Abstract
Photocatalytic carbon dioxide conversion is a promising method for generating carbon fuels, in which the most important thing is to adjust the catalyst material to improve the photocatalytic efficiency and selectivity to conversion products, but it is still very challenging. In order to enhance the efficiency of CO2 photoreduction, it is important to develop an appropriate photocatalyst. The present study focuses on developing a simple and effective hydrothermal reaction treatment to improve the catalytic efficiency of transition metal cobalt (Co) and organophosphonates. Photoexcited charge carriers are separated and transferred efficiently during this treatment, which enhances CO2 chemisorption. Under visible light exposure, the best performing catalyst, CoP-4, showed 2.4 times higher activity than Co3O4 (19.90 μmol h−1 g−1) for reducing CO2 into CO, with rates up to 47.16 μmol h−1 g−1. This approach provides a viable route to enhancing the efficiency of CO2 photoreduction.
1. Introduction
Carbon dioxide has been released into the atmosphere by fossil fuels, posing an imminent threat to the current energy system, as well as causing environmental problems [1,2,3,4]. In theory, transforming CO2 into fuel or chemical feedstock compounds could decrease the use of fossil fuels, alleviate atmospheric CO2 levels, and mitigate the greenhouse effect [5,6,7]. Efficient and selective photocatalytic CO2 conversion into valuable carbon-based fuels and chemicals, such as CO, HCOOH, CH3OH, and CH4, is a promising solution to environmental issues and the energy crisis. Despite the challenges posed by the high thermodynamic stability of the stable C-O double bond (750 kJ/mol) and the inefficient transportation of photoinduced carriers during photocatalysis, we are confident in our ability to achieve highly active and selective photocatalytic CO2 conversion, even in a diluted CO2 atmosphere. To overcome these bottlenecks, we have developed various strategies such as atom doping, designing peculiar nanoarchitecture, constructing heterojunctions, and defect engineering. These strategies aim to significantly enhance the adsorption and activation capability of CO2, enhance the accessibility of active sites, and shorten the charge transfer distances. Furthermore, the photocatalytic reduction of CO2 into CO offers a promising avenue for the efficient utilization of this greenhouse gas [8]. In addition to reducing atmospheric CO2 levels, photocatalytic CO2 reduction also provides a clean fuel source [9]. Among the existing CO2 conversion technologies, photocatalysis is highly advanced in converting CO2 into valuable solar fuels [10].
Because photocatalytic carbon dioxide reduction is a complex process and produces many kinds of products, it is important to study the reaction mechanism. The reaction pathway has a significant impact on the selectivity of the reduction products, especially for multi-carbon products [11,12]. The photocatalytic CO2 reduction pathways involve a series of elementary steps, including electron/proton transfer, C-O bond breaking, intermediate formation, and new bond formation [13,14]. Understanding the basic principle of the photocatalytic CO2 reduction reaction is of great importance to the development of photocatalysts. The mechanism of photocatalytic CO2 reduction can be summarized in three basic steps: (1) When the semiconductor is exposed to light, the absorbed energy triggers the excitation of electrons within the valence band, creating photogenerated electron (e−)–hole (h+) pairs; (2) The photogenerated electrons and holes are separated and transferred to the conduction band.; (3) There is a redox reaction between the photogenerated charge and the reactants adsorbed on the surface of the semiconductor [15,16]. By nature, the process of CO2 photoreduction is a multifaceted reaction that involves the coupled electron transfer of multiple protons, resulting in a range of potential products, including CO, CH4, HCHO, CH3OH, HCOOH, and compounds with multiple carbon atoms [17,18,19]. Specifically, CO serves as a crucial starting material for the production of a wide array of chemical compounds [20,21]. Therefore, it becomes extremely difficult to control the harvest of carbonaceous products. However, CO2 molecules are inert due to their inert chemistry [22], and the conversion efficiency of photocatalytic CO2 reduction has so far remained low. Therefore, the efficiency and selectivity of CO2 reduction using photocatalysts should be investigated.
So far, although numerous photocatalysts have been developed for CO2 reduction, such as TiO2 [23], Bi2MoO6 [24], g-C3N4 [25], CuIn5S8 [26], metal oxide, and carbide-surface-supported metal nanoparticles (NPs) [27], many still face challenges such as low generation rates, poor selectivity, and high costs. Cobalt-based [28,29] composites, including small atomic clusters [30], oxides, hydroxides [31], nitrides [32], and fractional complex fractions [33], are currently effective catalysts for CO2 reduction. Wang and colleagues reported cobalt-containing benzimidazole metal–organic frameworks (MOFs) as co-catalysts for enhanced CO2 capture and conversion. Out of the numerous options available, Co compounds stand out as one of the most promising catalysts. Li et al. [34] reported developing a new approach: using MOF-templated methods, they have designed innovative Fe-doped CoP hierarchical double-shelled nanocages for the highly effective reduction of CO2 using visible light. The distinctive double-shell hollow structure with layers can significantly decrease the charge transport distance and offer numerous reaction sites. Additionally, the incorporation of iron atom doping can decrease the activation energy of the CO2 by stabilizing the *COOH intermediate and facilitate CO2 desorption by disrupting the stability of the CO* adduct. Wang et al. [35,36] have created hierarchical N-doped carbon@NiCo2O4 nanoboxes using a multistep strategy involving a sacrificial template of Fe2O3 nanocubes. This unique architecture exhibited significantly improved activity and stability in the photocatalytic reduction of CO2. Hierarchical hollow heterostructures with ultrathin 2D nanosheet subunits are designed and synthesized for the photocatalytic reduction of carbon dioxide and water separation in suspension systems. This innovative material combines the structural and compositional benefits of hollow structures, ultrathin 2D nanosheets, and heterojunctions, resulting in significant performance enhancements for semiconductor photocatalysts in catalytic solar fuel production through CO2 conversion and water separation. However, the synthesis process reported above is divided into multiple steps, which is cumbersome, and the temperature requirement for synthesis is relatively high. Therefore, there is a requirement to discover a more straightforward approach for producing effective and durable catalysts for CO2 reduction.
