Poly[(N-acryloyl glycinamide)-co-(N-acryloyl l-alaninamide)] and Their Ability to Form Thermo-Responsive Hydrogels for Sustained Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
2.1. NAGA and NAlALA Monomers
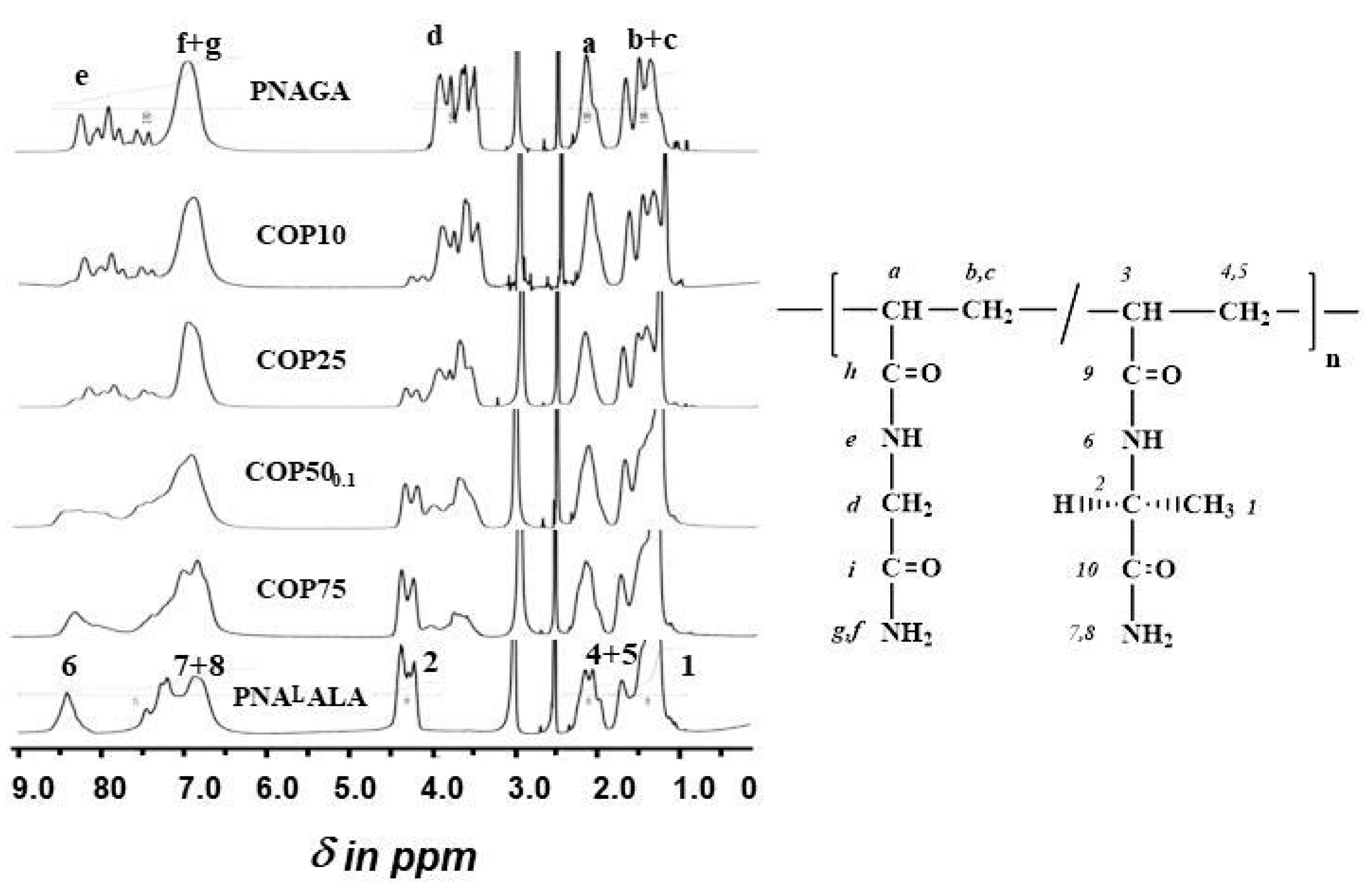
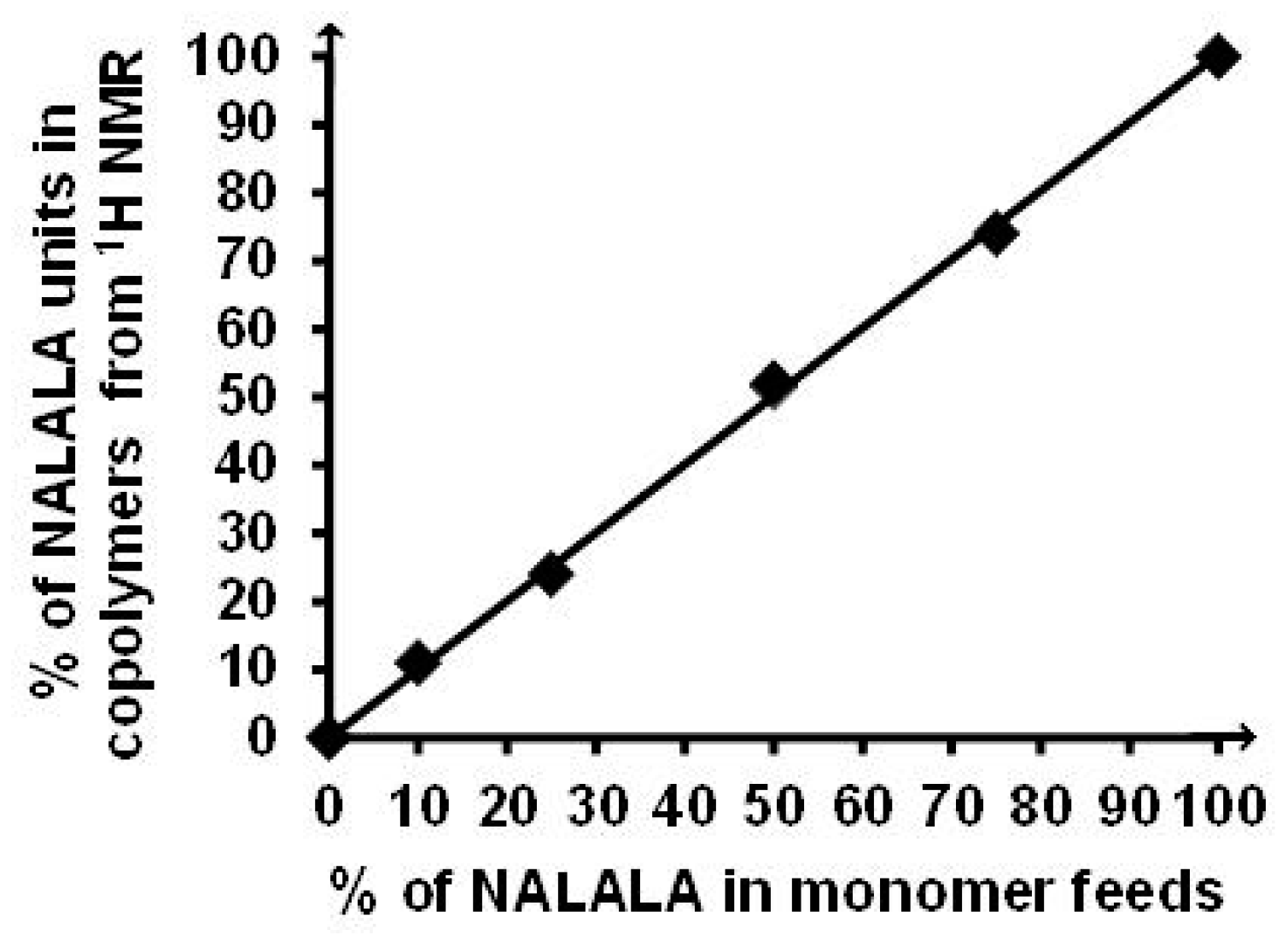
2.2. Synthesis and Characterization of Poly(NAGA-co-NAlALA) Copolymers
2.3. Fourrier Transform Infrared
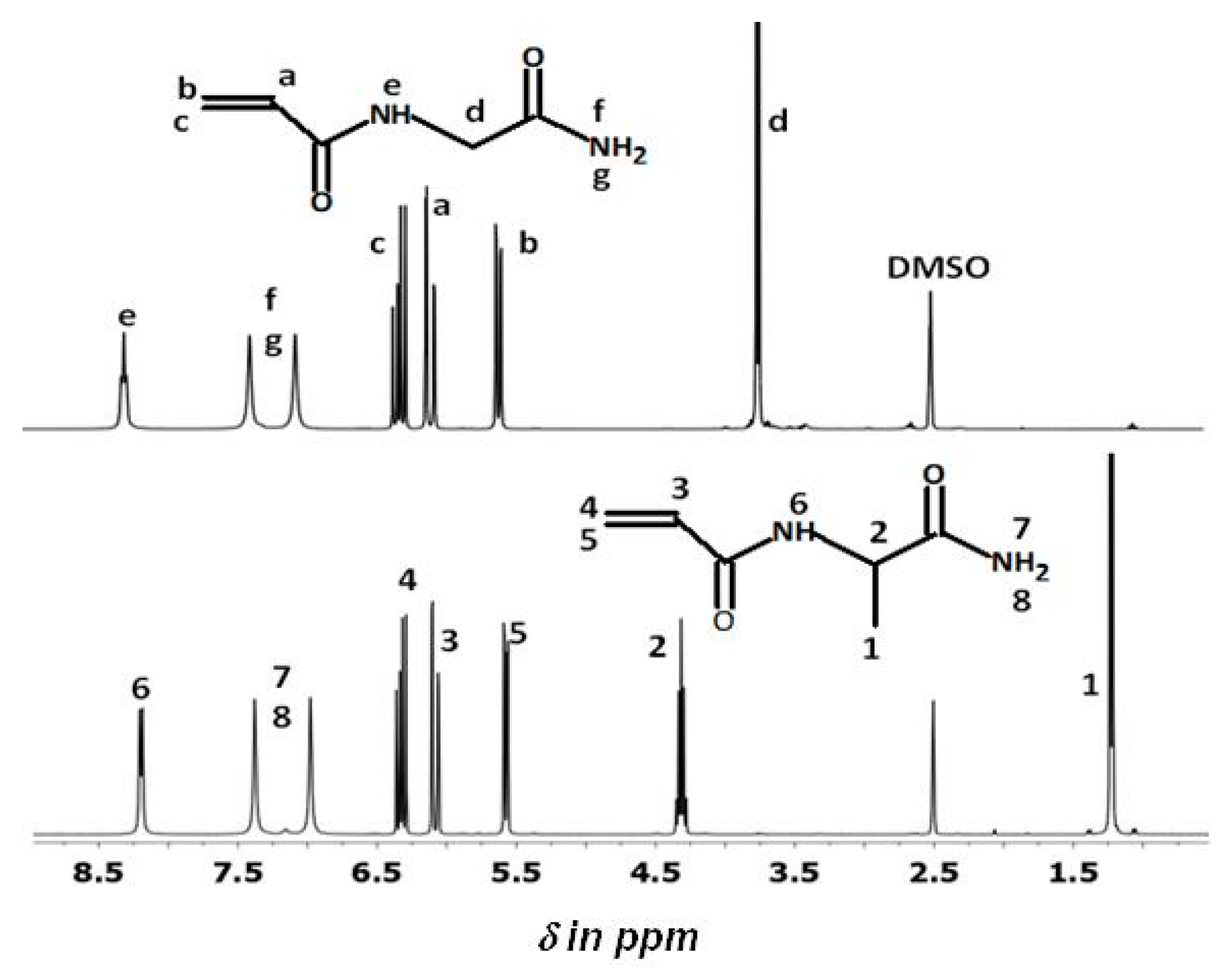
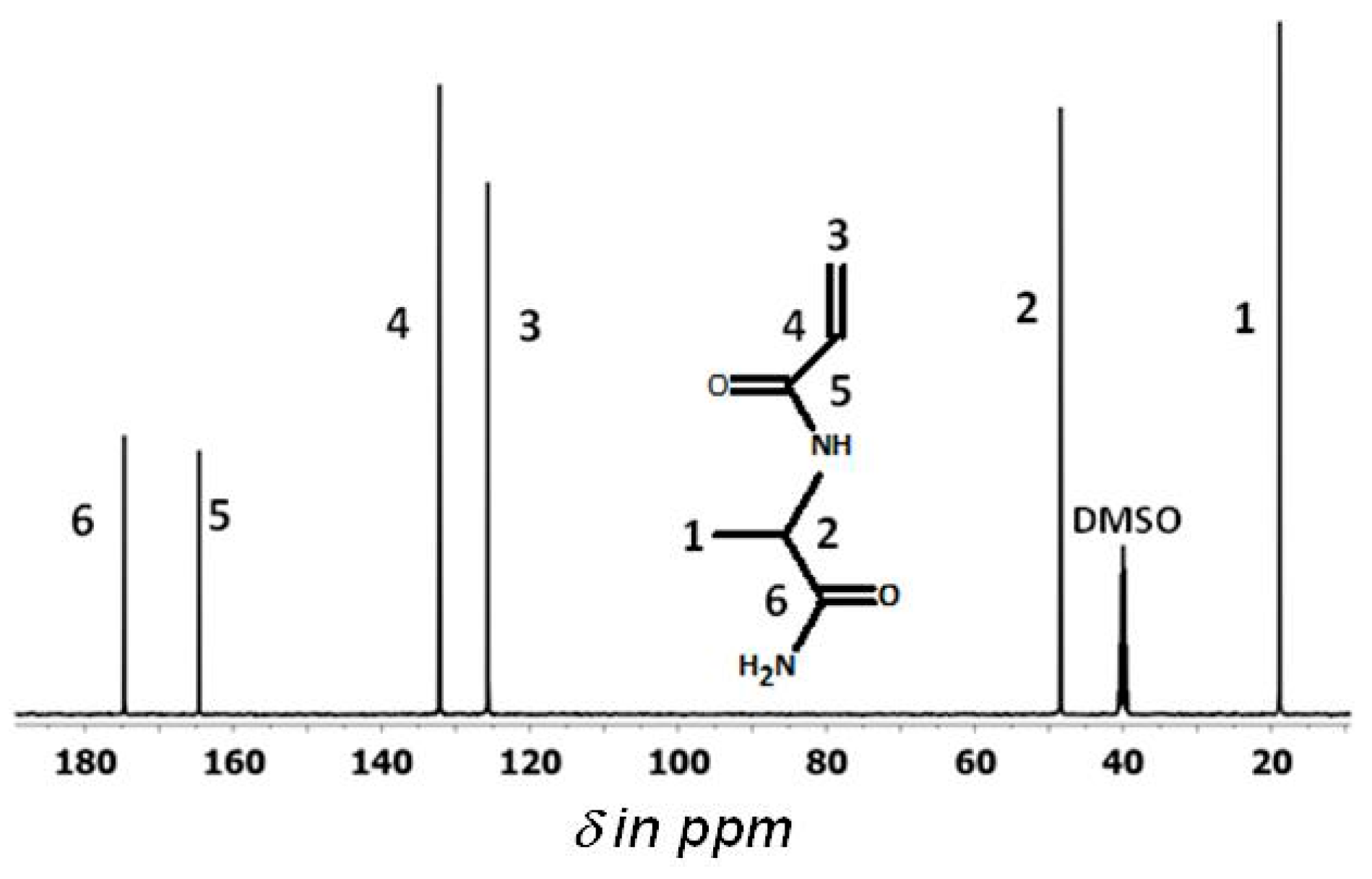
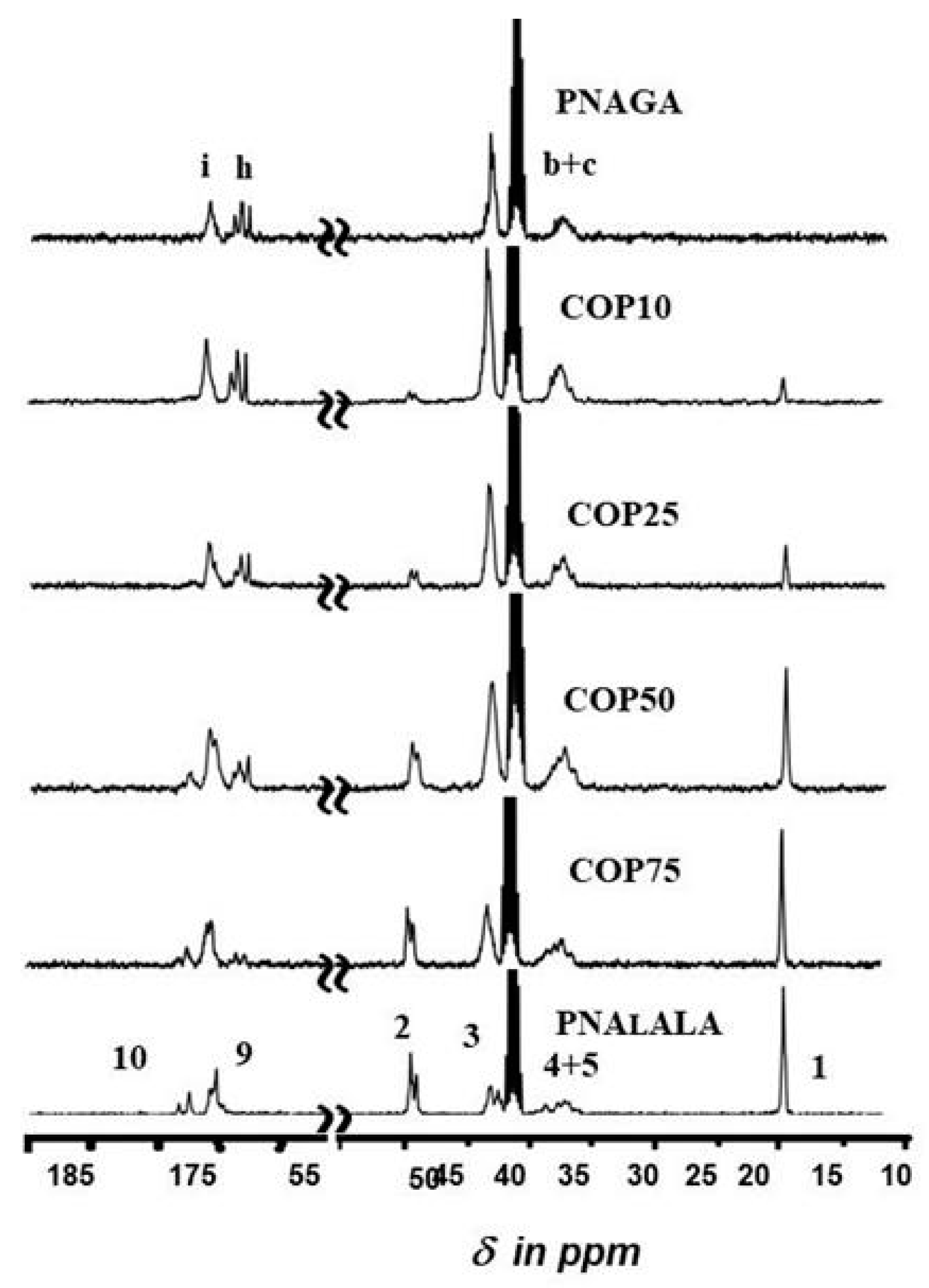
2.4. Nuclear Magnetic Resonance
2.5. Circular Dichroism
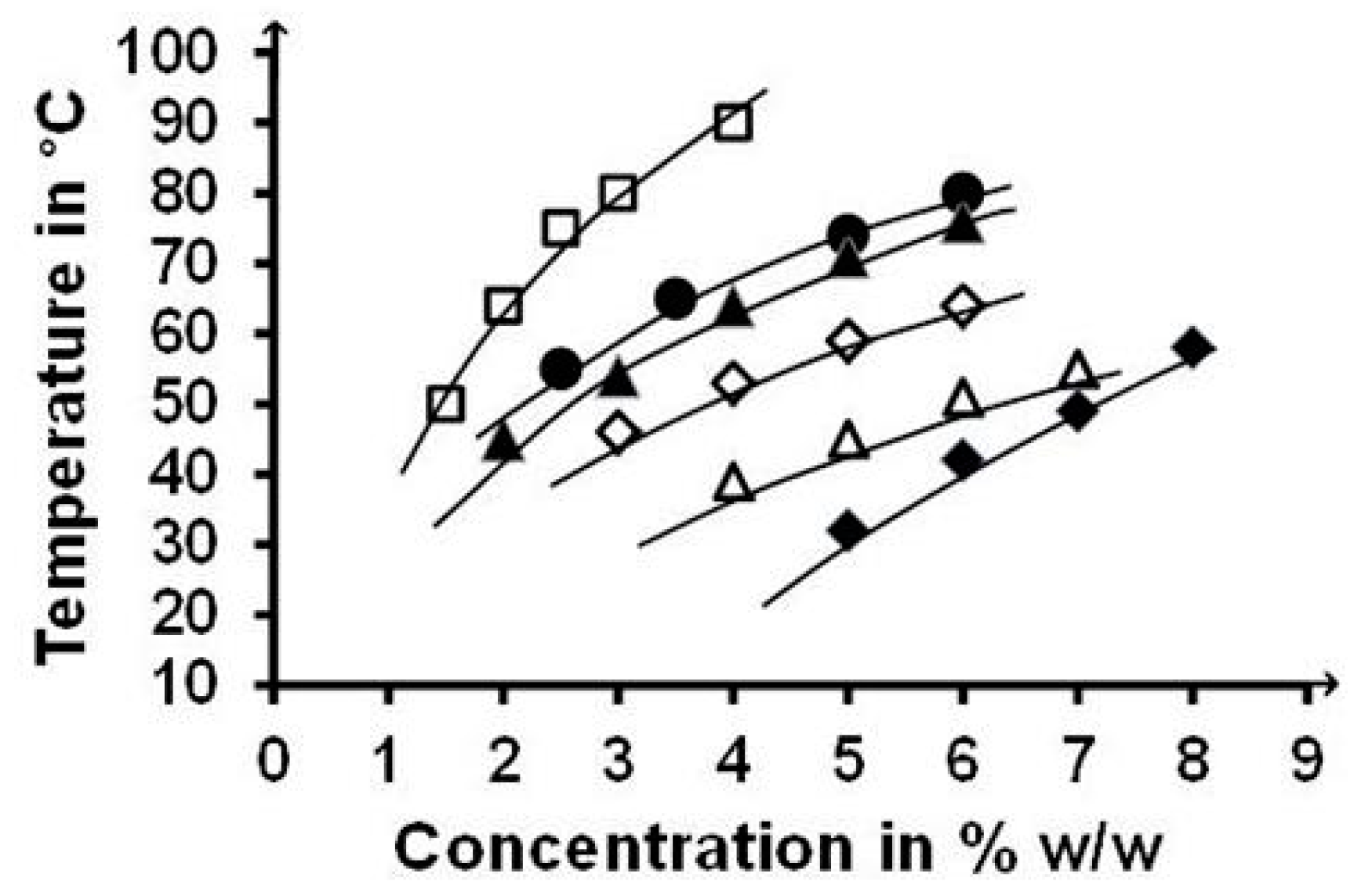
2.6. Reversible Gel↔Sol Transitions
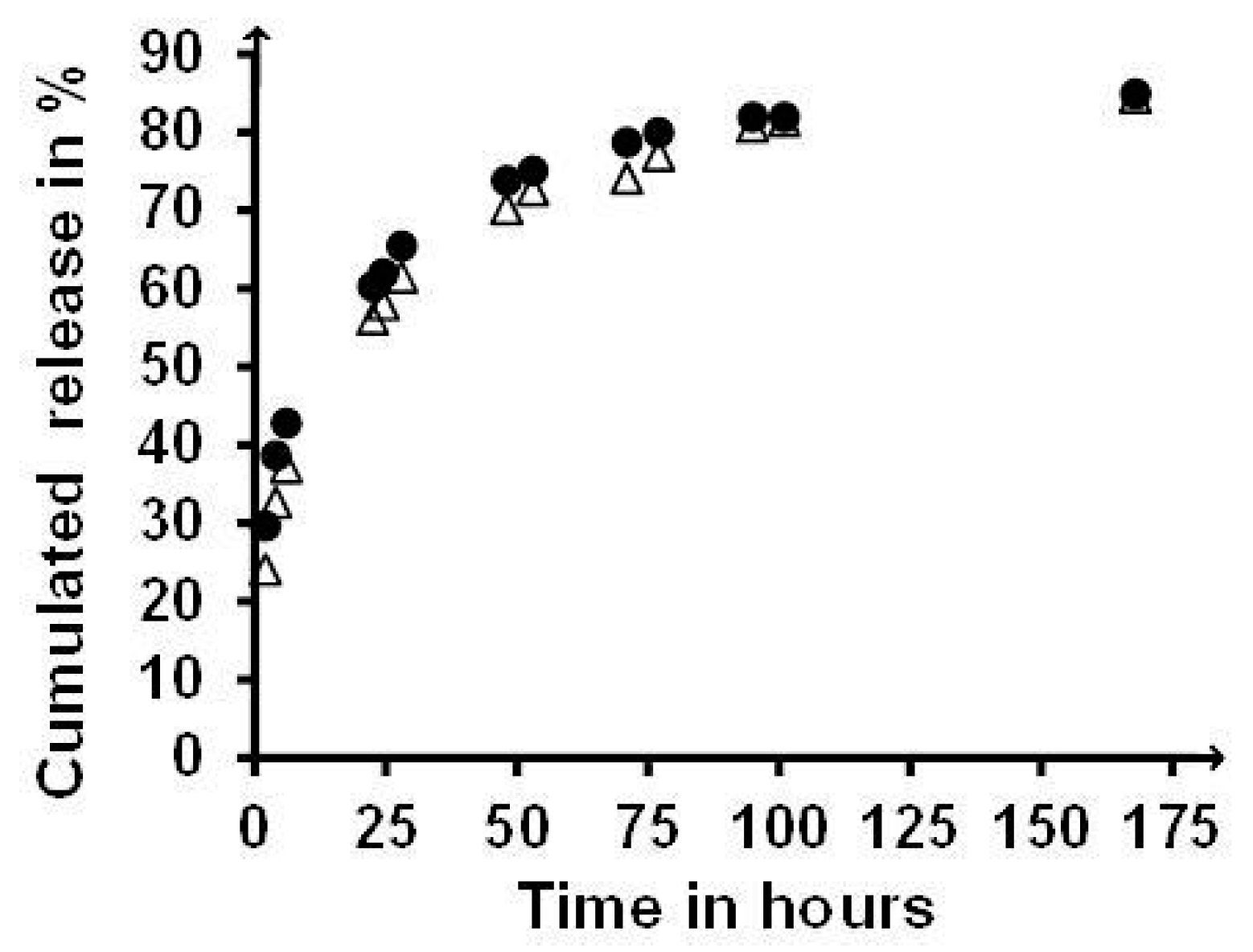
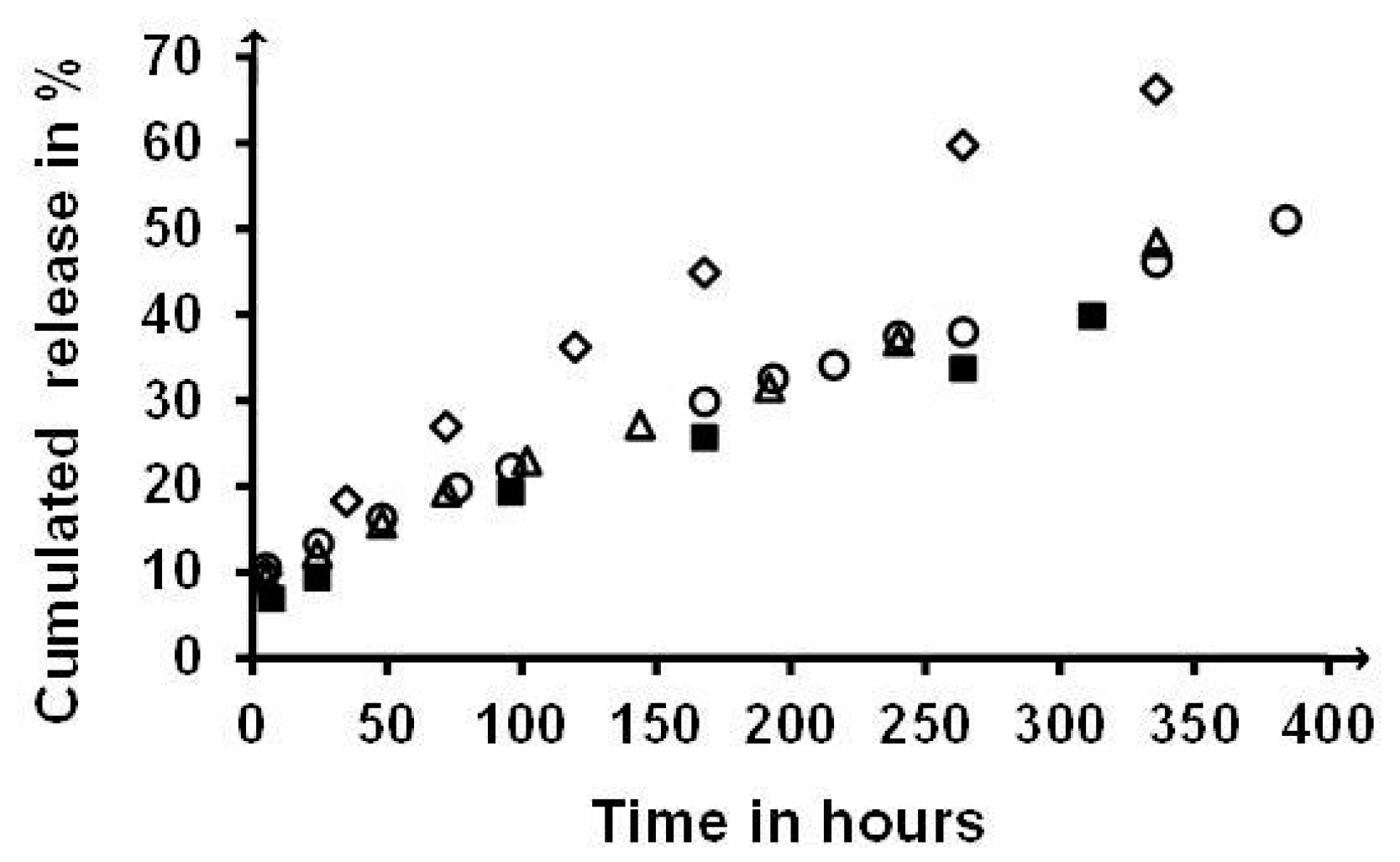
2.7. Release Sustaining
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.1.1. Synthesis of N-Acryloyl l-Alaninamide (NAlALA)
