that cannot be broken down into simpler sugar units. These types of sugar can influence the transport of targeted compound from the bottom phase to the top phase [18]. From the statistical analysis, all data collected for different types of MSA indicated p-value < 0.05. The observations of the study showed that fructose had the highest tendency to recover lipid from microalgae, followed by sucrose, glucose and maltose, with lipid yields of 16.17%, 16.03%, 12.49% and 11.37%, respectively (Figure 1).
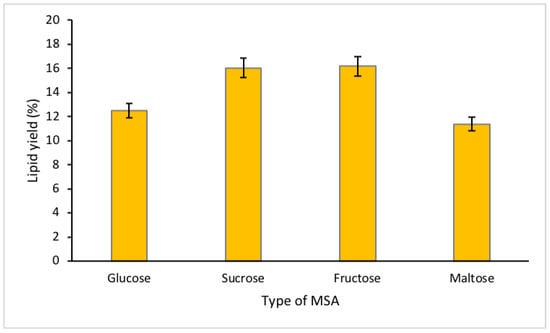
Figure 1.
Percentage of lipid yield for different types of sugar.
As mentioned above, sucrose is made of two monosaccharides. This combination of monosaccharides consisted of one fructose and glucose molecules. Fructose is categorized as a ketonic simple sugar due to the presence of ketone groups, while glucose is known as an aldose simple sugar, due to the presence of aldehyde groups in the molecular chain [18]. The presence of these two groups makes the MSA solutions highly polar, driven by the high electronegativity of the oxygen atom in C=O [22]. Lipid is a non-polar molecule, which may form the weakest chemical interaction (van der Waals forces) among the other compounds in the microalgae cell, such as protein, astaxanthin, lutein and chlorophylls. The highly electronegative oxygen atoms from the ketone and aldehyde may be more attracted to these polar compounds, forming a permanent dipole–dipole moment or hydrogen bonding in the bottom phase (Figure 2). Ketone has a slightly greater electronegativity than aldehyde [23], which may allow it to have stronger chemical interaction with the polar compounds, allowing the transport of non-polar lipids to the top phase through the air bubbles generated from the LBF system. Due to the presence of the ketone group, fructose and sucrose recovered the most lipid. A few other studies have observed highest protein recovery with glucose, this may due to weaker electronegativity of aldehyde compared to the ketone group, allowing high concentrations of proteins in the top phase [10,18]. Due to the emphasis on higher lipid recovery, fructose was used for the subsequent experiments.
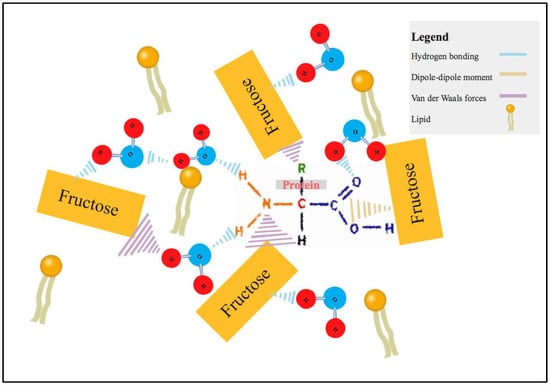
Figure 2.
Chemical interaction of protein with fructose and water molecule. The chemical clusters of protein with these molecules allow lipid to move from the bottom phase to the top phase of the LBF system.
3.1.2. Effect of Concentration of Mass Separating Agent
Similar to the salting-out two-phase extraction method, the concentration of the MSA plays a vital part in forming separation phase. In this study, the different concentration of fructose was used to understand the optimum MSA concentration for lipid recovery. The extraction of lipid in this study was carried out in an ambient room temperature to minimize the expenditure cost and extensive energy requirement. Wang et al. [21] observed the formation of instant separation phase when 50 g/L of glucose was added into 1:1 ACN-water solution ratio at 1 °C. The study claimed that higher sugar concentration is required in order to allow the formation two-phase layer in a system of higher temperature [21]. Therefore, it is important to understand the optimum concentration of sugar needed to form two phases in a system of a temperature between 26–28 °C to allow maximum lipid recovery.
