Adsorption Characteristics of Bacterial Cellulose Membranes Toward Methylene Blue Dye in Aqueous Environment
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorption Kinetics
2.1.1. FDBC
2.1.2. ODBC
2.1.3. RDBC
2.1.4. Summary and Comparisons
2.2. Adsorption Isotherms
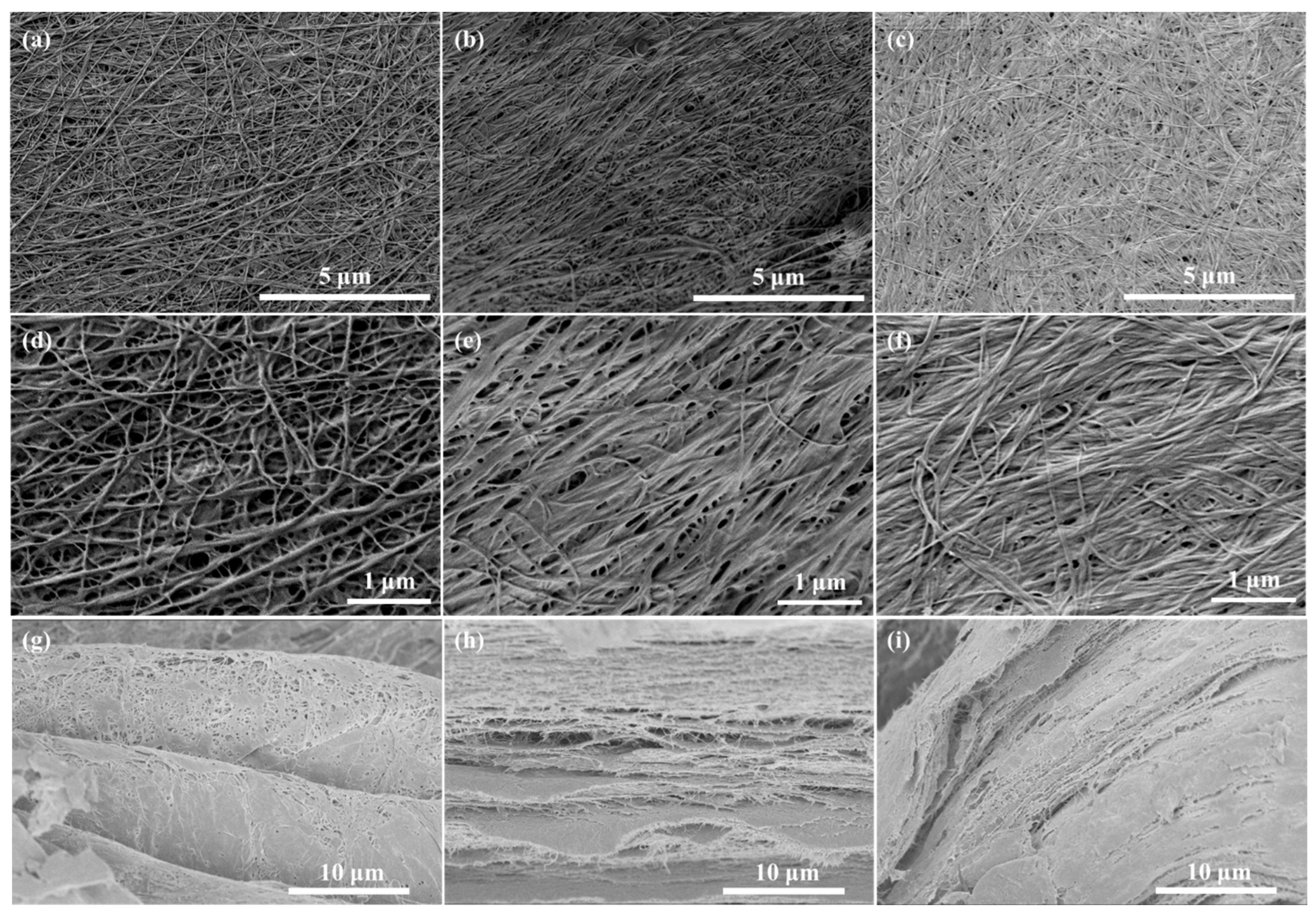
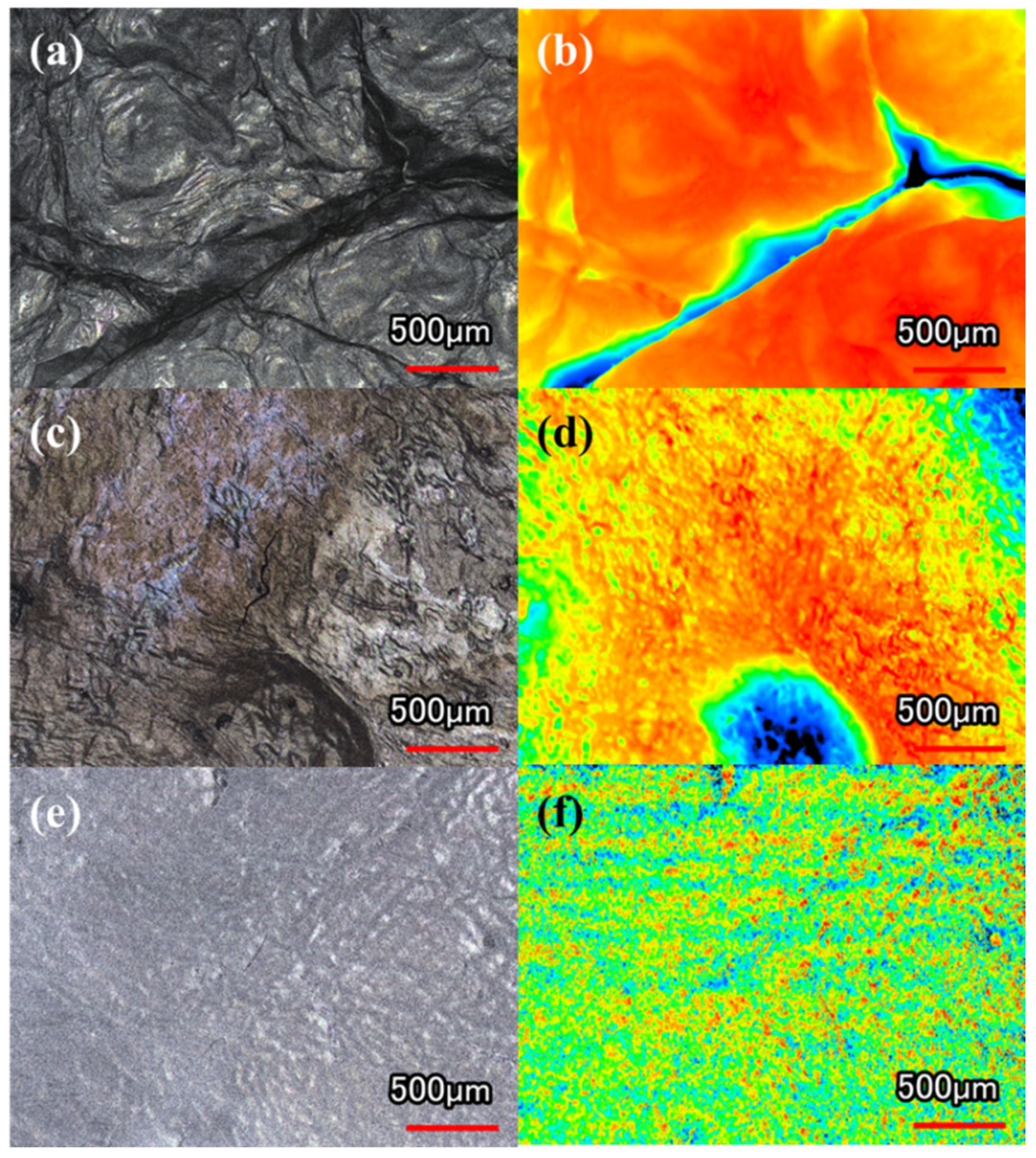
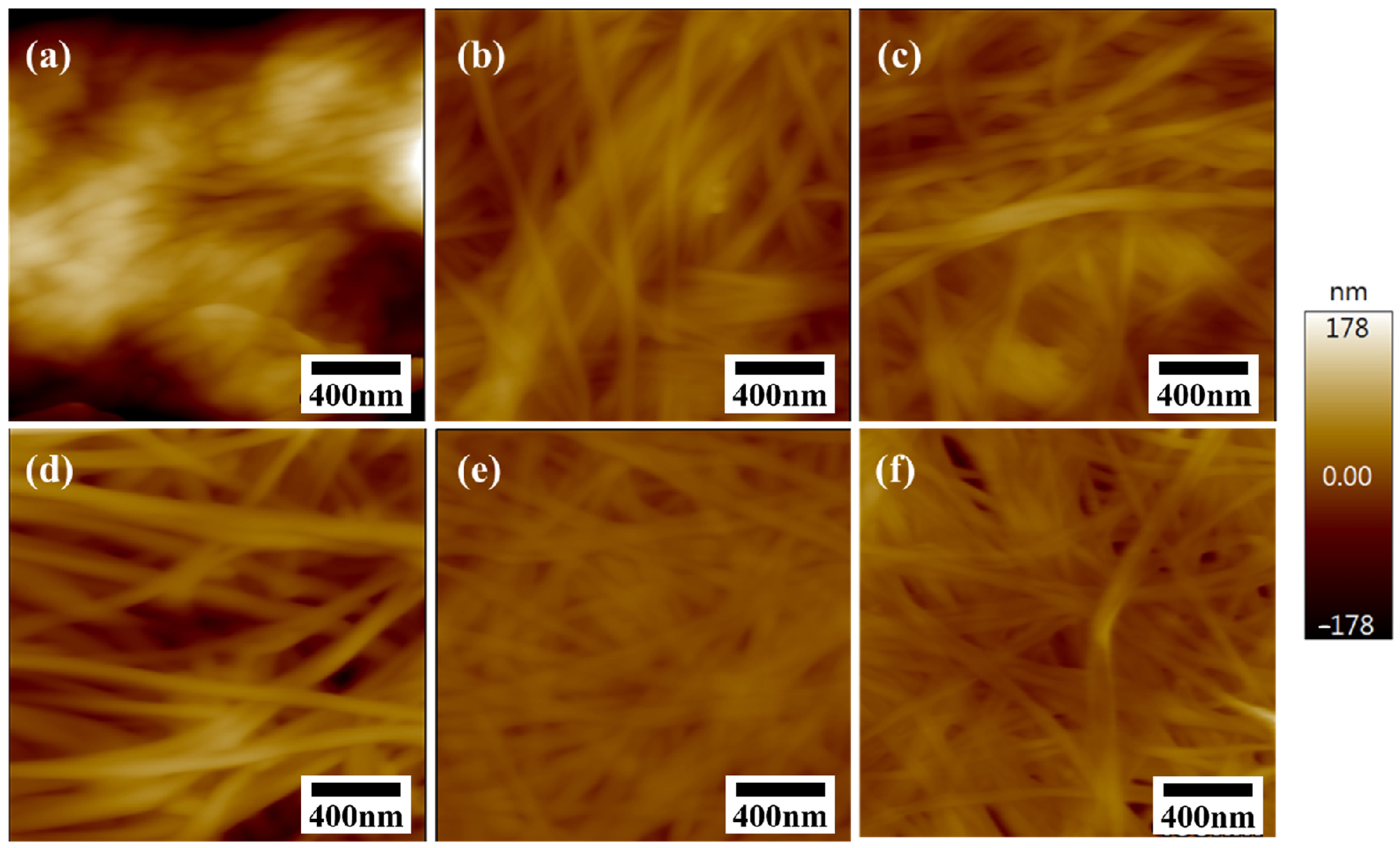
2.3. Morphology of Dried BC Membranes
2.3.1. FE-SEM
2.3.2. CLSM
2.3.3. AFM
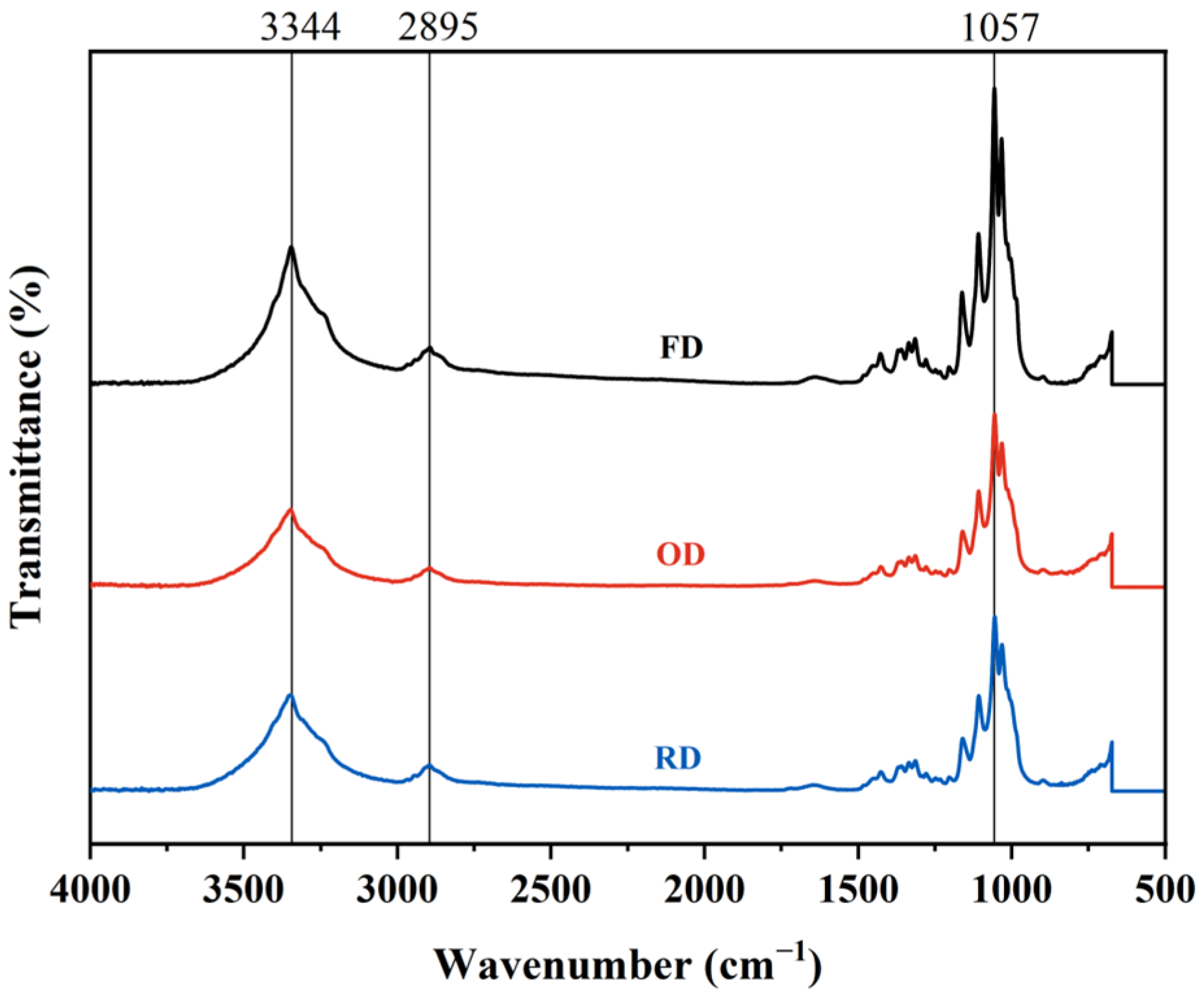
2.4. FT-IR
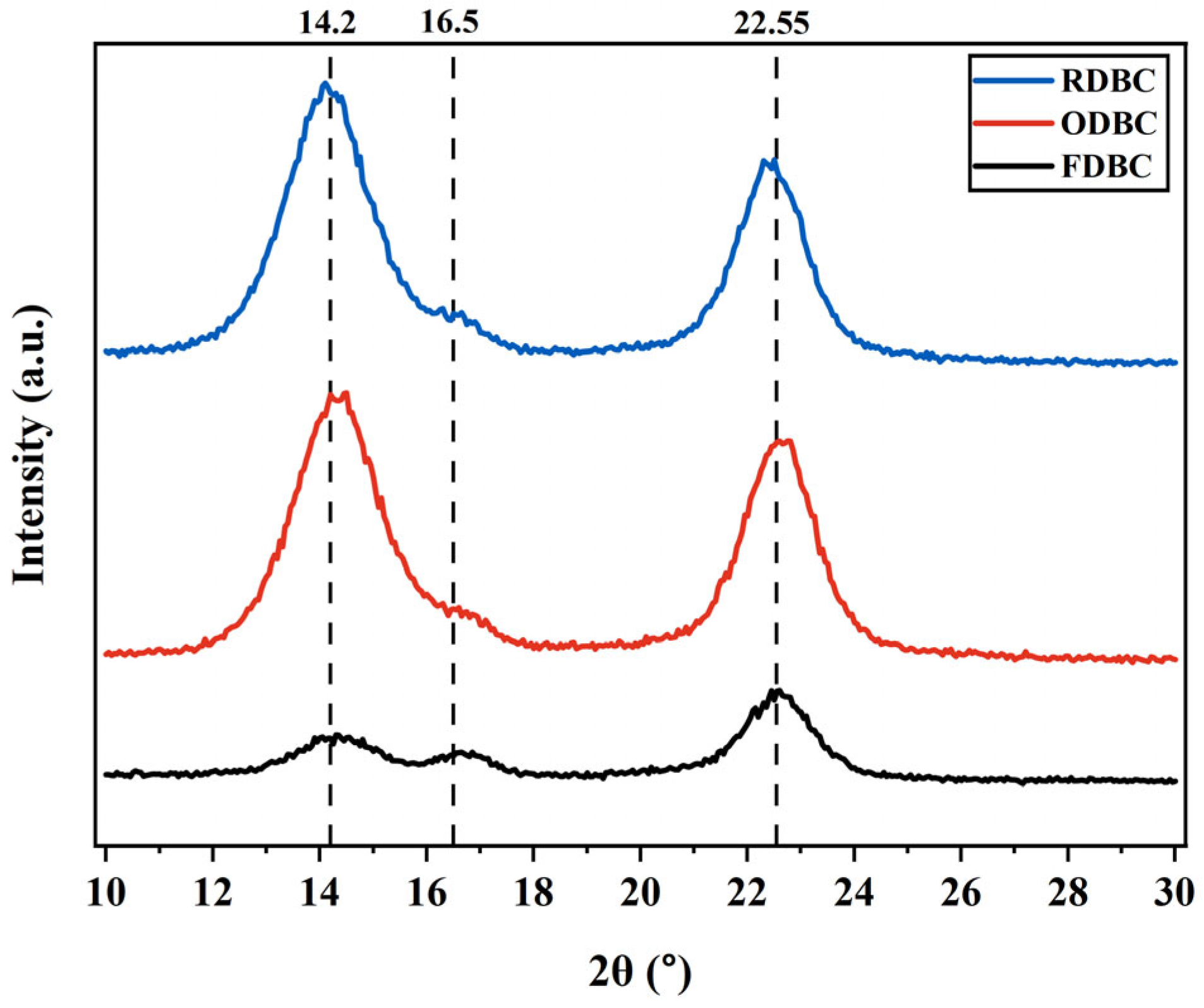
2.5. XRD
2.6. Thermal Analysis (DSC & TGA)
2.7. BET
2.8. PALS
2.9. Summary of Characterization Tests
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Bacterial Cellulose Biosynthesis
4.3. Bacterial Cellulose Purification
4.4. Preparation of Dried/Rehydrated Dried BC Samples
4.5. The UV-Vis Test: Methylene Blue Solution Adsorption Kinetics Study
4.6. Characterization Tests Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bacterial Cellulose |
| MB | Methylene Blue |
| FD | Freeze-Dried |
| OD | Oven-Dried |
| RD | Room Temperature-Dried |
| SCOBY | Symbiotic Culture of Bacteria and Yeast |
| FE-SEM | Field Emission Scanning Electron Microscopy |