Organophosphonic acid compounds are a class of compounds containing one or more methylphosphonic acid groups, and some studies have confirmed that organophosphonic acid compounds can be used to chelate and solidify heavy metal ions [37]. In transition-metal-catalyzed reactions, organophosphines serve primarily as ligands. Metal centers can be changed by altering the density of the electron clouds and the spatial distribution of the surrounding groups through the coordination of phosphine with the metal. As a result, the catalytic properties of the metal can be adjusted. Therefore, in transition-metal-catalyzed reactions, organophosphine ligands play a critical role and can be a decisive factor in determining the success or failure of the reaction or the selectivity of the product. Catalysts for CO2 reduction have been the subject of extensive research, involving the use of transition metal complexes modified with diverse ligands [38]. According to reports, tris(trimethylsilyl)phosphine is widely considered an efficient and stable Co catalyst for hydrogen precipitation, particularly for Ni2P, CoP, FeP, and MoP transition metal phosphides, due to its low potential and good electrical conductivity [39,40,41,42]. Inorganic–organic hybrid materials, known as metal phosphonates, have attracted great interest in coordination chemistry due to their versatility, exhibition of high stability, and potential applications in catalysis, adsorption, magnetic systems, and more. Their unique structural flexibility is particularly beneficial for promoting the formation of active catalytic sites in various reactions, including the notoriously difficult CO2 reduction process [43]. Cobalt phosphide (CoP) shows significant promise for enhancing CO2 photoreduction; it is characterized by high electrical conductivity and abundant redox-active sites. Fu et al. efficiently catalyzed the conversion of CO2 into CO using pure CoP, hybrid CoP/CNTs, and CoP/rGO in mixed aqueous solutions containing a Ru-based photosensitizer under visible light irradiation. The hybrid catalysts CoP/CNT and CoP/rGO show excellent catalytic activity in the reduction of CO2 into CO. The strong interactions between CoP and the carbon-supported materials as well as the partially adsorbed H2O molecules on the catalyst surface significantly increase the rates of CO generation [44,45].
In this study, a novel amorphous metal–organophosphonate photocatalyst (CoP-4) was successfully obtained using a simple solvent reaction in which the solvent was replaced with dimethylformamide (DMF). When exposed to visible light with [Ru(bpy)3]2+ as a photosensitizer, the resulting CoP-4 showed an excellent photocatalytic performance to promote CO2 reduction. We introduced a mechanism for CO2 reduction through photocatalysis. The selectivity and the entire process of photocatalytic CO2 reduction could be better understood. Physicochemical characterization showed that CoP-4 accelerated the photo-induced charge separation and migration, and the activity of the CoP-4 catalyst was increased 2.4-fold compared to that of Co3O4, and the rate of the reduction of CO2 into CO could reach 47.16 μmol h−1 g−1. This study opens up new possibilities for the synthesis of high-surface-area metal phosphonates using simple solvent reactions for catalysis and other applications.
2. Experimental
2.1. Materials
No additional purification was necessary since all chemicals were analytical-grade. The Co(NO3)2·6H2O and acetonitrile were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). The 3-phosphonopropionic acid (3-PPA), [Ru(bpy)3]Cl2·6H2O, and triethanolamine were bought from Aladdin Co., Ltd. (Shanghai, China). The N, N-dimethylformamide (DMF) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Preparation of CoP-N and Co3O4
The CoP photocatalysts were synthesized using the hydrothermal method. As a first step, 10.0 mL of DMF was dissolved with 2.0 mmol of Co(NO3)2·6H2O as solution A, while solution B was made by dissolving 2.0 mmol of 3-PPA in 20.0 mL of DMF. Subsequently, the aforementioned solutions were combined and stirred for 30 min. They were then heated at 180° for 2 h, 4 h, 8 h, and 12 h in a 100 mL Teflon-sealed autoclave, respectively, labeled as CoP-N (CoP-2, CoP-4, CoP-8, and CoP-12). Upon cooling the products to room temperature, following three rounds of washing with deionized water and ethanol, the final products were dried at 600 °C for 12 h. Co3O4 was obtained by directly heating 2 mmol Co(NO3)2·6H2O in a muffle furnace at 400 °C for 2 h at 2 °C per minute.
2.3. Material Characterization
To determine the crystal structure of the material, X-ray diffraction (XRD) analysis of the material was conducted using an X-ray powder diffractor (XRD-6100, Shimadzu, Kyoto, Japan) with a scanning rate of 5°/min. Furthermore, transmission electron microscopy (TEM, Hitachi, Tokyo, Japan, H-8100) and scanning electron microscopy (SEM, S-4800, Hitachi, Tokyo, Japan) were used to observe the morphology and dimensions of the materials. So as to determine the chemical state and elemental composition of the surface of the material, we conducted an analysis using the X-ray photoelectron spectra (XPS), carried out on an ESCALAB 250Xi spectrometer (Thermo Scientific Inc., Waltham, MA, USA). Fourier transform infrared spectroscopy (FT-IR, Avatar 360, Nicole, Brunswick, NC, USA) was used to identify the functional groups in the samples, ranging from 400 to 4000 cm−1. Additionally, an electrochemical workstation was utilized with a standard three-electrode system; the electrochemical impedance spectra (EIS) were examined along with photocurrents.
2.4. Characterization of CO2’s Photocatalytic Properties
In order to evaluate the catalyst’s photocatalytic CO2 reduction activity, the following steps were followed: firstly, 2 mg of the photocatalyst, 10 μmol of [Ru(bpy)3]Cl2·6H2O, 3 mL of acetonitrile, 2 mL of deionized water, and 1 mL of sacrificial agent were mixed thoroughly via sonication. CO2 was introduced into the sealed vessel, and other gases were purged to ensure continuous CO2 filling for 30 min. Subsequently, the sealed vessel was exposed to a 300 W Xenon lamp for 2 h. Gas chromatography (GC-7920) was used to measure the amount of photocatalysis products on the catalyst.
