Abstract
In this study, BiBaO3 perovskite was successfully synthesized via the sol–gel method and thoroughly characterized to evaluate its structural, microstructural, dielectric, electrical, and magnetic properties. X-ray diffraction (XRD) confirmed the formation of a single-phase perovskite structure with high crystallinity. Scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) revealed a uniform grain morphology and elemental composition consistent with the intended stoichiometry. Dielectric measurements exhibited strong frequency-dependent behavior, suggesting potential for capacitive applications. The electrical conductivity displayed thermally activated behavior, indicative of semiconducting characteristics. Magnetic measurements showed weak ferromagnetic behavior at room temperature, an unusual observation for undoped BaBiO3-based systems. This magnetism may stem from subtle structural distortions or compositional variations introduced during synthesis. Comparison with previously reported studies underscores the significant influence of the synthesis route and microstructural features on the multifunctional properties of BiBaO3. Overall, the results highlight the promise of sol–gel-derived BiBaO3 as a candidate for multifunctional electronic and magnetic applications.
1. Introduction
In recent years, the pursuit of multifunctional materials has intensified due to growing demands from smart technologies, sustainable energy, and high-performance electronics [1,2,3]. Perovskite ceramics have emerged as leading candidates because of their unique structural flexibility, thermal stability, and tunable electrical and magnetic properties [4,5]. Advanced synthesis approaches, such as in situ chlorination and green fabrication methods, have enabled breakthroughs in optoelectronics, including efficient deep-blue LEDs and fingerprint visualization devices [6,7]. At the same time, researchers have developed novel porous structures and dual-scale aerogels with impressive electromagnetic absorption capabilities, expanding the scope of multifunctional ceramic applications [8,9]. Modeling techniques using meshless and thermodynamic approaches further enhance the understanding of nonlinear wave behavior and particle interactions within complex material systems [10]. Environmental impact and resource sustainability have become essential considerations in materials design. Studies on embodied CO2 in trade [6], deforestation avoidance [7], and heavy metal transport in soils [11] demonstrate the importance of integrating ecological concerns into materials development. The reuse of industrial wastes particularly red mud and fly ash in geopolymer composites supports both sustainability and material performance, showing effectiveness in pollutant remediation and heavy metal immobilization [12,13,14,15]. Further work has introduced thermodynamic models that account for particle breakage, crystallization, and ion migration under harsh conditions, improving the reliability of ceramics in real-world environments [16,17,18,19]. These materials also show strong potential in contaminated soil treatment through flowing-water remediation and unified chemical biological technologies [15]. Recent multi-field models for saline soils reinforce the role of simulation in advancing sustainable ceramic systems [20,21].
Perovskite materials with the general formula ABO3 have attracted widespread research interest due to their exceptional range of physical and chemical properties [22,23]. These oxides often exhibit multifunctional characteristics, including ferroelectricity, magnetoresistance, superconductivity, and high dielectric constants, which make them valuable for a wide spectrum of technological applications such as capacitors, sensors, memory devices, and spintronic components [24,25,26,27,28]. One of the significant advantages of the perovskite structure is its ability to accommodate a wide variety of cations at the A and B sites, enabling extensive chemical substitution and property tuning. Bismuth-based perovskites are of particular interest because of the unique electronic configuration of the Bi3+ ion, which contributes to complex electronic and magnetic interactions. Among them, BaBiO3 has been studied extensively and is known for its insulating nature, which arises from charge disproportionation between Bi3+ and Bi5+ ions [29]. This unusual electronic structure gives rise to intriguing phenomena, such as polaron formation, breathing-mode lattice distortions, and, under appropriate doping conditions, superconductivity [30,31,32].