The concentrations of fructose were set to 50 g/L, 100 g/L, 150 g/L, 200 g/L, and 250 g/L, with 250 g/L as the initial concentration, determined from the previous study of optimum lipid recovery [9]. From the statistical analysis, all data collected for different concentrations of MSA indicated a p-value < 0.05. This study observed that sugar concentration of <50 g/L hinders the formation of separation phase, that support the claim by Wang et al. [21] of higher temperature system (26–28 °C) requires higher sugar concentration. The low sugar concentration (<50 g/L) may have caused the system to become unstable, leading to the unwanted mixing of the bottom and top phase solutions, which halts the occurrence of lipid extraction and separation processes [9].
Lipid yield was comparatively low using 200 g/L and 250 g/L of fructose, which can only recover 29.27–30.70% of lipids compared to using 100 g/L, and 150 g/L concentrations of fructose (Figure 3). The presence of sugar molecules in the bottom phase can contribute to the breaking of the microalgae cell wall, easing the release of bioactive compound into the bottom phase [18]. However, high concentration of fructose failed to recover high lipid yield, instead, 150 g/L of fructose concentration was seen to recover the highest lipid yield (54.06%) compared to the other concentration levels. The systems consisted of fructose concentration higher than 150 g/L may have lower content of water molecule than the fructose concentration of <150 g/L [18]. Therefore, it is sensible to say that lower water content may inhibit maximum release of bioactive compounds, as the medium is rich in fructose ions/molecules that can reduce the dispersion capacity of the bioactive compounds in the lower phase and vice versa [18].
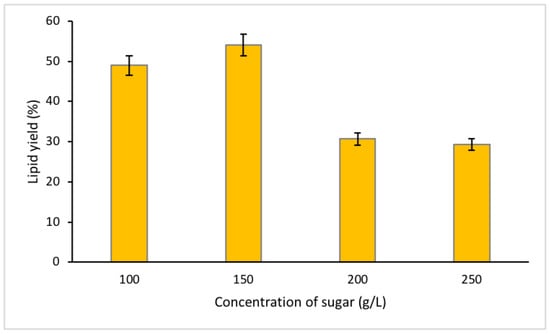
Figure 3.
Percentage of lipid yield for different concentrations of sugar.
3.1.3. Effect of Liquid Biphasic Volume Ratio
Due to exhibiting the highest lipid recovery, 150 g/L fructose concentration was selected for the following experiment, to understand the effect of volume ratio of the system on lipid recovery. This is a crucial step for understanding the most favorable ratio needed to excrete lipid from the microalgae biomass to ensure optimum lipid yield. Increasing volume ratio of acetonitrile could possibly improve the recovery of bioactive compounds [18]. In this study, the total working volume of 50 mL remained the same, while the ratio of the MSA solution to acetonitrile was adjusted to 1:0.5/1:0.75/1:1/1:1.25/1:1.50/1:1.75/1:2.0/1:2.5/1:2.75 (volume of bottom phase to volume of top phase). The initial volume ratio of 1:1 was determined from the previous study of optimal lipid recovery from Chlorella sorokiniana CY-1 [9].
