Ambient-Dried Silica Xerogels with Enhanced Strength and Thermal Insulation via Calcium Ion-Glycerol Synergistic Crosslinking
Abstract
1. Introduction
2. Results and Discussion
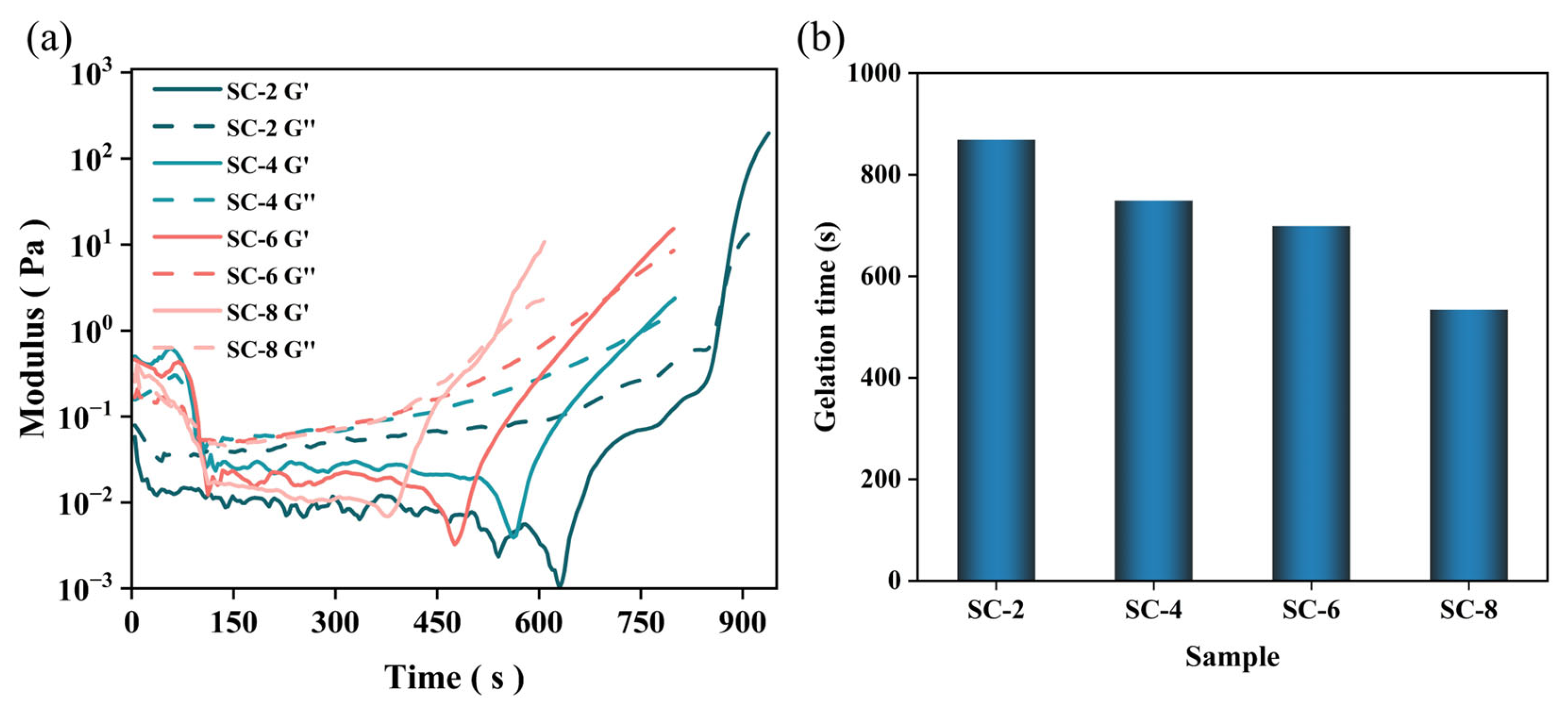
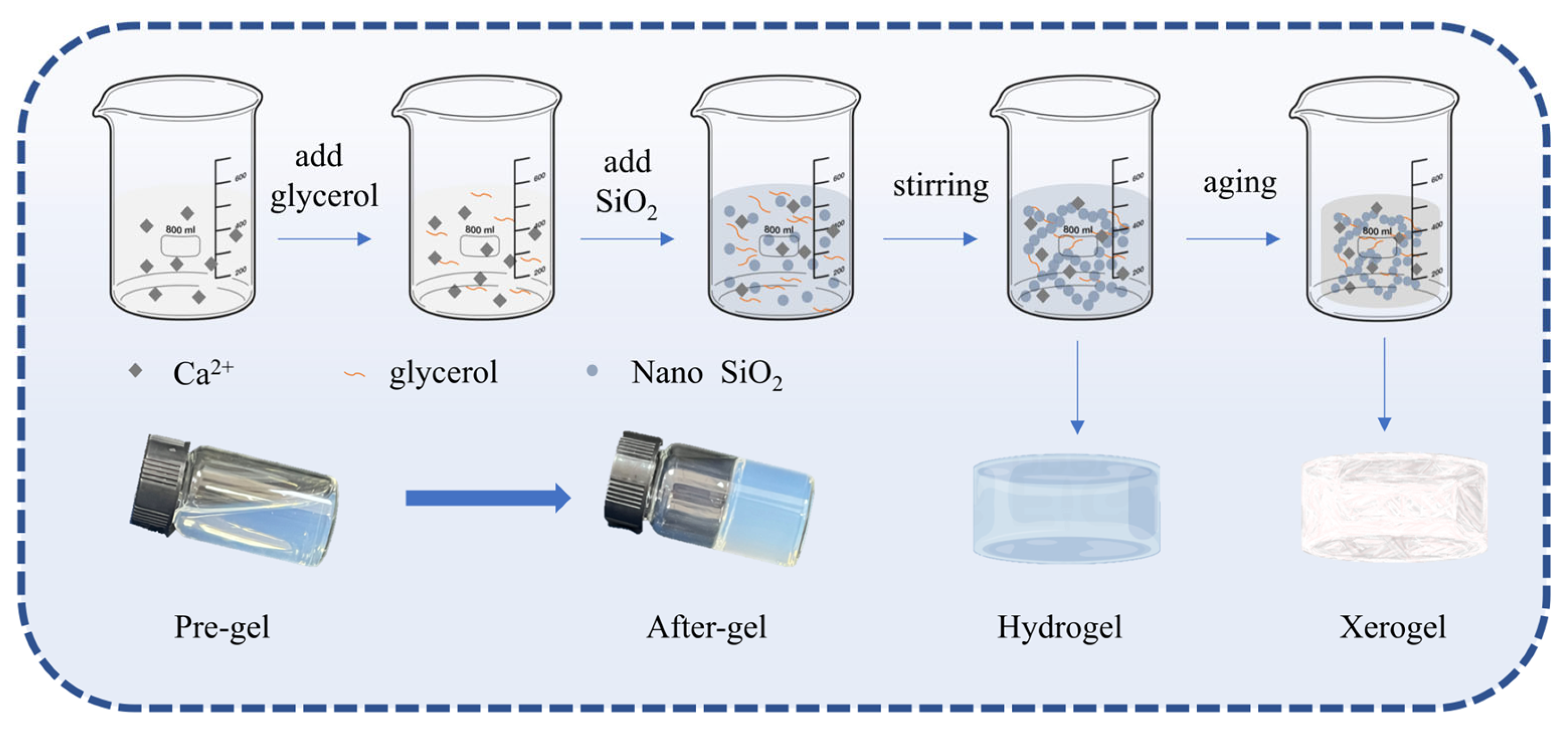
2.1. Gel Process
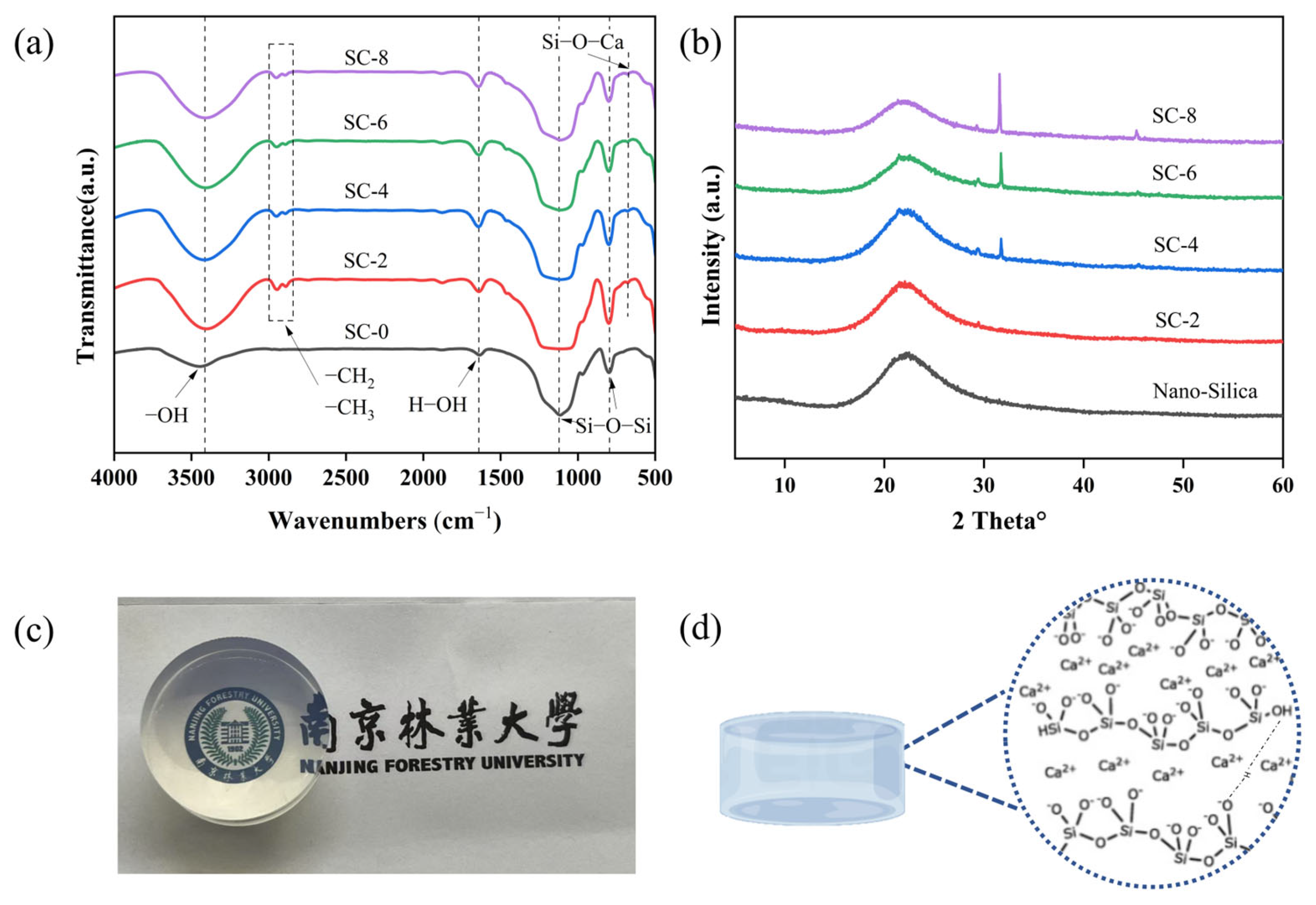
2.2. Structural and Morphological Characterization of SC Xerogel
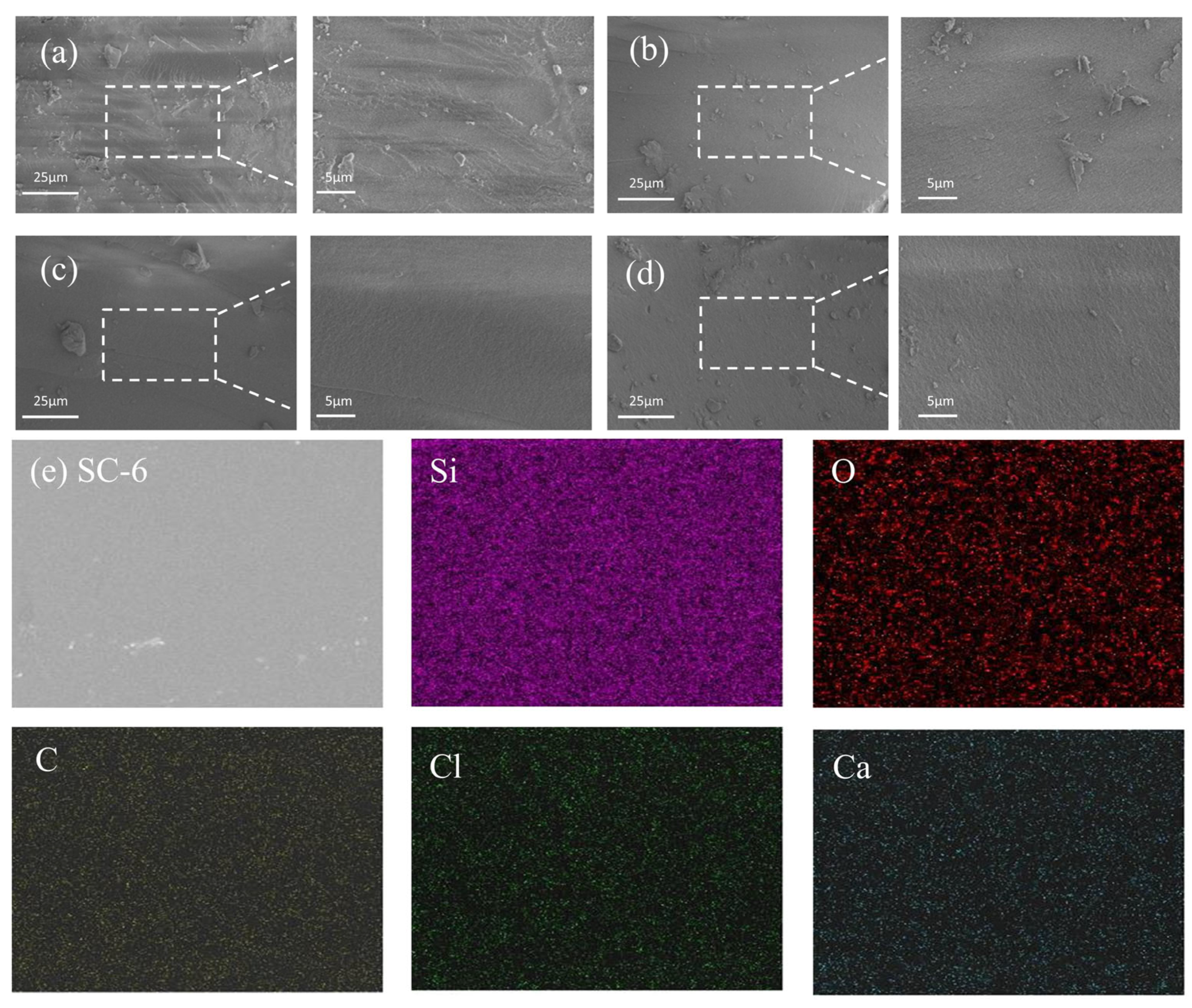
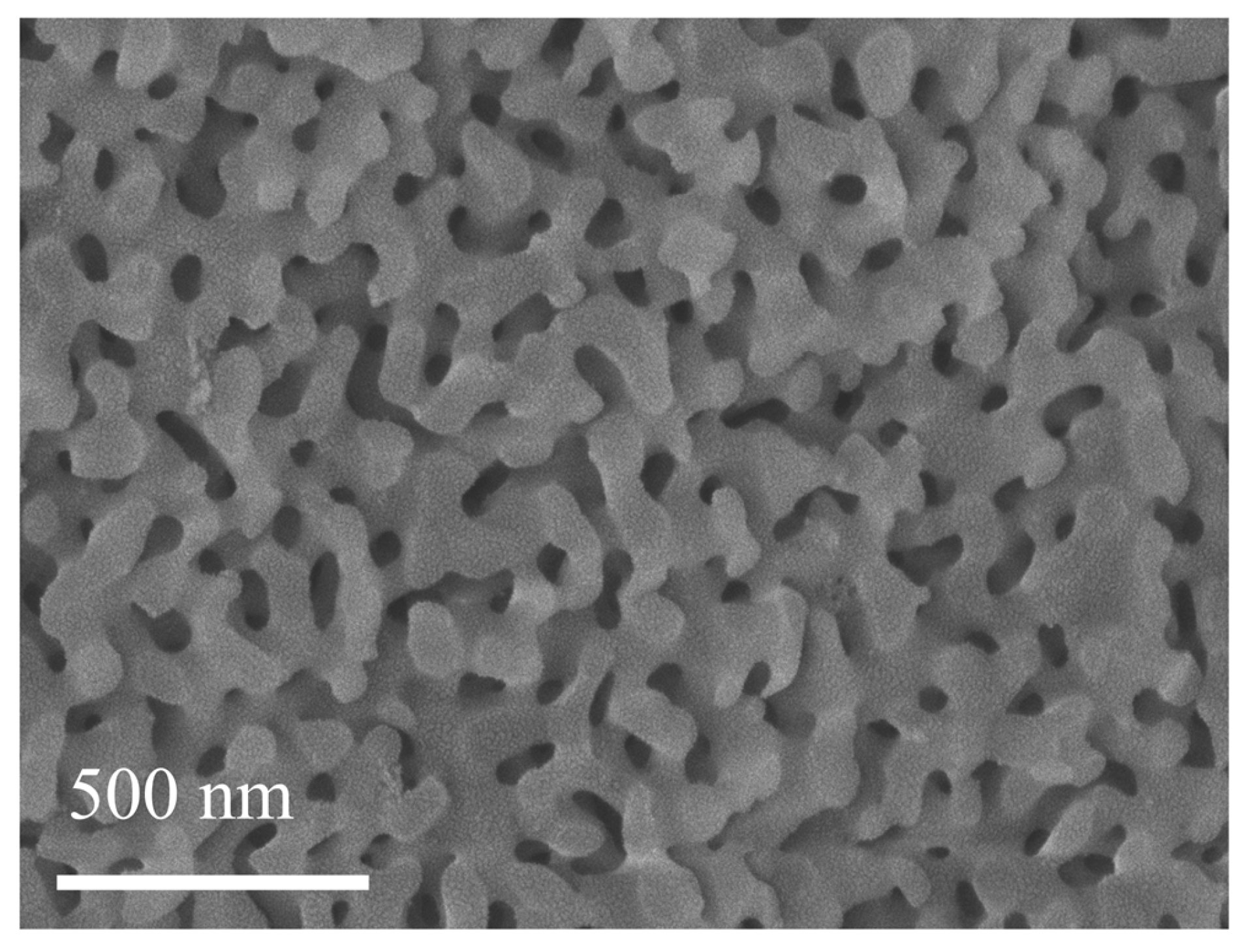
2.3. Microstructure Characterization
2.4. Nitrogen Sorption Analysis
2.5. Mechanical Properties of Silicon-Calcium Xerogels
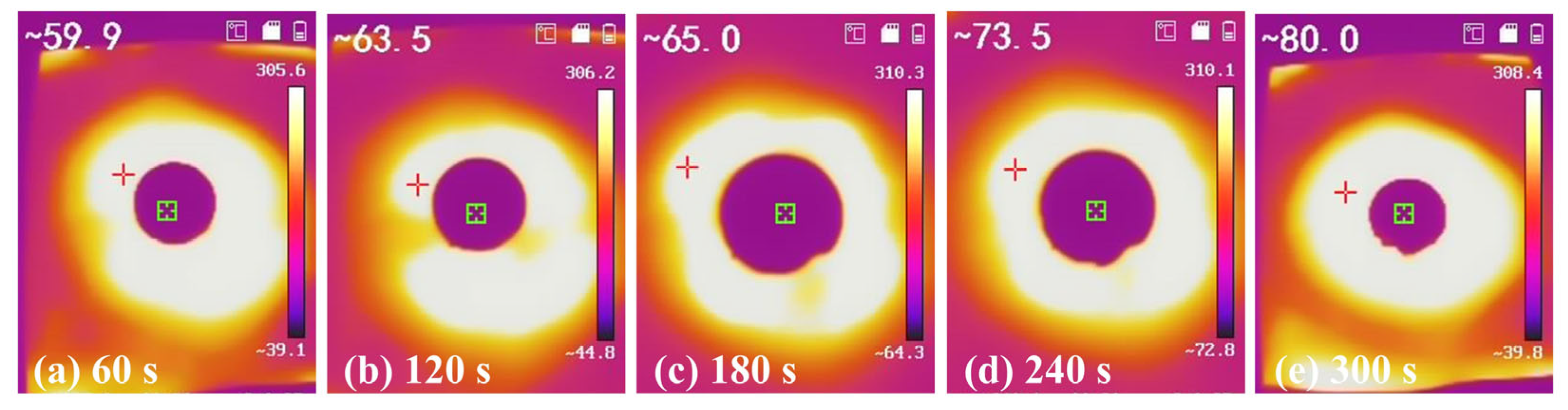
2.6. The Thermal Properties of Silicon-Calcium Xerogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Silicon-Calcium Xerogel
4.3. Characterization of Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Fiqi, A. Sol-Gel Synthesis, Physicochemical Characteristics and in Vitro pH-Responsive Metformin Drug Release of Uniformly Polydopamine Coated Mesoporous Silica Nanospheres. J. Non-Cryst. Solids 2024, 631, 122938. [Google Scholar] [CrossRef]
- Kovářík, T.; Bělský, P.; Křenek, T.; Deshmukh, K.; Forejtová, J.; Medlín, R.; Beneš, J.; Svoboda, M.; Kadlec, J.; Pola, M.; et al. Sol-Gel Derived Silicate-Phosphate Glass SiO2–P2O5–CaO–TiO2: The Effect of Titanium Isopropoxide on Porosity and Thermomechanical Stability. Microporous Mesoporous Mater. 2024, 374, 113138. [Google Scholar] [CrossRef]
- Ding, W.; Wang, X.; Chen, D.; Li, T.; Shen, J. Cast-In-Situ, Large-Sized Monolithic Silica Xerogel Prepared in Aqueous System. Molecules 2018, 23, 1178. [Google Scholar] [CrossRef] [PubMed]
- Akti, F.; Balci, S. Silica Xerogel and Iron Doped Silica Xerogel Synthesis in Presence of Drying Control Chemical Additives. Mater. Chem. Phys. 2023, 297, 127347. [Google Scholar] [CrossRef]