4.1.2. Synthesis of Poly(NAGA-co-NAlALA) Copolymers
4.2. Methods
4.2.1. Spectral Characterizations
4.2.2. Dynamic Viscosity
4.2.3. Thermal Characterizations
4.2.4. Chiroptical Characterization
4.2.5. Formulations of Copolymer/Model Drug Hydrogels
4.2.6. Release Profiles
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef]
- Amin, S.; Rajabnezhad, S.; Kohli, K. Hydrogels as potential drug delivery systems. Sci. Res. Essay 2009, 3, 1175–1183. [Google Scholar]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Macaya, D.; Spector, M. Injectable hydrogel material for spinal cord regeneration: A review. Biomed. Mater. 2012, 7, 012001. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.T.; Nguyen, M.K.; Lee, D.S. Injectable block copolymer hydrogels: Achievements and future challenges for biomedical applications. Macromolecules 2011, 44, 6629–6636. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermo-responsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Liang, Y.; Qiao, Y.; Guo, S.; Wang, L.; Xie, C.; Zhai, Y.; Deng, L.; Dong, A. Thermoreversible gelation of poly(ethylene glycol)/poly(ester anhydride) triblock copolymer nanoparticles for injectable drug delivery systems. Soft Matter 2010, 6, 1915–1922. [Google Scholar] [CrossRef]
- Shimada, N.; Kidoaki, S.; Maruyama, A. Smart hydrogels exhibiting UCST-type volume changes under physiologically relevant conditions. RSC Adv. 2014, 4, 52346–52348. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, W. Poly(N-acryloyl glycinamide): A fascinating polymer that exhibits a range of properties from UCST to high-strength hydrogels. Chem. Commun. 2018, 54, 10540–10553. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.C.; Schuler, N.W. Thermally reversible homopolymer gel systems. J. Polym. Sci. B 1964, 2, 1095–1096. [Google Scholar] [CrossRef]