The amount of lipid recovery peaked at a ratio of 1:2.0, with approximately 30% lipid yield. The observation of lipid recovery showed a gradually increasing trend when increasing the working volume of acetonitrile from a ratio of 1:0.5 to 1:2.0, but decreasing when altering the ratio from 1:2.0 to 1:2.75 ratio (Figure 4). This demonstrates the importance of understanding the optimum ratio value to ensure maximum lipid yield. Increasing the acetonitrile working volume may increase the capacity of the system to extract lipid as the medium of the top phase will increase, thus allowing higher retention of lipid extractant. The decreasing trend of lipid yield from 1:2.0 to 1:2.75 ratio may be explained by looking at the total working volume of sugar solution [24]. The decreasing trend of lipid yield from 1:2.0 to 1:2.75 ratio may be due to smaller working volume of the sugar solution. The result of this study showed a similar pattern to the study carried out by Chia et al. [18] on protein recovery. The working volume of sugar solution decreases with increasing working volume of acetonitrile, which may inhibit the dispersion capacity of the bioactive compounds that can reduce the maximum release of bioactive compounds [18,24]. Additionally, from that statistical analysis, this study observed no distinctive difference (p-value > 0.05) in the yield of lipid between ratio 1:2.0 to 1:2.75. This indicates that the full lipid content has been released from the microalgae biomass.
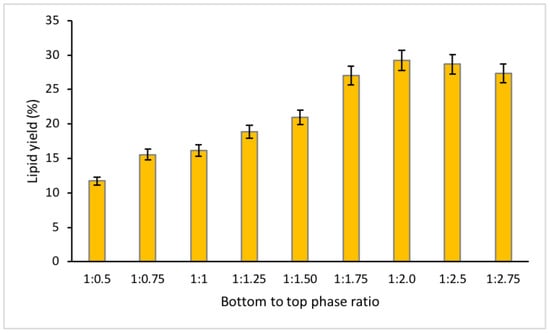
Figure 4.
Percentage of lipid recovered with different bottom to top phase ratio.
3.1.4. Effect of Flotation Time
Understanding the function of flotation time in the LBF system is important to determine the effective conditions for maximum lipid extraction. The mechanisms of the LBF system are greatly influenced by the transport of biomolecules through air bubbles from the lower phase to the upper phase [25]. Flotation time is a period of continuous supply of air bubbles in the system. The air bubbles have the ability to attract the hydrophobic non-polar tails of the biomolecules, leaving the hydrophilic polar heads on the surface of the air bubbles [9]. Without the air bubbles, the biomolecules will likely remain in the bottom phase. Therefore, flotation time is one of the crucial parameters that needed to be investigated in this study.
Several works have suggested that longer flotation time can increase the time of exposure of the biomolecules with the air bubbles that could increase the yield of the extractant [26,27]. However, a different trend was seen in this study, which observed the highest lipid recovery at flotation time of 15 min followed by 20 min with the lowest lipid yield at 10 min of approximately 62%, 58% and 48% respectively (Figure 5). All data collected for flotation time expressed p-value < 0.05. The observation of this study abides the claim by Koyande et al. [28] and Mat Aron et al. [9] that the duration of the interaction of the biomolecules with the air bubbles may not necessarily result in higher recovery of the biomolecules. The process of extraction may not be efficient at times of 5 min and 10 min due to insufficient separation time. However, the bubbles generated from the air pump may have caused agitation in the system, allowing a few biomolecules to move from the bottom phase to the top phase. The results of the lipid yield under the same conditions may vary in each factor which is due to the inconsistent composition of microalgae, where each batch may contain more or less of the lipid content within the cells.
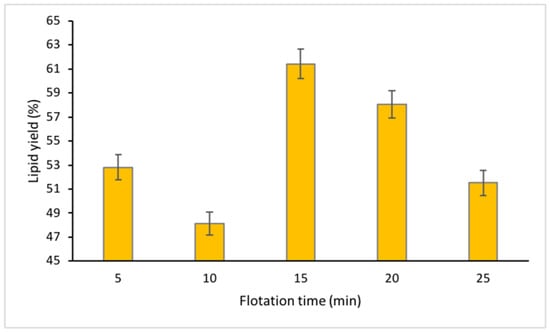
Figure 5.
Percentage of lipid recovered at different flotation time.