- Wiroonpochit, P.; Boonmee, P.; Kerdlap, W.; Chisti, Y.; Hansupalak, N. Synthesis of Low Crystalline Thermally Insulating Calcium Silicate Hydrate via a Simple Template-Assisted Sol–Gel Method. Constr. Build. Mater. 2022, 353, 129081. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Zhang, G.; Lin, L.; Ostrikov, K.K. Synthesis of High-Performance Polymethylsilsesquioxane Xerogels by Improving Acid Catalytic Conditions in Aluminum Chloride Aqueous Solution. Powder Technol. 2024, 443, 119877. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y. Iron/Silicon Oxy-Hydroxide Co-Precipitate Trace-Incorporated [-Fe-O]m-[Si-O-]n Colloids: A Facile Strategy for Silica Xerogel Strengthening and Toughening. Constr. Build. Mater. 2025, 461, 139895. [Google Scholar] [CrossRef]
- A’yuni, Q.; Rahmayanti, A.; Hartati, H.; Purkan, P.; Subagyo, R.; Fuadah, S.; Sholeha, N.A.; Bahruji, H.; Hikmat, H. Transforming Volcanic Mud into Mesoporous Silica Xerogel and Its Performance for Efficient Humidity Adsorption. J. Saudi Chem. Soc. 2023, 27, 101771. [Google Scholar] [CrossRef]
- Rosales-Reina, B.; Cruz-Quesada, G.; Pujol, P.; Reinoso, S.; Elosúa, C.; Arzamendi, G.; López-Ramón, M.V.; Garrido, J.J. Determination of Hazardous Vapors from the Thermal Decomposition of Organochlorinated Silica Xerogels with Adsorptive Properties. Environ. Res. 2024, 256, 119247. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Aznar, E.; Deveci, H.; Martínez-Máñez, R. Low-Cost Silica Xerogels as Potential Adsorbents for Ciprofloxacin Removal. Sustain. Chem. Pharm. 2021, 22, 100483. [Google Scholar] [CrossRef]
