Abstract
Citric acid production is generally carried out in an aqueous medium in stirred tank reactors (STR), where the solubility of oxygen is low and the oxygen demand of microbial cultures is high. Thus, for this bioprocess, providing adequate oxygen mass transfer rate (OTR) from the gas phase into the aqueous culture medium is the main challenge of bioreactor selection and operation. In this study, citric acid production by Yarrowia lipolytica W29 from crude glycerol, in batch cultures, was performed in two non-conventional bioreactors normally associated with high mass transfer efficiency: a pressurized STR and an airlift bioreactor. Increased OTR was obtained by raising the total air pressure in the pressurized STR and by increasing the aeration rate in the airlift bioreactor. An improvement of 40% in maximum citric acid titer was obtained by raising the air pressure from 1 bar to 2 bar, whereas, in the airlift bioreactor, a 30% improvement was attained by increasing the aeration rate from 1 vvm to 1.5 vvm. Both bioreactor types can be successfully applied for the citric acid production process using alternative ways of improving OTR than increasing mechanical stirring power input, thus leading to important operating saving costs.
1. Introduction
Citric acid, an intermediate in the tricarboxylic acid cycle, is extensively used in the pharmaceutical, food, detergent, biomedical, textile, and leather industries. The global market of citric acid will reach approximately EUR 4 billion by 2024 [1]. Originally produced by Aspergillus niger, essentially from molasses, it can also be obtained by Yarrowia lipolytica cultures [2,3]. This yeast secretes citric acid from several carbon sources, such as glucose, glycerol, sunflower oil, rapeseed oil, and ethanol. Besides pure substrates, Y. lipolytica is also able to metabolize crude glycerol—a low-cost by-product from the biodiesel industry—and produce several added-value compounds (organic acids, acetic acid, erythritol, mannitol, 1,3-propanediol, and poly(3-hydroxybutyrate)) and accumulating microbial lipids [4,5,6,7,8]. The production of citric acid by Y. lipolytica occurs under specific conditions, nitrogen limitation being the most important one [9]. Furthermore, pH, medium composition, and oxygen availability are important parameters that affect citric acid production by this yeast [6,10,11,12,13]. As citric acid production by Y. lipolytica is an aerobic process, oxygen is a crucial factor for the maximization of microbial growth and product formation [6,12,13]. The oxygen mass transfer from the gas phase to the liquid medium and the amount of oxygen available to the cells can directly affect the quantity and the type of organic acids produced [6,13].
The most common type of bioreactor used in citric acid production by Y. lipolytica is a stirred tank reactor (STR). Some disadvantages have been associated with traditional stirred tanks: (a) wide variation of shear forces inside the reactor, once the energy required to move the fluid is introduced into a single point of the reactor, which results in a higher dissipation near the stirrer and decreases from it towards the walls [14]; (b) due to a low oxygen mass transfer coefficient, high stirring rates are required to achieve a sufficient oxygen mass transfer; (c) the high mechanical power input usually results in overheating; (d) the increase of mechanical power input generates high shear stress that can damage [15] or change the cells’ morphology [16]; (e) owing to its complexity, STR bioreactors are more expensive, require higher maintenance costs, and are less robust than other types of reactors [16]. Considering these negative aspects of traditional STR bioreactors and the limitation of oxygen mass transfer that can occur at atmospheric pressure, other alternatives must be considered, such as pressurized and airlift bioreactors [17,18].
Pressurized reactors are of great interest to enhancing the oxygen mass transfer rate (OTR) from the gas phase to the liquid medium [19]. In these bioreactors, the enhancement of OTR is achieved by the increase in total air pressure and consequently, in oxygen partial pressure, leading to the increase in oxygen solubility [18]. High-pressure reactors are broadly used in the chemical industry, and the high mass transfer capacity and cost efficiency open new opportunities to adapt them to microbial cultures technology [20]. Published studies have already proven that pressurized bioreactors could be successfully applied to microorganism cultivation. Several authors have demonstrated the applicability of increased air pressure (up to 15 bar) for biomass production and metabolite secretion enhancement, such as extracellular lipase, homologous β-galactosidase, and heterologous proteins [18,21]. Moreover, it was shown that, when high OTR values are needed, the increase in air pressure could be a way of improving OTR, with energy cost efficiencies acceptable for industrial applications [20].