| CLSM | Confocal Laser Scanning Microscopy |
| AFM | Atomic Force Microscopy |
| TEM | Transmission Electron Microscopy |
| XRM | X-ray Microscopy |
| DSC | Differential Scanning Calorimetry |
| TGA | Thermogravimetric Analysis |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| XRD | X-ray Diffraction |
| PALS | Positron Annihilation Lifetime Spectroscopy |
| o-Ps | Ortho-positronium |
| BET | Brunauer–Emmett–Teller |
| CI | Crystallinity Index |
| DI | Deionized |
| RH | Rehydrated |
| PMTs | Photomultiplier Tubes |
| WOB | Without Bleach Treatment |
References
- Hu, J.; Wu, D.; Feng, Q.; Wei, A.; Song, B. Soft high-loading TiO2 composite biomaterial film as an efficient and recyclable catalyst for removing methylene blue. Fibers Polym. 2020, 21, 1760–1766. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Rashid, M.M.; Abir, N.; Kamal, S.A.B.; Al-Amin, M.; Ahamed, M.A.; Islam, M.T.; Iqbal, M.I. Functionalized cellulose for textile organic pollutant treatment: A comprehensive review. Water Conserv. Sci. Eng. 2024, 9, 11. [Google Scholar] [CrossRef]
- Elshahawy, M.F.; Ahmed, N.A.; Gad, Y.H.; Ali, A.E.-H. Efficient photocatalytic remediation of lerui acid brilliant blue dye using radiation-prepared carboxymethyl cellulose/acrylic acid hydrogel supported by ZnO@Ag. Int. J. Biol. Macromol. 2024, 262, 129946. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Van Tran, V.; Phung, V.-D.; Do, H.H. Advances and innovations in hydrogel particles for sustainable purification of contaminants in aqueous solutions. Chem. Eng. J. 2024, 486, 150324. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Liu, C.; Crini, G.; Wilson, L.D.; Balasubramanian, P.; Li, F. Removal of contaminants present in water and wastewater by cyclodextrin-based adsorbents: A bibliometric review from 1993 to 2022. Environ. Pollut. 2024, 348, 123815. [Google Scholar] [CrossRef]
- Bai, X.; Liu, Z.; Liu, P.; Zhang, Y.; Hu, L.; Su, T. An eco-friendly adsorbent based on bacterial cellulose and vermiculite composite for efficient removal of methylene blue and sulfanilamide. Polymers 2023, 15, 2342. [Google Scholar] [CrossRef]
- Chen, J.; Hong, F.; Zheng, H.; Zheng, L.; Du, B. Using static culture method to increase the production of Acetobacter xylinum bacterial cellulose. J. Nat. Fibers 2024, 21, 2288286. [Google Scholar] [CrossRef]