3.2. Microalgae Cell Disruption
Microalgae cells are surrounded by rigid cell walls constituted of polysaccharides and/or glycoproteins that restrict the release of bioactive compounds [18]. Deployment of cell disruption to the cell wall is important to break free of the bioactive compound. Cell disruption can be classified into three categories: physical, chemical and biological [29]. Recent studies have shown physical pre-treatment, such as exposure of the microalgae cell to microwave radiation, can lead to promising release of lipids that is sufficient for the recovery process [9,30,31,32]. However, such pre-treatment requires high energy input that disregards the sustainability and economic feasibility of the extraction process of lipid.
One of the promising methods for tackling energy intensiveness is the use of organic solvents as the means of cell disruption. The principle of the process involves dissolving the hydrophobic cell membrane of the microalgae that subsequently strike the lipid storage in the cell [33]. The most reliable and most common method using organic solvents for direct cell disruption and lipid extraction is the Bligh–Dyer method. The organic solvent functions as the source of the electrostatic forces and a hydrogen bond breakage between membrane lipid and integrated protein, followed by the ingress of non-polar component of the solvent into the cell to allow the extraction of natural lipid [33]. The Bligh–Dyer method, however, involves the use of highly toxic chemicals that are harmful to the environment and the health of living organisms. Therefore, in this study, a sugar solution was used as the organic solvent to overcome the limitation of the use of hazardous chemicals and the energy-intensive nature of physical methods of cell disruption.
This section focuses on the total lipid recovery using two different methods of pre-treatment: microwave and organic solvent (fructose solution). This is done to understand the effectiveness of the fructose solution acting as the agent of microalgae cell wall rupture. The optimal parameters described above were used in subsequent experiments. For microwave pre-treatment, the microalgae cell wall was distorted to 450 W microwave power for 90 s at 80% duty cycle [9]. The disrupted microalgae cells were then transferred into the LBF system. Meanwhile, for organic solvent pre-treatment, the microalgae was placed directly into the LBF system. All data collected from these two techniques expressed p-value < 0.05. In this study, a slightly greater recovery of lipids was observed from microwave-pre-treated microalgae cell. Disruption of microalgae cell using the organic solvent, however, showed promising performance in lipid recovery. Comparing the recovery of lipids between microwave and organic solvent cell disruptions, pre-treatment of microalgae using the organic solvent (fructose solution) resulted in only 2% lower of lipid yield. The organic solvent and microwave–pre-treated microalgae recovered about 74% and 76% of lipid yield, respectively. Although a slightly lower lipid recovery yield was obtained, the results were comparable with microwave pre-treatment. Furthermore, the use of fructose solution as the agent of cell disruption could reduce the energy requirement of the whole process that aligns with sustainable lipid extraction.
3.3. Lipid Profile
Microwave pre-treated and non-microwave pre-treated microalgae cells were used in this study to determine suitable candidates for biofuel production. Both of the lipids extracted from the microalgae cells were analyzed using the GC-FID to confirm the presence of lipid, and to subsequently understand the lipid profile. Min-Xi Wan et al. carried out a study on lipid extraction on Chlorella Sorokiniana using the Bligh and Dyer method. The study recovered 16 and 18 hydrocarbon chains of FAME [34]. The study reported the generation of two significant peaks of FAME at C5:0 and C14:0. FAME of both C5:0 and C14:0 were detected in microwave pre-treated and non-microwave pre-treated microalgae cells. The typical size of hydrocarbon chain for biofuel production ranges between 8 to 21-carbon molecule [9]. From the study, microwave pre-treated microalgae cell managed to extract a slightly higher percentage of C5:0 and C14:0 but comparable with the non-microwave pre-treated microalgae cells.
The size of the carbon chain can determine the cetane number that influences the biofuel flammability. Higher cetane numbers allow faster ignition, which supports smoother combustion process and has higher flammability efficiency [35]. The cetane number should at least be 47 and 51 to meet the minimum United States (US) and European Union (EU) fuel quality standards, respectively [36]. Giakoumis et al. [36] summarized the cetane numbers of hydrocarbon chain of different lengths. From the study, the authors found the average cetane number of C14:0 was 71.9, which meets both the US and EU fuel quality standards.