Airlift bioreactors are pneumatically agitated with unique hydrodynamic characteristics and often employed in bioprocesses wherein the gas–liquid mass transfer is an important parameter. This type of bioreactor presents some advantages compared with conventional STR: (a) uniform shear distribution; (b) high liquid velocity and intensity of turbulence, which allows an increase in heat transfer capacity, mass transfer rate, and good mixing properties at low energy consumption; (c) both the aeration and agitation of production medium are due to the gas phase; (d) low shear stress [22]. Studies conducted in airlift bioreactors showed the great potential of this type of bioreactor for the development of bioprocesses based on Y. lipolytica, namely the biotransformation of methyl ricinoleate and castor oil into lactones [16,23] and citric acid production with immobilized cells [24,25]. However, no recent works regarding the use of airlift bioreactors for Y. lipolytica cultivation are found in the literature.
In this work, the production of citric acid in batch cultures by Y. lipolytica W29 from crude glycerol was studied in two bioreactor types—pressurized STR and airlift bioreactors. To our best knowledge, this is the first time that citric acid production is evaluated in a pressurized bioreactor under increased air pressure or in an airlift bioreactor with suspended cells. Thus, in this work, the effect of increased air pressure (in a pressurized reactor) and aeration rate (in an airlift reactor) on citric acid production was evaluated.
2. Materials and Methods
2.1. Microorganism and Culture Conditions
Yarrowia lipolytica W29 (ATCC 20460) was maintained in YPDA medium (yeast extract 10 g∙L−1, peptone 20 g∙L−1, glucose 20 g∙L−1, and agar 20 g∙L−1) at 4 °C for a maximum of 2 weeks. Cells of Y. lipolytica were pre-grown in 500 mL Erlenmeyer flasks with 200 mL of medium (pure glycerol 20 g∙L−1, peptone 20 g∙L−1, and yeast extract 10 g·L−1) for 18 h, at 27 °C in an orbital incubator at 200 rpm.
2.2. Pressurized Bioreactor
To evaluate the effect of increased air pressure on citric acid production, several batch cultures were performed in a stainless stirred tank bioreactor (PARR 4563, Parr Instruments, Moline, IL, USA) with 600 mL of capacity and a working volume of 400 mL. The production medium had the following composition (g∙L−1): crude glycerol 20; yeast extract 0.5; MgSO4∙H2O 1.5; KH2PO4 24; Na2HPO4 2; salts solution (CaCl2 0.75; FeCl3∙6H2O 0.75; ZnSO4∙7H2O 0.1; MnSO4∙H2O 0.3). Batch cultures started with 0.5 g∙L−1 of cells and were performed at 27 °C, 400 rpm, and initial pH of 5. Compressed air was continuously sparged into the culture at an aeration rate of 1 vvm (under standard temperature and pressure conditions). The reactor pressure was set by manipulating inlet air pressure and the regulatory valve in the exit gas line. The bioreactor was equipped with a pressure transducer (Parr 4842, Parr Instruments, Moline, IL, USA) to monitor the total internal pressure. The values of total air pressure studied were 1 bar, 2 bar, and 4 bar.
2.3. Airlift Bioreactor
Several experiments were carried out in an airlift bioreactor, varying the aeration rate, and evaluating its effect on citric acid production. The airlift bioreactor was constructed in glass with a working volume of 4 L and an inside diameter of 0.7 m. The riser tube had 0.37 m of height and an inside diameter of 0.032 m. Air was used as the gas stream in the gas–liquid contactor, and it was fed at the bottom of the bioreactor using a five-hole sparger. The dissolved oxygen concentration (DO) in the medium was measured with a polarographic-membrane probe and monitored with a computer interface (CIODAS08JR, Computer Boards, Huntington Beach, CA, USA) using the LABtech Notebook software (Datalab Solution, Germantown, MD, USA).
After pre-growth overnight, yeast cells were collected and transferred to the production medium composed of (g∙L−1): crude glycerol 50; yeast extract 0.5; MgSO4 H2O 1.5; KH2PO4 6; Na2HPO4 0.5; salts solution (CaCl2 0.75; FeCl3∙6H2O 0.75; ZnSO4∙7H2O 0.1; MnSO4∙H2O 0.3). The medium was inoculated with 1.5 g·L−1 of cells, and the experiments were performed at 27 °C and a controlled pH of 5.0.
2.4. OTR Calculation
For the pressurized bioreactor, OTR values were estimated by the sulfite oxidation method [26], which measures the maximum possible value of OTR at the operating conditions used. The static gassing-out technique was used to determine OTR in the airlift bioreactor [6].
