Microfluidic Synthesis of Magnetic Silica Aerogels for Efficient Pesticide Removal from Water
Abstract
1. Introduction
2. Results and Discussion
2.1. Nanoparticle Characterization
2.2. Aerogel Characterization
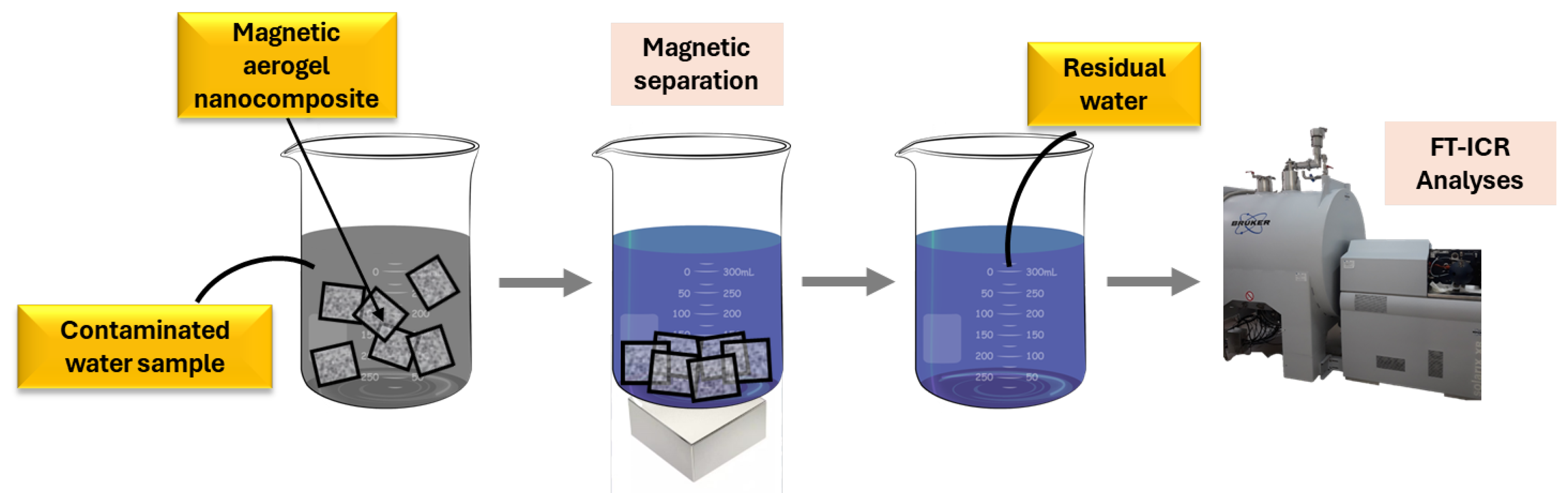
2.3. Decontamination Capacity
2.4. Discussion
3. Conclusions
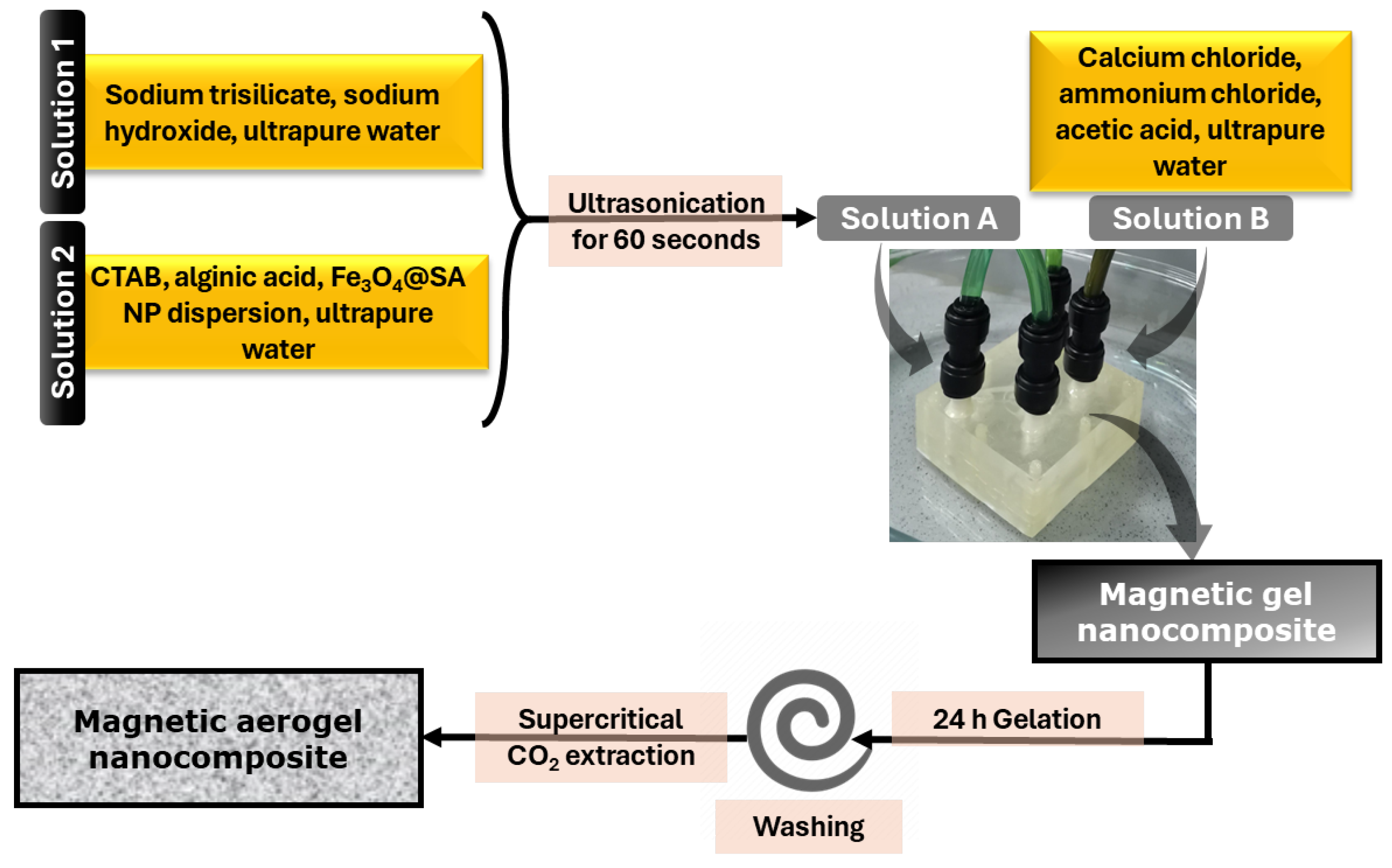
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Nanoparticle Synthesis
4.2.2. Aerogel Synthesis
4.2.3. Nanoparticle Characterization
4.2.4. Aerogel Characterization
4.2.5. Water Decontamination Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Amreen, K.; Ramya, K.; Goel, S. Microfluidic Devices for Synthesizing Nanomaterials. In Microfluidics in Food Processing: Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2025; p. 106. [Google Scholar]
- Yao, F.; Zhu, P.; Chen, J.; Li, S.; Sun, B.; Li, Y.; Zou, M.; Qi, X.; Liang, P.; Chen, Q. Synthesis of nanoparticles via microfluidic devices and integrated applications. Microchim. Acta 2023, 190, 256. [Google Scholar] [CrossRef] [PubMed]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Wittmann, L.; Krucker-Velasquez, E.; Schaupp, J.; Westphal, L.; Swan, J.W.; Alexander-Katz, A.; Bazant, M.Z.; Schwaminger, S.P.; Berensmeier, S. Influence of magnetic convection on separation efficiency in magnetophoretic microfluidic processes: A combined simulation and experimental study. Nanoscale 2025, 17, 1574–1584. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Moroșan, A.; Bîrcă, A.C.; Gherasim, O.; Oprea, O.C.; Vasile, B.Ș.; Purcăreanu, B.; Mihaiescu, D.E.; Rădulescu, M.; Grumezescu, A.M. Microwave-Assisted Silanization of Magnetite Nanoparticles Pre-Synthesized by a 3D Microfluidic Platform. Nanomaterials 2023, 13, 2795. [Google Scholar] [CrossRef]
- Mittal, T.; Kant, R. Functional Nanohybrid and Nanocomposite Coatings for Sustainable Environmental Remediation. Lett. Appl. NanoBioScience 2024, 13, 181. [Google Scholar] [CrossRef]
- Rodrigues, A.R.d.S.P. Application of Nanotechnological Compounds in the Removal of Heavy Metals. Mater. Int. 2024, 6, 15. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Munteanu, O.M.; Bîrcă, A.C.; Moroșan, A.; Purcăreanu, B.; Vasile, B.Ș.; Istrati, D.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. New 3D Vortex Microfluidic System Tested for Magnetic Core-Shell Fe3O4-SA Nanoparticle Synthesis. Nanomaterials 2024, 14, 902. [Google Scholar] [CrossRef]
- Stiufiuc, G.F.; Stiufiuc, R.I. Magnetic Nanoparticles: Synthesis, Characterization, and Their Use in Biomedical Field. Appl. Sci. 2024, 14, 1623. [Google Scholar] [CrossRef]
- Ismail, A.M.; Tiama, T.M.; Farghaly, A.; Elhaes, H.; Ibrahim, M.A. Assessment of the Functionalization of Chitosan/Iron Oxide Nanoparticles. Biointerface Res. Appl. Chem. 2023, 13, 582. [Google Scholar] [CrossRef]
- Salehirozveh, M.; Dehghani, P.; Mijakovic, I. Synthesis, Functionalization, and Biomedical Applications of Iron Oxide Nanoparticles (IONPs). J. Funct. Biomater. 2024, 15, 340. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.; Ahmad, R.; Tanweer, M.S.; Azam, I. Magnetic Iron Oxide Nanoparticles as a Tool for the Advancement of Biomedical and Environmental Application: A Review. Biomed. Mater. Devices 2024, 2, 139–157. [Google Scholar] [CrossRef]