- Feng, X.; Ge, Z.; Wang, Y.; Xia, X.; Zhao, B.; Dong, M. Production and characterization of bacterial cellulose from kombucha-fermented soy whey. Food Prod. Process. Nutr. 2024, 6, 20. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; El-Malkey, S.E.; Abu-Saied, M.; Mohammed, A.A. Exploration of a novel and efficient source for production of bacterial nanocellulose, bioprocess optimization and characterization. Sci. Rep. 2022, 12, 18533. [Google Scholar] [CrossRef]
- Zeng, M.; Laromaine, A.; Roig, A. Bacterial cellulose films: Influence of bacterial strain and drying route on film properties. Cellulose 2014, 21, 4455–4469. [Google Scholar] [CrossRef]
- Arkell, A.; Olsson, J.; Wallberg, O. Process performance in lignin separation from softwood black liquor by membrane filtration. Chem. Eng. Res. Des. 2014, 92, 1792–1800. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent advances in bacterial cellulose: A low-cost effective production media, optimization strategies and applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Tang, L.; Jin, Y.; He, X.; Huang, R. Biodegradable poly(ethylene glycol-glycerol-itaconate-sebacate) copolyester elastomer with significantly reinforced mechanical properties by in-situ construction of bacterial cellulose interpenetrating network. Sci. Rep. 2024, 14, 7172. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, J.