2.5. Analytical Methods
Samples were collected for the determination of biomass concentration (optical density at 600 nm and converted to dry cell weight (g L−1)), glycerol consumption, and citric acid production. Glycerol concentration was measured by high-performance liquid chromatography (HPLC) using a Metacarb 87H (Varian) column (300 mm × 7.7 mm) and a RI detector. The column was eluted with H2SO4 0.005 M at a flow rate of 0.5 mL·min−1, and the temperature was maintained at 60 °C. The citric acid quantification by HPLC was performed using a YMC ODS-Aq (250 × 4.6 mm) reverse-phase column and a UV detector (214 nm). The eluent was KH2PO4 0.02 M, pH 2.8 at a 0.7 mL·min−1 flow rate, and the column was maintained at room temperature.
2.6. Statistical Analysis
One-way analysis of variance (ANOVA) was performed, and Tukey’s test was used to detect significant differences among means (p < 0.05). All analyses were performed in GraphPad Prism 7 software (Dotmatics, San Diego, CA, USA).
3. Results and Discussion
3.1. Effect of Operating Conditions on OTR
For a specific bioreactor and culture medium, the increase in the aeration rate (airflow rate), stirring rate, and oxygen solubility in the medium results in an OTR enhancement. Increasing air pressure from 1 bar to 4 bar led to a 2-fold improvement in OTR (Figure 1). This result is in accordance with Henry’s law, in which increases in air pressure increase the oxygen solubility in the medium and consequently improve OTR [18]. In the airlift bioreactor, the airflow rate promotes aeration and stirring; thus, OTR increases with the aeration rate [17]. The increase in the aeration rate from 1 vvm to 2 vvm led to a 2.5-fold OTR improvement (Figure 1). In the pressurized bioreactor, OTR values were higher than in the airlift bioreactor at operating conditions used on each bioreactor. This shows that an air pressure increase may be used to obtain high OTR values. Nevertheless, it must be stressed that the OTR obtained by the sulfite method may be overestimated, mainly due to the differences in physicochemical properties between the aqueous sulfite solution and the cultivation medium used in the static method, which affects bubbles’ coalescence and consequently the interfacial area for mass transfer [27].
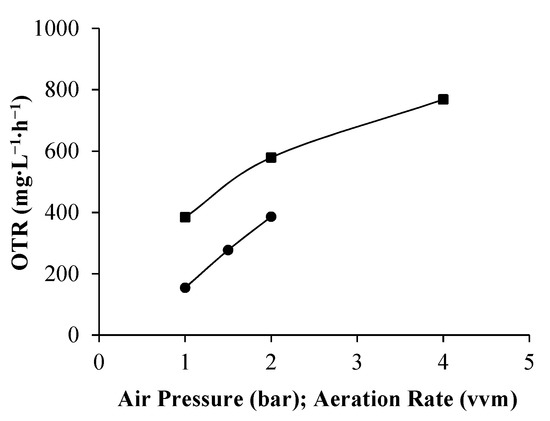
Figure 1.
Variation of oxygen mass transfer rate (OTR) under different operating conditions: pressurized bioreactor (■)—OTR vs. air pressure; airlift bioreactor (●)—OTR vs. aeration rate.
3.2. Effect of Increased Air Pressure on Citric Acid Production
To study the effect of increased air pressure on citric acid production by Y. lipolytica W29, several experiments were conducted in a pressurized bioreactor under total air pressure of 1 bar (equivalent to atmospheric pressure), 2 bar, and 4 bar. The increase in air pressure had no significant effect on cellular growth (Figure 2a); thus, no inhibitory effects were observed under air pressure of 4 bar compared to 1 bar. Lopes et al. [28] and Fernandes et al. [26] reported an enhancement in the cellular growth of Y. lipolytica W29 and Aureobasidium pullulans, respectively, and an increase in the carbon source consumption rate under 4 bar of air pressure. However, those studies were carried out in a rich medium (without nitrogen limitation) with glucose as a carbon source. The maximum citric acid concentration was obtained in the experiments conducted at 2 bar of air pressure, and no significant differences were obtained in the final citric acid concentration attained under 4 bar and atmospheric pressure (Figure 2c). The citric acid profiles are in accordance with those obtained in an STR operating at atmospheric pressure, in which citric acid synthesis by Y. lipolytica W29 from crude glycerol was more pronounced after the exponential growth phase [6].