- Upadhyay, J.; Singh, A. Lignin Derived Biofabrication of Magnetic Iron Nanoparticles from Moringa oleifera. Lett. Appl. NanoBioScience 2024, 13, 104. [Google Scholar] [CrossRef]
- Urbina, A.D.; Sridhara, H.; Scholtz, A.; Armani, A.M. Synthesis and Characterization of Superparamagnetic Iron Oxide Nanoparticles: A Series of Laboratory Experiments. J. Chem. Educ. 2024, 101, 2039–2044. [Google Scholar] [CrossRef]
- Kara, G.; Ozpolat, B. SPIONs: Superparamagnetic iron oxide-based nanoparticles for the delivery of microRNAi-therapeutics in cancer. Biomed. Microdevices 2024, 26, 16. [Google Scholar] [CrossRef]
- Nedylakova, M.; Medinger, J.; Mirabello, G.; Lattuada, M. Iron oxide magnetic aggregates: Aspects of synthesis, computational approaches and applications. Adv. Colloid Interface Sci. 2024, 323, 103056. [Google Scholar] [CrossRef]
- Tiama, T.M.; Ismail, A.M.; Elhaes, H.; Ibrahim, M.A. Structural and Spectroscopic Studies for Chitosan/Fe3O4 Nanocomposites as Glycine Biosensors. Biointerface Res. Appl. Chem. 2023, 13, 547. [Google Scholar] [CrossRef]
- Dong, L.; Chen, G.; Liu, G.; Huang, X.; Xu, X.; Li, L.; Zhang, Y.; Wang, J.; Jin, M.; Xu, D.; et al. A review on recent advances in the applications of composite Fe3O4 magnetic nanoparticles in the food industry. Crit. Rev. Food Sci. Nutr. 2024, 64, 1110–1138. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Giove, A.; Laatikainen, M.; Branger, C.; Laatikainen, K. Benefit of ion imprinting technique in solid-phase extraction of heavy metals, special focus on the last decade. J. Environ. Chem. Eng. 2021, 9, 106548. [Google Scholar] [CrossRef]
- da Silva Sousa, J.; do Nascimento, H.O.; de Oliveira Gomes, H.; do Nascimento, R.F. Pesticide residues in groundwater and surface water: Recent advances in solid-phase extraction and solid-phase microextraction sample preparation methods for multiclass analysis by gas chromatography-mass spectrometry. Microchem. J. 2021, 168, 106359. [Google Scholar] [CrossRef]
- Tang, K.H.D. Environmental Co-existence of Microplastics and Perfluorochemicals: A Review of Their Interactions. Biointerface Res. Appl. Chem. 2023, 13, 587. [Google Scholar] [CrossRef]
- Kannabiran, K. A Critical Review on Effect of Macro, Micro and Nanoplastics Pollution on Human Health and their Control Measures. Mater. Int. 2024, 6, 31. [Google Scholar] [CrossRef]
- Brewer, A.; Florek, J.; Kleitz, F. A perspective on developing solid-phase extraction technologies for industrial-scale critical materials recovery. Green Chem. 2022, 24, 2752–2765. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Liang, Q.; Zhang, X.; Yang, L.; Ding, M. Graphene aerogel based monolith for effective solid-phase extraction of trace environmental pollutants from water samples. J. Chromatogr. A 2016, 1447, 39–46. [Google Scholar] [CrossRef]
- Zhu, F.; Lu, H.; Lu, Y. Effective solid phase extraction for the enrichment of p-nitrophenol in water using microwave-assisted synthesized fly ash@ p-nitrophenol surface molecular imprinted polymer. J. Mater. Sci. 2023, 58, 4399–4415. [Google Scholar] [CrossRef]
- Büyüktiryaki, S.; Keçili, R.; Hussain, C.M. Functionalized nanomaterials in dispersive solid phase extraction: Advances & prospects. TrAC Trends Anal. Chem. 2020, 127, 115893. [Google Scholar] [CrossRef]
