Abstract
Nitrogen-rich carbon nanotubes NCNT700 and NCNT800 were prepared using the chemical vapor deposition method (CVD). The catalysts were characterized via high-resolution transmission electron microscopy (HRTEM) and X-ray photoelectron spectroscopy (XPS) analysis. Both the catalysts were found to have an inverted cup-stack-like morphology. The XPS analysis revealed that the catalysts are rich in pyridinic sites with variable amounts of nitrogen on their surface. The NCTN700, with a higher nitrogen content and more pyridinic sites on its surface, was found to be a good catalyst for the oxidation of benzyl and veratryl alcohols into respective aldehydes. It was observed that toluene and 4-methyl veratrole were also produced in this reaction. The amount of toluene produced was as high as 21%, with 99% conversion of benzaldehyde in the presence of NCNTs-700. The mechanistic pathway was revealed through DFT studies, where the unusual product formation of aromatic alkanes such as toluene and 4-methyl veratrole was explained during the reaction. It was astonishing to observe the reduced product in the reaction that proceeds in the forward direction in presence of a peroxide (tert-butyl hydroperoxide, TBHP). During the computational analysis, it was revealed that the reduced product observed in the reaction did not appear to proceed through a direct disproportionation reaction. Rather, the benzyl alcohol (the reactant) used in the reaction may undergo oxidation by releasing the hydrogen radicals. The hydrogen atoms released during the oxidation reaction appear to have been trapped on pyrrolic sites on the surface of catalyst and later transferred to the reactant molecules to produce toluene as a side product.
1. Introduction
Nowadays, there is a consensus that petroleum-based chemicals and fuels are unsustainable, thus increasing the attention paid to renewable resources. Replacing these products with biomass resources is important to attain economical, scientific, social, and political goals [1]. Waste biomass resources such as fallen leaves, waste wood, and non-edible plants are mainly rich in hemicelluloses, cellulose, and lignin. The amount of these components varies based on the plant type and origin. The utilization of these components has been made possible by the emergence of bio-refineries [2]. The pyrolysis of ligno-cellulosic biomass normally affords liquid oils that are rich in phenolic compounds. Normally, the components of bio-oil need efficient separation and up-gradation technology to make them usable as fine chemicals. The most commonly used processes are oxidation [3] and hydrodeoxygenation [4], which generally require metal-based homogenous or heterogeneous catalysts. Again, the use of metal-based catalysts is often avoided for the sake of sustainability. Therefore, the use of metal-free catalysts for valorization processes is highly recommended.
The past decade has seen extensive efforts toward the depolymerization of lignin via reductive and oxidative processes [4]. Transition-metal-based catalysts are the first choice for this [4]. Ni@C is used for the hydrogenolytic cleavage of lignin derived from native birch wood lignin [5,6]. A high chemoselectivity has been achieved for monomeric phenols using an Ni-based carbon catalyst, and the lignin was cleaved into propylsyringol and propylguaiacol with a selectivity of more than 50%. Similarly, cobalt chloride in ionic liquid 1-ethyl-3-methylimidazolium diethylphosphate has been used for the oxidative cleavage of model lignin compounds using molecular oxygen [7]. The catalyst was able to oxidize the benzyl alcoholic group in lignin molecules, and the phenolic –OH, along with the 5–5′, β-O-4, and phenylcoumaran groups, remained intact. The nitrogen-rich carbon nanomaterials act as good support for depositing metals on its surface, thereby increasing the efficiency of the catalyst. Wang et al. synthesized N-doped CNTs to support Ni nanoparticles on its surface, and the resulting catalyst was used for the hydrogenolysis of lignin [8]. The doped nitrogen atoms provided anchoring sites for Ni metal, promoting its dispersion and forming stable Ni–N bonds on the support. The electron transfer between Ni and N atoms facilitated hydrogen activation, thus proving useful for hydrogenolysis. DFT calculations have revealed the role of doped nitrogen atoms in promoting electron-rich states for enhanced hydrogen adsorption. Similarly, the catalytic depolymerization and hydrodeoxygenation of Kraft lignin with bimettalic Zn–O–Co deposited on nitrogen-doped carbon nanotubes was performed in water [9]. The catalyst exhibited exceptional catalytic activity, achieving excellent lignin conversion with a small amount of residue and high bio-oil yield. This process yielded cyclohexanone derivatives and alkylated phenols as major