- Review
Mechanisms at the Intersection of lncRNA and m6A Biology
- Samuel J. Gonzalez,
- Edgardo Linares and
- Aaron M. Johnson
- + 1 author
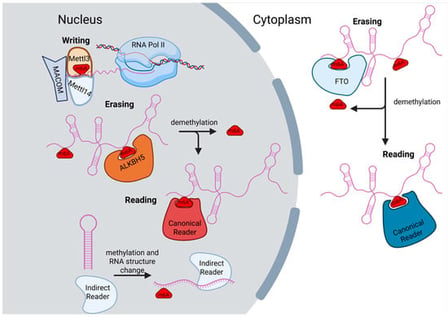
This review provides a thorough survey of long noncoding RNAs that bear the RNA modification N6-methyladenosine (m6A) and current work to understand the resulting mechanistic and biological consequences. We give an overview of lncRNA and m6A biology first, describing the writers, erasers, and readers of m6A and their targeting of lncRNAs. Next, we give an in-depth review of the field of nuclear lncRNAs that regulate chromatin and their regulation via m6A. We then describe the growing appreciation of liquid–liquid phase separation properties in lncRNA and m6A biology. Finally, we cover examples of cytoplasmic lncRNAs regulated by m6A. Overall, this review aims to emphasize how epitranscriptomics influences noncoding RNA mechanisms to provide additional layers of regulation, integrated into downstream biological processes.
31 January 2026



![miRNAs are transcribed by RNA polymerase II as primary transcripts (pri-miRNAs), which are then processed in the nucleus by the enzyme Drosha along with its cofactor DGCR8 into precursor miRNAs (pre-miRNAs). These pre-miRNAs are transported to the cytoplasm via exportin 5, where they associate with the Dicer/TRBP complex and are cleaved into short double-stranded RNA molecules. One strand of this miRNA duplex is then incorporated into the Argonaute protein to form the RNA-induced silencing complex (RISC). RISC then binds to specific target mRNAs, leading to their degradation, destabilization, or inhibition of translation. In addition to the canonical pathway, miRNAs can be generated through non-canonical biogenesis routes, including Drosha-independent mechanisms (where pri-miRNAs are processed by the spliceosome) as well as Dicer-independent pathways. Abbreviations: Ago2: Argonaute 2, DGCR8: DiGeorge syndrome critical region 8, RISC: RNA-induced silencing complex, TRBP: Transactivation response element RNA-binding protein. Created in https://BioRender.com (accessed on 8 July 2025). Modified from Seyhan, Attila 2023 [51].](https://mdpi-res.com/cdn-cgi/image/w=281,h=192/https://mdpi-res.com/ncrna/ncrna-12-00002/article_deploy/html/images/ncrna-12-00002-g001-550.jpg)