- Lewińska, I.; Bącal, P.; Tymecki, Ł. Hydrogen Peroxide Stabilization with Silica Xerogel for Paper-Based Analytical Devices and Its Application to Phenolic Compounds Determination. Anal. Chim. Acta 2024, 1320, 343028. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Torris, A.; Suresha, P.R.; Jadhav, S.; Badiger, M.V.; Ghormade, V. Design and Synthesis of a New Topical Agent for Halting Blood Loss Rapidly: A Multimodal Chitosan-Gelatin Xerogel Composite Loaded with Silica Nanoparticles and Calcium. Colloids Surf. B Biointerfaces 2021, 198, 111454. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Hu, D.; Liu, Z.; Zhang, X.; Yu, K.; Ma, W. A Simple and Efficient in Situ Polymerization of Silica Xerogel-Acrylic Thermal Insulation Coatings. Prog. Org. Coat. 2024, 187, 108142. [Google Scholar] [CrossRef]
- Khan, N.R.; Sharmin, T.; Bin Rashid, A. Exploring the Versatility of Aerogels: Broad Applications in Biomedical Engineering, Astronautics, Energy Storage, Biosensing, and Current Progress. Heliyon 2024, 10, e23102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive Manufacturing of Silica Aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef]
- Priya, A.K.; Alghamdi, H.M.; Kavinkumar, V.; Elwakeel, K.Z.; Elgarahy, A.M. Bioaerogels from Biomass Waste: An Alternative Sustainable Approach for Wastewater Treatment. Int. J. Biol. Macromol. 2024, 282, 136994. [Google Scholar] [CrossRef]
- Yang, B.; Lin, Y.; Chen, C. Greener Production of High-Performance Silica Xerogel: Upcycling Waste Glass Using Deep Eutectic Solvent-Assisted Extraction of Residual Na+. J. Clean. Prod. 2024, 448, 141401. [Google Scholar] [CrossRef]
- Wu, X.; Fan, M.; Mclaughlin, J.F.; Shen, X.; Tan, G. A Novel Low-Cost Method of Silica Aerogel Fabrication Using Fly Ash and Trona Ore with Ambient Pressure Drying Technique. Powder Technol. 2018, 323, 310–322. [Google Scholar] [CrossRef]