products. Furthermore, Li et al. investigated the impact of nitrogen species and content on the catalytic activity of C–O bond cleavage in lignin using N-doped carbon nanosheets supported by Ru-based catalysts [10]. The catalyst, obtained via the pyrolysis of glucosamine hydrochloride and melamine, featured highly dispersed and small-sized Ru nanoparticles. This catalyst efficiently converted lignin, yielding 40.70% aromatic monomers—2.3 times higher than a commercial catalyst (Ru/C). The presence of pyridinic nitrogen in the support not only stabilized and dispersed the Ru nanoparticles but also enhanced the proportions of Ru(0) through electronic interactions, contributing to improved catalytic activity. The study demonstrated the potential of nitrogen-rich carbon nanotubes in promoting lignin conversion to valuable aromatic monomers. Three metal-based catalysts based on copper(II) bromide complexes were synthesized for the eco-friendly aerobic oxidation of veratryl alcohol in water. The reaction was performed in ambient conditions with a copper catalyst and TEMPO in the presence of air as an oxidant. The presence of a base was necessary to ensure a good yield of aldehyde, and over oxidation took place in its absence [11]. However, no base is required for the aerobic oxidation of vanillyl alcohol in presence of a copper (II)-based complexes and TEMPO, which mainly provided vanillin as an end product [12].
Though metal-based catalysts are capable of performing the oxidative/reductive cleavage of lignin, metal-free catalysts are promoted to achieve sustainability. S. S. Stahl and coworkers have performed the metal-free oxidation of lignin using a catalytic system consisting of 4-acetamido-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) with HCl and HNO3 [13]. The aerobic oxidation proceeded with the chemoselective oxidation of secondary alcoholic groups in the presence of unprotected –OH groups. The method was first tested on model molecules, which was followed by a study of the catalytic activity on real lignin.
In the last few decades, metal-free carbocatalysts have attracted a lot of attention as substitutes to metal-based heterogeneous catalysts for several reactions [14,15,16,17], including alkene and alkane dehydrogenation [18] and liquid-phase dehydrogenation [19,20], oxidation [21,22,23], and hydrogenation [24]. In particular, metal-free nitrogen-functionalized carbons have already been reported to be effective catalysts for the oxidation of alcohols to carbonyl compounds, and pyridinic nitrogen species were believed to be the active sites for these reactions. For example, Su et al. demonstrated that the pyridine group was the main active site in aerobic benzyl alcohol oxidation by forming an N–O group, while graphitic nitrogen was less active [25]. The catalytic activity of graphitic nitrogen was attributed to the formation of a sp2 N–O2 transition state in the oxidation of alcohol [25].
As has been previously demonstrated, CNTs act as electron “reservoirs” in oxidative dehydrogenation reactions of alkanes, promoting the stabilization of intermediate radicals and guiding them to the selective pathways for functionalized molecules instead of non-selective full oxidation [26]. The use of TEMPO, a well-known spin trap, as shown above, underlines the importance of controlling radical pathways in contrast with conventional acid/base ones, since they might lead to both unique and undesirable products. Hence, special attention should be given to the role of spin chemistry in the catalytic conversion of lignin and its by-products.
A complete analysis of the literature reveals that metal-based catalysts on carbon support, including the nitrogen-rich CNTs, are the first choice of researchers for conducting the hydrogenolysis and/or oxidation of lignin or its monomers. There are few reports of metal-free catalytic systems for this particular conversion. Keeping in mind the importance of metal-free catalytic systems for the oxidation of lignin (or related model molecules), we have used nitrogen-containing carbon nanotubes for this purpose. The present work focused on the preparation of two different nitrogen-functionalized carbon nanotubes, NCNT700 and NCNT800, prepared using the chemical vapor deposition method and tested in the oxidation of benzyl and veratryl alcohol. The nature of the nitrogen groups was modulated by varying the reaction temperature (700 °C and 800 °C for NCNT700 and NCNT800, respectively). The functionalized carbo-catalysts were tested in the alcohol oxidation in the presence of tert-butyl hydroperoxide (TBHP) as the oxidant. To understand the effect of the nitrogen species on the carbonaceous materials, the catalysts were thoroughly characterized through TEM and XPS in combination with computational techniques (DFT) to unveil the mechanism of the reaction.