-J. Improvement of bacterial cellulose fermentation by metabolic perturbation with mixed carbon sources. Process Biochem. 2022, 122, 95–102. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Fatima, A.; Ullah, M.W.; Manan, S. Cost-effective synthesis of bacterial cellulose and its applications in the food and environmental sectors. Gels 2022, 8, 552. [Google Scholar] [CrossRef]
- Dáger-López, D.; Chenché, Ó.; Ricaurte-Párraga, R.; Núñez-Rodríguez, P.; Bajaña, J.M.; Fiallos-Cárdenas, M. Advances in the production of sustainable bacterial nanocellulose from banana leaves. Polymers 2024, 16, 1157. [Google Scholar] [CrossRef]
- Walling, B.; Borah, A.; Hazarika, S.; Bharali, P.; Ramachandran, D.; Kanagasabai, V.; Dutta, N.; Maadurshni, G.B.; Manivannan, J.; Mudoi, P. Production of bacterial nanocellulose as green adsorbent matrix using distillery wastes for dye removal: A combined approach for waste management and pollution mitigation. Biomass Convers. Biorefinery 2025, 15, 7265–7281. [Google Scholar] [CrossRef]
- Marchiori, L.; Santos, L.S.; Schuler, T.; Bernardes, J.C.; Mattos, B.O.; Onishi, B.S.D.; Bortoletto-Santos, R.; Rodrigues-Filho, U.P.; Domeneguetti, R.R.; Ullah, S. Effect of drying methods on the structure and properties of bacterial nanocellulose/MoS2 hybrid gel membranes and sphere-like particles for enhanced adsorption and photocatalytic applications. J. Sol-Gel Sci. Technol. 