- Maranata, G.J.; Surya, N.O.; Hasanah, A.N. Optimising factors affecting solid phase extraction performances of molecular imprinted polymer as recent sample preparation technique. Heliyon 2021, 7, e05934. [Google Scholar] [CrossRef]
- Maftouh, A.; El Fatni, O.; El Hajjaji, S.; Jawish, M.W.; Sillanpää, M. Comparative Review of Different Adsorption Techniques Used in Heavy Metals Removal in Water. Biointerface Res. Appl. Chem. 2023, 13, 397. [Google Scholar] [CrossRef]
- Sun, M.; Li, C.; Feng, J.; Sun, H.; Sun, M.; Feng, Y.; Ji, X.; Han, S.; Feng, J. Development of aerogels in solid-phase extraction and microextraction. TrAC Trends Anal. Chem. 2022, 146, 116497. [Google Scholar] [CrossRef]
- Zhuang, Y.; Tang, S.; Shen, W.; Yang, F.; Lee, H.K. Recent progress of graphene aerogel as sorbent in solid-phase extraction: A review. TrAC Trends Anal. Chem. 2023, 168, 117352. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Tudorache, D.-I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, S.; Sheikh, J.N. Silica centered aerogels as advanced functional material and their applications: A review. J. Non-Cryst. Solids 2023, 611, 122322. [Google Scholar] [CrossRef]
- Jinde, P.D.; Gudiyawar, M.Y. Silica aerogel in textiles and nanofibers: A comprehensive review of synthesis techniques and embedding strategies. J. Text. Inst. 2024, 115, 1962–1982. [Google Scholar] [CrossRef]
- Firoozi, A.A.; Firoozi, A.A.; El-Abbasy, A.A.; Aati, K. Enhanced perspectives on silica aerogels: Novel synthesis methods and emerging engineering applications. Results Eng. 2025, 25, 103615. [Google Scholar] [CrossRef]
- Ben Rejeb, Z.; Abidli, A.; Zaoui, A.; Fashandi, M.; Selka, A.; Naguib, H.E.; Park, C.B. One-pot synthesis of rationally-designed flexible, robust, and hydrophobic ambient-dried molecularly-bridged silica aerogels with efficient and versatile oil/water separation applications. Adv. Compos. Hybrid Mater. 2024, 7, 188. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, K.; Chen, J.; Xia, M.; Li, M.; Liu, K.; Wang, D.; Wu, C.; Xie, Y. Recent advances in novel aerogels through the hybrid aggregation of inorganic nanomaterials and polymeric fibers for thermal insulation. Aggregate 2021, 2, e30. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in carbon nanostructure–silica aerogel composites: A review. J. Mater. Chem. A 2018, 6, 1340–1369. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Q.; Wang, L.; Cao, J.; Song, J.; Song, L.; Zhang, Y. Recent advances in the synthesis and application of graphene aerogel and silica aerogel for environment and energy storage: A review. J. Environ. Manag. 2025, 377, 124668. [Google Scholar] [CrossRef]
- Akhter, F.; Jamali, A.R.; Abbasi, M.N.; Mallah, M.A.; Rao, A.A.; Wahocho, S.A.; Anees-ur-Rehman, H.; Chandio, Z.A. A comprehensive review of hydrophobic silica and composite aerogels: Synthesis, properties and recent progress towards environmental remediation and biomedical applications. Environ. Sci. Pollut. Res. 2023, 30, 11226–11245. [Google Scholar] [CrossRef]