3. Discussion and Results
3.1. Morphology and Structure Characteristics
The XRD patterns of the prepared CoP-N (N = 2, 4, 8, 12) and the type of photocatalyst Co3O4 are shown in Figure 1a. Based on the diffraction patterns observed from the Co3O4 samples., the peaks appearing at 19.0°, 31.3°, 36.9°, 59.5°, and 65.4° correspond to the (111), (220), (311), (511), and (440) crystal planes of Co3O4, which are consistent with the standard phase Co3O4 (PDF No. 43-1003) [46]. The almost featureless XRD patterns of CoP synthesized in DMF demonstrate its amorphous nature (Figure 1a). By using DMF as a solvent instead of water, the growth of the compound was disrupted [47], and after hydrothermal treatment, the Co structure was destroyed, resulting in a decrease in crystallinity and the near-complete disappearance of the diffraction peaks, exhibiting an amorphous nature. Figure 1b shows the results of the FT-IR analysis of the prepared catalysts. The broad peak at 3460 cm−1 is associated with the O-H bond vibrations present in the OH functional group on the catalyst surface and in the adsorbed interlayer water molecules. The peak at 1633 cm−1 corresponds to the bending mode of the water molecules. The tensile vibration in the P=O bond and the vibration and bending modes in the CO32− are represented by peaks at 1073 cm−1 and 1462 cm−1, respectively. In the case of metal–oxygen (Co-O), the 576 cm−1 peak can be attributed to the stretching and bending vibrations, respectively. This phenomenon indicates that the CoP photocatalysts were successfully prepared in the absence of other impurities.
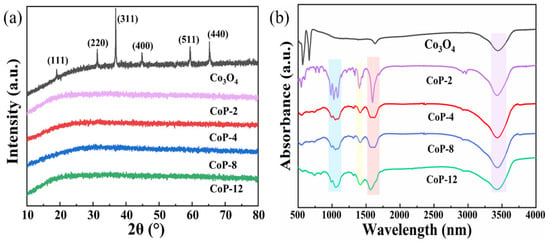
Figure 1.
XRD patterns (a) and FT-IR spectra (b) of CoP-N and Co3O4.
The preparation of the photocatalysts and their morphological properties were investigated by hand using SEM and TEM. According to Figure 2a, the SEM images show that the CoP-4 photocatalyst exhibited an irregular particle structure and a high utilization rate due to its large specific surface area. Further details of the photocatalyst structure were observed in the TEM images. As shown in Figure 2b, the CoP-4 exhibits an ultrathin and nearly transparent morphology.
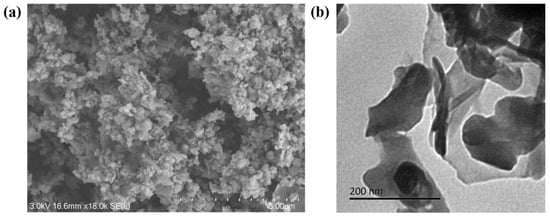
Figure 2.
SEM (a) and TEM (b) image of CoP-4 sample, respectively.
X-ray photoelectron spectra (XPS) were used to investigate the CoP nanophotocatalysts’ surface structure and chemical state. As shown in Figure 3a, the measured spectra proved the presence of Co, P, C, and O in the CoP-4 nanophotocatalyst. As presented in Figure 2a, the high-resolution XPS of Co 2p in CoP@C can be separated into four peaks: the main peaks at 780.6 and 796.4 eV in the spectrum of Co 2p’s XPS of CoP correspond to Co 2p3/2 and Co 2p1/2 of CoP, respectively, while the oscillatory satellite peak is attributed to 786.5 and 801.6 eV [48]. It can be seen from Figure 3b that the low-energy peak of CoP-4, with a binding energy of 782.8 eV, is positively shifted compared to that of metallic Co (778.1–778.2 eV) [49], indicating that the Co-to-P charge transfer is reduced, aligned with Co 2p3/2, and the higher-energy peak at 796.4 eV represents the Co 2p1/2 electron [50]. Furthermore, the two remaining peaks at 782.8 and 796.4 eV are associated with the Co-O species, which could result from the partial oxidation of the surface CoP-4 when exposed to air [51]. Accordingly, the binding energies of the P 2p XPS spectra at 133.0 and 131.9 eV correspond to the P-O and P-C bonds in organophosphine ligands (Figure 3c) [52]. This can be further demonstrated by the O1s spectra (Figure 3d): the P-O bonds are represented by the peak at 532.0 eV. Furthermore, H2O and O-H are responsible for the binding energy at 534.2 eV.
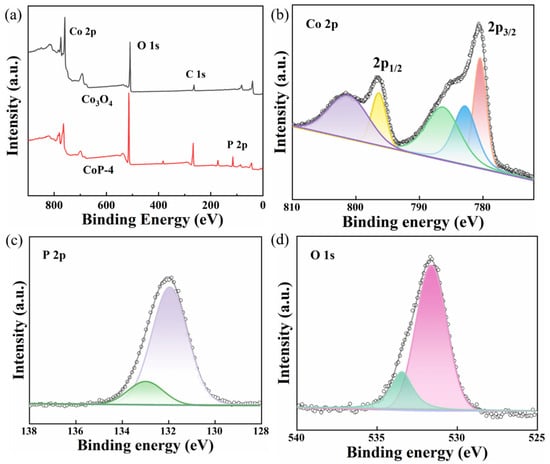
Figure 3.
Photocatalyst XPS spectra (a) and as-prepared XPS spectra of Co 2p (b), P 2p (c), and O 1s (d).