- Haas, H.C.; Moreau, R.D.; Schuler, D.W. Synthetic thermally reversible gel systems. II. J. Polym. Sci. Polym. Phys. Ed. 1967, 5, 915–927. [Google Scholar] [CrossRef]
- Haas, H.C.; Chiklis, C.K.; Moreau, R.D. Synthetic thermally reversible gel systems. III. J. Polym. Sci. Part A Polym. Chem. 1970, 8, 1131–1145. [Google Scholar] [CrossRef]
- Seuring, J.; Bayer, F.M.; Huber, K.; Agarwal, S. Upper Critical Solution Temperature of Poly(N-acryloyl glycinamide) in water: A Concealed Property. Macromolecules 2012, 45, 374–384. [Google Scholar] [CrossRef]
- Glatzel, S.; Badi, N.; Pach, M.; Laschewsky, A.; Lutz, J.-F. Well-defined synthetic polymers with a protein-like gelation behavior in water. Chem. Commun. 2010, 46, 4517–4519. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, S.; Laschewsky, A.; Lutz, J.-F. Well-Defined Uncharged Polymers with a Sharp UCST in Water and in Physiological Milieu. Macromolecules 2011, 44, 413–415. [Google Scholar] [CrossRef]
- Boustta, M.; Colombo, P.-J.; Vert, M. Combination of Poly(N-Acryloyl Glycinamide) with at Least One Active Principle. WO 2013128373, 6 September 2013. [Google Scholar]
- Boustta, M.; Colombo, P.-E.; Lenglet, S.; Poujol, S.; Vert, M. Versatile UCST-based thermoresponsive hydrogels for loco-regional sustained drug delivery. J. Control. Release 2014, 174, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Boustta, M.; Vert, M. A method to slow down the ionizationdependent release of risperidone loaded in a thermoresponsive poly(N-acryloylglycinamide) hydrogel. Drug Deliv. Transl. Res. 2017, 7, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Braud, C.; Vert, M. Chiroptical