- Rashid, A.B.; Shishir, S.I.; Mahfuz, M.A.; Hossain, M.T.; Hoque, M.E. Silica Aerogel: Synthesis, Characterization, Applications, and Recent Advancements. Part. Part. Syst. Charact. 2023, 40, 2200186. [Google Scholar] [CrossRef]
- Hou, X.; Chen, J.; Chen, Z.; Yu, D.; Zhu, S.; Liu, T.; Chen, L. Flexible Aerogel Materials: A Review on Revolutionary Flexibility Strategies and the Multifunctional Applications. ACS Nano 2024, 18, 11525–11559. [Google Scholar] [CrossRef] [PubMed]
- Muringayil Joseph, T.; Azat, S.; Kianfar, E.; Joshy, K.S.; Moini Jazani, O.; Esmaeili, A.; Ahmadi, Z.; Haponiuk, J.; Thomas, S. Identifying gaps in practical use of epoxy foam/aerogels: Review—Solutions and prospects. Rev. Chem. Eng. 2025, 41, 269–308. [Google Scholar] [CrossRef]
- Kapadnis, P.S.; Nam, K.-S.; Kim, H.-Y.; Park, H.-H.; Hwang, H. Facile Synthesis of Surface-Modified Hollow-Silica (SiO2) Aerogel Particles via Oil–Water–Oil Double Emulsion Method. Gels 2024, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Z.; Gong, J.; Zhou, X.; Li, J.; Lyu, Z. 3D printing of graphene-based aerogels and their applications. FlatChem 2024, 47, 100731. [Google Scholar] [CrossRef]
- Istiqomah, N.I.; Sabarman, H.; Suharyadi, E. Synthesis of varied oleic acid-coated Fe3O4 nanoparticles using the co-precipitation technique for biosensor applications. Sens. Int. 2025, 6, 100295. [Google Scholar]
- Bhole, R.; Gonsalves, D.; Murugesan, G.; Narasimhan, M.K.; Srinivasan, N.R.; Dave, N.; Varadavenkatesan, T.; Vinayagam, R.; Govarthanan, M.; Selvaraj, R. Superparamagnetic spherical magnetite nanoparticles: Synthesis, characterization and catalytic potential. Appl. Nanosci. 2023, 13, 6003–6014. [Google Scholar] [CrossRef]
- Mercan, D.-A.; Niculescu, A.-G.; Bîrcă, A.C.; Cristea, D.-E.; Moroșan, A.; Tudorache, D.-I.; Purcăreanu, B.; Vasile, B.Ș.; Radu, D.; Grigoroscuta, M.A.; et al. Vortex-Mixing Microfluidic Fabrication of Micafungin-Loaded Magnetite–Salicylic Acid–Silica Nanocomposite with Sustained-Release Capacity. Materials 2024, 17, 5816. [Google Scholar] [CrossRef]
- Mercan, D.-A.; Tudorache, D.-I.; Niculescu, A.-G.; Mogoantă, L.; Mogoşanu, G.D.; Bîrcă, A.C.; Vasile, B.Ș.; Hudiță, A.; Voinea, I.C.; Stan, M.S.; et al. Antimicrobial Coatings Based on Hybrid Iron Oxide Nanoparticles. Nanomaterials 2025, 15, 637. [Google Scholar] [CrossRef]
- Sodipo, B.K.; Azlan, A.A. Superparamagnetic iron oxide nanoparticles incorporated into silica nanoparticles by inelastic collision via ultrasonic field: Role of colloidal stability. AIP Conf. Proc. 2015, 1657, 100002. [Google Scholar] [CrossRef]
- Purcăreanu, B.; Ene, M.D.; Moroșan, A.; Mihaiescu, D.E.; Florea, M.A.; Ghica, A.; Nita, R.A.; Drumea, V.; Grigoroscuta, M.A.; Kuncser, A.; et al. Mesoporous Composite Bioactive Compound Delivery System for Wound-Healing Processes. Pharmaceutics 2023, 15, 2258. [Google Scholar] [CrossRef]
- Waseem, M.; Mustafa, S.; Naeem, A.; Shah, K.; Shah, I.; Ihsan Ul, H. Synthesis and characterization of silica by sol-gel method. J. Pak. Mater. Soc. 2009, 3, 19–21. [Google Scholar]
- Handzo, B.; Peters, J.; Kalyanaraman, R. A fingerprint in a fingerprint: A Raman spectral analysis of pharmaceutical ingredients. Spectroscopy 2022, 37, 24–30; 45. [Google Scholar] [CrossRef]