2. Materials and Methods
2.1. Chemicals and Reagents
All the chemicals such as veratryl alcohol, xylene, tert-Butyl hydroperoxide (TBHP), benzaldehyde, benzoic acid, toluene, and imidazole (99% purity) used for the preparation of NCNTs were purchased from Sigma Aldrich and were of laboratory reagent grade. They were used as such without any further purification.
2.2. Synthesis of Catalyst
CNTs with a significant nitrogen content were synthesized using a catalytic chemical vapor deposition process. The method involved the use of imidazole as the source for carbon and nitrogen over a Fe–Mo–Al catalyst. The catalytic CVD growth was achieved on 10 mg of the catalyst dispersed evenly on a quartz boat, which was positioned at the center of a horizontal quartz tube. Subsequently, the furnace was heated by adopting the following program: the heating was started at 25 °C and gradually increased to 700 °C at a heating rate of 10 °C min−1. The heating was performed under ammonia flow at a rate of 0.4 mL min−1 and a 700 °C temperature was maintained for a duration of 35 min, before the furnace was cooled to room temperature. The resulting nanotubes, enriched with nitrogen, were denoted as “NCNT700”. Another catalyst, “NCNT800”, was prepared by adopting a similar procedure and keeping the temperature constant at 800 °C for 35 min. Commercial CNT (Pyrolytically Striped Carbon Nanofibers, PS-CNFs Applied Science) were used as reference.
2.3. Catalytic Oxidation Test
The catalytic oxidation tests were performed in a hydrothermal reactor. The veratryl alcohol (0.3 M) solution in xylene (10 mL) was mixed with tert-Butyl hydroperoxide (TBHP) (6 mMol, 70% in decane), which was followed by the addition of the catalyst, ensuring a 25:1 weight-to-weight ratio of alcohol to catalyst. The contents were stirred at 100 °C for the specified period of time mentioned in Table 1. After the desired reaction time, the reactor was cooled and the reaction mixture was analyzed by GC analysis, using a HP 7820A gas chromatograph equipped with a capillary column HP-5 30 m × 0.32 mm, 0.25 µm Film, by Agilent Technologies. Quantitative analyses using an external standard method (n-octanol) were carried out. Identification of the products was performed using a Thermo Scientific Trace ISQ QD Single Quadrupole GC-MS equipped with a capillary column HP-5 30 m × 0.32 mm × 0.25 m Film, by Agilent Technologies. Recycling tests were carried out under the same experimental conditions. The catalyst was filtered and recycled in the subsequent run after filtration without any further treatment.

Table 1.
Composition of different elements on the surface of catalysts.
2.4. Characterization of the Catalysts
Transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), and scanning transmission electron microscopy (STEM) were performed on a FEI Tecnai G2 F20 microscope operated at 200 kV. Specimens were prepared by ultrasonically suspending the sample in ethanol and depositing a drop of the suspension onto a TEM holey carbon grid. The X-ray photoelectron spectroscopy (XPS) measurements were carried out using an ultra-high vacuum ESCALAB 250 set-up equipped with a monochromatic Al Kα X-ray source (1486.6 eV; anode operating at 15 kV and 20 mA). The XPS spectra were fitted using mixed Gaussian–Lorentzian component profiles after the subtraction of a Shirley background using XPSPEAK41 software. The specific surface area was measured according to the BET method using nitrogen adsorption isotherms obtained on a Micrometrics ASAP 2020 system. Metal content was verified via atomic absorption spectroscopy (AAS) using a Perkin Elmer 3100 (PerkinElmer, Waltham, MA, USA).
2.5. Quantum Chemical Calculations
Quantum chemical calculations were performed within the framework of density functional theory (DFT), employing the three-parameter hybrid functional by Becke–Lee–Yang–Parr (B3LYP) [27] together with Ahlrichs’ double-zeta split-valence basis set augmented by Coulomb fitting (def2-SVP) [28]. The unrestricted Kohn–Sham (UKS) formalism was employed for open-shell states. Molecular geometries were optimized for various relevant multiplicities to gradients of 5 × 10−6 Eh/bohr or less. The ORCA ab initio, DFT, and semiempirical SCF-MO package [29] was used for all calculations. The resolution of identity with “chain of spheres exchange” (RIJCOSX) algorithm for the calculation of the exchange terms was applied [30].