- Mazraeh-shahi, Z.T.; Shoushtari, A.M.; Abdouss, M.; Bahramian, A.R. Relationship Analysis of Processing Parameters with Micro and Macro Structure of Silica Aerogel Dried at Ambient Pressure. J. Non-Cryst. Solids 2013, 376, 30–37. [Google Scholar] [CrossRef]
- Si, Q.L.; Tang, G.H.; Yang, M.Y.; Yang, R.; Hu, Y.; Du, M.; Zhang, H. Ambient-Dried Hydrophobic Silica Aerogels for Both Enhanced Transparency and Thermal Insulation. Ceram. Int. 2024, 50, 48680–48691. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Q.; Wang, L.; Cao, J.; Song, J.; Song, L.; Zhang, Y. Recent Advances in the Synthesis and Application of Graphene Aerogel and Silica Aerogel for Environment and Energy Storage: A Review. J. Environ. Manag. 2025, 377, 124668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, C.; He, J.; Liu, L.; Yao, J.; Yang, Y.; Xu, K.; Feng, W.; Du, G.; Zhang, L. Cellulose/Covalent Organic Framework Aerogel for Efficient Removal of Cr(VI): Performance and Mechanism Study. Int. J. Biol. Macromol. 2025, 300, 140243. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, A.A.; Firoozi, A.A.; El-Abbasy, A.A.; Aati, K. Enhanced Perspectives on Silica Aerogels: Novel Synthesis Methods and Emerging Engineering Applications. Results Eng. 2025, 25, 103615. [Google Scholar] [CrossRef]
- Wang, H.; Cao, M.; Zhao, H.-B.; Liu, J.-X.; Geng, C.-Z.; Wang, Y.-Z. Double-Cross-Linked Aerogels towards Ultrahigh Mechanical Properties and Thermal Insulation at Extreme Environment. Chem. Eng. J. 2020, 399, 125698. [Google Scholar] [CrossRef]
- Wassgren, J.; Clarke, B.R.; Messikh, M.B.; Ho, C.-H.; Crosby, A.J.; Tew, G.N.; Carter, K.R. Enhancement of Mechanical Properties of Nanocellulose Xerogels Using TEMPO-Oxidized Fibers. Carbohydr. Polym. 2025, 348, 122839. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Z.; Hu, J.; Wang, W.; Li, W. Facile Preparation of Lignocellulosic Xerogels by Alkali Freezing and Ambient Drying. Green Chem. 2024, 26, 6501–6510. [Google Scholar] [CrossRef]