2024, 110, 635–653. [Google Scholar] [CrossRef]
- Shen, W.; Chen, S.; Shi, S.; Li, X.; Zhang, X.; Hu, W.; Wang, H. Adsorption of Cu (II) and Pb (II) onto diethylenetriamine-bacterial cellulose. Carbohydr. Polym. 2009, 75, 110–114. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, C.; Yang, L.; Cui, J.; Hao, Q.; Sun, D. Handy purifier based on bacterial cellulose and Ca-montmorillonite composites for efficient removal of dyes and antibiotics. Carbohydr. Polym. 2019, 222, 115017. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Mohammed, M.; Shitu, A.; Ibrahim, A. Removal of methylene blue using low cost adsorbent: A review. Res. J. Chem. Sci. ISSN 2014, 2231, 606X. [Google Scholar]
- Tran, G.T.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. Bacterial cellulose and composites for the treatment of water pollution: A review. Environ. Chem. Lett. 2025, 23, 707–732. [Google Scholar] [CrossRef]
- de Brito Alves, G.; Beverari, S.F.; Florentino, L.C.; Guerrero, A.S.; de Almeida Silva, M.A. Biosynthesis and characterization of bacterial cellulose from the kombucha tea. Periódico Tchê Química 2019, 16, 395–405. [Google Scholar] [CrossRef]