3.2. Photocatalytic Activity Investigation
The catalytic activity of the CoP-4 catalyst for CO2 reduction was assessed under visible light and mild reaction conditions. A solution of H2O/acetonitrile and TEOA were used as the reaction solvent and photoactivator, respectively, for the reaction of [Ru(bpy)3]2+, H2O, and TEOA. CO was produced without measurable hydrocarbons from the CO2 photoreduction system (Figure 4a,b), in line with previous findings in comparable systems [45]. This is due to the fact that the CoP-4 sample produces the highest CO release rate of 47.16 μmol h−1 g−1, which is about 2.4 times higher than that of Co3O4. Based on these results, we conclude that CoP catalysts are effective and selective in reducing CO2 and can be significantly improved after long-term high-temperature treatment. The reaction mixture was repeatedly cycled with dispersed catalysts as a cycling test, thereby exposing fewer catalytically active sites in the redox reaction [53]. The introduction of phosphonates led to a further enhancement in photocatalytic performance. To check the stability of the CoP-4 catalyst for the CO2 reduction reaction, the reaction mixture was cycled repeatedly with dispersed catalysts as a cycling test. The performance of CoP-4 in reducing CO2 emissions did not decrease significantly in five consecutive runs (Figure 4c), demonstrating the excellent continuity of CoP-4 in the photo-oxidation system for reduction. It is noteworthy that CoP contributes to considerable CO production (47.16 μmol h−1g−1), which is superior to the reported multiphase catalysts under comparable conditions (Figure 4d and Table 1). The CO2 reduction reaction was then studied without [Ru(bpy)3]2+. The findings revealed that the rate of CO precipitation decreased in the absence of the photosensitizer (Figure S1), suggesting that the CO2 reduction catalysis is a visible-light-excited dye sensitization process. Only adding [Ru(bpy)3]2+, the efficiency of CO is close to 0, indicating that Ru plays a small role in CO2 reduction. Moreover, the rate of CO production increased upon the addition of CoP-4, emphasizing the crucial role of transition metal phosphide co-catalysts in promoting efficient CO2 reduction. We synthesized organophosphate photocatalysts for catalytic carbon dioxide reduction using the hydrothermal method to form CoP photocatalysts in the complexes. The prepared CoP exhibited a good photocatalytic performance for carbon dioxide reduction. Among the Co3O4 and CoP-4 photocatalysts, CoP-4 obtained excellent photoactivity. A series of characterizations showed that the introduction of organophosphates enhanced the active site, as well as the charge separation/transfer; resulted in the outstanding photoreduction of CO2; and effectively lowered the energy barrier for the formation of the intermediates required for CO2 adsorption.
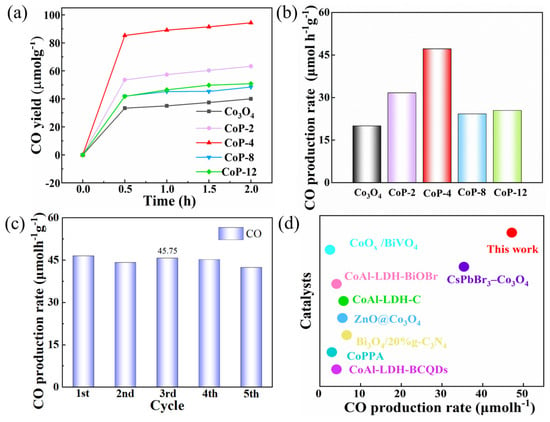
Figure 4.
(a) Photocatalytic CO2 reduction CO yield, (b) photocatalytic CO2 reduction production rate, (c) CO production rate in stability testing of CoP-4, (d) comparison of CO2 photoreduction performance between the CoP and previously reported photocatalysts.

Table 1.
Comparison of CO2 photoreduction performance between the CoP-4 and previously reported photocatalysts.
3.3. Electrocatalytic Activity Investigation
Generally speaking, the higher the photocurrent, the higher the electron–hole separation efficiency and the higher the photocatalytic activity. To investigate the migration and separation behavior, as well as to verify the photoreactivity, we measured the photocurrent in CoP-4 and Co3O4 with photogenic supports and holes. CoP-4 and Co3O4 in response to visible light irradiation are shown in Figure 5a under four intermittent photocurrent response cycles. As the photocurrent increases, the separation efficiency increases [62]. In terms of the photocurrent response, CoP-4 shows a greater response: CoP-4 exhibited a current density as high as ca. 3.2 μA/cm2 under visible light irradiation, outperforming the other counterparts, which indicates a lower carrier complexation rate, enhancing photocatalysis greatly [63]. This result indicated the effective transport of light-induced charge carriers, which is also highly consistent with the PL measurements. CoP-4 displays a good photophysical stability after several cycles, indicating its stability. Electrochemical impedance spectroscopy (EIS) was employed to examine the photocatalyst’s charge transfer capacity. From Figure 5b, it can be concluded that CoP-4 has a small arc radius, which indicates a low carrier complexation rate. This may be due to the fact that organophosphorides act as effective electron transfer channels in the CoP-4 nanoparticles, promoting charge separation and thus significantly reducing the carrier complexation rate to improve the photocatalytic performance. Among the comparison samples, CoP-4 has the smallest semicircle diameter, indicating the lowest charge transfer resistance. The above results confirm that the successful recombination of transition metals and organic phosphonates can promote the separation and transfer of photogenerated carriers. As photogenerated electrons and holes complex in the photoluminescence (PL) spectra, photoluminescence analysis reveals how charge carriers trap, transfer, and separate, as well as revealing the lifetime of the charge carriers in the samples. Conversely, the lower photoluminescence intensity implies that more carriers are involved in the process of photocatalysis. In Figure 5c, the CoP-4 and Co3O4 photoluminescence spectra are shown. The photoluminescence intensity of CoP-4 is lower than that of Co3O4, with the peak concentrated at 430 nm. Lower electron–hole complexation results in weaker PL emission, so photoexcited electrons can be transferred faster at the interface, inhibiting the complexation of electrons and holes. CoP-4 exhibits a significantly smaller photoluminescence strength compared to individual Co3O4, indicating that the fabrication of photocatalysts can effectively reduce the recombination of photogenerated charge carriers during photochemical reactions.
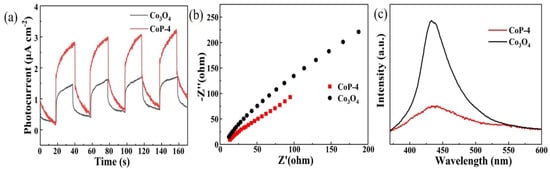
Figure 5.
(a) Photocurrent response images, (b) electrochemical impedance spectra images, and (c) PL spectra of CoP and Co3O4 samples, respectively.
3.4. Mechanism of the Photocatalytic Reduction of CO2
For Co3O4 and CoP-4, the VB values are determined to be 1.28 eV and 1.04 eV for the XPS valence band (VB) spectra in Figure 6a. According to the Kubelka–Munk function (αhν)2 against Eg, Co3O4 and CoP have Eg values of 1.78 and 1.62 eV, respectively (Figure 6b).
