Abstract
Probiotics exert beneficial effects on health improvement, infection prevention, and disease management. This study investigated the probiotic characteristics and safety parameters of Levilactobacillus brevis ZG2488, a novel strain isolated from healthy human feces. The strain exhibited robust tolerance to simulated gastrointestinal conditions, maintaining survival rates of 87.20% in artificial gastric juice (pH 3.0; 3 h) and 95.32% in 0.3% bile salt (24 h). Notably, L. brevis ZG2488 displayed superior microbial adhesion properties with high cell surface hydrophobicity (87.32%), auto-aggregation (81.15% at 24 h), and co-aggregation capacities with Escherichia coli ATCC 43895 (63.90%) and Salmonella typhimurium SL1344 (59.28%). Its adhesion to HT-29 cells (7.15%) surpassed that of the reference strain Lactobacillus rhamnosus GG (1.26%). Antimicrobial testing revealed broad-spectrum inhibitory effects against multidrug-resistant Klebsiella pneumoniae NK04152 and other pathogens. Comprehensive safety assessments confirmed the absence of hemolytic or DNase activity, along with appropriate antibiotic susceptibility to most antibiotics, except kanamycin, streptomycin, vancomycin, and penicillin G. Furthermore, L. brevis ZG2488 significantly enhanced nitric oxide production and upregulated the gene expression of nitric oxide synthase (iNOS) and proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in RAW264.7 macrophages. These findings underscore L. brevis ZG2488 as a promising probiotic candidate with functionality in pathogen inhibition and immune modulation.
1. Introduction
Probiotics, which are live microorganisms that confer health benefits to the host when administered in adequate amounts, modulate immune responses through multiple mechanisms, including pathogen inhibition, microbiota balance regulation, and epithelial barrier enhancement [,,]. Moreover, research has demonstrated that probiotics can enhance both innate and adaptive immune responses [,,,] through interactions with intestinal immune cells and commensal microflora to modulate specific immune functions and maintain immune homeostasis []. Additionally, these beneficial microorganisms further augment nonspecific cellular immune responses, which are characterized by the activation of macrophages, natural killer cells, and antigen-specific cytotoxic T-lymphocytes, as well as the secretion of various cytokines [].
Notably, probiotics exhibit marked immunomodulatory effects on RAW264.7 macrophages, including enhanced phagocytic activity [,], cytokine regulation [,], and modulation of key signaling pathways, such as NF-κB and MAPK []. These properties highlight their potential as functional foods or therapeutic agents in modulating immune responses and managing inflammatory conditions.
In this study, we characterized a novel L. brevis ZG2488 strain isolated from healthy human feces. Since probiotic functions are strain-specific, even within the same species. It was essential to evaluate the potential probiotic properties and functions of L. brevis ZG2488 in this study. Researchers have demonstrated that L. brevis has potential probiotic properties [], including antimicrobial, antioxidant, and anti-inflammatory activities stimulated by LPS or pathogenic bacteria [,,]. Furthermore, researchers have suggested that L. brevis has immune-stimulating activities through improving the production of TNF-α, IL-6, and nitric oxide in macrophages [,].
Several criteria should be examined before strains are used as probiotic agents []. Therefore, the essential characteristics of L. brevis ZG2488 as a potential probiotic were evaluated in this study, which including the following: (i) tolerance to acid and bile salt stress conditions, (ii) a high ability to adhere to intestinal epithelial cells, (iii) the ability to aggregate with itself or pathogenic bacteria, and (ⅳ) the inhibition of pathogenic bacteria. Meanwhile, the immunomodulatory effect of L. brevis ZG2488 on the production of nitric oxide and the gene expression of cytokines was investigated in RAW 264.7 macrophages.
2. Materials and Methods
2.1. Isolation and Identification of L. brevis ZG2488
L. brevis ZG2488 was isolated from healthy human feces within 1 year of collection. The participant was screened to ensure the absence of clinical or subclinical diseases, they had not received antibiotics or probiotics, and they had passed a medical examination. Written informed consent was obtained from the participant prior to this study. The strain was identified through the amplification of the 16S rRNA gene using universal primers (27F and 1492R) [], followed by a BLAST+ 2.15.0 search in the NCBI database. The sequences of L. brevis ZG2488 and other representative strains were aligned using the computer software MEGA_11.0.13_win64, and a phylogenetic tree was constructed based on the 16S rRNA gene sequence utilizing the maximum likelihood method [].
2.2. Strains and Growth Conditions
Lactobacillus strains were cultivated on De Man–Rogosa–Sharpe (MRS) agar plates at 37 °C for 24 h under aerobic conditions. Pathogenic bacteria (Escherichia coli ATCC 43895, Salmonella typhimurium SL1344, Staphylococcus aureus ATCC 25923, Listeria monocytogenes ATCC BAA-679, and multidrug-resistant Klebsiella pneumoniae NK04152) were grown on brain heart infusion (BHI) agar plates overnight at 37 °C under aerobic conditions.
2.3. Cell Culture
The HT-29 cell line, a human colon adenocarcinoma cell line, was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. The cells were cultured at 37 °C in a humidified incubator with 5% CO2 and were sub-cultured 2 to 3 times per week.