- Birdsong, B.K.; Capezza, A.J.; Mensah, R.A.; Elf, P.; Hedenqvist, M.S.; Nilsson, F.; Olsson, R.T. Flexible Fire-Safe Hybrid Organic–Inorganic Cellulose Aerogels from Sol–Gel Casting. RSC Sustain. 2025, 3, 1009–1018. [Google Scholar] [CrossRef]
- Ul Haq, E.; Zaidi, S.F.A.; Zubair, M.; Abdul Karim, M.R.; Padmanabhan, S.K.; Licciulli, A. Hydrophobic Silica Aerogel Glass-Fibre Composite with Higher Strength and Thermal Insulation Based on Methyltrimethoxysilane (MTMS) Precursor. Energy Build. 2017, 151, 494–500. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, Drying Process and Medical Application of Polysaccharide-Based Aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Seraji, M.M.; Seifi, A.; Bahramian, A.R. Morphology and Properties of Silica/Novolac Hybrid Xerogels Synthesized Using Sol–Gel Polymerization at Solvent Vapor-Saturated Atmosphere. Mater. Des. 2015, 69, 190–196. [Google Scholar] [CrossRef]
- Ostovar, S.; Moussavi, G.; Mohammadi, S.; Marin, M.L.; Bosca, F.; Diego-Lopez, A.; Giannakis, S. Rapid Degradation of Omeprazole and Highly Effective Inactivation of E. coli in the UVA-Light Photocatalytic Process with Cu-Doped in Spinel-Structured ɣAl2O3 as a Stable Catalyst. Chem. Eng. J. 2024, 479, 147536. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Chen, J.; He, J. Thermal Reduction Triggered Red–Orange to Blue-Violet Color-Tunable Eu Doped Bulk Transparent Nanoporous Al2O3-SiO2 Glass for LEDs. Mater. Sci. Eng. B 2024, 305, 117446. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Liu, T.; Liu, L.; Yu, C.; Tian, Y.; Zhang, X.; Shen, J. Al3+ Coordinated Chitosan Hydrogel with Ultrahigh Water Absorbency and Environmental Response. Mater. Des. 2022, 214, 110390. [Google Scholar] [CrossRef]