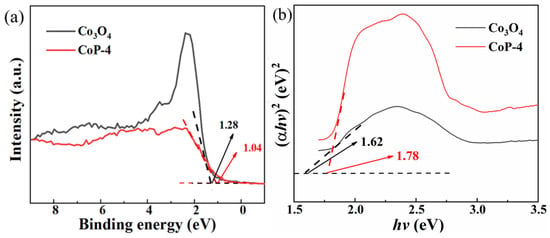
Figure 6.
(a) VB XPS spectra and (b) Kubelka–Munk plots of the Co3O4 and CoP-4.
Last but not least, we measured the flat band potential of CoP-4 in order to better understand how the reaction occurs. To evaluate the potential of Co-based materials for the photocatalytic reduction of CO2, Mott–Schottky tests were performed. Mott–Schottky analysis reveals that the flat band potential for the CoP-4 photocatalyst is −0.56 V (relative to NHE at pH 7.0, Figure 7a), which is appropriate for accepting electrons from the Ru complex in order to drive the CO2-to-CO conversion reaction (Equations (2) and (3)), as seen in Figure 7b [64]. shows the schematic of the band structure. This indicates that the CoP-4 catalysts have the appropriate potential to accept the photogenerated electrons of the excited photosensitizer and thus facilitate the catalytic reduction of CO2 into CO. As shown in Figure 7a, the measured flat band potential of CoP-4 is about −0.56 V (versus the normal Ag/AgCl electrode, NHE), which is more negative than the reduction potential of CO2 into CO (−0.53 V) on the part of the NHE [65]. In contrast to Co3O4, CoP-4 has a negative CB, indicating a strong photocatalytic CO2 reduction capability. The porous nanomaterials are beneficial to the adsorption of CO2 [66]. Photoinduced electrons were transferred to the CoP-4 cocatalyst following the visible light irradiation of the Ru photosensitizer (Equation (1)). The CoP-4 solid is then reduced into CO by the CO2 molecules adsorbed on the surface (Equations (2) and (3)). Photocatalysis is completed by quenching the oxidized Ru dye with TEOA, which serves as an electron donor (Equation (4)).
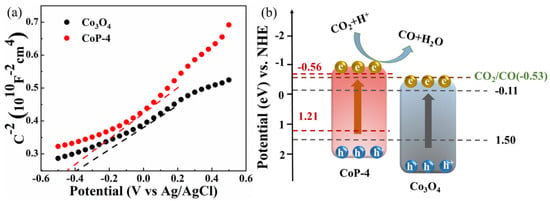
Figure 7.
(a) Mott–Schottky plots and (b) band structure diagrams of CoP-4 and Co3O4.
Hence, we propose a potential mechanism for photosensitized CO2 reduction (Figure 8). Photoinduced energy transfer occurs when the photosensitizer [Ru(bpy)3]2+ is exposed to visible light and is then reduced by TEOA to generate [Ru(bpy)3]+, the reduced photosensitizer. Subsequently, electrons from the π orbital of [Ru(bpy)3] + are transferred to CoP-4 and rapidly move to the Co site within CoP, facilitated by the space charge region. Electrophilic CO2 is activated by accepting electrons from high-electron-density Co sites and subsequently reduced into CO through an electron transfer process [67]. Additionally, the desorption of products from the active site is a crucial characteristic of catalytically active materials.
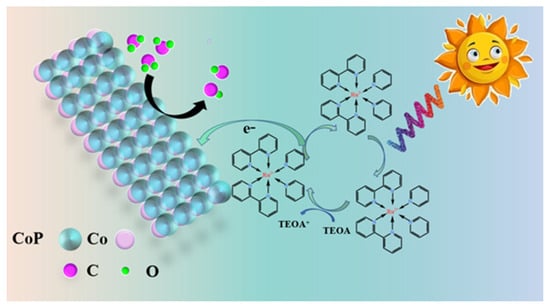
Figure 8.
Schematic diagram of the process mechanism of photodecomposition of CO2 into CO using CoP catalyst.
4. Conclusions
In conclusion, we synthesized organophosphate photocatalysts for catalytic carbon dioxide reduction using the hydrothermal method to form CoP photocatalysts in complexes. The prepared CoP exhibited a good photocatalytic performance for carbon dioxide reduction. Among the Co3O4 and CoP-4 photocatalysts, CoP-4 obtained an excellent photoactivity with a CO release rate of 47.16 μmol−1 h−1g. A series of characterizations showed that the introduction of organophosphates enhanced the active site, as well as the charge separation/transfer; resulted in the outstanding photoreduction of CO2; and effectively lowered the energy barrier for the formation of the intermediates required for CO2 adsorption. This study may offer innovative concepts for the advancement of novel composite photocatalysts for the photocatalytic reduction of CO2. The photocatalytic reduction of carbon dioxide into fuel products may not only reduce carbon dioxide emissions but also allow clean solar energy to be used to provide green chemical energy and to achieve a high catalytic efficiency and high selectivity toward high value-added products. A lot of effort needs to be invested for the exploration and fulfillment of these four points. This work may shed some light on the precise modulation of catalytic materials for CO2 reduction in order to achieve high efficiency in the production of carbon fuels.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/c10010012/s1, Figure S1: Photocatalytic CO2 performance under different conditions.
Author Contributions
Conceptualization, methodology, and formal analysis were carried out by C.Z., F.W., Y.T., B.C. and J.L.; material characterization was undertaken by J.H., W.X. and Y.H.; writing—original draft preparation, manuscript review, supervision, and funding acquisition were the responsibility of C.Z. and G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (21901119), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (21KJB530010), the Open Project of Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology (2023K004, 2021K006), Student Practice Innovation and Training Program of Nanjing Forestry University (2023NFUSPITP0617), and the Start-up Fund from Nanjing Forestry University.
Data Availability Statement
The data can be made available upon request.