2.4. Tolerance to Artificial Gastric Juice and Bile Salts
The tolerance to artificial gastric juice and bile salts was evaluated as previously described [], with slight modifications. During the exponential growth phase, the L. brevis ZG2488 and L. rhamnosus GG (LGG) strains were harvested when the optical density at 600 nm (OD600) reached 0.6. The strains were collected via centrifugation at 4000 rpm for 10 min and subsequently washed twice with phosphate-buffered saline (PBS). To evaluate the resistance to artificial gastric juice, the cell pellets were incubated in MRS broth containing 0.3% (w/v) pepsin (pH 3.0) for 3 h at 37 °C. The resistance to bile salts was assessed by incubating the cells in MRS broth supplemented with 0.1% (w/v) trypsin and 0.3% cow bile salts (pH 8.0) for 24 h at 37 °C. The initial cell numbers (N0) and the numbers of cells treated with artificial gastric juice or bile salts (Nt) were calculated by counting the surviving cells on the MRS agar plates. The survival rate (%) of the strains was calculated as follows:
Survival rate (%) = Log Nt/Log N0 × 100
2.5. Cell Surface Hydrophobicity
The cell surface hydrophobicity of L. brevis ZG2488 was evaluated by measuring the level of microbial adhesion to hydrocarbons, as described by Zibaei-Rad et al. []. L. brevis ZG2488 or LGG was resuspended in 3 mL of PBS, and the optical density of the cell suspension (OD600) was recorded (A1). Subsequently, chloroform (1 mL) was added to this cell suspension, which was vortexed for 2 min and incubated at 25 °C for 30 min. The aqueous phase was then transferred to a new tube, and its optical density was measured (A2). The percentage of the cell surface hydrophobicity was calculated using the following formula:
Hydrophobicity (%) = (A1 − A2)/A1 × 100
2.6. Assays for Auto-Aggregation and Co-Aggregation
The bacterial aggregation, including auto-aggregation and co-aggregation, was investigated following the methods outlined by Fang et al. []. Cells of L. brevis ZG2488, E. coli ATCC 43895, and S. typhimurium SL1344 were harvested by centrifugation for 10 min at 4000 rpm during the exponential growth phase. Next, the cell pellets were washed twice with PBS and resuspended in PBS to achieve an OD600 value of 0.6 (Ai). The absorbance of the upper suspension (At) was measured at various time intervals (i.e., 1, 2, 3, 5, 7, 17, and 24 h). For the co-aggregation assay, 2 mL samples of the adjusted suspensions of L. brevis ZG2488 (Aprobio) and the aforementioned pathogenic bacteria (Apat) were mixed in equal volumes and incubated without agitation. The absorbance of the bacterial mixture (Amix) was measured at 600 nm using a spectrophotometer at specified time intervals (0, 1, 2, 3, 5, 7, 17, and 24 h). The percentages of auto-aggregation and co-aggregation were calculated using the following formula:
Auto-aggregation (%) = (Ai − At)/Ai × 100
Co-aggregation (%) = [(Apat + Aprobio)/2 − (Amix)]/[(Apat + Aprobio)/2] × 100
2.7. Adhesion Ability
HT-29 cell lines were seeded at 2 × 105 cells per well in 24-well plates and incubated for 24 h at 37 °C in a 5% CO2 atmosphere. After washing with PBS, antibiotic-free DMEM medium containing either L. brevis ZG2488 or LGG (7 log CFU/mL) was added to each well and incubated for 3 h. Then, the cells were washed twice with PBS, and the attached bacteria were harvested using PBS supplemented with 0.1% Triton X-100. The collected bacteria were subsequently diluted and inoculated on MRS agar plates.
2.8. Antimicrobial Activities
An antimicrobial assay was conducted utilizing the agar spot-on-lawn method. L. brevis ZG2488 was grown in MRS broth at 37 °C overnight. Sterile Oxford cups were placed on MRS agar plates, and 10 µL of the bacterial solution was dropped into the cups, which were then incubated under aerobic conditions for 24 h at 37 °C. The plates were subsequently overlaid with 10 mL of 0.8% semi-solid BHI agar mixed with indicator bacteria at a final concentration of 107 colony-forming units/mL and cultured at 37 °C for 12 h. The indicator bacteria included E. coli ATCC 43895, S. typhimurium SL1344, S. aureus ATCC 25923, L. monocytogenes ATCC BAA-679, and multidrug-resistant K. pneumoniae NK04152. After the removal of the Oxford cups, the diameters of the inhibition zones surrounding the spots were measured using a SCAN 1200 (Interscience, Saint Nom la Bretêche, France).
2.9. Hemolytic Activity
L. brevis ZG2488 and Staphylococcus aureus were seeded onto Columbia agar plates supplemented with sheep blood and incubated for 48 h and 24 h, respectively, at 37 °C under aerobic conditions. Staphylococcus aureus served as a positive control. Following incubation, the presence of clear colorless zones around the colonies was assessed as β-hemolysis, indicating hemolytic activity.
2.10. Deoxyribonuclease (DNase) Assay
A DNase assay was performed to determine the capacity of L. brevis ZG2488 to hydrolyze DNA. The strain was inoculated onto DNase agar plates and incubated at 37 °C under aerobic conditions. After 24 h of incubation, 1 N HCl (2 mL) was added to the medium. Meanwhile, Staphylococcus aureus served as a positive control in this experiment. The presence of a clear zone surrounding the colony was interpreted as a positive result.