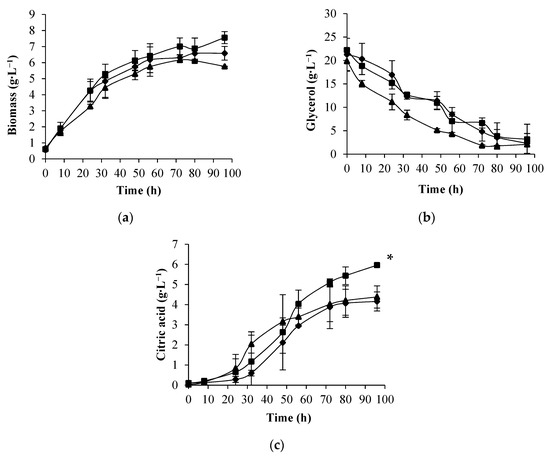
Figure 2.
Biomass concentration (a), glycerol consumption (b), and citric acid production (c) in batch cultures of Y. lipolytica W29 at 1 bar (♦), 2 bar (■), and 4 bar (▲) of total air pressure. The error bars represent the standard deviation of two independent replicates. * Statistically different (p < 0.05).
The increase in total air pressure from 1 bar to 2 bar led to a 40% improvement in citric acid concentration, but above this value of air pressure, a decrease in acid production was observed. The increase in citric acid production with the increase in air pressure up to 2 bar can be due to the enhancement of the activity of several enzymes of the tricarboxylic acid and glyoxylate cycles, which are involved in citric acid production. The increase in air pressure led to an increase in oxygen solubility in the culture, which may enhance some enzymes’ activity, such as citrate synthase, isocitrate lyase, aconitate hydrate, and NAD-dependent isocitrate dehydrogenase, according to previously reported works performed at atmospheric pressure [29,30]. The slight decrease in citric acid concentration and maximum productivity obtained at 4 bar of air pressure is probably due to a shift in yeast metabolism, mainly in the tricarboxylic acid cycle. Aguedo et al. [31] also reported a change in the metabolic pathway of γ-decalactone production when cells of Y. lipolytica W29 were growing under 10 bar of total air pressure. Other authors reported that highly aerated cultures at atmospheric pressure led to a metabolic shift in Y. lipolytica cells toward the production of citric acid instead of polyols synthesis using crude glycerol as substrate. In conditions of a high agitation rate, citric acid production by Y. lipolytica DSM 8218 was favored over mannitol biosynthesis [32]. During flask batch cultures (low dissolved oxygen concentration) of Y. lipolytica LMBF Y-46, polyols (mannitol, arabitol, and erythritol) were the major metabolites produced, whereas in the STR experiments (higher oxygen saturation), insignificant quantities of polyols were obtained and citric acid reached 42 g·L−1 [33]. The same behavior was found for Y. lipolytica ACA YC 5029 cultures, in which no citric acid was produced in the flask experiments and mannitol and erythritol were synthesized in significant amounts. The scale-up to a bench-top bioreactor led to a metabolic transition and citric acid was the main compound produced, while no erythritol at all and a low quantity of mannitol was obtained [34].
Among the values of air pressure tested in the herein presented work, a slight increase in the specific growth rate, citric acid yield, and maximum specific citric acid productivity (qCA) was observed by raising the air pressure from 1 bar to 2 bar but was still statistically insignificant (Table 1). No significant differences were observed in biomass yield at different pressures in spite of the slightly higher value observed at 2 bar. However, a positive effect on biomass yield and specific growth rate of Y. lipolytica W29 cultures under increased air pressure was described by other authors [28,31]. On the other hand, Lopes et al. [21] observed no effect on the maximum specific growth rate, biomass yield, and specific consumption rate with the increased air pressure of up to 5 bar in batch cultures of two recombinant Pichia pastoris strains. Previous reports proved that the effect of increased air pressure was dependent not only on yeast strain but also on operational conditions. The increase in air pressure in Saccharomyces cerevisiae batch cultures led to a decrease in biomass productivity [35] but had a positive effect in fed-batch cultures [36].

Table 1.
Effect of increased air pressure on maximum specific growth rate (μmax), biomass yield (YX/S), specific consumption rate (qS), citric acid yield (YCA/S), and maximum specific citric acid productivity (qCA) during bath cultures of Y. lipolytica W29 in a pressurized bioreactor. Data are presented as the average and standard deviation of two independent experiments.
3.3. Effect of Aeration Rate on Citric Acid Production in an Airlift Bioreactor
Airlift bioreactors are used in some microbial processes due to their high oxygen transfer capacity and less shear stress imposed on the cells. There are few studies regarding the production of citric acid by Y. lipolytica in airlift bioreactors and only with immobilized cells [24,25]. Moreover, none of them studied the effect of the aeration rate on bioprocess yield and productivity. To evaluate the effect of aeration rate on citric acid production by Y. lipolytica W29 from crude glycerol in an airlift batch bioreactor, several experiments were performed with three aeration rates (1 vvm, 1.5 vvm, and 2 vvm).