3. Results
The first task was to prepare the metal-free catalyst rich in nitrogen content. The NCNTs were prepared with variable nitrogen content using the chemical vapor deposition method (see experimental details). The two metal-free catalysts NCNT700 and NCNT800 were prepared at two different temperatures. The difference in temperature ensured that a variable amount of nitrogen was incorporated into the carbon matrix. Once the catalysts were prepared, the morphology of NCNT 700 and NCNT800 was observed by HRTEM analysis (Figure 1). Both of the nitrogen-rich carbon nanotubes exhibited an inverted-cup-shaped structure, with ~20 nm average diameter. The ends of the tubes appeared to be open, whereas the inner portions appeared to be blocked by a carbon wall. This structure appears to be beneficial because some impurities, which might have been trapped inside the NCNT, can remain inaccessible during the catalytic test runs. The external metal impurities in both the NCNTs were removed after their treatment with concentrated HCl, followed by subsequent rinsing with demineralised water until the washings became pH neutral.
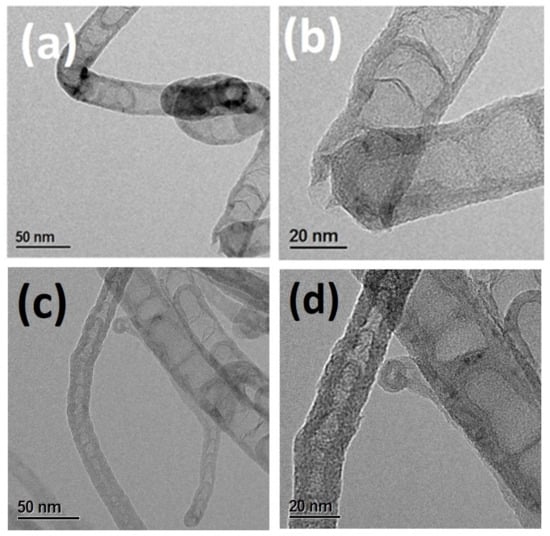
Figure 1.
(a,b) HRTEM images of NCNT700 and (c,d) HRTEM images of NCNT800.
X-ray photoelectron spectroscopy (XPS) was used to determine the surface functional groups, and the analysis revealed the presence of nitrogen, carbon, and oxygen on the catalyst surface. The HRTEM images did not show any metal particles on the surface; therefore, atomic absorption spectroscopy (AAS) was used to determine the metal content. It displayed 0.1% contents of Fe, but as the structure was shaped like an inverted cup, this much impurity might have remained trapped inside the catalyst. The probable nitrogen-containing functional groups were determined by the deconvolution of the N 1s spectrum. The elemental percentage and the percentage of each individual functional group are tabulated in Table 1. The nitrogen content determined was higher for NCNT700, whereas NCNT800 showed lower N content: 13.7 atom% for NCNT700 and 2.3 atom% for NCNT800. The nitrogen peak was further deconvoluted into four sub-peaks. The pyridinic nitrogen peak at 398.1 eV, the pyrrolic nitrogen peak at 400.0 eV, the quaternary nitrogen peak at 400.9 eV, and the pyridinic N-oxide peak at 404.1 eV were deconvoluted (Figure 2). All the nitrogen functional groups were present on the catalyst surface, with the maximum concentration for pyridinic groups in NCT700 (45.8%), followed by NCNT800 (22.6%).
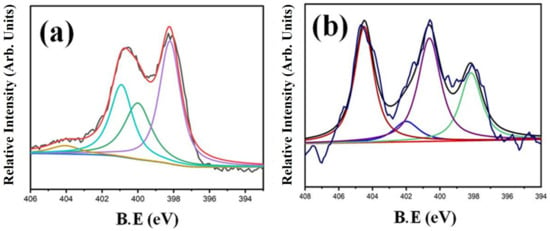
Figure 2.
XPS analyses of (a) NCNT700 and (b) NCNT800.
Once the catalysts were prepared and characterized, they were used for the oxidation of aromatic alcohol (benzyl alcohol) with tert-butyl hydrogen peroxide (TBHP). The reaction was performed by taking a ratio of 25:1 (alcohol: catalyst wt/wt) at 100 °C, and the results are tabulated in Table 2. Blanck experiments performed in the absence of either the catalyst or the oxidant did not show any significant activity. The reaction produced benzaldehyde as the main product by all the NCNTs, with a minor content of benzoic acid. Hence, nitrogen-rich carbon nanotubes were selectively providing aldehyde instead of carboxylic acid (the usual end product in most of the oxidation reactions). The reaction almost did not proceed for CNTs without any nitrogen content in them. Surprisingly, two catalysts displayed the formation of toluene in the reaction mixture. It was ascertained that no toluene was used in the reaction; therefore, it is generated in the course of the reaction.