2.11. Antibiotic Susceptibility Evaluation
In total, 3–4 colonies of L. brevis ZG2488 were selected from an MRS agar plate and resuspended in 4 mL of PBS. The bacterial suspension was then evenly distributed onto MRS agar plates using a swab. Fifteen commonly utilized antibiotic discs were employed, including imipenem (10 µg), chloramphenicol (30 µg), erythromycin (15 µg), clindamycin (10 µg), azithromycin (15 µg), minocycline (30 µg/disk), gentamycin (10 µg), netilmicin (30 µg), amoxicillin and clavulanic acid (30 µg), trimethoprim (5 µg/disk), penicillin G (10 µg), kanamycin (30 µg), tetracycline (30 µg), streptomycin (10 µg), and vancomycin (30 µg), which were placed on the surfaces of the MRS agar plates. Then, the plates were incubated at 37 °C for 24 h. The diameters of the inhibition zones surrounding the antibiotic discs were particularly measured by a vernier caliper. The antibiotic susceptibility was subsequently evaluated in accordance with the guidelines established by the Clinical and Laboratory Standards Institute (CLSI) in 2024 [].
2.12. Measurement of Cell Viability
The cytotoxicity of L. brevis ZG2488 in RAW264.7 cells was evaluated by an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium assay (M1020, Solarbio, Beijing, China). RAW264.7 cells were seeded at a density of 3 × 104 cells per well in a 96-well plate and incubated for 24 h at 37 °C in a 5% CO2 humidified incubator. Following this, the cells were treated with serially diluted doses of L. brevis ZG2488 (bacteria: cells = 10, 20, and 40) or 1 µg/mL of lipopolysaccharide (LPS) (L8880, Solarbio, Beijing, China) and incubated for 24 h at 37 °C with 5% CO2. The cells were then washed twice with PBS, and 100 µL of 10% MTT solution was added to each well, followed by 4 h of incubation at 37 °C. After removing the supernatant, 110 µL of formazan-dissolving solution was added to each well. The plate was placed on a shaker and agitated at a low speed for 10 min to ensure the complete dissolution of the crystals. The optical density (OD) levels were subsequently measured by a microplate reader (Bio-Tek, Winooski, VT, USA) at a wavelength of 490 nm.
2.13. Measurement of Nitric Oxide
RAW264.7 cells were seeded in a 96-well plate and incubated for 24 h at 37 °C in a humidified atmosphere containing 5% CO2. Following the aforementioned treatments, the cells were further incubated for 24 h under the same conditions. Subsequently, the supernatant was analyzed for nitric oxide levels using the Griess reaction system (S0021S, Beyotime, Shanghai, China), with measurements taken at 540 nm using a microplate reader (Bio-Tek, Winooski, VT, USA).
2.14. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
RAW264.7 cells were seeded at a density of 1 × 106 cells per well in a 6-well plate and cultured for 24 h at 37 °C with 5% CO2. Then, the cells were incubated with serial dilutions of L. brevis ZG2488 or 100 ng/mL of LPS for 24 h at 37 °C with 5% CO2. Following treatment, the cells were washed with PBS. Total RNA was extracted using TRIzol reagent (15596018CN, Invitrogen, Whitefield, Bangalore). After determining the RNA concentration, 1 µg of RNA was reverse-transcribed into complementary DNA (cDNA). The relative expression levels of each gene were quantified using the Bio-Rad CFX-96 system (Bio-Rad Laboratories, Hercules, CA, USA) with the SYBR Green method (1725122, Bio-Rad) and normalized to mouse β-actin as the reference gene []. The specific oligonucleotide primers were as follows: for IL-1β, (forward) 5′-GGGCCTCAAAGGAAAGAATC-3′ and (reverse) 5′-TACCAGTTGGGGAACTCTGC-3′; for IL-6, (forward) 5′-AGTTGCCTTCTTGGGACTGA-3′ and (reverse) 5′-CAGAATTGCCATTGCACAAC-3′; for TNF-α, (forward) 5′-ATGAGCACAGAAAGCATGATC-3′ and (reverse) 5′-TACAGGCTTGTCACTCGAATT-3′; for iNOS, (forward) 5′-TTCCAGAATCCCTGGACAAG-3′ and (reverse) 5′-TGGTCAAACTCTTGGGGTTC-3′; and for β-actin, (forward) 5′- CCACAGCTGAGAGGGAAATC-3′ and (reverse) 5′-AAGGAAGGCTGGAAAAGAGC-3′.
2.15. Data Analysis
The data from the experiments are presented as means ± standard deviations (SDs). To assess the differences between groups, data from two groups were analyzed by two-tailed unpaired t-tests with Welch’s correction, while data from multiple groups were analyzed by one-way ANOVA followed by Dunnett’s multiple comparison test, employing the GraphPad Prism software Version 8.0 (GraphPad Inc., San Diego, CA, USA). A p-value of <0.05 was regarded as statistically significant, with significance levels indicated as * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. Identification of L. brevis ZG2488
L. brevis ZG2488 was isolated from healthy human feces and identified by 16S rRNA gene sequencing. Sanger sequencing of the 16S rRNA amplicon, followed by comparative sequence alignment (99.86% identity match), confirmed the isolate’s classification within the Levilactobacillus brevis species. A phylogenetic tree analysis was performed to verify the isolate’s evolutionary position, which was constructed based on the 16s rRNA gene retrieved from the GenBank database (Figure 1). The 16S rRNA gene sequence of L. brevis ZG2488 has been deposited in GenBank under the accession number PQ849070 and designated as L. brevis ZG2488 PQ849070.