The increase of the aeration rate from 1 vvm to 2 vvm had a positive effect on cellular growth (Figure 3a) and a 40% improvement in the final biomass concentration was obtained compared to 1 vvm. Crude glycerol consumption (Figure 3b) was slightly enhanced with the increase of the aeration rate from 1 vvm to 2 vvm. However, citric acid production was only improved with the increase of the aeration rate from 1 vvm to 1.5 vvm (Figure 3c). Above this value, a decrease in citric acid concentration was observed.
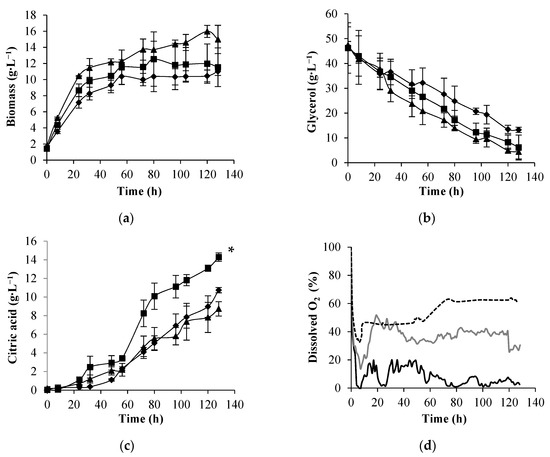
Figure 3.
Biomass concentration (a), glycerol consumption (b), citric acid production (c), and dissolved oxygen concentration profiles (d) obtained in batch cultures of Y. lipolytica W29 in an airlift bioreactor at 1 vvm (♦, black line), 1.5 vvm (■, grey line), and 2 vvm (▲, dashed line). The error bars represent the standard deviation of two independent replicates. * Statistically different (p < 0.05).
A 30% improvement in citric acid concentration was obtained by increasing the aeration rate from 1 vvm to 1.5 vvm (Figure 3c). The increase in the aeration rate from 1.5 vvm to 2 vvm resulted in a decrease in citric acid concentration. Similar behavior was described by Yuguo et al. [37] that reported a slight decrease in citric acid production by A. niger in an external-loop airlift bioreactor, raising the aeration rate from 1.3 vvm to 1.4 vvm. Furthermore, Braga et al. [16] described a decrease in γ-decalactone maximum concentration with the increase in the aeration rate in batch cultures of Y. lipolytica W29 performed in an airlift bioreactor. Nevertheless, the citric acid concentration obtained herein was 1.4-fold higher than that attained by the same yeast strain in a mechanically agitated bioreactor operating at 2 vvm and 400 rpm [6]. In a fed-batch mode of operation, Y. lipolytica DSM 8218 cells only synthesized 10 g L−1 of citric acid from crude glycerol after 256 h of cultivation in an STR operating at 1 vvm and 800 rpm [32]. This is an important advantage for the industrial production of citric acid since the costs imputed to the aeration and mechanical agitation will decrease.
There was no relevant effect of aeration rate on biomass yield and specific consumption rate, and the values of these parameters were similar for all the conditions tested (Table 2). Analogous to citric acid concentration, both citric acid yield and maximum specific citric acid productivity were slightly enhanced with the increase in the aeration rate from 1 vvm to 1.5 vvm and decreased above this value.

Table 2.
Effect of aeration rate on maximum specific growth rate (μmax), biomass yield (YX/S), specific consumption rate (qS), citric acid yield (YCA/S), and maximum specific citric acid productivity (qCA) during bath culture of Y. lipolytica W29 in an airlift bioreactor. Data are presented as the average and standard deviation of two independent experiments.