Acknowledgments
The authors also would like to thank Shiyanjia Lab (https://www.shiyanjia.com) and the Analysis Test Center of Nanjing Forestry University for their help with the testing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hu, X.M.; Daasbjerg, K. Carbon dioxide efficiently converted to methanol. Nature 2019, 575, 598–599. [Google Scholar] [CrossRef]
- Lei, Q.; Yuan, H.; Du, J.; Ming, M.; Yang, S.; Chen, Y.; Lei, J.; Han, Z. Photocatalytic CO2 reduction with aminoanthraquinone organic dyes. Nat. Commun. 2023, 14, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated Bilayer MOF Enables High-Selectivity Photocatalytic Reduction of CO2 to CO. Adv. Mater. 2023, 35, 2209814. [Google Scholar] [CrossRef]
- Lobus, N.V.; Knyazeva, M.A.; Popova, A.F.; Kulikovskiy, M.S. Carbon Footprint Reduction and Climate Change Mitigation: A Review of the Approaches, Technologies, and Implementation Challenges. C J. Carbon Res. 2023, 9, 120–147. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.M.; Ng, B.J.; Er, C.C.; Kong, X.Y.; Tan, L.L.; Mohamed, A.R.; Chai, S.P. Synergistic Hybridization of Twin-Induced Red/Black Phosphorus and Tungsten Oxide as Homo–Hetero Dynamic Dual Junctions for Z-Scheme CO2 Photoreduction. ACS Appl. Energy Mater. 2022, 5, 15257–15268. [Google Scholar] [CrossRef]
- Zhai, R.; Zhang, L.; Gu, M.; Zhao, X.; Zhang, B.; Cheng, Y.; Zhang, J. A review of phosphorus structures as CO2 reduction photocatalysts. Small 2023, 19, 2207840. [Google Scholar] [CrossRef]
- Qiu, L.-Q.; Yao, X.; Zhang, Y.-K.; Li, H.-R.; He, L.-N. Advancements and challenges in reductive conversion of carbon dioxide via thermo-/photocatalysis. J. Org. Chem. 2022, 88, 4942–4964. [Google Scholar] [CrossRef]
- Dong, K.; Razzaq, R.; Hu, Y.; Ding, K. Homogeneous reduction of carbon dioxide with hydrogen. Chem. Transform. Carbon Dioxide 2018, 376, 203–228. [Google Scholar]
- Abbas, T.; Yahya, H.S.M.; Amin, N.A.S. Insights and Progress on Photocatalytic and Photoelectrocatalytic Reactor Configurations and Materials for CO2 Reduction to Solar Fuels. Energy Fuels 2023, 37, 18330–18368. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, M.; Zheng, N.; He, X.; Hu, R.; Wang, R.; Zhou, Q.; Hu, Z. Promoting the protonation step on the interface of titanium dioxide for selective photocatalytic reduction of CO2 to CH4 by using red phosphorus quantum dots. Nano Res. 2022, 15, 3042–3049. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, J.; Zhu, X.; Li, Q.; Yu, Q.; El-alami, W.; Wang, C.; She, Y.; Qian, J.; Xu, H. Cryo-induced closely bonded heterostructure for effective CO2 conversion: The case of ultrathin BP nanosheets/g-C3N4. J. Energy Chem. 2020, 49, 89–95. [Google Scholar] [CrossRef]
- Li, L.; Guo, H.; Yao, G.; Hu, C.; Liu, C.; Tian, Z.; Li, B.; Zhang, Q.; Chen, L. Visible/infrared light-driven high-efficiency CO2 conversion into ethane based on a B–Co synergistic catalyst. J. Mater. Chem. A 2020, 8, 22327–22334. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Z.; Wang, L. Photocatalytic and photoelectrochemical carbon dioxide reductions toward value-added multicarbon products. ACS EST Eng. 2021, 2, 975–988. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: Achievements, challenges, and opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, B.; Ran, J.; Davey, K.; Qiao, S.Z. Atomic-level reactive sites for semiconductor-based photocatalytic CO2 reduction. Adv. Energy. Mater. 2020, 10, 1903879. [Google Scholar] [CrossRef]
- Han, C.; Wang, B.; Wu, N.; Shen, S.; Wang, Y. Deep and selective photoreduction of CO2 to CH4 over ultrafine Pt nanoparticles-decorated SiC nanosheets. Appl. Surf. Sci. 2020, 515, 1459522. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Xu, J.; Shao, Y.; Wu, J.; Xu, X.; Pan, Y.; Ju, H.; Zhu, J.; Xie, Y. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 2019, 4, 690–699. [Google Scholar] [CrossRef]
- Yao, S.; Liu, J.; Liu, F.; Wang, B.; Ding, Y.; Li, L.; Liu, C.; Huang, F.; Fang, J.; Lin, Z. Robust route to photocatalytic nitrogen fixation mediated by capitalizing on defect-tailored InVO4 nanosheets. Environ. Sci. Nano 2022, 9, 1996–2005. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Lai, C.; Xia, T.; Zhang, B.; Zhou, X. Challenges and perspectives of covalent organic frameworks for advanced alkali-metal ion batteries. Sci. China Chem. 2021, 64, 1267–1282. [Google Scholar] [CrossRef]
- Yao, S.; He, J.; Gao, F.; Wang, H.; Lin, J.; Bai, Y.; Fang, J.; Zhu, F.; Huang, F.; Wang, M. Highly selective semiconductor photocatalysis for CO2 reduction. J. Mater. Chem. A 2023, 11, 12539–12558. [Google Scholar] [CrossRef]
- Tan, L.; Xu, S.M.; Wang, Z.; Xu, Y.; Wang, X.; Hao, X.; Bai, S.; Ning, C.; Wang, Y.; Zhang, W. Highly selective photoreduction of CO2 with suppressing H2 evolution over monolayer layered double hydroxide under irradiation above 600 nm. Angew. Chem. Int. Ed. 2019, 58, 11860–11867. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Sheng, W.; Fu, Q.; Ge, J. Enhancement of CO2 photoreduction efficiency by supporting blue TiO2 with photonic crystal substrate. Nano Res. 2023, 16, 9310–9317. [Google Scholar] [CrossRef]