Figure 1.
The phylogenetic relationships of L. brevis ZG2488 in comparison with other strains were constructed using the 16S rRNA gene sequence by the maximum likelihood method. The percentage values at the nodes represent the levels of bootstrap support based on 100 replications. Bar: 0.06 nucleotide substitutions per site.
3.2. L. brevis ZG2488 Characters with Potential Probiotic Properties
Due to the species-specific and even strain-specific effects of probiotic bacteria on the host’s health, it is essential to evaluate the probiotic potential of wild or novel strains. Therefore, the probiotic characteristics of L. brevis ZG2488 were assessed, including its tolerance to artificial gastric juice and bile salts, hydrophobicity, auto-aggregation, co-aggregation, adhesion, and antimicrobial activity.
Probiotics in the stomach and intestines are exposed to low pH, bile salts, and various digestive enzymes, which can threaten their viability and function. To address this, the resistance to artificial gastric juice and bile salts was evaluated. We found that the survival rate of L. brevis ZG2488 decreased to 87.20% after 3 h of incubation with artificial gastric juice, which was slightly lower than that of LGG (95.82%), a commercial probiotic. However, after treatment with 0.3% bile salts for 24 h, the survival rate of L. brevis ZG2488 was 95.32%, which was significantly higher than that of LGG (77.55%) (Figure 2A).
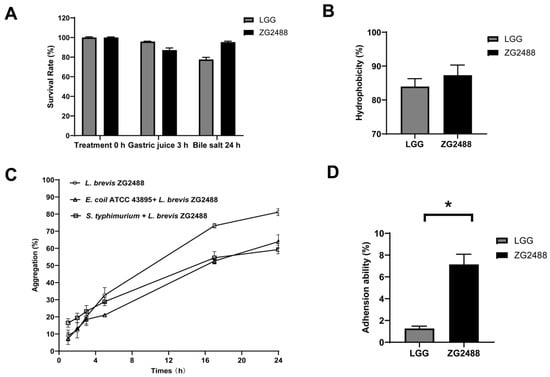
Figure 2.
L. brevis ZG2488 possessed potential probiotic properties. (A) Tolerance of L. brevis ZG2488 and LGG to artificial gastric juice for 3 h and bile salts for 24 h. (B) Cell surface hydrophobicity of L. brevis ZG2488 and LGG. (C) Auto-aggregation of L. brevis ZG2488 and co-aggregation of ZG2488 with pathogens. Open symbols: circle, L. brevis ZG2488; upper triangle, E. coli ATCC 43895 + L. brevis ZG2488; square, S. typhimurium SL1344 + L. brevis ZG2488. (D) Adhesion ability of L. brevis ZG2488 and LGG to HT-29 cells. Statistical significance was determined by * p < 0.05.
The efficacy of probiotics depends on whether they can successfully adhere to the gastrointestinal tract of the host. Their adhesion capability can be indirectly confirmed through the examination of their hydrophobicity and auto-aggregation activities. In addition, the coaggregation capacity with pathogens serves as an important probiotic attribute by enhancing microbial competition and colonization resistance []. In our study, the cell surface hydrophobicity of L. brevis ZG2488 was shown at 87.32%, marginally exceeding LGG (83.95%) (Figure 2B). We observed that the auto-aggregation capability of L. brevis ZG2488 was increased in a time-dependent manner and reached 81.15% at 24 h. Furthermore, the co-aggregation percentages of L. brevis ZG2488 with E. coli ATCC 43895 and S. typhimurium SL1344, respectively, were 63.9% and 59.28% after 24 h of incubation (Figure 2C). Notably, the adhesion rate of L. brevis ZG2488 to HT-29 intestinal epithelial cells was 7.15%, markedly higher than that of LGG (1.26%) (Figure 2D).
Afterward, the antimicrobial potential of L. brevis ZG2488 was assessed using an agar diffusion assay, with its inhibitory activity quantified through the measurement of the inhibition zone diameters. The results revealed that L. brevis ZG2488 showed broad-spectrum antimicrobial efficacy against both Gram-negative pathogens (E. coli ATCC 43895 and S. typhimurium SL1344) and Gram-positive species (S. aureus ATCC 25923 and L. monocytogenes ATCC BAA-679). Notably, the inhibitory effects extended to multidrug-resistant K. pneumoniae NK04152 (Table 1). Altogether, the results collectively indicate that L. brevis ZG2488 possesses significant probiotic potential through its antimicrobial properties.

Table 1.
The antimicrobial activity of L. brevis ZG2488 against the indicator test bacteria, which was determined with the agar spot-on-lawn test.
3.3. In Vitro Safety Evaluation of L. brevis ZG2488
As probiotics should not be virulent, we examined whether L. brevis ZG2488 produced virulence-related enzymes, such as hemolysin and DNase. The hemolytic and DNase activity assessment revealed that L. brevis ZG2488 did not possess hemolytic and DNase activity, contrasting with the positive control, S. aureus ATCC 25923 (Figure 3A,B).