As expected, according to OTR values, different dissolved oxygen profiles were observed in batch cultures of Y. lipolytica in the airlift bioreactor (Figure 3d). A decrease in oxygen concentration was observed in the first hours of yeast cultivation (corresponding to the exponential growth phase). This decrease was more pronounced for 1 vvm, which, in the first hours, led to a complete depletion of oxygen in the medium. At the citric acid production phase (after the nitrogen source had been completely consumed), the oxygen demand is lower, resulting in an increase in oxygen concentration in the medium [38]. In the experiments carried out at 1 vvm of aeration rate, the DO concentration dropped to zero in the first hours and stabilized around 10% during the citric acid production. For the other aeration conditions, the oxygen concentration in the medium never reached zero and stabilized around 35% and 60% of air saturation for 1.5 vvm and 2 vvm, respectively. Some authors reported a decrease in the activity of some enzymes involved in the citric acid production at DO concentration close to 5% of air saturation, which leads to a decrease in citric acid concentration [29,30]. This observation can explain the lower citric acid production obtained at 1 vvm. An optimum DO concentration of around 50% to 60% of air saturation was reported in the literature for citric acid production processes [38,39]. The maximum citric acid concentration in the airlift bioreactor was attained in the experiments performed at 1.5 vvm, in which the DO remained near 35% air saturation during the citric acid production; above this value (DO around 55%–60% air saturation), a citric acid concentration decrease was observed. Anastassiadis and Rehm [39] reported that, for DO concentrations lower or higher than 20% air saturation, the citric acid production decreased in Candida oleophila ATCC 20177 continuous cultures. The authors suggested a “kind of Crabtree effect” since a high glycolytic flow rate was attained, simulating an anaerobic glycolytic pathway under aerobic conditions. In Y. lipolytica cultures growing in crude glycerol, a differentiation in the metabolic response to the oxygenation conditions has been observed: DO concentrations between 20% and 50% air saturation favored the synthesis of polyols, whereas DO concentrations above 40% air saturation enhanced the production of citric acid [33].
In this work, the maximum specific citric acid productivity of 0.012 g g−1 h−1 was reached in both bioreactors by oxygenation conditions improvement. A 30% enhancement in maximum specific citric acid productivity resulted either from the increase in total air pressure from 1 bar to 4 bar in the pressurized bioreactor or from the aeration rate increase from 1 vvm to 1.5 vvm in the airlift bioreactor. The amount of citric acid produced herein, as well as the global citric acid yield, are comparable or even higher than others found in the literature for Y. lipolytica strains growing in crude glycerol (Table 3). Using alternative ways of improving OTR, both bioreactor types can be successfully implemented in the citric acid production process with important operating saving costs. In particular, the use of pressurized bioreactors will also reduce the need for high aeration rates that present limitations by causing turbulence and foam problems in bioreactors’ operation.

Table 3.
Maximum citric acid production (CAmax, g·L−1) and global conversion yield of citric acid produced per crude glycerol consumed (YAC/S, g·g−1), obtained in several wild-type Y. lipolytica strain cultures carried out in lab-scale bioreactors. Comparisons with the current work.
4. Conclusions
Citric acid production from crude glycerol by Y. lipolytica W29 batch cultures was studied in pressurized and airlift bioreactors. No cellular growth inhibition was observed raising the total air pressure from 1 bar to 4 bar, but the maximum citric acid concentration was obtained under 2 bar of total air pressure. In the airlift bioreactor, the increase in the aeration rate up to 2 vvm had a clear positive effect on the final biomass concentration, but the maximum citric acid concentration was attained at 1.5 vvm. These results show that oxygenation is a crucial optimization parameter in different bioreactor types. Nevertheless, in the range of conditions tested in this work, it was possible to reach a similar maximum specific citric acid productivity in both bioreactors, proving the applicability of both bioreactor types in the citric acid production process using Y. lipolytica cultures and crude glycerol (a by-product of the biodiesel industry) as a low-cost substrate. Both alternatives for OTR improvement will lead to important operating cost savings through the reduction of power input consumption. Additionally, pressurized bioreactors will allow the reduction of foam and hydrodynamic stress caused by the use of high aeration rates.