- Liu, H.; Hahn, S.H.; Ren, M.; Thiruvillamalai, M.; Gross, T.M.; Du, J.; van Duin, A.C.T.; Kim, S.H. Searching for correlations between vibrational spectral features and structural parameters of silicate glass network. J. Am. Ceram. Soc. 2020, 103, 3575–3589. [Google Scholar] [CrossRef]
- Ru, Y.; Guoqiang, L.; Min, L. Analysis of the effect of drying conditions on the structural and surface heterogeneity of silica aerogels and xerogel by using cryogenic nitrogen adsorption characterization. Microporous Mesoporous Mater. 2010, 129, 1–10. [Google Scholar] [CrossRef]
- Anderson, A.M.; Carroll, M.K. Rapid aerogel fabrication facilitates a range of applications. J. Sol-Gel Sci. Technol. 2025, 1–16. [Google Scholar] [CrossRef]
- Meti, P.; Wang, Q.; Mahadik, D.B.; Lee, K.-Y.; Gong, Y.-D.; Park, H.-H. Evolutionary Progress of Silica Aerogels and Their Classification Based on Composition: An Overview. Nanomaterials 2023, 13, 1498. [Google Scholar] [CrossRef]
- Koreeda, H.; Ishijima, M.; Kajihara, K. Cosolvent-free sol–gel synthesis of macroporous silica gels from tetramethoxysilane–tetraethoxysilane mixtures. J. Sol-Gel Sci. Technol. 2025, 113, 48–55. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Q.; Shi, Y.; Yang, D.; Lan, Y.; Wang, T. A facile strategy for freeze-drying preparation of silica aerogel from sodium silicate. Ceram. Int. 2025, 51, 5342–5350. [Google Scholar] [CrossRef]
- Sun, W.; Yu, L.; Su, J.; Liu, R.; Yan, X.; Su, D.; Zhang, P.; Li, X. Preparation of SiO2 aerogel by water glass: Effect of different sodium removal methods on aerogel properties. J. Sol-Gel Sci. Technol. 2025, 113, 274–284. [Google Scholar] [CrossRef]
- Minju, N.; Nair, B.N.; Savithri, S. Sodium silicate-derived aerogels: Effect of processing parameters on their applications. RSC Adv. 2021, 11, 15301–15322. [Google Scholar] [CrossRef]
- Xiang, L.; Zheng, S.; Lei, J.; Pan, D.; Li, S.; Wang, J.; Wang, Y.; Liu, C. Research Progress on Preparation and Application of Sodium Alginate Aerogel. ES Mater. Manuf. 2024, 25, 1215. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Quan, F.; Chen, Z.; Xia, Y.; Xiong, Z. Wide-Spectrum Dye Adsorption Performance of Alginic Acid Carbon Aerogel. Water Air Soil Pollut. 2024, 235, 375. [Google Scholar] [CrossRef]
- El-Desouky, M.G.; Alayyafi, A.A.; Al-Hazmi, G.A.A.M.; El-Bindary, A.A. Effect of metal organic framework alginate aerogel composite sponge on adsorption of tartrazine from aqueous solutions: Adsorption models, thermodynamics and optimization via Box-Behnken design. J. Mol. Liq. 2024, 399, 124392. [Google Scholar] [CrossRef]
- Shang, Z.; Lei, E.; Chen, Y.; Zhao, D.; Wei, Y.; Chai, Q.; Wei, H.; Zhao, Y. Sepiolite fiber reinforced TiO2/SiO2 composite aerogel based on CTAB “template” effect. J. Mater. Sci. Mater. Electron. 2024, 35, 1894. [Google Scholar] [CrossRef]
- Song, Y.; Li, K.; Sun, W.; Zhao, S.; Zhang, Z.; Li, X.; Zhang, P.; Gan, Z.; Yang, Z. Synergistic strategy of hard-template etching and soft-template protection for rapid fabrication of aerogel short fibers. Appl. Mater. Today 2025, 42, 102537. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, X.; Feng, Y.; Yang, X. Adsorption of methylene blue dye on feather keratin/silk fibroin porous aerogels. J. Taiwan Inst. Chem. Eng. 2025, 166, 105298. [Google Scholar] [CrossRef]