Table 2.
Catalytic activity of nitrogen-rich carbon nanotubes and commercial CNTs for the benzyl alcohol oxidation.
After the optimization of benzaldehyde oxidation, the catalysts were tested for the metal-free oxidation of a model lignin molecule veratryl alcohol using TBHP as an oxidant. Veratryl alcohol is an aromatic compound containing ether and primary alcohols. It has one alcoholic and two methoxy groups that represent alcohol and ether linkage present in the lignin. Its oxidation can generate three compounds that are shown in Scheme 1. The catalytic tests were performed using the optimized method, and the results are shown in Table 3.
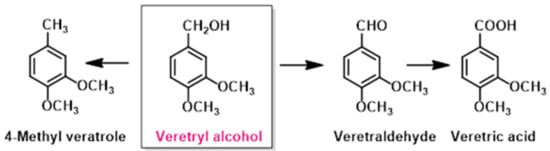
Scheme 1.
Oxidation of veratryl alcohol to different products (the reaction also involved the formation of 4-methyl veratrole).

Table 3.
Catalytic activity of nitrogen-rich carbon nanotubes and commercial CNTs for veratryl alcohol oxidation.
The stability of N-CNTs 700 was evaluated in the veratryl alcohol oxidation. The catalyst was found to exhibit almost the same conversion and selectivity after eight cycles, and the results are summarized in Figure 3.
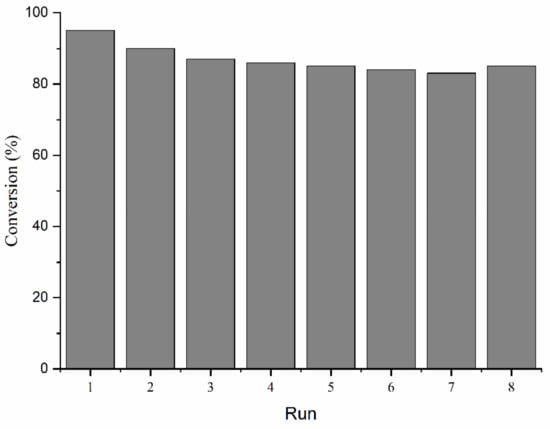
Figure 3.
Stability tests of NCNT700 in the veratryl alcohol oxidation.
4. Discussion
The formation of toluene under oxidative conditions is quite unique for base-free carbo-catalysts; therefore, the mechanism of veratryl alcohol oxidation was investigated using DFT calculations. The analysis was performed by choosing benzyl alcohol (BA) as a model compound in comparison to veratryl alcohol due to its facilitation of easily running the calculations, and the results are shown in Scheme 2. The conversion of BA into benzaldehyde is a facile process and easily proceeds in a forward direction. The formation of toluene in this oxidation reaction may be explained by the self-oxidation and reduction reaction of BA [31]. While investigating toluene formation via the disproportionation reaction, the barriers were found to be very high, and this pathway did not appear to be feasible. Therefore, it appears that there is no direct pathway from BA to toluene. Hence, the second option for toluene formation was considered. It was observed that toluene was formed through the formation of intermediate PhCH2*, which involves water formation. The formation of H2 is kinetically preferable (although another pathway leads directly to PhCH2* + 2 H2O). The results are shown in Scheme 2.
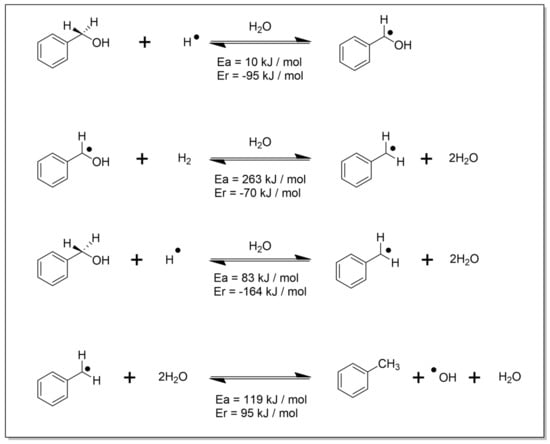
Scheme 2.