Author Contributions
Conceptualization, I.B.; methodology, P.F. and M.L.; validation, I.B.; investigation, P.F.; resources, I.B.; writing—original draft preparation, P.F. and M.L.; writing—review and editing, I.B. and M.L.; supervision, I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit; by LABBELS—Associate Laboratory in Biotechnology, Bioengineering and Microelectromechanical Systems, LA/P/0029/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reena, R.; Sindhu, R.; Balakumaran, P.A.; Pandey, A.; Awasthi, M.K.; Binod, P. Insight into citric acid: A versatile organic acid. Fuel 2002, 327, 125181. [Google Scholar]
- Nangare, S.; Vispute, Y.; Tade, R.; Dugam, S.; Patil, P. Pharmaceutical applications of citric acid. Future J. Pharm. Sci. 2021, 7, 54. [Google Scholar] [CrossRef]
- Mores, S.; Vandenberghe, L.P.S.; Júnior, A.I.M.; Carvalho, J.C.; Mello, A.F.M.; Pandey, A.; Soccol, C.R. Citric acid bioproduction and downstream processing: Status, opportunities, and challenges. Bioresour. Technol. 2021, 320, 124426. [Google Scholar] [CrossRef] [PubMed]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric Acid Production by Yarrowia lipolytica Yeast on Different Renewable Raw Materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Yan, S.; Tyagi, R.D.; Drogui, P. Elucidating the effect of impurities present in different crude glycerol sources on lipid and citric acid production by Yarrowia lipolytica SKY7. J. Chem. Technol. Biotechnol. 2021, 96, 227–240. [Google Scholar] [CrossRef]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen mass transfer impact on citric acid production by Yarrowia lipolytica from crude glycerol. Biochem. Eng. J. 2016, 110, 35–42. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S. Biotechnological production of sugar-alcohols: Focus on Yarrowia lipolytica and edible/medicinal mushrooms. Process Biochem. 2023, 124, 113–131. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Costa, A.R.; Pereira, A.S.; Belo, I. Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization—Challenges and opportunities. Crit. Rev. Biotechnol. 2022, 42, 163–183. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Screening various Yarrowia lipolytica strains for citric acid production. Yeast 2019, 36, 319–327. [Google Scholar] [CrossRef]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen transfer rate and pH as major operating parameters of citric acid production from glycerol Yarrowia lipolytica W29 and CBS 2073. Chem. Pap. 2016, 70, 869–876. [Google Scholar] [CrossRef]
- Zhang, S.; Jagtap, S.S.; Deewan, A.; Rao, C.V. pH selectively regulates citric acid and lipid production in Yarrowia lipolytica W29 during nitrogen-limited growth on glucose. J. Biotechnol. 2019, 290, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Agirman, B.; Erten, H. Citric Acid Production by Yarrowia lipolytica. In Non-Conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 91–117. [Google Scholar]
- Timoumi, A.; Guillouet, S.E.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Impacts of environmental conditions on product formation and morphology of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2018, 102, 3831–3848. [Google Scholar] [CrossRef] [PubMed]
- Merchuk, J. Why use air-lift bioreactors? Trends Biotechnol. 1990, 8, 66–71. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Simadri, D. Stress response in fungal system. In New and Future Developments in Microbial Biotechnology and Bioengineering. Recent Advances in Application of Fungi and Fungal Metabolites: Environmental and Industrial Aspects; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–58. [Google Scholar]
- Braga, A.; Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C.; Belo, I. Aroma production by Yarrowia lipolytica in airlift and stirred tank bioreactors: Differences in yeast metabolism and morphology. Biochem. Eng. J. 2015, 93, 55–62. [Google Scholar] [CrossRef]
- Vial, C.; Poncin, S.; Wild, G.; Midoux, N. Experimental and theoretical analysis of the hydrodynamics in the riser of an external loop airlift reactor. Chem. Eng. Sci. 2002, 57, 4745–4762. [Google Scholar] [CrossRef]
- Lopes, M.; Belo, I.; Mota, M. Over-pressurized bioreactors: Application to microbial cell cultures. Biotechnol. Prog. 2014, 30, 767–775. [Google Scholar] [CrossRef]
- Lopes, M.; Mota, M.; Belo, I. Oxygen mass transfer rate in a pressurized lab-scale stirred bioreactor. Chem. Eng. Technol. 2013, 36, 1779–1784. [Google Scholar] [CrossRef]
- Knoll, A.; Maier, B.; Tscherrig, H.; Büchs, J. The oxygen mass transfer, carbon dioxide inhibition, heat removal, and the energy and cost efficiencies of high pressure fermentation. Adv. Biochem. Eng. Biotechnol. 2005, 92, 77–99. [Google Scholar]