However, C5:0 was observed to be the dominant component detected by the GC-FID of about 90.6% coverage, followed by C14:0. This study suggests that extraction of lipid using the LBF-sugaring-out method is more suitable for recovering shorter carbon chains of FAME. FAME of C5:0 has the characteristics of fruity odour, colourless at room temperature and low solubility in water that makes them suitable for the making of perfume and food-flavoring [37]. Apart from that, this carbon chain of FAME acts as an excellent surrogate fuel. Surrogate fuel is a mixture of one or more simple fuel that is intended to mimic the physical properties of the combustion of complex fuel, improving the ignition capacity [37]. The cetane number of surrogate fuels depends on the mixture of the one or more simple fuels. A study by Al-Esawi and Al Qubeissi reported up to 60.9 cetane number of surrogate fuels [38]. Thus, the type of FAME extracted using the method of sugaring-out-assisted LBF is not only suitable for biofuel production, but also appropriate for the perfume and food-flavoring industry.
4. Conclusions
This study is the first to investigate the potential of integrating LBF systems with the sugaring-out method in the recovery of microalgae lipids. The highest lipid recovery yield was obtained using fructose as the mass separating agent with the solution concentration of 150 g/L, at 15 min with volume ratio of 1:2.0. Apart from that, in this study, the effectiveness of sugar as the agent of cell-disruption was also observed, whereby the amount of lipid extracted was comparable to that extracted from microwave pre-treated microalgae cell. Both microwave pre-treated and non-microwave pre-treated microalgae cells managed to recover C5:0 and C14:0 hydrocarbons in FAME from Chlorella sorokiniana CY-1, with C5:0 being the dominant component, which favors not only the biofuel industry but also the perfume and food-flavoring industries.
Author Contributions
N.S.M.A.: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft. K.W.C.: Conceptualization, Formal analysis, Methodology, Project administration, Writing—original draft. Z.M. and Y.T.: Methodology, Supervision, Writing—review and editing. M.S.: Formal analysis, Visualization. I.S.T. and C.N.M.: Visualization, Writing—review and editing. A.X.: Methodology, Visualization. T.A.K.: Conceptualization, Writing—review and editing. P.L.S.: Funding acquisition, Project administration, Resources, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Grant Scheme, Malaysia [FRGS/1/2019/STG05/UNIM/02/2] and MyPAIR-PHC-Hibiscus Grant [MyPAIR/1/2020/STG05/UNIM/1].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing interests of financial, personal, or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, the work.
References
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels—A review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- EPA, U.S.E.P.A. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2018; U.S.E.P.A.: Washington, DC, USA, 2020; Volume 76.