Plausible pathway leading to the formation of toluene in the reaction of BA with TBHP over NCNT. The homolytic cleavage of the –OH bond by free radicals or the homolytic cleavage of the C–H bond during oxidation reaction can generate H* in the reaction. These radicals can be stabilized on the catalyst surface during the reaction. During DFT analysis, stabilization from water molecules in the reaction mixture was considered (H3O*) for obtaining the reported results. The *OH generated in the end can be reused for generating the peroxide reagent.
The reaction involves the formation of radical intermediates, which are preferred in comparison to their ionic or charged analogues. The hydrogen radical that is involved in the reaction may appear during the BA oxidation process. The radical species may also be formed in presence of a radical initiator TBHP. Once this radical PhCH2* is formed, it may be stabilized on the catalyst surface along with other radical species. Recently, the formation of radicals has also been explained during the self-oxidation and reduction reaction of 5-HMF [32]. The reaction mechanism of 5-HMF reduction with HI involves a substitution–reduction process [32]. Initially, the -OH group is replaced by iodide, which is further reduced to form an alkane. During this process, hydrogen iodide is used, and iodine is generated simultaneously. The reaction also follows the radical pathway. Initially, iodine radicals are formed from iodine, which then attack the alcoholic group. This attack leads to the formation of a hydrogen radical and other reaction intermediates. Benzyl alcohol was used as a model substrate instead of unstable 5-hydroxymethylfurfural in this work, and it supports the claim for the evolution of H*. Another work by J. A. Puértolas has demonstrated that multi-walled carbon nanotubes (MWCNTS) have the tendency to combine with free radicals. MWCNTs were incorporated into ultrahigh molecular weight polyethylene via a ball milling process. MWCNTs were found to exhibit radical scavenging behavior. Electron spin resonance detection confirmed the radical scavenging characteristics of MWCNTs in response to radiation-induced radicals [33]. As the concentration of nanotubes increased, there was a significant reduction in the quantity of radicals generated during the gamma irradiation process. This observation validates the ability of MWCNTs to effectively scavenge radicals, demonstrating a concentration-dependent impact on the radical generation associated with gamma irradiation. Therefore, it is highly likely that the radicals formed in the reaction may be stabilized on catalyst’s surface. Moreover, Xiong et al. demonstrated that N-doped carbon nanotubes are efficient in nitrobenzene hydrogenation [34]. In particular, they showed that pyrrolic groups are the main active center of hydrogen chemisorption during the reaction. Therefore, we attribute the formation of toluene to the presence of pyrrolic groups on the NCNT catalysts, chemisorbing the H-specie generated during the dehydrogenation of benzyl alcohol.
5. Future Perspective
The development of a metal-free catalytic system based on nanocarbon materials, especially NCNTs, is very promising for sustainable catalytic systems. The metal-free nature of this catalytic system aligns perfectly with green chemistry principles and offers a potential avenue for environmentally friendly lignin valorization. The use of toxic metals in the design of catalysts can be minimized, and the fear of them leaching into the environment can be resolved. Future research should include attaining better control over surface functional groups for enhanced yields and the system’s capability to work effectively on lignin molecule. Moreover, the bulk reactions need to be optimized for increasing the industrial applicability of these catalysts or related NCNTs. The development of such catalytic systems can avoid waste production (especially regarding toxic metals). However, more insights are required to unravel the true behavior of this catalyst during the conversion. The radical formation needs to be ascertained in the near future to make them viable.