- Seljelid, K.K.; Neto, O.T.; Akanno, A.N.; Ceccato, B.T.; Ravindranathan, R.P.; Azmi, N.; Cavalcanti, L.P.; Fjelde, I.; Knudsen, K.D.; Fossum, J.O. Growth Kinetics and Structure of a Colloidal Silica-Based Network: In Situ RheoSAXS Investigations. Eur. Phys. J. Spec. Top. 2024, 233, 2757–2773. [Google Scholar] [CrossRef]
- Pougher, N.; Vollmer, A.H.; Sharma, P. Effect of pH and Calcium Chelation on Cold Gelling Properties of Highly Concentrated-Micellar Casein Concentrate. LWT 2024, 214, 117136. [Google Scholar] [CrossRef]
- Gogajeh, N.N.; Yekta, B.E.; Javadpour, J.; Eslaminejad, M.B. Structural Characterization, in Vitro Bioactivity, and Cytotoxicity Evaluation of Sol-Gel Derived SiO2-CaO-Na2O-P2O5 Based Glass- Ceramics in the Presence of Manganese. Mater. Today Chem. 2025, 45, 102701. [Google Scholar] [CrossRef]
- Rashid, I.; Omari, M.H.A.; Leharne, S.A.; Chowdhry, B.Z.; Badwan, A. Starch Gelatinization Using Sodium Silicate: FTIR, DSC, XRPD, and NMR Studies. Starch-Stärke 2012, 64, 713–728. [Google Scholar] [CrossRef]
- Nayak, P.P.; Datta, A.K. Synthesis of SiO2-Nanoparticles from Rice Husk Ash and Its Comparison with Commercial Amorphous Silica through Material Characterization. Silicon 2021, 13, 1209–1214. [Google Scholar] [CrossRef]
- Niu, Z.; He, X.; Huang, T.; Tang, B.; Cheng, X.; Zhang, Y.; Shao, Z. A Facile Preparation of Transparent Methyltriethoxysilane Based Silica Xerogel Monoliths at Ambient Pressure Drying. Microporous Mesoporous Mater. 2019, 286, 98–104. [Google Scholar] [CrossRef]