- Aron, N.S.M.; Khoo, K.S.; Chew, K.W.; Veeramuthu, A.; Chang, J.-S.; Show, P.L. Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies. J. Water Process Eng. 2020, 39, 101701. [Google Scholar] [CrossRef]
- Elrayies, G.M. Microalgae: Prospects for greener future buildings. Renew. Sustain. Energy Rev. 2018, 81, 1175–1191. [Google Scholar] [CrossRef]
- Massimi, R.; Kirkwood, A.E. Screening microalgae isolated from urban storm- and wastewater systems as feedstock for biofuel. PeerJ 2016, 4, e2396. [Google Scholar] [CrossRef]
- Breuer, G.; Evers, W.A.C.; de Vree, J.H.; Kleinegris, D.M.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Analysis of fatty acid content and composition in microalgae. J. Vis. Exp. 2013, 5, e50628. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Leong, W.H.; Chai, Y.H.; Show, P.L.; Chen, W.H. Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresour. Technol. 2020, 304, 122996. [Google Scholar] [CrossRef]
- Mat Aron, N.S.; Chew, K.W.; Ang, W.L.; Ratchahat, S.; Rinklebe, J.; Show, P.L. Recovery of microalgae biodiesel using liquid biphasic flotation system. Fuel 2022, 317, 123368. [Google Scholar] [CrossRef]
- Sankaran, R.; Manickam, S.; Yap, Y.J.; Ling, T.C.; Chang, J.S.; Show, P.L. Extraction of proteins from microalgae using integrated method of sugaring-out assisted liquid biphasic flotation (LBF) and ultrasound. Ultrason. Sonochem. 2018, 48, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, I.A.; Hammad, S.F.; Bedair, A.; Mansour, F.R. Sugaring-out induced homogeneous liquid-liquid microextraction as an alternative mode for biological sample preparation: A comparative study. J. Sep. Sci. 2021, 44, 3117–3125. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chuang, H.Y.; Chen, H.H.; Wu, Y.W.; Cheng, S.H.; Huang, T.C. Application of sugaring-out extraction for the determination of sulfonamides in honey by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 2010, 1217, 7812–7815. [Google Scholar] [CrossRef]
- Dhamole, P.B.; Mahajan, P.; Feng, H. Sugaring out: A new method for removal of acetonitrile from preparative RP-HPLC eluent for protein purification. Process Biochem. 2010, 45, 1672–1676. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, K.; Yu, P.; Liu, H. Sugaring-out three-liquid-phase extraction and one-step separation of Pt(IV), Pd(II) and Rh(III). Sep. Purif. Technol. 2012, 87, 127–134. [Google Scholar] [CrossRef]
- Zhang, J.; Myasein, F.; Wu, H.; El-Shourbagy, T.A. Sugaring-out assisted liquid/liquid extraction with acetonitrile for bioanalysis using liquid chromatography-mass spectrometry. Microchem. J. 2013, 108, 198–202. [Google Scholar] [CrossRef]
- Fu, C.; Li, Z.; Sun, Z.; Xie, S. A review of salting-out effect and sugaring-out effect: Driving forces for novel liquid-liquid extraction of biofuels and biochemicals. Front. Chem. Sci. Eng. 2021, 15, 854–871. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Chia, S.R.; Chew, K.W.; Show, P.L.; Sivakumar, M.; Ling, T.C.; Tao, Y. Isolation of protein from Chlorella sorokiniana CY1 using liquid biphasic flotation assisted with sonication through sugaring-out effect. J. Oceanol. Limnol. 2019, 37, 898–908. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Lim, J.W.; Lee, S.Y.; Lam, M.K.; Show, P.L. Optimization of Protein Extraction from Chlorella Vulgaris via Novel Sugaring-out Assisted Liquid Biphasic Electric Flotation System. Eng. Life Sci. 2019, 19, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Yew, G.Y.; Chew, K.W.; Malek, M.A.; Ho, Y.C.; Chen, W.H.; Ling, T.C.; Show, P.L. Hybrid liquid biphasic system for cell disruption and simultaneous lipid extraction from microalgae Chlorella sorokiniana CY-1 for biofuel production. Biotechnol. Biofuels 2019, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ezejias, T.; Feng, H.; Blaschek, H. Sugaring-out: A novel phase separation and extraction system. Chem. Eng. Sci. 2008, 63, 2595–2600. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. Structure of Organic Compounds, 1st ed.; Ouellette, R.J., Rawn, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128024447. [Google Scholar]