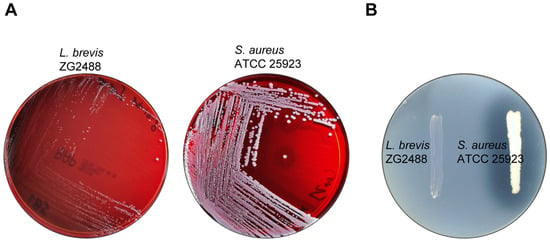
Figure 3.
L. brevis ZG2488 did not possess hemolytic (A) and DNase (B) activity. S. aureus ATCC 25923 was used as a positive control.
A critical safety consideration of probiotics that should be examined is their antibiotic susceptibility profiles, which determine their safety for food consumption. In our study, the antibiotic susceptibility of L. brevis ZG2488 was evaluated against 15 clinically relevant antibiotics, with the inhibition zone diameters detailed in Table 2. Moreover, the antibiotic susceptibility evaluation was conducted following CLSI (2024) guidelines []. The results indicate that L. brevis ZG2488 demonstrated susceptibility to most of the clinical antibiotics, except kanamycin, streptomycin, vancomycin, and penicillin G.

Table 2.
Antibiotic susceptibility of L. brevis ZG2488 determined with the disk diffusion assay.
3.4. Immune-Stimulating Effect of L. brevis ZG2488 on RAW 264.7 Cells
The RAW 264.7 cell line was utilized to investigate the immune-stimulating effects of LAB []. Initially, the cell viability of L. brevis ZG2488 was evaluated in the RAW 264.7 cell line using the MTT assay. LPS was employed as a positive control for immune stimulation. The results indicate that the dose treatment of L. brevis ZG2488 did not exhibit any significant inhibitory effects, suggesting that L. brevis ZG2488 does not possess cytotoxic properties toward RAW 264.7 macrophages (Figure 4A). Subsequently, we assessed the production of nitric oxide in the cell culture medium, which is recognized as a biomarker for immune response in macrophages []. The treatment of RAW 264.7 macrophage cells with L. brevis ZG2488 resulted in a significant increase in nitric oxide levels in a dose-dependent manner (Figure 4B). These findings imply that L. brevis ZG2488 treatment activates RAW 264.7 macrophage cells, thereby contributing to an immune response.
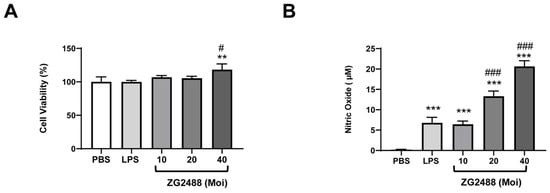
Figure 4.
The immune-stimulating effects of L. brevis ZG2488 on RAW 264.7 cells. (A) The cell viability was assessed using the MTT assay, and (B) the stimulatory effect on nitric oxide production was evaluated by the Griess reaction system after 24 h of treatment with varying doses of L. brevis ZG2488 (Moi = 10, 20, and 40). Statistical significance was determined by ** p < 0.01, and *** p < 0.001 compared with the PBS group, and # p < 0.05 and ### p < 0.001 compared with the LPS group.
3.5. Overexpression of Proinflammatory Gene for Immune Stimulation by L. brevis ZG2488 in RAW 264.7 Cells
To further validate the association between elevated nitric oxide levels and the increase in proinflammatory cytokines induced by L. brevis ZG2488, we conducted a gene expression analysis of iNOS, IL-1β, IL-6, and TNF-α through qRT-PCR. The results demonstrate that the treatment of RAW 264.7 cells with L. brevis ZG2488 resulted in a dose-dependent enhancement of mRNA gene expression for iNOS, IL-1β, and IL-6 (Figure 5A–C). Conversely, while treatment with L. brevis ZG2488 also augmented the expression of TNF-α, the increase exhibited a diminishing trend with higher doses (Figure 5D). These results suggest that L. brevis ZG2488 treatment significantly stimulates immune responses in RAW 264.7 macrophage cells.
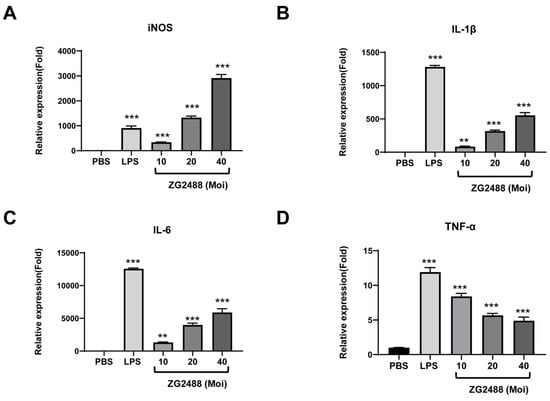
Figure 5.
L. brevis ZG2488 evoked proinflammatory genes for immune stimulation in RAW 264.7 cells. The mRNA expression levels for (A) iNOS, (B) IL-1β, (C) IL-6, and (D) TNF-α were quantified using qRT-PCR and normalized to β-actin expression. Statistical significance was denoted by ** p < 0.01, and *** p < 0.001 compared with the PBS group.