- Lin, S.-P.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
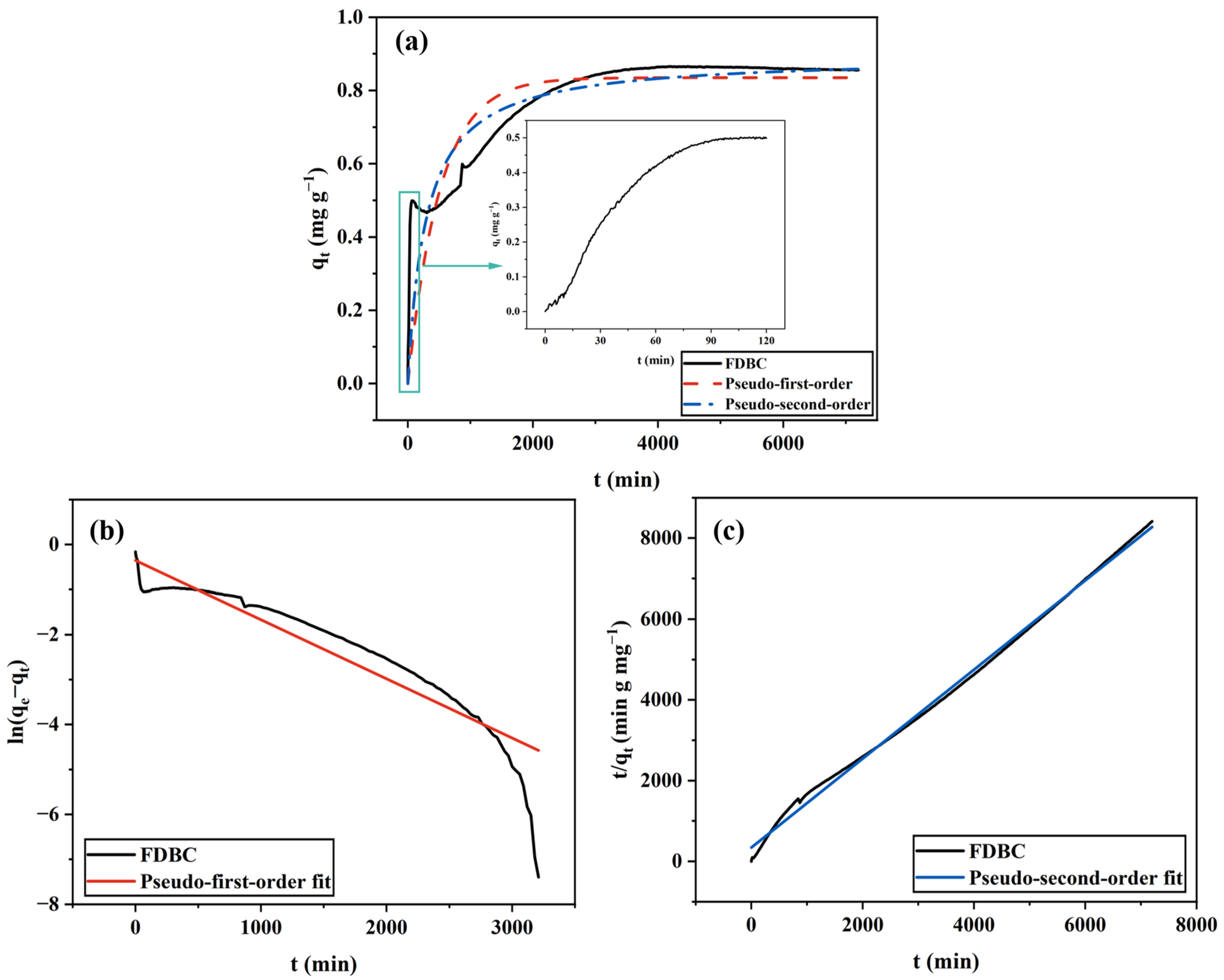
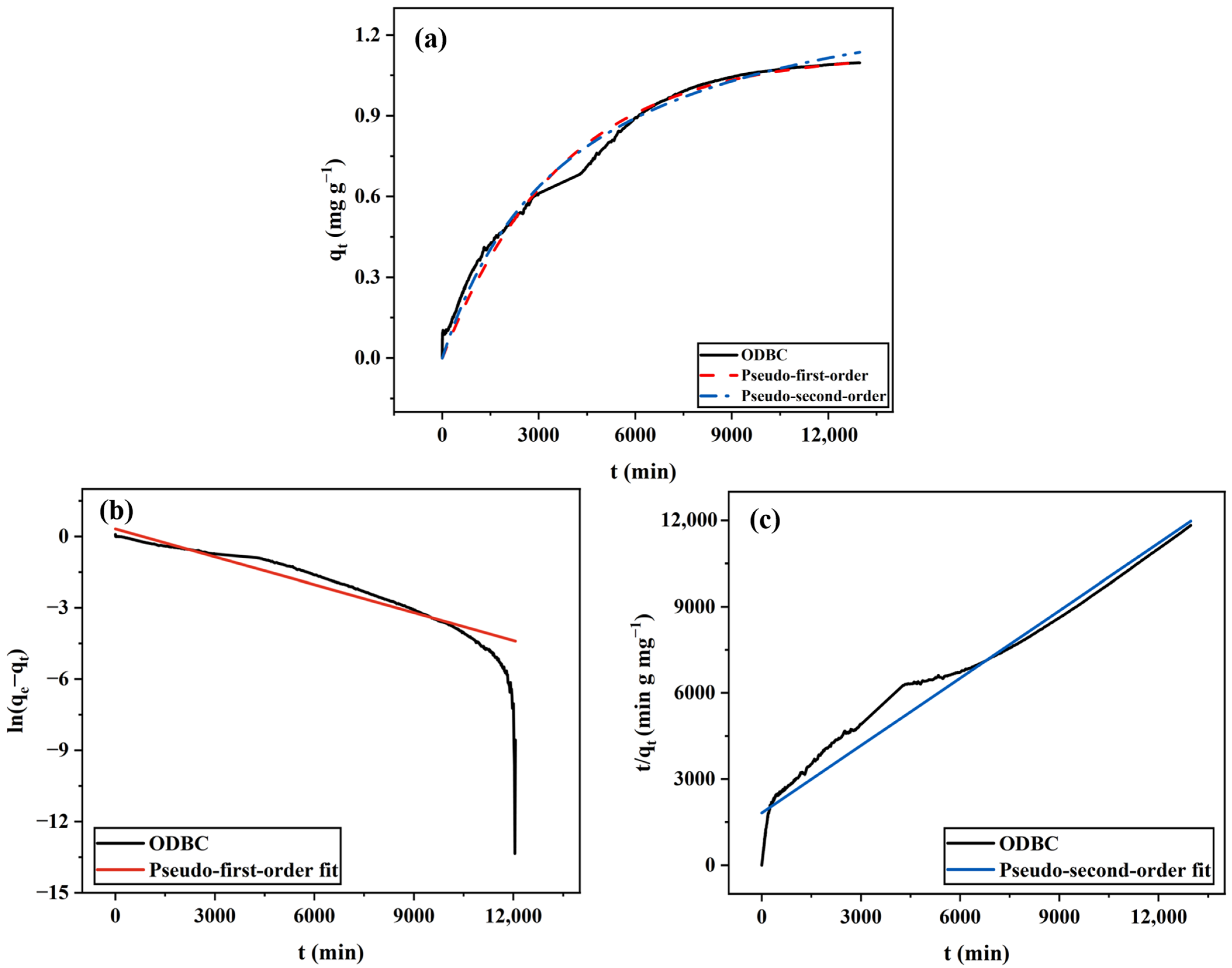
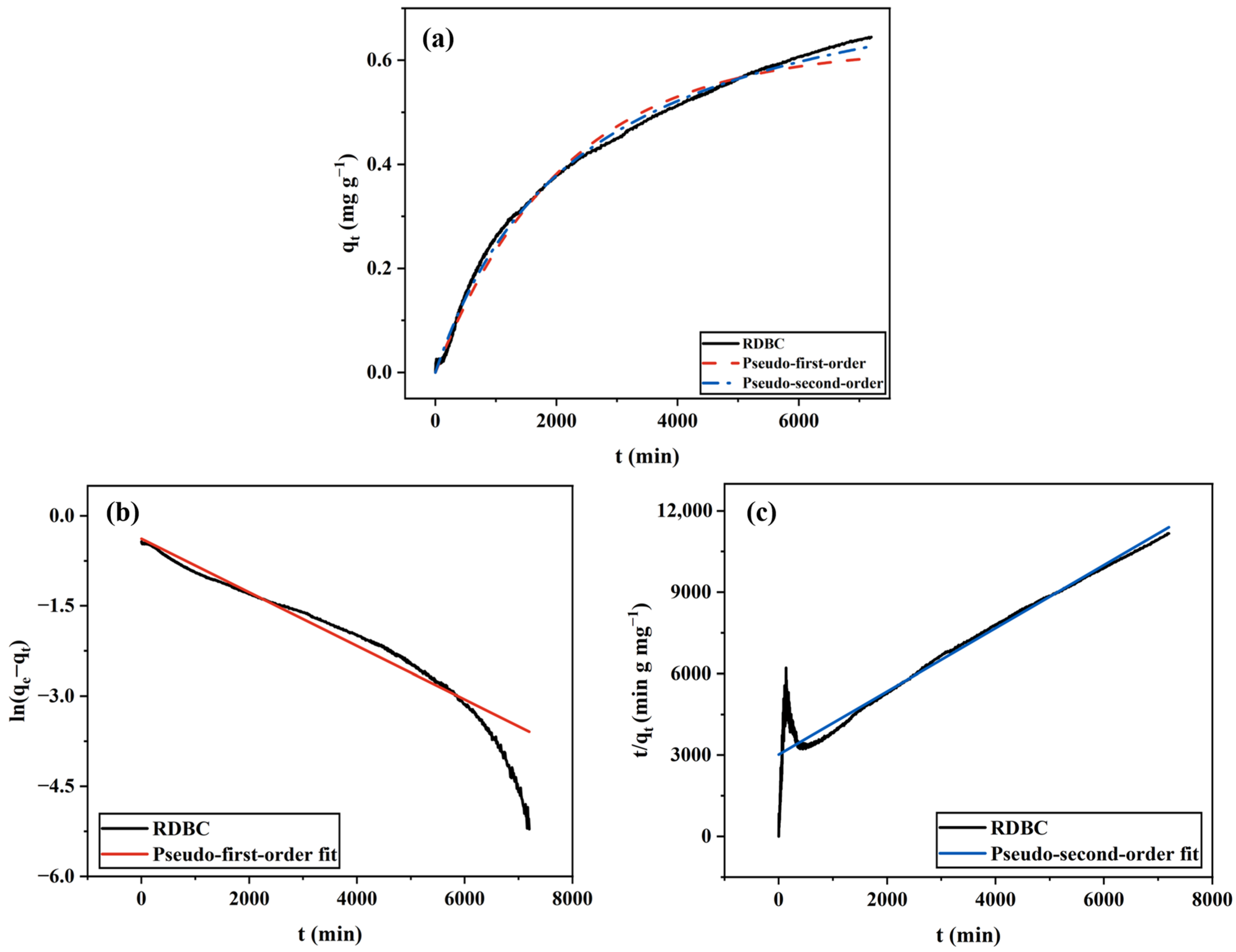
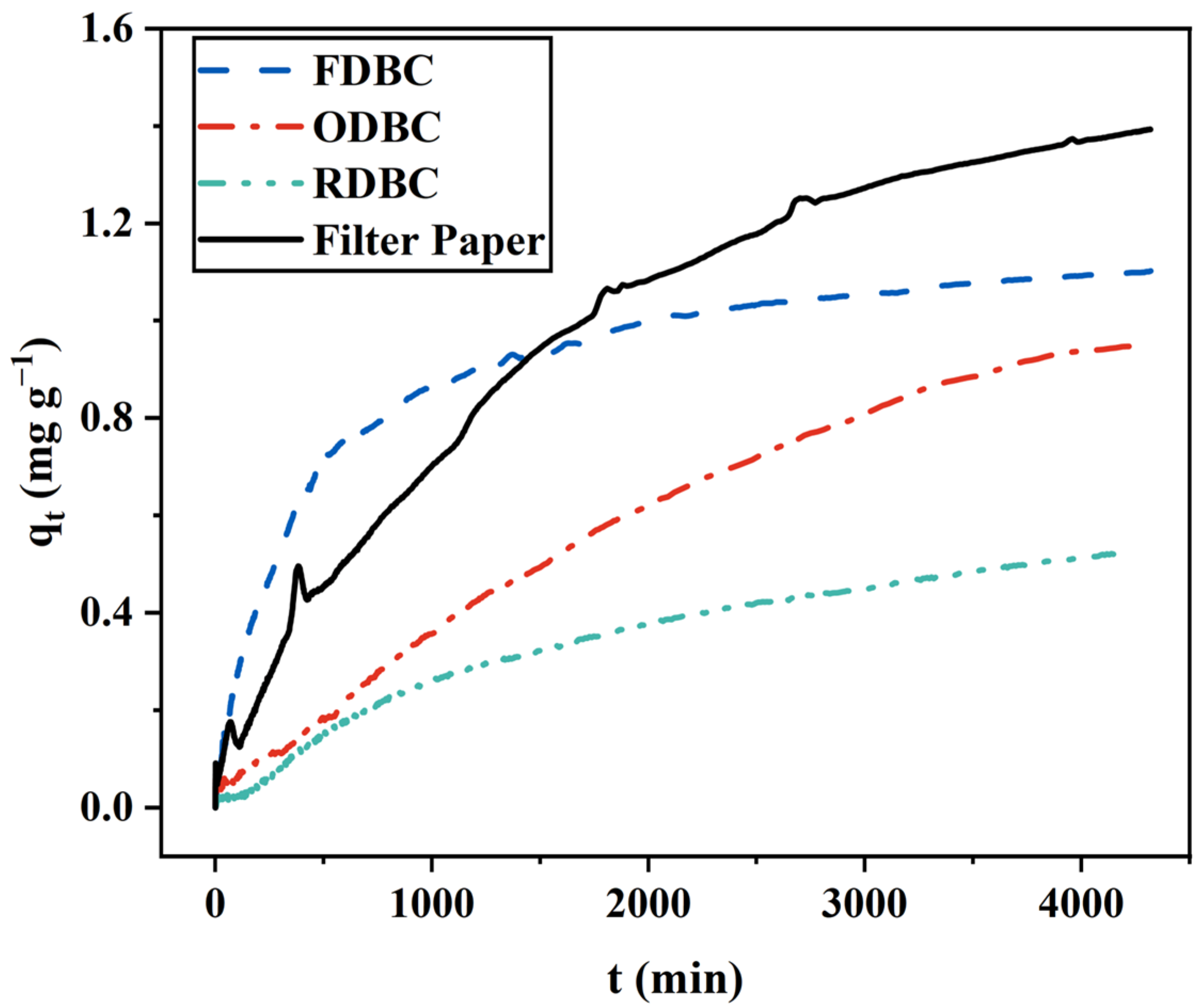


| BC Type | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| FDBC | 0.0020 | 0.84 | 0.726 | 0.0038 | 0.89 | 0.829 |
| ODBC | 0.0002 | 0.91 | 0.995 | 0.0001 | 1.30 | 0.997 |
| RDBC | 0.0005 | 0.62 | 0.994 | 0.0005 | 0.84 | 0.998 |
| BC Type | Langmuir Parameter | Freundlich Parameter | ||||
|---|---|---|---|---|---|---|
| FDBC | 0.067 | 14.25 | 0.276 | 0.868 | 1.01 | 0.954 |
| ODBC | −0.074 | −25.77 | 0.047 | 2.172 | 0.94 | 0.946 |
| RDBC | −0.036 | −20.75 | 0.210 | 0.783 | 0.93 | 0.986 |
| BC Type | CI (%) |
|---|---|
| FDBC | 86.92 |
| ODBC | 98.56 |
| RDBC | 98.14 |
| BC Type | BET Surface Area (m2 g−1) |
|---|---|
| FDBC | 5.31 ± 0.04 |
| ODBC | 3.60 ± 0.03 |
| RDBC | 3.25 ± 0.05 |
| BC Type | (ps) | (%) | (ps) | (%) | (ns) | (%) | (nm) |
|---|---|---|---|---|---|---|---|
| FDBC | 208 | 46 | 452 ± 1 | 44.4 ± 0.3 | 2.53 ± 0.01 | 9.7 ± 0.2 | 0.661 ± 0.001 |
| ODBC | 267 | 52 | 535 ± 3 | 38.9 ± 0.8 | 2.04 ± 0.01 | 9.5 ± 0.5 | 0.579 ± 0.001 |