- Abedpour, H.; Moghaddas, J.S.; Borhani, M.N.; Borhani, T.N. Separation of toxic contaminants from water by silica aerogel-based adsorbents: A comprehensive review. J. Water Process Eng. 2023, 53, 103676. [Google Scholar] [CrossRef]
- Rad, S.M.; Ray, A.K.; Barghi, S. Water Pollution and Agriculture Pesticide. Clean Technol. 2022, 4, 1088–1102. [Google Scholar] [CrossRef]
- Kiran, P.S.; Mandal, P.; Jain, M.; Ghosal, P.S.; Gupta, A.K. A comprehensive review on the treatment of pesticide-contaminated wastewater with special emphasis on organophosphate pesticides using constructed wetlands. J. Environ. Manag. 2024, 368, 122163. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Nguyen, K.T.; Le, B.H.; Nghiem, X.T.; La, D.D.; Nguyen, D.K.; Nguyen, H.P.T. Synthesis of magnetic Fe3O4/graphene aerogel for the removal of 2,4-dichlorophenoxyacetic acid herbicide from water. RSC Adv. 2024, 14, 22304–22311. [Google Scholar] [CrossRef]
- Ali, I.; Neskoromnaya, E.A.; Melezhik, A.V.; Babkin, A.V.; Kulnitskiy, B.A.; Burakov, A.E.; Burakova, I.V.; Tkachev, A.G.; Almalki, A.S.A.; Alsubaie, A. Magnetically active nanocomposite aerogels: Preparation, characterization and application for water treatment. J. Porous Mater. 2022, 29, 545–557. [Google Scholar] [CrossRef]
- Yang, L.; Han, Q.; Sun, S.; Ding, M. Evaluation of Graphene Aerogel Monolith-Based Solid-Phase Extraction for the Separation of Pyrethroids from Water Samples. Chromatographia 2017, 80, 1781–1787. [Google Scholar] [CrossRef]
- Mahpishanian, S.; Sereshti, H. Three-dimensional graphene aerogel-supported iron oxide nanoparticles as an efficient adsorbent for magnetic solid phase extraction of organophosphorus pesticide residues in fruit juices followed by gas chromatographic determination. J. Chromatogr. A 2016, 1443, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Emam, H.E. Macroporous Cu-MOF@cellulose acetate membrane serviceable in selective removal of dimethoate pesticide from wastewater. J. Environ. Chem. Eng. 2021, 9, 105121. [Google Scholar] [CrossRef]
- Yang, A.; Wang, T.; Wu, J.; Hao, C.; Gan, W.; Ran, Z.; Tan, X.; Wang, Z.; Lu, K. Efficient adsorption of pesticides by porous organic polymers based on imine/aminal linkages. J. Water Process Eng. 2024, 64, 105681. [Google Scholar] [CrossRef]
- Feng, T.; Ye, X.; Zhao, Y.; Zhao, Z.; Hou, S.; Liang, N.; Zhao, L. Magnetic silica aerogels with high efficiency for selective adsorption of pyrethroid insecticides in juices and tea beverages. New J. Chem. 2019, 43, 5159–5166. [Google Scholar] [CrossRef]
- Roostaie, A.; Mohammadiazar, S.; Bargozin, H.; Ehteshami, S. A Modified Nanoporous Silica Aerogel as a New Sorbent for Needle Trap Extraction of Chlorobenzenes from Water Samples. Chromatographia 2018, 81, 649–655. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Zhang, Z.; Wang, X.; Shen, J. Adsorption of cationic dyes from aqueous solution using hydrophilic silica aerogel via ambient pressure drying. Chin. J. Chem. Eng. 2020, 28, 2467–2473. [Google Scholar] [CrossRef]
- Liu, G.; Yang, R.; Li, M. Liquid adsorption of basic dye using silica aerogels with different textural properties. J. Non-Cryst. Solids 2010, 356, 250–257. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, J.; Wei, W.; Ma, L.; Zhu, J.; Xie, J. Comparative study of modified/non-modified aluminum and silica aerogels for anionic dye adsorption performance. RSC Adv. 2018, 8, 29129–29140. [Google Scholar] [CrossRef]