- Morsch, L.; Andrews, M. Che 269 (Morsch and Andrews), 1st ed.; Morsch, L., Andrews, M., Eds.; Springfield: Geneseo, IL, USA, 2019; Volume 269. [Google Scholar]
- Naveena, B.; Armshaw, P.; Tony Pembroke, J. Ultrasonic intensification as a tool for enhanced microbial biofuel yields. Biotechnol. Biofuels 2015, 8, 140. [Google Scholar] [CrossRef]
- Sankaran, R.; Parra Cruz, R.A.; Show, P.L.; Haw, C.Y.; Lai, S.H.; Ng, E.P.; Ling, T.C. Recent advances of aqueous two-phase flotation system for the recovery of biomolecules. Fluid Phase Equilibria 2019, 501, 112271. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Teh, W.H.; Teh, T.X.; Lim, H.M.Y.; Nazri, N.S.B.; Tan, C.H.; Chang, J.S.; Ling, T.C. Proteins recovery from wet microalgae using liquid biphasic flotation (LBF). Bioresour. Technol. 2017, 244, 1329–1336. [Google Scholar] [CrossRef]
- Show, P.L.; Tan, C.P.; Anuar, M.S.; Ariff, A.; Yusof, Y.A.; Chen, S.K.; Ling, T.C. Direct recovery of lipase derived from Burkholderia cepacia in recycling aqueous two-phase flotation. Sep. Purif. Technol. 2011, 80, 577–584. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Show, P.L.; Munawaroh, H.S.H.; Chang, J.S. Liquid triphasic systems as sustainable downstream processing of Chlorella sp. biorefinery for potential biofuels and feed production. Bioresour. Technol. 2021, 333, 125075. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Mat Aron, N.S.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R.; Chew, K.W.; Show, P.L. A review on bioconversion processes for hydrogen production from agro-industrial residues. Int. J. Hydrogen Energy 2021, 47, 37302–37320. [Google Scholar] [CrossRef]
- Dai, Y.M.; Chen, K.T.; Chen, C.C. Study of the microwave lipid extraction from microalgae for biodiesel production. Chem. Eng. J. 2014, 250, 267–273. [Google Scholar] [CrossRef]
- Khoomrung, S.; Chumnanpuen, P.; Jansa-Ard, S.; Staìšhlman, M.; Nookaew, I.; Borén, J.; Nielsen, J. Rapid quantification of yeast lipid using microwave-assisted total lipid extraction and HPLC-CAD. Anal. Chem. 2013, 85, 4912–4919. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, W.; Tu, R.; Guo, Q.; Han, S.F.; Chen, C.; Wang, Q.; Liu, W.; Jensen, P.D.; Wang, Q. Optimization of microwave assisted lipid extraction from microalga Scenedesmus obliquus grown on municipal wastewater. J. Clean. Prod. 2019, 221, 502–508. [Google Scholar] [CrossRef]
- Nagappan, S.; Devendran, S.; Tsai, P.C.; Dinakaran, S.; Dahms, H.U.; Ponnusamy, V.K. Passive cell disruption lipid extraction methods of microalgae for biofuel production—A review. Fuel 2019, 252, 699–709. [Google Scholar] [CrossRef]
- Wan, M.X.; Wang, R.M.; Xia, J.L.; Rosenberg, J.N.; Nie, Z.Y.; Kobayashi, N.; Oyler, G.A.; Betenbaugh, M.J. Physiological evaluation of a new Chlorella sorokiniana isolate for its biomass production and lipid accumulation in photoautotrophic and heterotrophic cultures. Biotechnol. Bioeng. 2012, 109, 1958–1964. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.W. Fuel characteristics of biodiesel produced from a high-acid oil from soybean soapstock by supercritical-methanol transesterification. Energies 2012, 5, 2370–2380. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. A comparative assessment of biodiesel cetane number predictive correlations based on fatty acid composition. Energies 2019, 12, 422. [Google Scholar] [CrossRef]
- Djojoputro, H.; Ismadji, S. Density and viscosity of binary mixtures of ethyl-2-methylbutyrate and ethyl hexanoate with methanol, ethanol, and 1-propanol at (293.15, 303.15, and 313.15) K. J. Chem. Eng. Data 2005, 50, 1343–1347. [Google Scholar] [CrossRef]
- Al-Esawi, N.; Al Qubeissi, M. A new approach to formulation of complex fuel surrogates. Fuel 2021, 283, 118923. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).