- Kazancioglu, M.; Tsilomelekis, G.; Lehman, R.; Hara, M. FTIR Studies on Plasticization of Silicate Glass with Ionic Liquids (Conversion to Silicate Polymers). J. Non-Cryst. Solids 2021, 561, 120757. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Huang, H.; Yao, M.; Li, S.; Li, J.; Zhang, W.; Yin, J. Rapid and Well-Controlled Degradation of Polylactic Acid Materials with Bio-Based GEL(Pectin/α-Cellulose/SiO2/CaCl2). Int. J. Biol. Macromol. 2025, 291, 139099. [Google Scholar] [CrossRef] [PubMed]
- Guzel Kaya, G.; Yilmaz, E.; Deveci, H. Synthesis of Sustainable Silica Xerogels/Aerogels Using Inexpensive Steel Slag and Bean Pod Ash: A Comparison Study. Adv. Powder Technol. 2020, 31, 926–936. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, W.; Lv, G.; Liu, J.; Peng, H.; Fang, T.; Tan, X.; Chen, Z. Preparation and Properties of CaCl2·6H2O/Silica Aerogel Composite Phase Change Material for Building Energy Conservation. J. Mol. Liq. 2023, 382, 121986. [Google Scholar] [CrossRef]
- Gediz Ilis, G. Influence of New Adsorbents with Isotherm Type V on Performance of an Adsorption Heat Pump. Energy 2017, 119, 86–93. [Google Scholar] [CrossRef]
- Chang, C.; Tao, S.; Xu, C.; Zhang, Y.; Jiang, W.; Cao, Y.; Ma, C. Highly Transparent Polymethylsilsesquioxane Xerogel Monoliths with Nanopores Around 10 Nm via Ambient Pressure Drying: A Potential Host for Nano-Functional Materials. J. Lumin. 2025, 286, 121353. [Google Scholar] [CrossRef]
- Klomkliang, N.; Do, D.D.; Nicholson, D. Effects of Temperature, Pore Dimensions and Adsorbate on the Transition from Pore Blocking to Cavitation in an Ink-Bottle Pore. Chem. Eng. J. 2014, 239, 274–283. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, W.; Chen, Z.; Su, D. Double-Network MK Resin-Modified Silica Aerogels for High-Temperature Thermal Insulation. ACS Appl. Mater. Interfaces 2023, 15, 44238–44247. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, X.; Yu, H.; Tong, Z. Preparation of High-Strength Silica Aerogels by Two-Step Surface Modification via Ambient Pressure Drying. J. Porous Mater. 2024, 28, 651–659. [Google Scholar] [CrossRef]