- Tang, R.; Hong, W.; Srinivasakannan, C.; Liu, X.; Wang, X.; Duan, X. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep. Purif. Technol. 2022, 281, 119950. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Mamane, H.; Vadivel, V.K.; Avisar, D. Ethanol-activated granular aerogel as efficient adsorbent for persistent organic pollutants from real leachate and hospital wastewater. J. Hazard. Mater. 2020, 384, 121396. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.L.; Gozlan, I.; Kaplan, A.; Zachor-Movshovitz, D.; Avisar, D. Solid phase extraction based on trimethylsilyloxy silica aerogel. RSC Adv. 2021, 11, 18617–18622. [Google Scholar] [CrossRef] [PubMed]
- Mohseni-Bandpei, A.; Eslami, A.; Kazemian, H.; Zarrabi, M.; Al-Musawi, T.J. A high density 3-aminopropyltriethoxysilane grafted pumice-derived silica aerogel as an efficient adsorbent for ibuprofen: Characterization and optimization of the adsorption data using response surface methodology. Environ. Technol. Innov. 2020, 18, 100642. [Google Scholar] [CrossRef]
- Faghihian, H.; Nourmoradi, H.; Shokouhi, M. Removal of copper (II) and nickel (II) from aqueous media using silica aerogel modified with amino propyl triethoxysilane as an adsorbent: Equilibrium, kinetic, and isotherms study. Desalination Water Treat. 2014, 52, 305–313. [Google Scholar] [CrossRef]
- Ghahremani, P.; Vakili, M.H.; Nezamzadeh-Ejhieh, A. Optimization of Pb(II) removal by a novel modified silica aerogel using Quince seed mucilage with response surface methodology. J. Environ. Chem. Eng. 2021, 9, 106648. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Naeimirad, M.; Peterek, S.; Begum, H.; Galmarini, S.; Pursche, F.; Baskin, E.; Zhao, S.; Gries, T.; Malfait, W.J. Multiple assembly strategies for silica aerogel-fiber combinations—A review. Mater. Des. 2022, 223, 111228. [Google Scholar] [CrossRef]
- Tai, M.H.; Kumar, P.S. Harnessing the power of silica aerogels for applications in energy and water sustainability. J. Mater. Chem. A 2024, 12, 18879–18900. [Google Scholar] [CrossRef]
- Hayat, M.; Manzoor, S.; Raza, N.; Raza, H.; Javid, A.; Ali, Z.; Khan, M.I.; Algethami, F.K.; AlMasoud, N.; Alomar, T.S. Amine-functionalized organically modified silica for the effective adsorption of Chlorpyrifos and Triazophos Residues from Orange juice. Food Chem. 2025, 465, 141967. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, S.; Ma, Y.; Duo, H.; Wu, W.; Yang, Q.; Hou, X. An efficient PCN-224/graphene aerogel-based extraction method for monitoring the degradation of organophosphorus pesticides in juice. J. Chromatogr. A 2024, 1738, 465500. [Google Scholar] [CrossRef]
- Sun, P.; Gao, Y.L.; Xu, C.; Lian, Y.F. Determination of six organophosphorus pesticides in water samples by three-dimensional graphene aerogel-based solid-phase extraction combined with gas chromatography/mass spectrometry. RSC Adv. 2018, 8, 10277–10283. [Google Scholar] [CrossRef]
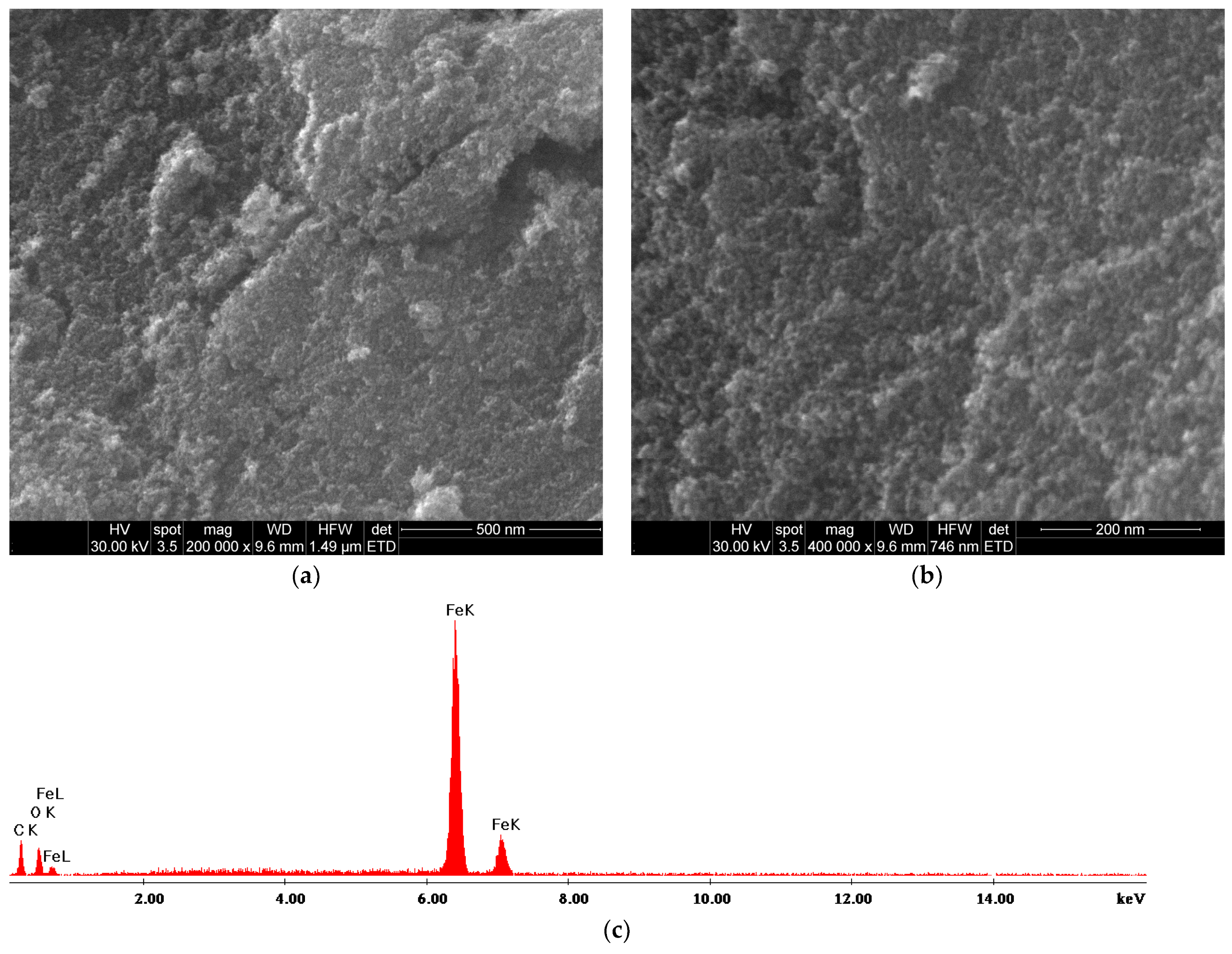
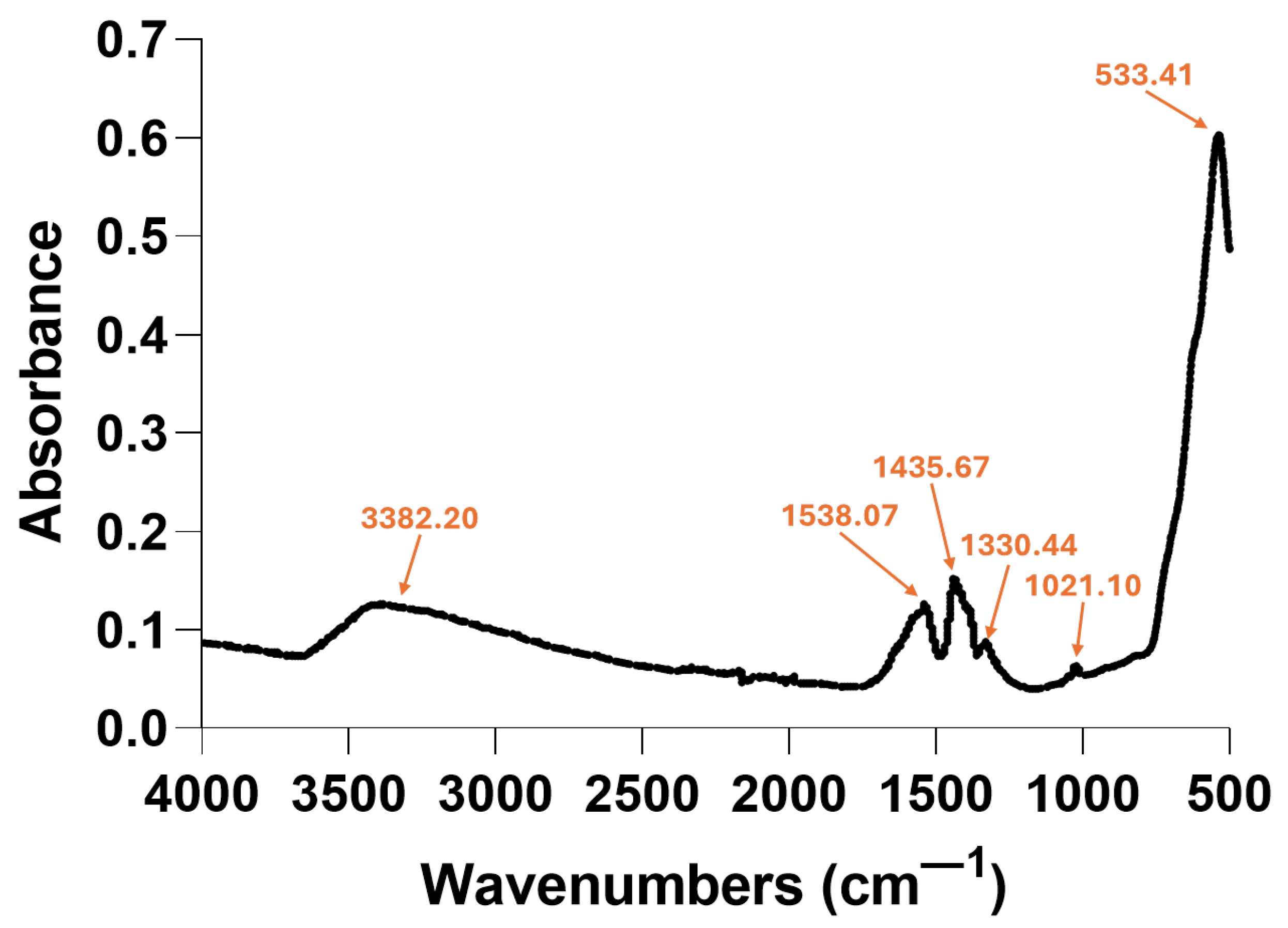
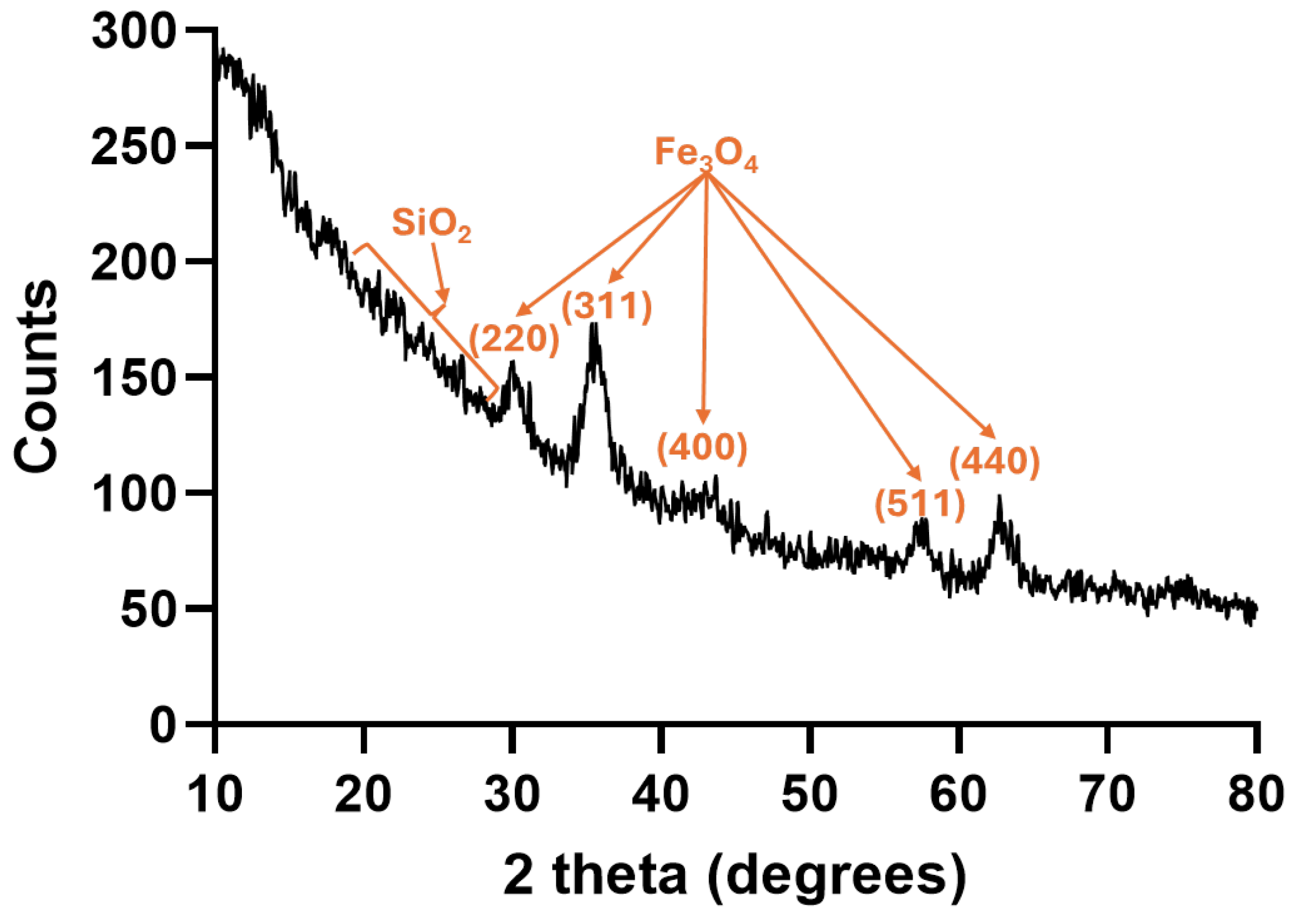