4. Discussion
Previous studies have indicated that L. brevis possesses probiotic characteristics, suggesting its potential use as a probiotic supplement [,,,,]. In the present study, L. brevis ZG2488, a strain isolated from healthy human feces, showed probiotic properties, including resistance to artificial gastric juice and bile salts, adherence to colonic epithelial cells, and the ability to inhibit pathogenic bacteria. Furthermore, L. brevis ZG2488 exhibited a relative safety profile for use as a probiotic supplement, as it displayed no hemolytic activity and limited antibiotic resistance. In a similar study, the researchers demonstrated that the L. brevis MYSN105 strain, derived from an Indian traditional fermented food, Pozha, exhibited significant probiotic properties, particularly its superior stability in acidic and bile-rich environments [].
Probiotics are known to adhere to the host, which can lead to transient colonization, thereby promoting immunomodulatory effects and enhancing the gut barrier []. The ability to adhere is a traditional criterion for selecting potential probiotic bacteria. Previous studies have shown that in vitro experiments utilizing cell lines such as HT-29, HT29-MTX, Caco-2, or H357 can effectively predict the adhesion capabilities of probiotics under gastrointestinal conditions [,,,]. In the HT-29 cell line experiment, L. brevis ZG2488 exhibited a superior adhesion capacity to HT-29 cells (7.15% vs. LGG’s 1.26%), which is consistent with previous findings regarding L. brevis strains KU15152 [] and KU15147 [], suggesting strain-specific adaptations for intestinal colonization.
Additionally, hydrophobicity and auto-aggregation activity can serve as indirect indicators of adhesion capability []. L. brevis ZG2488 showed remarkable hydrophobicity (87.32%) and an auto-aggregation value of 81.25% after 24 h. In a comparable study, L. brevis MYSN105 displayed hydrophobicity and auto-aggregation capacities of 65.90% and 58.99%, respectively []. The co-aggregation ability of probiotics refers to their capacity to attach to pathogens, thereby inhibiting these pathogens from adhering to the gut []. Notably, L. brevis ZG2488 displayed co-aggregation capacities of 63.9% with E. coli ATCC 43895 and 59.28% with S. typhimurium SL1344 after 24 h of incubation, indicating its potential ability to eliminate pathogens from the digestive system. Similarly, other studies have reported that a wide range of co-aggregation capacities (23.10–55%) was observed for L. brevis [,].
L. brevis ZG2488 exhibited antimicrobial effects against both Gram-positive (L. monocytogenes and S. aureus) and Gram-negative (E. coli and S. typhimurium) bacteria. In addition, it demonstrated antimicrobial activity against multidrug-resistant K. pneumoniae. Similar to our findings, the L. brevis fg104 also showed antibacterial effects against E. coli ATCC 25922, P. aeruginosa PTCC 1707, S. typhimurium PTCC 1609, and S. aureus ATCC 25923 [].
L. brevis ZG2488 was evaluated for the presence of virulence-related enzymes, specifically hemolysin and DNase, through phenotypic assays. The findings revealed that this strain did not exhibit hemolytic or DNase activity. This observation aligns with previous studies indicating that L. brevis strains generally do not produce hemolysis or DNase [,,,]. While phenotypic assays confirmed the absence of hemolytic/DNase activity, further genomic analysis is necessary to rule out the possibility of horizontal gene transfer of virulence factors.
The recent literature has indicated that commercial strains utilized in industrial dairy production often exhibit resistance to antibiotics such as gentamycin, kanamycin, chloramphenicol, and tetracycline []. In our study, L. brevis ZG2488 was sensitive to the majority of the antibiotics tested, including imipenem, chloramphenicol, erythromycin, clindamycin, azithromycin, netilmicin, amoxicillin combined with clavulanic acid, minocycline, trimethoprim, and tetracycline. However, it displayed intermediate sensitivity to gentamicin and resistance to kanamycin, streptomycin, vancomycin, and penicillin G. A similar study corroborated that various lactobacillus species, including 10 isolates from fermented palm sap, exhibited resistance to vancomycin, kanamycin, and streptomycin []. Nonetheless, multiple studies have indicated that the antibiotic sensitivity among different L. brevis strains is strain-specific [,,,,]. Therefore, it is imperative to thoroughly identify and characterize the genetic determinants of antibiotic resistance in this strain.
Probiotics are recognized for their potential immune-modulating effects, which are considered beneficial for human health []. In this study, the immune-stimulating effects of L. brevis ZG2488 were assessed using RAW 264.7 macrophage cell lines. The results demonstrated that L. brevis ZG2488 enhanced nitric oxide production in RAW 264.7 cells without exhibiting cytotoxic effects. Previous research has established that activated macrophages produce nitric oxide, along with proinflammatory cytokines or tumor necrosis factor []. For instance, researchers have found that Lactobacillus sakei K040706, Lacticaseibacillus rhamnosus LM1019, and Weissella cibaria JW15 stimulate nitric oxide production and enhance the gene expression of iNOS, TNF-α, and IL-6 in macrophages [,,], indicating the immune-enhancing properties of these strains. In this study, it was confirmed that L. brevis ZG2488 increased the mRNA expression levels of iNOS, IL-1β, IL-6, and TNF-α, suggesting its potential role in immunomodulation within macrophage cells. Nevertheless, future studies should validate these effects in vivo and evaluate the genomic safety of this strain.