properties of optically active (−) poly N-sec-butyl-N-methyl acrylamide] with regard to amide chromophores. Isr. J. Chem. 1976, 15, 39–45. [Google Scholar] [CrossRef]
- Braud, C.; Vert, M. Poly(β-malic acid) as a source of polyvalent drug carriers: Possible effects of hydrophobic substituents in aqueous media. In Polymers as Biomaterials; Shalaby, S.W., Hoffman, A.S., Ratner, B.D., Horbett, T.A., Eds.; Plenum Publ. Co.: New York, NY, USA, 1984. [Google Scholar]
- Vert, M. Polyvalent polymeric drug carriers. In CRC Critical Reviews—Therapeutic Drug Carrier Systems; Bruck, S.D., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 291–327. [Google Scholar]
- Domurado, D.; Vert, M. Bioresorbable polyelectrolyte amphiphiles as nanosized carriers for lipophilic drug solubilization and delivery. J. Biomater. Sci. Polym. Ed. 2007, 18, 287–301. [Google Scholar] [CrossRef] [PubMed]
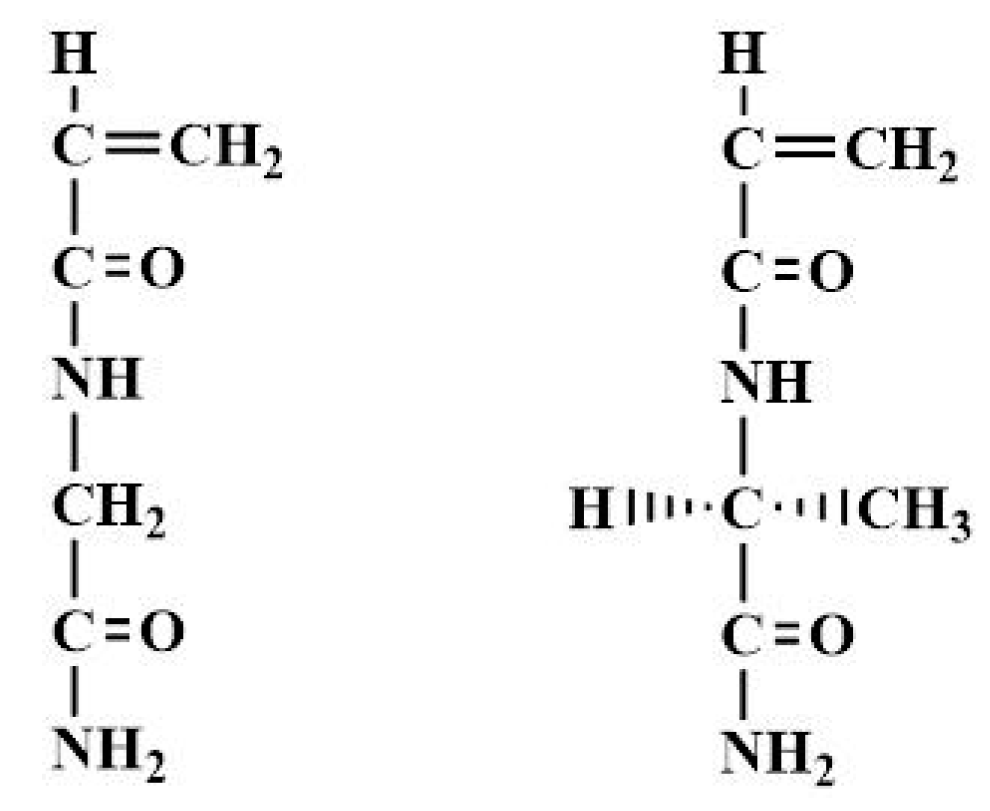

| Polymer | NAlALA in the Monomer Feed W/W % | Isopropanol mol/dm3 | Yield % | Behavior in Saline at Room Temperature | |||
|---|---|---|---|---|---|---|---|
| Medium Aspect 2 | Dynamic Viscosity Pa·s | ||||||
| Feed | Polymer 1 | 1% W/V 3 | 5% W/V 3 | 1% W/V 3 | |||
| PNAGA1 | 0 | 0 | 1 | 93 | 0 | 5 | 1.401 4 |
| COP101 | 10 | 9 ± 1 | 1 | 95 | 0 | 5 | 1.411 |
| COP250.25 | 25 | 22 ± 2 | 0.25 | 93 | 0 | 6 | 1.778 |
| COP500.1 | 50 | 53 ± 3 | 0.1 | 95 | 2 | 6 | 8.534 |
| COP500.25 | 50 | - | 0.25 | 96 | 0 | 5 | 1.810 |
| COP501 | 50 | 53 ± 3 | 1 | 93 | 0 | 2 | 1.322 |
| COP750.25 | 75 | 73 ± 5 | 0.25 | 96 | 0 | 4 | 1.918 |
| PNAlALA1 | 100 | 100 | 1 | 90 | 1 | 3 | n.a. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boustta, M.; Vert, M. Poly[(N-acryloyl glycinamide)-co-(N-acryloyl l-alaninamide)] and Their Ability to Form Thermo-Responsive Hydrogels for Sustained Drug Delivery. Gels 2019, 5, 13. https://doi.org/10.3390/gels5010013
Boustta M, Vert M. Poly[(N-acryloyl glycinamide)-co-(N-acryloyl l-alaninamide)] and Their Ability to Form Thermo-Responsive Hydrogels for Sustained Drug Delivery. Gels. 2019; 5(1):13. https://doi.org/10.3390/gels5010013
Chicago/Turabian StyleBoustta, Mahfoud, and Michel Vert. 2019. "Poly[(N-acryloyl glycinamide)-co-(N-acryloyl l-alaninamide)] and Their Ability to Form Thermo-Responsive Hydrogels for Sustained Drug Delivery" Gels 5, no. 1: 13. https://doi.org/10.3390/gels5010013
APA StyleBoustta, M., & Vert, M. (2019). Poly[(N-acryloyl glycinamide)-co-(N-acryloyl l-alaninamide)] and Their Ability to Form Thermo-Responsive Hydrogels for Sustained Drug Delivery. Gels, 5(1), 13. https://doi.org/10.3390/gels5010013