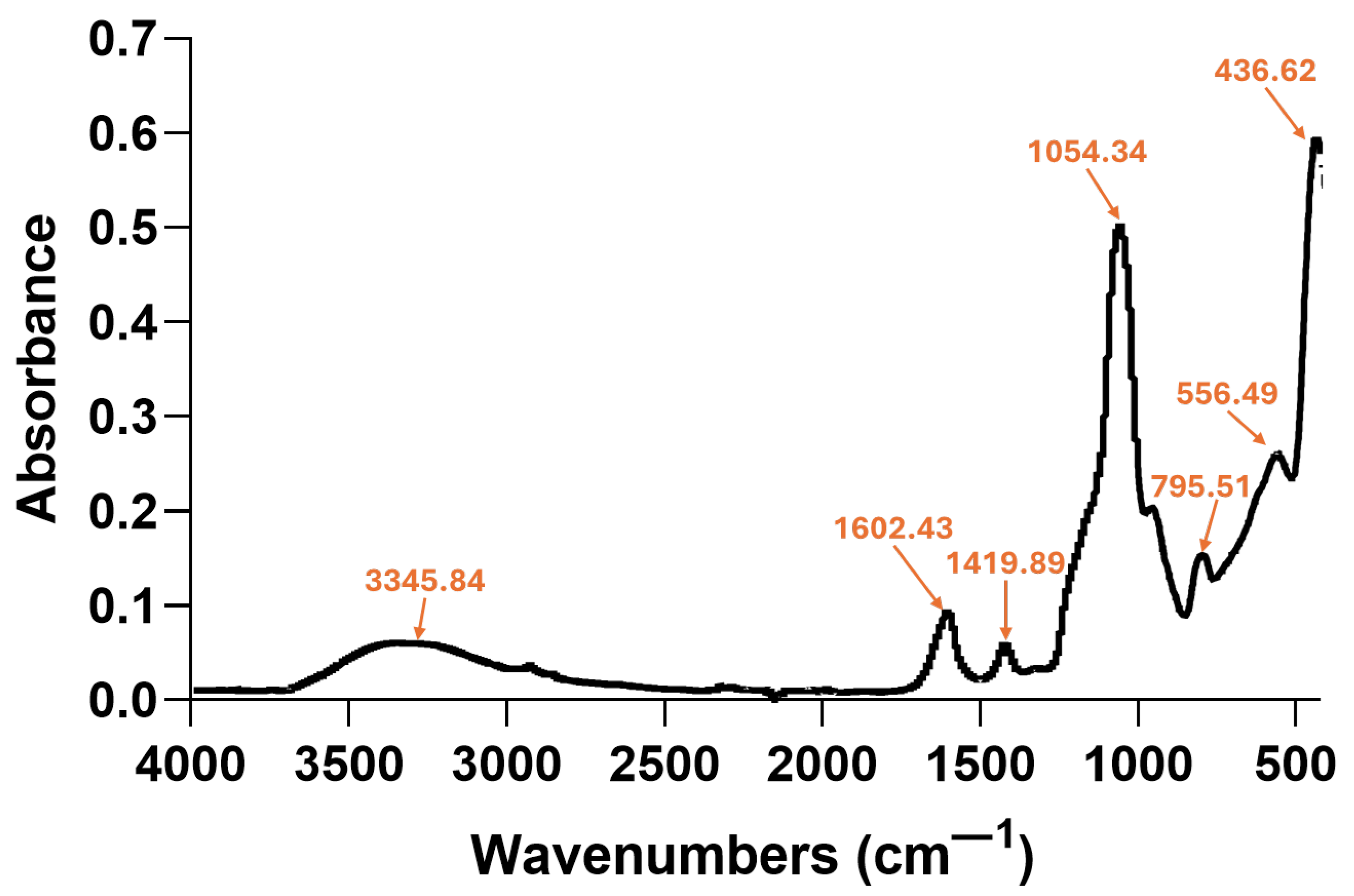
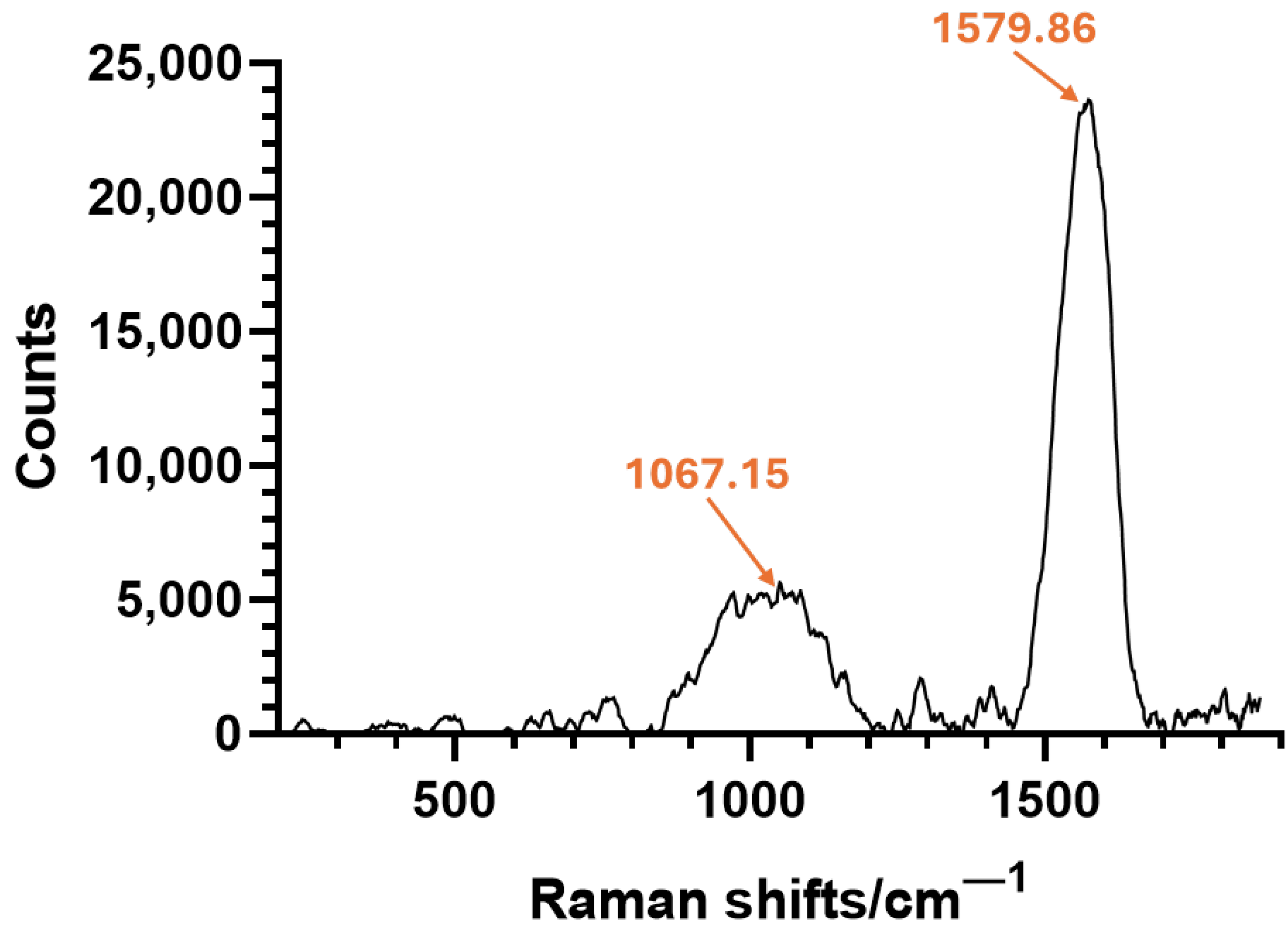

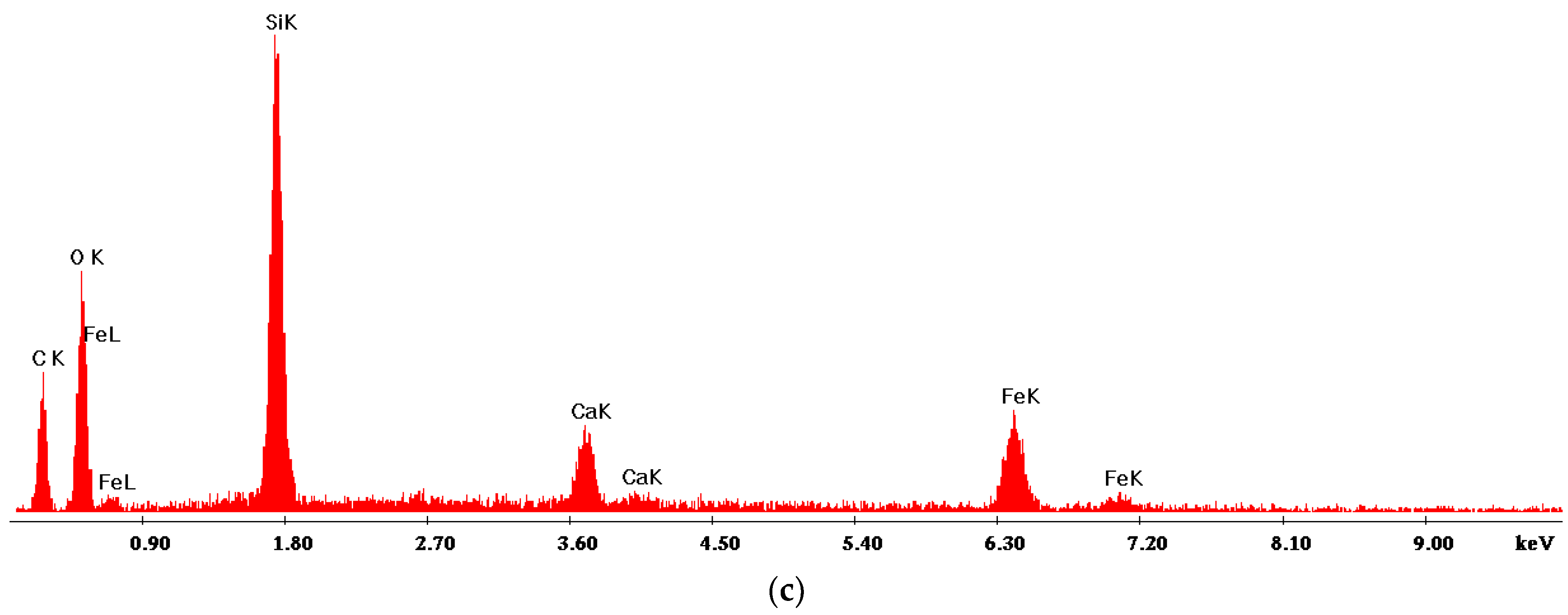

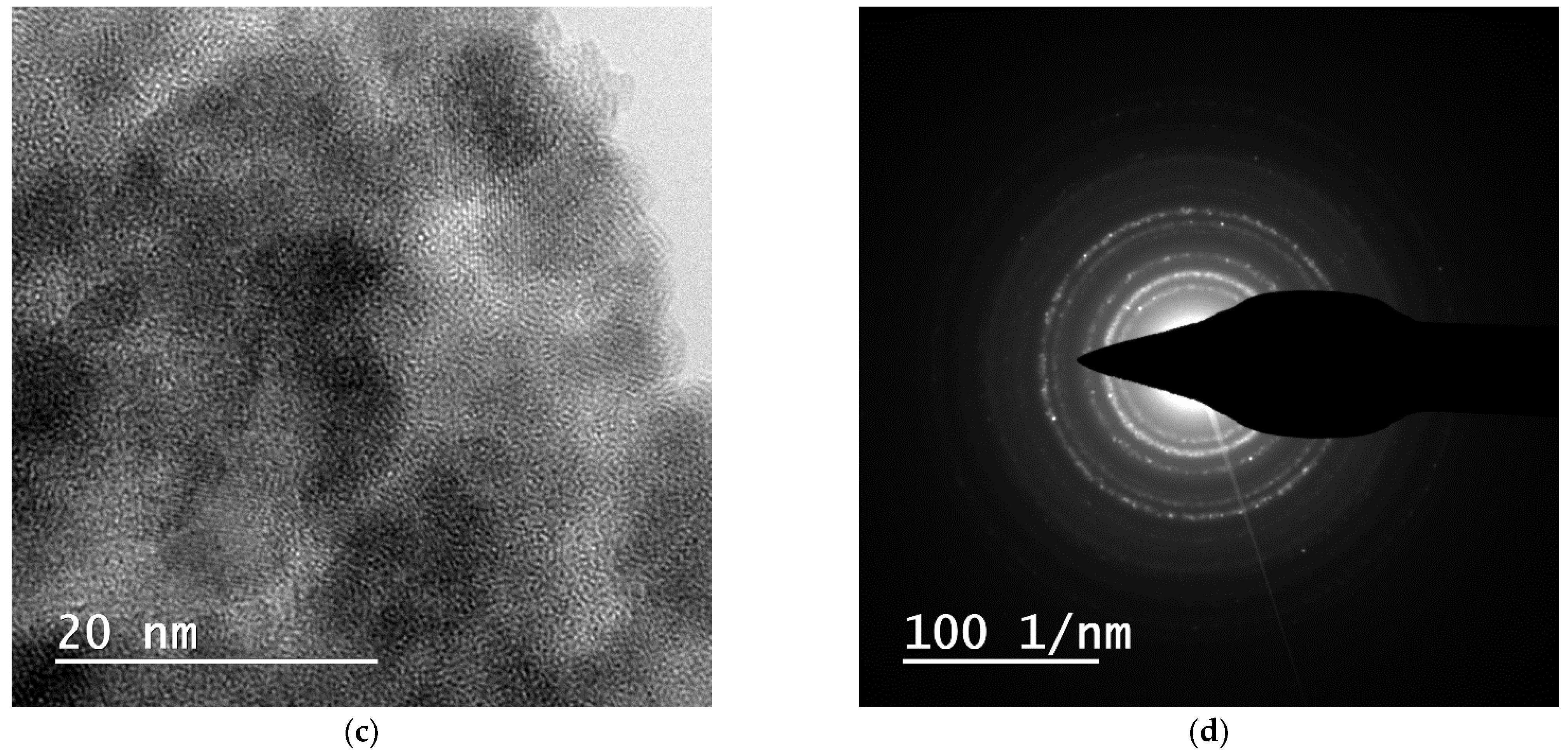
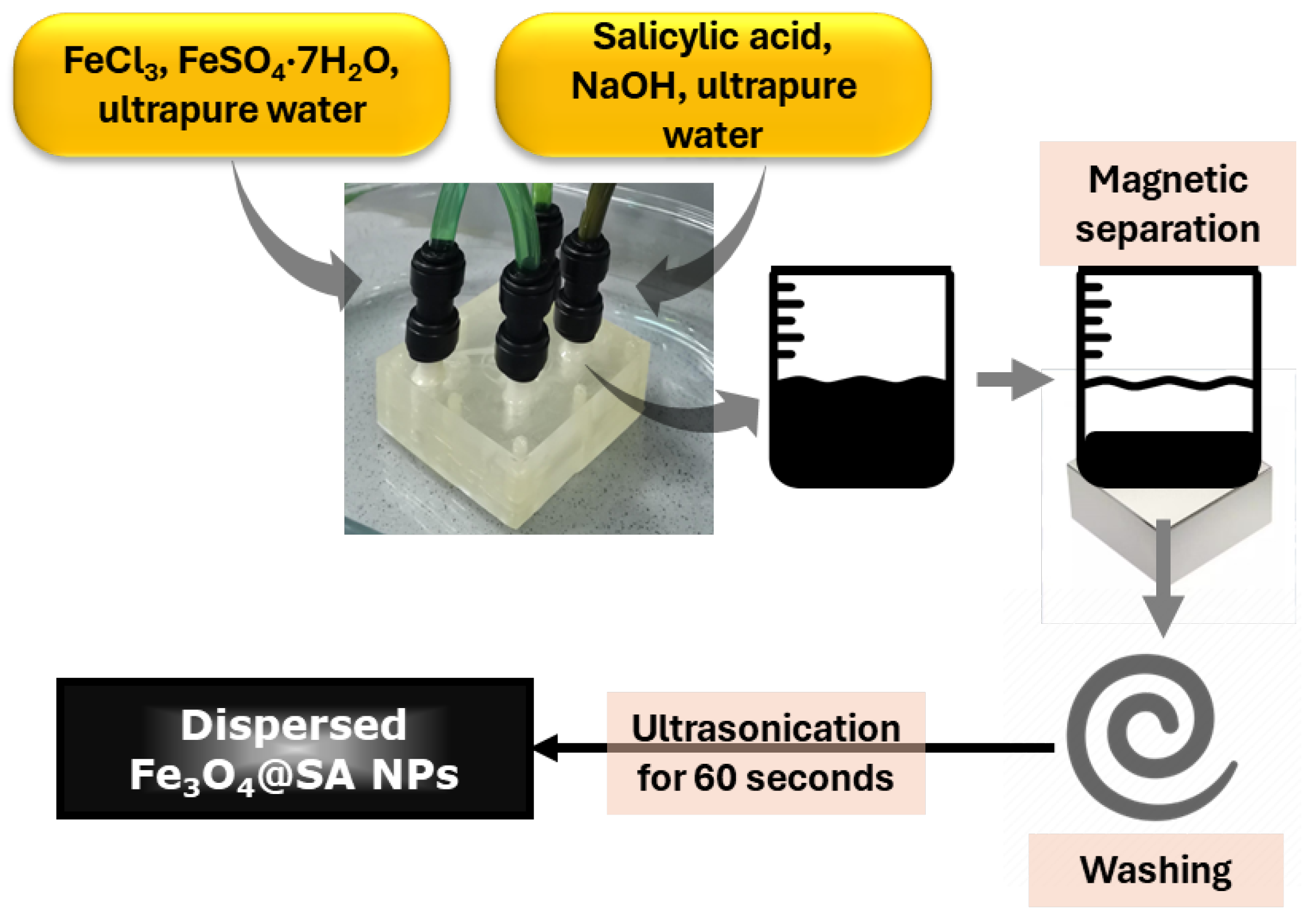
| Zeta Potential (mV) | St. Dev. (mV) | Diameter (nm) | St. Dev. (nm) |
|---|---|---|---|
| 61.271 | 1.26 | 92.288 | 0.40 |
| Zeta (mV) | St. Dev. | Hydrodynamic Radius (nm) | St. Dev. |
|---|---|---|---|
| −25.86 | 1.73 | 4038.17 | 372.42 |
| Pesticide | Molecular Formula | Chemical Structure | m/z | z | Resolution | Initial Conc. (ppb) | Residual Conc. (ppb) | Extraction Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| Chlorthal-dimethyl | C10H6Cl4O4 | 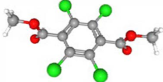 | 332.9061 | 1+ | 375,885 | 1.011 | 0.370 | 63.39 |
| Chlorpropham | C10H12ClNO2 | 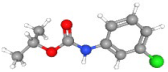 | 214.06289 | 1+ | 539,082 | 0.9925 | 0.507 | 48.93 |
| Fenitrothion | C9H12NO5PS | 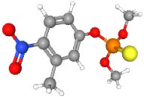 | 278.0245 | 1+ | 474,842 | 1.0095 | 0.629 | 37.68 |
| Fenson | C12H9ClO3S | 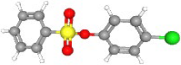 | 269.00327 | 1+ | 464,620 | 0.9976 | 0.210 | 78.94 |
| Fenthion | C10H15O3PS2 | 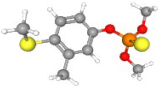 | 279.02709 | 1+ | 438,185 | 1.2664 | 0.505 | 60.12 |
| Mevinphos | C7H13O6P | 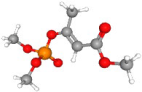 | 225.05167 | 1+ | 586,354 | 2.0058 | 0.860 | 57.12 |
| Propyzamide | C12H11Cl2NO |  | 256.02891 | 1+ | 528,620 | 0.9931 | 0.647 | 34.84 |
| Prothiofos | C11H15Cl2O2PS2 |  | 344.96992 | 1+ | 355,745 | 1.0059 | 0.518 | 48.48 |
| Tolclofos-methyl | C9H11Cl2O3PS |  | 300.96150 | 1+ | 429,356 | 0.9907 | 0.424 | 57.21 |
| Triazophos | C12H16N3O3PS |  | 314.07211 | 1+ | 436,730 | 0.9948 | 0.063 | 93.67 |
| Trifluralin | C13H16F3N3O4 | 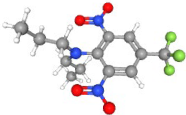 | 336.11638 | 1+ | 342,422 | 0.9893 | 0.538 | 45.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudorache, D.-I.; Niculescu, A.-G.; Bîrcă, A.-C.; Florea, D.A.; Rădulescu, M.; Vasile, B.-Ș.; Trușcă, R.; Mihaiescu, D.-E.; Hadibarata, T.; Grumezescu, A.-M. Microfluidic Synthesis of Magnetic Silica Aerogels for Efficient Pesticide Removal from Water. Gels 2025, 11, 463. https://doi.org/10.3390/gels11060463
Tudorache D-I, Niculescu A-G, Bîrcă A-C, Florea DA, Rădulescu M, Vasile B-Ș, Trușcă R, Mihaiescu D-E, Hadibarata T, Grumezescu A-M. Microfluidic Synthesis of Magnetic Silica Aerogels for Efficient Pesticide Removal from Water. Gels. 2025; 11(6):463. https://doi.org/10.3390/gels11060463
Chicago/Turabian StyleTudorache (Trifa), Dana-Ionela, Adelina-Gabriela Niculescu, Alexandra-Cătălina Bîrcă, Denisa Alexandra Florea, Marius Rădulescu, Bogdan-Ștefan Vasile, Roxana Trușcă, Dan-Eduard Mihaiescu, Tony Hadibarata, and Alexandru-Mihai Grumezescu. 2025. "Microfluidic Synthesis of Magnetic Silica Aerogels for Efficient Pesticide Removal from Water" Gels 11, no. 6: 463. https://doi.org/10.3390/gels11060463
APA StyleTudorache, D.-I., Niculescu, A.-G., Bîrcă, A.-C., Florea, D. A., Rădulescu, M., Vasile, B.-Ș., Trușcă, R., Mihaiescu, D.-E., Hadibarata, T., & Grumezescu, A.-M. (2025). Microfluidic Synthesis of Magnetic Silica Aerogels for Efficient Pesticide Removal from Water. Gels, 11(6), 463. https://doi.org/10.3390/gels11060463