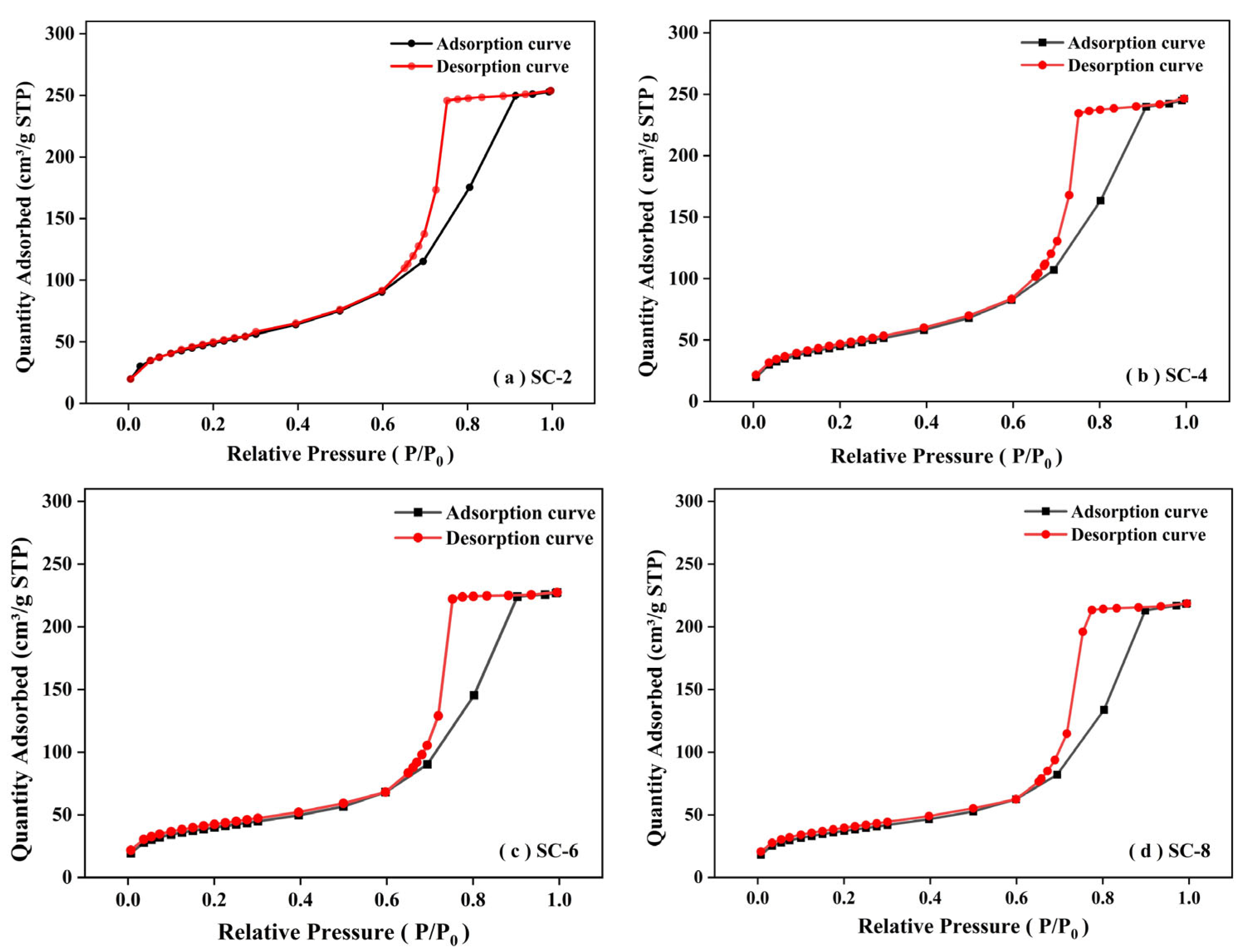


| Sample ID | Surface Area m2/g | Pore Volume cm3/g |
|---|---|---|
| SC-2 | 177.26 | 74.95 |
| SC-4 | 162.22 | 74.66 |
| SC-6 | 139.54 | 74.97 |
| SC-8 | 130.80 | 75.15 |
| Sample | Silicon Sol (g) | Glycerin (g) | Calcium Chloride Solution (g) | Calcium Ion Content (wt.%) |
|---|---|---|---|---|
| SC-2 | 20 | 0.3 | 0.6 | 2% |
| SC-4 | 20 | 0.3 | 1.2 | 4% |
| SC-6 | 20 | 0.3 | 1.8 | 6% |
| SC-8 | 20 | 0.3 | 2.4 | 8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Zhu, Z.; Meng, Y.; Wang, L.; Zhao, F.; Chen, L.; Jiang, L.; Yan, M.; Zhou, X. Ambient-Dried Silica Xerogels with Enhanced Strength and Thermal Insulation via Calcium Ion-Glycerol Synergistic Crosslinking. Gels 2025, 11, 462. https://doi.org/10.3390/gels11060462
Xie X, Zhu Z, Meng Y, Wang L, Zhao F, Chen L, Jiang L, Yan M, Zhou X. Ambient-Dried Silica Xerogels with Enhanced Strength and Thermal Insulation via Calcium Ion-Glycerol Synergistic Crosslinking. Gels. 2025; 11(6):462. https://doi.org/10.3390/gels11060462
Chicago/Turabian StyleXie, Xiaoyu, Zilin Zhu, Yu Meng, Lijia Wang, Fuquan Zhao, Lingqing Chen, Lijie Jiang, Ming Yan, and Xiaofan Zhou. 2025. "Ambient-Dried Silica Xerogels with Enhanced Strength and Thermal Insulation via Calcium Ion-Glycerol Synergistic Crosslinking" Gels 11, no. 6: 462. https://doi.org/10.3390/gels11060462
APA StyleXie, X., Zhu, Z., Meng, Y., Wang, L., Zhao, F., Chen, L., Jiang, L., Yan, M., & Zhou, X. (2025). Ambient-Dried Silica Xerogels with Enhanced Strength and Thermal Insulation via Calcium Ion-Glycerol Synergistic Crosslinking. Gels, 11(6), 462. https://doi.org/10.3390/gels11060462