- Lopes, M.; Oliveira, C.; Domingues, L.; Mota, M.; Belo, I. Enhanced heterologous protein production in Pichia pastoris under increased air pressure. Biotechnol. Prog. 2014, 30, 1040–1047. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Wang, W.; Lau, R.; Wang, C.-H. Hydrodynamics and mass transfer of concentric-tube internal loop airlift reactors: A review. Bioresour. Technol. 2022, 359, 127451. [Google Scholar] [CrossRef]
- Escamilla-García, E.; O’Riordan, S.; Gomes, N.; Aguedo, M.; Belo, I.; Teixeira, J.; Belina, J.-M.; Waché, Y. An air-lift biofilm reactor for the production of γ-decalactones by Yarrowia lipolytica. Process Biochem. 2014, 49, 1377–1382. [Google Scholar] [CrossRef]
- Kautola, H.; Rymowicz, W.; Linko, Y.-Y.; Linko, P. Production of citric acid with immobilized Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 1991, 35, 447–449. [Google Scholar] [CrossRef]
- Rymowicz, W.; Kautola, H.; Wojtatowicz, M.; Linko, Y.-Y.; Linko, P. Studies on citric acid production with immobilized Yarrowia lipolytica in repeated batch and continuous air-lift bioreactors. Appl. Microbiol. Biotechnol. 1993, 39, 1–4. [Google Scholar] [CrossRef]
- Fernandes, S.; Belo, I.; Lopes, M. Highly aerated cultures boost gluconic acid production by the yeast-like fungus Aureobasidium pullulans. Biochem. Eng. J. 2021, 175, 108133. [Google Scholar] [CrossRef]
- Belo, I.; Pinheiro, R.; Mota, M. Response of the thermophile Thermus sp. RQ-1 to hyperbaric air in batch and fed-batch cultivation. Appl. Microbiol. Biotechnol. 2000, 53, 517–524. [Google Scholar] [CrossRef]
- Lopes, M.; Gomes, N.; Mota, M.; Belo, I. Yarrowia lipolytica growth under increased air pressure: Influence on enzyme production. Appl. Biochem. Biotechnol. 2009, 159, 46–53. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shishkanova, N.V.; Morgunov, I.G.; Finogenova, T.V. Oxygen requirements for growth and citric acid production of Yarrowia lipolytica. FEMS Yeast Res. 2003, 3, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Finogenova, T.V.; Kamzolova, S.V.; Dedyukhina, E.G.; Shishkanova, N.V.; Il’chenko, A.P.; Morgunov, I.G.; Chernyavskaya, O.G.; Sokolov, A.P. Biosynthesis of citric and isocitric acids from ethanol by mutant Yarrowia lipolytica N 1 under continuous cultivation. Appl. Microbiol. Biotechnol. 2002, 59, 493–500. [Google Scholar] [PubMed]
- Aguedo, M.; Gomes, N.; Garcia, E.E.; Waché, Y.; Mota, M.; Teixeira, J.A.; Belo, I. Decalactone production by Yarrowia lipolytica under increased O2 transfer rates. Biotechnol. Lett. 2005, 27, 1617–1621. [Google Scholar] [CrossRef][Green Version]
- Giacomobono, R.; Albergo, R.; Valerio, V.; Caporusso, A.; De Bari, I. Modelling of the citric acid production from crude glycerol by wild-type Yarrowia lipolytica DSM 8218 using response surface methodology (RSM). Life 2022, 12, 621. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological characterization of a novel wild-type Yarrowia lipolytica strain grown on glycerol: Effects of cultivation conditions and mode on polyols and citric acid production. Appl. Sci. 2020, 10, 7373. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid. Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Pinheiro, R.; Belo, I.; Mota, M. Physiological behaviour of under increased air and oxygen pressures. Biotechnol. Lett. 1997, 7, 703–708. [Google Scholar] [CrossRef]
- Belo, I.; Pinheiro, R.; Mota, M. Fed-batch cultivation of Saccharomyces cerevisiae in a hyperbaric bioreactor. Biotechnol. Prog. 2003, 19, 665–671. [Google Scholar] [CrossRef]
- Yuguo, Z.; Zhao, W.; Xiaolong, C. Citric acid production from the mash of dried sweet potato with its dregs by Aspergillus niger in an external-loop airlift bioreactor. Process Biochem. 1999, 35, 237–242. [Google Scholar] [CrossRef]
- Rywińska, A.; Musiał, I.; Rymowicz, W.; Żarowska, B.; Boruczkowski, T. Effect of agitation and aeration on the citric acid production by Yarrowia lipolytica grown on glycerol. Prep. Biochem. Biotechnol. 2012, 42, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadis, S.; Rehm, H.J. Oxygen and temperature effect on continuous citric acid secretion in Candida oleophila. Electron. J. Biotechnol. 2006, 9, 341–350. [Google Scholar] [CrossRef][Green Version]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuka, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kuttiraja, M.; Dhouha, A.; Tyagi, R.D. Harnessing the effect of pH on lipid production in batch cultures of Yarrowia lipolytica SKY7. Appl. Biochem. Biotechnol. 2018, 184, 1332–1346. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Drogui, P. Purified crude glycerol by acid treatment allows to improve lipid productivity by Yarrowia lipolytica SKY7. Process Biochem. 2020, 96, 165–173. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).