| RDBC | 274 | 54 | 558 ± 9 | 36 ± 2 | 2.19 ± 0.01 | 10.0 ± 1.2 | 0.606 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Brewer, C.R.; Pyrch, A.J.; Wang, Z.; Jamadgni, D.U.; Krause, W.E.; Lucia, L.A. Adsorption Characteristics of Bacterial Cellulose Membranes Toward Methylene Blue Dye in Aqueous Environment. Gels 2025, 11, 721. https://doi.org/10.3390/gels11090721
Hu Z, Brewer CR, Pyrch AJ, Wang Z, Jamadgni DU, Krause WE, Lucia LA. Adsorption Characteristics of Bacterial Cellulose Membranes Toward Methylene Blue Dye in Aqueous Environment. Gels. 2025; 11(9):721. https://doi.org/10.3390/gels11090721
Chicago/Turabian StyleHu, Zimu, Christopher R. Brewer, Austin J. Pyrch, Ziyu Wang, Dhanush U. Jamadgni, Wendy E. Krause, and Lucian A. Lucia. 2025. "Adsorption Characteristics of Bacterial Cellulose Membranes Toward Methylene Blue Dye in Aqueous Environment" Gels 11, no. 9: 721. https://doi.org/10.3390/gels11090721
APA StyleHu, Z., Brewer, C. R., Pyrch, A. J., Wang, Z., Jamadgni, D. U., Krause, W. E., & Lucia, L. A. (2025). Adsorption Characteristics of Bacterial Cellulose Membranes Toward Methylene Blue Dye in Aqueous Environment. Gels, 11(9), 721. https://doi.org/10.3390/gels11090721