6. Conclusions
To conclude, nitrogen-rich carbon nanotubes NCNT700 and NCNT800 were prepared as metal-free catalysts for the oxidation of benzyl alcohol and veratryl alcohol. Nitrogen-rich sites such as pyridinic or pyrrolic sites in the carbon matrix were targeted to achieve the desirable conversion. The concentration of these active sites led to the different selectivity of the product. NCNT700 was found to be the best catalyst in terms of activity (186 h−1 and 104 h−1 for benzyl and veratryl alcohols, respectively) compared to NCT800 (54 h−1 and 37 h−1 for benzyl and veratryl alcohols, respectively), whereas the non-functionalized carbon nanotubes showed negligible activity for both alcohols. In terms of selectivity, the aldehyde was the main product in all cases. NCN700 showed a selectivity of 76% to benzaldehyde and 75% to veratraldehyde, whereas NCN800 showed a selectivity of 67% to benzaldehyde and 63% to veratraldehyde. Surprisingly, in addition to the oxidized products, both catalysts also promoted the formation of reduced product, such as toluene 4-methyl veratrol. In particular, NCNT 800, which presented a higher number of pyrrolic groups, showed a selectivity of 28% and 32% to toluene and 4-methyl veratrol, respectively, whereas NCNT700 showed a selectivity of 21% and 23% to toluene and 4-methyl veratrol, respectively. These products are unlikely to be formed by a disproportionation reaction and are probably formed by the transfer of H* trapped on the pyrrolic groups present on the catalyst surface. The catalyst can be found to be effective for the oxidation of lignin-derived alcohols into aldehydes and aromatic hydrocarbons, also showing good stability upon deactivation during recycling tests.
Author Contributions
Conceptualization, N.G. and A.V.; validation, O.K and I.B.; formal analysis, O.K. and I.B.; writing—original draft preparation, N.G.; writing—review and editing, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
N.G. thanks Chinese Academy of Sciences for the postdoctoral award (Grant number 2014FFGB0006).
Data Availability Statement
The data presented in this study are available from the authors.
Acknowledgments
A special thanks to Late Dang Sheng Su in IMR-SYNAL Shenyang for providing lab space to work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Getahun, T.; Verma, M.; Villa, A.; Gupta, N. Carbon Based Catalysts for the Hydrodeoxygenation of Lignin and Related Molecules: A Powerful Tool for the Generation of Non-Petroleum Chemical Products Including Hydrocarbons. Renew. Sustain. Energy Rev. 2020, 133, 110280. [Google Scholar] [CrossRef]
- Klein, I.; Saha, B.; Abu-Omar, M.M. Lignin Depolymerization over Ni/C Catalyst in Methanol, a Continuation: Effect of Substrate and Catalyst Loading. Catal. Sci. Technol. 2015, 5, 3242–3245. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin Depolymerization (LDP) in Alcohol over Nickel-Based Catalysts via a Fragmentation-Hydrogenolysis Process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Zakzeski, J.; Jongerius, A.L.; Weckhuysen, B.M. Transition Metal Catalyzed Oxidation of Alcell Lignin, Soda Lignin, and Lignin Model Compounds in Ionic Liquids. Green Chem. 2010, 12, 1225–1236. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Xie, X.; Yang, S.; Sun, L.; Li, T.; Chen, L.; Hua, D. Synthesis of Aromatic Monomers via Hydrogenolysis of Lignin over Nickel Catalyst Supported on Nitrogen-Doped Carbon Nanotubes. Fuel Process. Technol. 2023, 248, 107810. [Google Scholar] [CrossRef]
- Mushtaq, U.; Park, J.; Riaz, A.; Ranaware, V.; Khan, M.K.; Verma, D.; Kim, J. High-Yield Production of Deoxygenated Monomers from Kraft Lignin over ZnO-Co/N-CNTs in Water. ACS Sustain. Chem. Eng. 2021, 9, 3232–3245. [Google Scholar] [CrossRef]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z.; Ruan, T. Impact of Nitrogen Species and Content on the Catalytic Activity to C–O Bond Cleavage of Lignin over N-Doped Carbon Supported Ru-Based Catalyst. Fuel 2020, 278, 118324. [Google Scholar] [CrossRef]
- Jana, N.C.; Behera, S.; Maharana, S.K.; Behera, R.R.; Bagh, B. Selective Aerobic Oxidation of Biomass Model Compound Veratryl Alcohol Catalyzed by Air-Stable Copper(Ii) Complexes in Water. Catal. Sci. Technol. 2023, 13, 5422–5434. [Google Scholar] [CrossRef]