- Li, S.; Bai, L.; Ji, N.; Yu, S.; Lin, S.; Tian, N.; Huang, H. Ferroelectric polarization and thin-layered structure synergistically promoting CO2 photoreduction of Bi2MoO6. J. Mater. Chem. A 2020, 8, 9268–9277. [Google Scholar] [CrossRef]
- Raza, A.; Haidry, A.A.; Yao, Z.; Saleem, M.F.; Alothman, A.A.; Mohammad, S. Synergistic effect of CuO and Sr doped g-C3N4 for CO2 photoreduction into hydrocarbon fuels. Chem Eng. J. 2023, 480, 148162. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Liu, T.; Cai, S.; Zou, X.; Jiang, J.; Mei, Z.; Gao, Z.; Guo, H. Rich S vacant g-C3N4@ CuIn5S8 hollow heterojunction for highly efficient selective photocatalytic CO2 reduction. Chem. Eng. J. 2021, 424, 130325. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Solà, M.; Poater, A. Carbon dioxide conversion on supported metal nanoparticles: A brief review. Catalysts 2023, 13, 305. [Google Scholar] [CrossRef]
- Parastaev, A.; Muravev, V.; Osta, E.H.; van Hoof, A.J.; Kimpel, T.F.; Kosinov, N.; Hensen, E.J. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 2020, 3, 526–533. [Google Scholar] [CrossRef]
- Ruland, H.; Song, H.; Laudenschleger, D.; Stürmer, S.; Schmidt, S.; He, J.; Kähler, K.; Muhler, M.; Schlögl, R. CO2 hydrogenation with Cu/ZnO/Al2O3: A benchmark study. ChemCatChem 2020, 12, 3216–3222. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Huang, L.B.; Liu, X.Z.; Zhang, Q.H.; He, C.; Wu, Z.Y.; Zhang, L.J.; Wu, J.; Yang, W. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10, 1278. [Google Scholar] [CrossRef]
- Sun, X.; Chen, C.; Liu, S.; Hong, S.; Zhu, Q.; Qian, Q.; Han, B.; Zhang, J.; Zheng, L. Aqueous CO2 Reduction with High Efficiency Using α-Co(OH)2-Supported Atomic Ir Electrocatalysts. Angew. Chem. Int. Ed. 2019, 58, 4669–4673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Zhang, S.L.; Lou, X.W. Formation of hierarchical FeCoS2–CoS2 double-shelled nanotubes with enhanced performance for photocatalytic reduction of CO2. Angew. Chem. Int. Ed. 2020, 59, 11918–11922. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Cheng, S.; Cometto, C.; Anxolabehere-Mallart, E.; Ng, S.M.; Ko, C.C.; Liu, G.; Chen, L.; Robert, M.; Lau, T.C. Highly efficient and selective photocatalytic CO2 reduction by iron and cobalt quaterpyridine complexes. J. Am. Chem. Soc. 2016, 38, 9413–9416. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Qian, G.; Kong, X.-P.; Zhao, X.; Hou, T.; Chen, L.; Fang, R.; Li, Y. Hierarchical double-shelled CoP nanocages for efficient visible-light-driven CO2 reduction. ACS Appl. Mater. Inter. 2021, 13, 45609–45618. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, B.Y.; Wang, X.; Lou, X.W.D. Formation of hierarchical Co9S8@ZnIn2S4 heterostructured cages as an efficient photocatalyst for hydrogen evolution. J. Am. Chem. Soc. 2018, 140, 15145–15148. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zang, S.Q.; Lou, X.W. Hierarchical hollow heterostructures for photocatalytic CO2 reduction and water splitting. Small Methods 2020, 4, 1900586. [Google Scholar] [CrossRef]
- Lancellotti, I.; Ponzoni, C.; Barbieri, L.; Leonelli, C. Alkali activation processes for incinerator residues management. Waste Manag. 2013, 33, 1740–1749. [Google Scholar] [CrossRef]
- Leung, C.F.; Ho, P.Y. Molecular catalysis for utilizing CO2 in fuel electro-generation and in chemical feedstock. Catalysts 2019, 9, 760. [Google Scholar] [CrossRef]
- Dong, Y.; Kong, L.; Wang, G.; Jiang, P.; Zhao, N.; Zhang, H. Photochemical synthesis of CoxP as cocatalyst for boosting photocatalytic H2 production via spatial charge separation. Appl. Catal. B Environ. 2017, 211, 245–251. [Google Scholar] [CrossRef]
- Fung, C.M.; Er, C.C.; Tan, L.L.; Mohamed, A.R.; Chai, S.P. Red phosphorus: An up-and-coming photocatalyst on the horizon for sustainable energy development and environmental remediation. Chem. Rev. 2021, 122, 3879–3965. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, Q.; Tang, C.; Asiri, A.M.; Sun, X. Ni2P nanoparticle films supported on a Ti plate as an efficient hydrogen evolution cathode. Nanoscale 2014, 6, 11031–11034. [Google Scholar] [CrossRef]
- Wang, P.; Wu, T.; Wang, C.; Hou, J.; Qian, J.; Ao, Y. Combining heterojunction engineering with surface cocatalyst modification to synergistically enhance the photocatalytic hydrogen evolution performance of cadmium sulfide nanorods. ACS Sustain. Chem. Eng. 2017, 5, 7670–7677. [Google Scholar] [CrossRef]
- Liu, G.; Sheng, Y.; Ager, J.W.; Kraft, M.; Xu, R. Research advances towards large-scale solar hydrogen production from water. Energy Chem. 2019, 1, 100014. [Google Scholar] [CrossRef]
- Fu, Z.C.; Xu, R.C.; Moore, J.T.; Liang, F.; Nie, X.C.; Mi, C.; Mo, J.; Xu, Y.; Xu, Q.Q.; Yang, Z. Highly efficient photocatalytic system constructed from CoP/Carbon nanotubes or graphene for visible-light-driven CO2 reduction. Chem. A Eur. J. 2018, 24, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Lou, X.W. Dispersed nickel cobalt oxyphosphide nanoparticles confined in multichannel hollow carbon fibers for photocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2019, 59, 17236–17240. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, P.; Zhang, H.; Zhao, J.; Shi, H.; Huang, Y.; Yang, H. Oxygen vacancies in Co3O4 promote CO2 photoreduction. Appl. Catal. B Environ. 2022, 300, 120729. [Google Scholar] [CrossRef]