5. Conclusions
This study elucidated that L. brevis ZG2488 exhibits several critical probiotic characteristics, including resistance to artificial gastric juice and bile salts, a high capacity for auto-aggregation and co-aggregation, adhesion capabilities, and antimicrobial activity against both Gram-positive and Gram-negative bacteria. Furthermore, the strain demonstrated the absence of hemolytic/DNase activity and showed susceptibility to the majority of clinically relevant antibiotics. In addition, the findings indicated that L. brevis ZG2488 possesses immune-modulating properties, as evidenced by its ability to stimulate the production of nitric oxide and proinflammatory cytokines in RAW264.7 macrophage cells. Consequently, L. brevis ZG2488 may serve as a promising probiotic candidate and could play a significant role in enhancing the immunostimulatory response in macrophages.
Author Contributions
Conceptualization, Z.C.; methodology, Y.C.; software, M.C.; validation, Z.C., M.C., and Y.C.; formal analysis, M.C.; investigation, Y.C.; resources, H.S.; data curation, Z.C.; writing—original draft preparation, Z.C.; writing—review and editing, Z.C.; visualization, Z.C.; supervision, H.S.; project administration, Z.C.; funding acquisition, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project of the Youth Fund for Enhancing Capability of Infectious Disease Surveillance and Prevention of the National Institute for Communicable Disease Control and Prevention, the Chinese Center for Disease Control and Prevention (102393240020020000003).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the National Institute for Communicable Disease Control and Prevention, the Chinese Center for Disease Control and Prevention, China (no. ICDC2016007).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data about the 16S rRNA gene sequences have been deposited in NCBI GenBank under the accession number PQ849070.1 (https://www.ncbi.nlm.nih.gov/nuccore/PQ849070.1/). Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Gui Zhang at the Infection Management Office, the Shandong Provincial Hospital affiliated with Shandong First Medical University, for isolating the strain of Levilactobacillus brevis ZG2488. We sincerely acknowledge Jianguo Xu at the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, for generously providing the bacterial strains essential to this investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M.; Shanahan, F. Probiotics in Health Care: A Critical Appraisal. Annu. Rev. Med. 2025, 76, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Kim, H.J.; Hong, S.K.; Lee, D.H.; Lee, S.W.; Song, C.S.; Kim, K.T.; Choi, I.S.; Lee, J.B.; Park, S.Y. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J. Microbiol. Immunol. Infect. 2016, 49, 16–23. [Google Scholar] [CrossRef]
- He, G.; Long, H.; He, J.; Zhu, C. The Immunomodulatory Effects and Applications of Probiotic Lactiplantibacillus plantarum in Vaccine Development. Probiotics Antimicrob. Proteins 2024, 16, 2229–2250. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.F.; Yu, W.R.; Sun, S.Y.; Lei, Y.M.; Li, Y.X.; Lu, C.X.; Zhai, J.N.; Bai, F.R.; Ren, F.; et al. Roles of Probiotics, Prebiotics, and Postbiotics in B-Cell-Mediated Immune Regulation. J. Nutr. 2025, 155, 37–51. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Noh, H.J.; Park, J.M.; Kwon, Y.J.; Kim, K.; Park, S.Y.; Kim, I.; Lim, J.H.; Kim, B.K.; Kim, B.Y. Immunostimulatory Effect of Heat-Killed Probiotics on RAW264.7 Macrophages. J. Microbiol. Biotechnol. 2022, 32, 638–644. [Google Scholar] [CrossRef]
- Ali, M.S.; Lee, E.B.; Quah, Y.; Sayem, S.A.J.; Abbas, M.A.; Suk, K.; Lee, S.J.; Park, S.C. Modulating effects of heat-killed and live Limosilactobacillus reuteri PSC102 on the immune response and gut microbiota of cyclophosphamide-treated rats. Vet. Q. 2024, 44, 1–18. [Google Scholar] [CrossRef]
- Park, H.E.; Do, K.H.; Lee, W.K. The immune-modulating effects of viable Weissella cibaria JW15 on RAW 264.7 macrophage cells. J. Biomed. Res. 2019, 34, 36–43. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Kim, S.H.; Kim, C.H.; Kim, I.H.; Shin, Y.; Kim, T.R.; Sohn, M.; Park, J. Immune-Stimulating Potential of Lacticaseibacillus rhamnosus LM1019 in RAW 264.7 Cells and Immunosuppressed Mice Induced by Cyclophosphamide. Microorganisms 2023, 11, 2312. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Xu, J.; Li, Q.; Xia, X.; Du, G. Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol. 2018, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.H.; Yu, H.S.; Woo, I.K.; Lee, G.W.; Lee, N.K.; Paik, H.D. Anti-inflammatory activities of Levilactobacillus brevis KU15147 in RAW 264.7 cells stimulated with lipopolysaccharide on attenuating NF-kappaB, AP-1, and MAPK signaling pathways. Food Sci. Biotechnol. 2023, 32, 2105–2115. [Google Scholar] [CrossRef]