- Jana, N.C.; Sethi, S.; Saha, R.; Bagh, B. Aerobic Oxidation of Vanillyl Alcohol to Vanillin Catalyzed by Air-Stable and Recyclable Copper Complex and TEMPO under Base-Free Conditions. Green Chem. 2022, 24, 2542–2556. [Google Scholar] [CrossRef]
- Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S.S. Chemoselective Metal-Free Aerobic Alcohol Oxidation in Lignin. J. Am. Chem. Soc. 2013, 135, 6415–6418. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; Antonietti, M. Chemistry and Materials Options of Sustainable Carbon Materials Made by Hydrothermal Carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.-C. A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research. C 2018, 4, 54. [Google Scholar] [CrossRef]
- Mestl, G.; Maksimova, N.I.; Keller, N.; Roddatis, V.V.; Schlögl, R. Carbon Nanofilaments in Heterogeneous Catalysis: An Industrial Application for New Carbon Materials? Angew. Chem.—Int. Ed. 2001, 40, 2066–2068. [Google Scholar] [CrossRef]
- Su, D.S.; Wen, G.; Wu, S.; Peng, F.; Schlögl, R. Carbocatalysis in Liquid-Phase Reactions. Angew. Chem.—Int. Ed. 2017, 56, 936–964. [Google Scholar] [CrossRef]
- Zhang, J.; Su, D.S.; Blume, R.; Schlögl, R.; Wang, R.; Yang, X.; Gajović, A. Surface Chemistry and Catalytic Reactivity of a Nanodiamond in the Steam-Free Dehydrogenation of Ethylbenzene. Angew. Chem.—Int. Ed. 2010, 49, 8640–8644. [Google Scholar] [CrossRef]
- Barlocco, I.; Bellomi, S.; Tumiati, S.; Fumagalli, P.; Dimitratos, N.; Roldan, A.; Villa, A. Selective Decomposition of Hydrazine over Metal Free Carbonaceous Materials. Phys. Chem. Chem. Phys. 2022, 24, 3017–3029. [Google Scholar] [CrossRef]
- Barlocco, I.; Capelli, S.; Lu, X.; Tumiati, S.; Dimitratos, N.; Roldan, A.; Villa, A. Role of Defects in Carbon Materials during Metal-Free Formic Acid Dehydrogenation. Nanoscale 2020, 12, 22768–22777. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jia, H.P.; Bielawski, C.W. Graphene Oxide: A Convenient Carbocatalyst for Facilitating Oxidation and Hydration Reactions. Angew. Chem.—Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Luo, F.; Khoshi, M.R.; Rabie, E.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Garfunkel, E.; Szostak, M.; He, H. P-Doped Porous Carbon as Metal Free Catalysts for Selective Aerobic Oxidation with an Unexpected Mechanism. ACS Nano 2016, 10, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Sun, G.; Gao, Y.; Zhao, H.; Tang, P.; Tan, J.; Lu, A.H.; Ma, D. Direct Catalytic Oxidation of Benzene to Phenol over Metal-Free Graphene-Based Catalyst. Energy Environ. Sci. 2013, 6, 793–798. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, D.; Wang, C.; Guan, J.; Bao, X. Reduced Graphene Oxide as a Catalyst for Hydrogenation of Nitrobenzene at Room Temperature. Chem. Commun. 2011, 47, 2432–2434. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Su, D. Fabrication of Nitrogen-Modified Annealed Nanodiamond with Improved Catalytic Activity. ACS Nano 2014, 8, 7823–7833. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Gambarotti, C.; Carroccio, S.; Filippone, G.; Cicogna, F.; Guenzi, M. α-Tocopherol-Induced Radical Scavenging Activity in Carbon Nanotubes for Thermo-Oxidation Resistant Ultra-High Molecular Weight Polyethylene-Based Nanocomposites. Carbon N. Y. 2014, 74, 14–21. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree-Fock and Hybrid DFT Calculations. A “chain-of-Spheres” Algorithm for the Hartree-Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, Y.; Li, T.; Rong, N.; Jiang, H.; Shi, H.; Yang, W. Radical Induced Disproportionation of Alcohols Assisted by Iodide under Acidic Conditions. Green Chem. 2021, 23, 8108–8115. [Google Scholar] [CrossRef]
- Martínez-Morlanes, M.J.; Castell, P.; Alonso, P.J.; Martinez, M.T.; Puértolas, J.A. Multi-Walled Carbon Nanotubes Acting as Free Radical Scavengers in Gamma-Irradiated Ultrahigh Molecular Weight Polyethylene Composites. Carbon N. Y. 2012, 50, 2442–2452. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Z.; He, S.; Hao, F.; Yang, Y.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H. Nitrogen-Doped Carbon Nanotubes as a Highly Active Metal-Free Catalyst for Nitrobenzene Hydrogenation. Appl. Catal. B 2020, 260, 118105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).