- Xing, W.; Yin, S.; Tu, W.; Liu, G.; Wu, S.; Wang, H.; Kraft, M.; Wu, G.; Xu, R. Rational synthesis of amorphous iron-nickel phosphonates for highly efficient photocatalytic water oxidation with almost 100% yield. Angew. Chem. Int. Ed. 2020, 59, 1171–1175. [Google Scholar] [CrossRef]
- Pan, Z.; Niu, P.; Hou, Y.; Fang, Y.; Liu, M.; Wang, X. LiCl as Phase-Transfer Catalysts to Synthesize Thin Co2P Nanosheets for Oxygen Evolution Reaction. ChemSusChem 2019, 12, 1911–1915. [Google Scholar] [CrossRef]
- Hu, E.; Ning, J.; Zhao, D.; Xu, C.; Lin, Y.; Zhong, Y.; Zhang, Z.; Wang, Y.; Hu, Y. A Room-Temperature Postsynthetic Ligand Exchange Strategy to Construct Mesoporous Fe-Doped CoP Hollow Triangle Plate Arrays for Efficient Electrocatalytic Water Splitting. Small 2018, 14, 1704233. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Y.; Wang, C.J.; Lv, X.J.; Fu, W.F. Spectacular photocatalytic hydrogen evolution using metal-phosphide/CdS hybrid catalysts under sunlight irradiation. Chem. Commun. 2015, 51, 8708–8711. [Google Scholar] [CrossRef]
- Boppella, R.; Tan, J.; Yang, W.; Moon, J. Homologous CoP/NiCoP heterostructure on N-doped carbon for highly efficient and pH-universal hydrogen evolution electrocatalysis. Adv. Funct. Mater. 2019, 29, 1807976. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Yuan, Z.Y.; Zhu, Y.P.; Ma, T.Y. Titanium phosphonate based metal–organic frameworks with hierarchical porosity for enhanced photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2018, 57, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Pan, Z.; Wang, S.; Wang, X. Tuning crystallinity and surface hydrophobicity of a cobalt phosphide cocatalyst to boost CO2 photoreduction performance. ChemSusChem 2021, 14, 1302–1307. [Google Scholar] [CrossRef]
- Li, J.; Gao, G.; Liu, Y.; Li, Y.; Liu, Z. Highly-interspersed biomass-derived carbon quantum dots onto floral CoAl-LDH for significantly enhanced CO2 photoreduction into CO and CH4. J. CO2 Util. 2022, 65, 102257. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Qin, X.; Zhang, X.; Dai, Y.; Huang, B. Two transition metal phosphonate photocatalysts for H2 evolution and CO2 reduction. Chem. Commun. 2018, 54, 7195–7198. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, X.; Ge, T.; Xie, H.; Sun, R.; Su, F.; Li, X.; Ye, L. Realizing efficient CO2 photoreduction in Bi3O4Cl: Constructing van der Waals heterostructure with g-C3N4. Chem. Eng. J. 2021, 409, 128178. [Google Scholar] [CrossRef]
- Wang, T.; Shi, L.; Tang, J.; Malgras, V.; Asahina, S.; Liu, G.; Zhang, H.; Meng, X.; Chang, K.; He, J. A Co3O4-embedded porous ZnO rhombic dodecahedron prepared by the use of zeolitic imidazolate frameworks as precursors for CO2 photoreduction. Nanoscale 2016, 8, 6712–6720. [Google Scholar] [CrossRef]
- Yang, J.; Gao, G.; Zhu, Z.; Yu, X. Biochar modified Co-Al LDH for enhancing photocatalytic reduction CO2 performance and mechanism insight. Res. Chem. Intermed. 2022, 48, 2313–2323. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, D.; Qin, Y.; Xie, Y.; Ling, Y.; Ye, H.; Zhang, Y. Facile construction of BiOBr/CoAl-LDH heterojunctions with suppressed Z-axis growth for efficient photoreduction of CO2. Sep. Purif. Technol. 2022, 302, 122090. [Google Scholar] [CrossRef]
- Zhong, X.; Liang, X.; Lin, X.; Wang, J.; Shahid, M.Z.; Li, Z. A new 0D-2D CsPbBr3-Co3O4 heterostructure photocatalyst with efficient charge separation for photocatalytic CO2 reduction. Inorg. Chem. Front. 2023, 10, 3273–3283. [Google Scholar] [CrossRef]
- Yu, X.; Ordomsky, V.; Khodakov, A. Selective Deposition of Cobalt and Copper Oxides on BiVO4 Facets for Enhancement of CO2 Photocatalytic Reduction to Hydrocarbons. ChemCatChem 2020, 12, 740–749. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, E.; Shi, J.; Lin, X.; Sheng, L.; Zhang, M.; Wang, L.; Chen, J. A direct one-step synthesis of ultrathin g-C3N4 nanosheets from thiourea for boosting solar photocatalytic H2 evolution. Int. J. Hydrog. Energy 2019, 44, 7194–7204. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W.; Gao, Y.; Liu, S. Effects of sodium oleate on synthesis and photocatalytic activity of Bi2WO6/Bi2O3@RGO. J. Mater. Sci. Mater. Electron. 2017, 28, 14949–14953. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Wang, X. Development of a stable MnCo2O4 cocatalyst for photocatalytic CO2 reduction with visible light. ACS Appl. Mater. Inter. 2015, 7, 4327–4335. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, P.; Tian, D.; Zhang, M.; Dai, W.; Zou, J.; Luo, S.; Luo, X. Co engineered CoP catalyst for photochemical CO2 reduction with accelerated electron transfer endowed by the space-charge region. J. Colloid Interface Sci. 2023, 648, 389–396. [Google Scholar] [CrossRef]
- Reljic, S.; Martinez-Escandell, M.; Silvestre-Albero, J. Effect of porosity and surface chemistry on CO2 and CH4 adsorption in S-doped and S-/O-co-doped porous carbons. C J. Carbon Res. 2022, 8, 41–59. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, S.; Luo, M.; Wei, Q.; Chen, E.; He, L.; Lin, Q. Photochemical in-situ exfoliation of metal-organic frameworks for enhanced visible-light-driven CO2 reduction. Angew. Chem. Int. Ed. 2020, 52, 23588–23592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).