- Han, X.; Ding, S.; Ma, Y.; Fang, J.; Jiang, H.; Li, Y.; Liu, G. Lactobacillus plantarum and Lactobacillus brevis Alleviate Intestinal Inflammation and Microbial Disorder Induced by ETEC in a Murine Model. Oxid. Med. Cell Longev. 2021, 2021, 6867962. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.K.; Paik, H.D. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 1521–1528. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, J.H.; Lee, J.S.; Kang, S.D.; Shim, S.; Jung, M.Y.; Yang, H.; Byun, S.; Lee, K.W. Heat-Killed Lactobacillus brevis Enhances Phagocytic Activity and Generates Immune-Stimulatory Effects through Activating the TAK1 Pathway. J. Microbiol. Biotechnol. 2020, 30, 1395–1403. [Google Scholar] [CrossRef]
- Kim, K.T.; Yang, S.J.; Paik, H.D. Probiotic properties of novel probiotic Levilactobacillus brevis KU15147 isolated from radish kimchi and its antioxidant and immune-enhancing activities. Food Sci. Biotechnol. 2021, 30, 257–265. [Google Scholar] [CrossRef]
- Verdenelli, M.C.; Ghelfi, F.; Silvi, S.; Orpianesi, C.; Cecchini, C.; Cresci, A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur. J. Nutr. 2009, 48, 355–363. [Google Scholar] [CrossRef]
- Kunduhoglu, B.; Hacioglu, S. Probiotic Potential and Gluten Hydrolysis Activity of Lactobacillus brevis KT16-2. Probiotics Antimicrob. Proteins 2021, 13, 720–733. [Google Scholar] [CrossRef]
- Lo Presti, A.; Del Chierico, F.; Altomare, A.; Zorzi, F.; Monteleone, G.; Putignani, L.; Angeletti, S.; Cicala, M.; Guarino, M.P.L.; Ciccozzi, M. Phylogenetic analysis of Prevotella copri from fecal and mucosal microbiota of IBS and IBD patients. Therap. Adv. Gastroenterol. 2023, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zibaei-Rad, A.; Rahmati-Joneidabad, M.; Behbahani, B.A.; Taki, M. Assessing the protection mechanisms on Enterobacter aerogenes ATCC 13048 by potentially probiotic strain Lacticaseibacillus casei XN18: An experimental and modeling study. Microb. Pathog. 2023, 181, 106177. [Google Scholar] [CrossRef]
- CLSI. CLSI M100. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2024. [Google Scholar]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Hacıoglu, S.; Kunduhoglu, B. Probiotic Characteristics of Lactobacillus brevis KT38-3 Isolated from an Artisanal Tulum Cheese. Food Sci. Anim. Resour. 2021, 41, 967–982. [Google Scholar] [CrossRef]
- Somashekaraiah, R.; Mottawea, W.; Gunduraj, A.; Joshi, U.; Hammami, R.; Sreenivasa, M.Y. Probiotic and Antifungal Attributes of Levilactobacillus brevis MYSN105, Isolated from an Indian Traditional Fermented Food Pozha. Front. Microbiol. 2021, 12, 696267. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Sophatha, B.; Piwat, S.; Teanpaisan, R. Adhesion, anti-adhesion and aggregation properties relating to surface charges of selected Lactobacillus strains: Study in Caco-2 and H357 cells. Arch. Microbiol. 2020, 202, 1349–1357. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Coconnier, M.H.; Klaenhammer, T.R.; Kerneis, S.; Bernet, M.F.; Servin, A.L. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl. Environ. Microbiol. 1992, 58, 2034–2039. [Google Scholar] [CrossRef]
- Kim, W.J.; Hyun, J.H.; Lee, N.K.; Paik, H.D. Protective Effects of a Novel Lactobacillus brevis Strain with Probiotic Characteristics against Staphylococcus aureus Lipoteichoic Acid-Induced Intestinal Inflammatory Response. J. Microbiol. Biotechnol. 2022, 32, 205–211. [Google Scholar] [CrossRef]
- Hojjati, M.; Behabahani, B.A.; Falah, F. Aggregation, adherence, anti-adhesion and antagonistic activity properties relating to surface charge of probiotic Lactobacillus brevis gp104 against Staphylococcus aureus. Microb. Pathog. 2020, 147, 104420. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Jooyandeh, H.; Hojjati, M.; Ghodsi Sheikhjan, M. Evaluation of probiotic, safety, and anti-pathogenic properties of Levilactobacillus brevis HL6, and its potential application as bio-preservatives in peach juice. Lwt 2024, 191, 115601. [Google Scholar] [CrossRef]
- Nunziata, L.; Brasca, M.; Morandi, S.; Silvetti, T. Antibiotic resistance in wild and commercial non-enterococcal Lactic Acid Bacteria and Bifidobacteria strains of dairy origin: An update. Food Microbiol. 2022, 104, 103999. [Google Scholar] [CrossRef] [PubMed]
- Sornsenee, P.; Singkhamanan, K.; Sangkhathat, S.; Saengsuwan, P.; Romyasamit, C. Probiotic Properties of Lactobacillus Species Isolated from Fermented Palm Sap in Thailand. Probiotics Antimicrob. Proteins 2021, 13, 957–969. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, W.; Shin, W.R.; Kim, Y.H.; Min, J. Enhanced immune response by vacuoles isolated from Saccharomyces cerevisiae in RAW 264.7 macrophages. Biosci. Rep. 2021, 41, BSR20211158. [Google Scholar] [CrossRef]
- Jung, J.Y.; Shin, J.S.; Lee, S.G.; Rhee, Y.K.; Cho, C.W.; Hong, H.D.; Lee, K.T. Lactobacillus sakei K040706 evokes immunostimulatory effects on macrophages through TLR 2-mediated activation. Int. Immunopharmacol. 2015, 28, 88–96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).