Several researchers have reported superconducting behavior in Ba1−xKxBiO3 and BaPb1−xBixO3 systems with transition temperatures up to 30 K [33,34,35]. These materials are among the earliest examples of non-cuprite oxide superconductors. Moreover, BaBiO3 and its derivatives explored for photocatalytic applications, with Bi3+ playing a role in facilitating visible-light absorption due to 6s2 lone pair effects. Although BiBaO3 has received significant attention, reports on BiBaO3 in the reverse stoichiometry are non-existent, due to challenges in stabilizing the structure or limited exploration in the literature. Investigating BiBaO3 may reveal new structural or functional characteristics not observed in the traditional BaBiO3 compound [36]. The synthesis method plays a crucial role in determining the structural integrity and properties of perovskite materials. Traditional solid-state synthesis methods require elevated temperatures and often result in inhomogeneous products and large particle sizes. In contrast, the sol–gel method provides better control over stoichiometry, particle morphology, and lower crystallization temperatures. This approach enables molecular-level mixing of precursors and often leads to improved material properties [37].
An array of studies has demonstrated the advantages of the sol–gel method in preparing perovskites. For example, LaFeO3 synthesized using the sol–gel route and reported enhanced magnetic and dielectric properties compared to solid-state methods. Similarly, material BiFeO3 nanoparticles prepared via sol–gel experienced increased ferroelectricity and reduced leakage current due to improved microstructural control [38]. In terms of characterization, X-ray diffraction remains the primary technique for confirming phase formation, crystal structure, and crystallite size. For perovskites, a typical observation includes sharp diffraction peaks corresponding to a cubic, tetragonal, or orthorhombic symmetry, depending on the ionic radii and distortion factors [39,40]. For example, BaBiO3 commonly crystallizes in a monoclinic structure at room temperature, although reports also exist of orthorhombic phases under specific conditions. Scanning electron microscopy allows for detailed visualization of the surface morphology, particle size, and porosity. The microstructure has a significant impact on dielectric and electrical behavior, with smaller grains contributing to higher dielectric constants due to increased grain boundary polarization. Energy-dispersive X-ray spectroscopy, typically coupled with SEM, helps confirm the elemental composition and uniformity across the sample [41].
Dielectric measurements provide insight into the polarizability and insulation of materials. For perovskite oxides, high dielectric constants are often observed, especially in systems where lattice distortions or dipolar defects contribute to polarization mechanisms [42,43]. Frequency-dependent dielectric studies are essential to identify relaxer behavior, Maxwell–Wagner polarization, or interfacial effects. Materials like BaTiO3 and SrBi2Nb2O9 have shown such frequency dispersion and are used in capacitors and microwave devices [44,45]. Electrical conductivity in perovskites is often influenced by oxygen vacancies, carrier hopping, and polaronic transport. In Bi-containing perovskites, the mixed valence nature of bismuth can facilitate small polaron hopping, which manifests as thermally activated conduction. Studies on La1−xSrxMnO3 and BiFeO3 have shown that the nature of charge carriers can be drastically modified by doping, sintering temperature, and synthesis route [46].
Magnetic characterization is equally important, especially for multifunctional materials. While BaBiO3 is typically non-magnetic, doping or deviation from stoichiometry can lead to the appearance of weak ferromagnetism or antiferromagnetism [47,48,49]. BiFeO3 is a classic example of a multiferroic perovskite that exhibits both ferroelectric and antiferromagnetic behavior at room temperature. The observation of magnetic ordering in unexpected systems like BiBaO3 may suggest defect-mediated magnetism, a phenomenon observed in many oxide systems [50]. In recent years, the demand for multifunctional materials that combine electric, dielectric, and magnetic properties has led to a surge in research on novel or modified perovskite compositions. Materials exhibiting more than one ferroic order are especially interesting for sensors, memory devices, and spintronic applications. Despite the wealth of information available on BaBiO3 and other bismuth-based perovskites, very limited work has been reported on BiBaO3 [51,52,53,54]. This suggests a significant research gap, and exploring this composition could contribute to the discovery of new structural phases and property combinations. Furthermore, no prior reports exist, to the best of our knowledge, on BiBaO3 synthesized via the sol–gel route, making this investigation both novel and potentially impactful.
In addition to the sol–gel method explored in this study, Bi-based perovskites such as BaBiO3 are commonly synthesized using solid-state reaction, hydrothermal, and combustion techniques [55]. However, these methods often require higher temperatures and may result in phase inhomogeneities, large grain sizes, or incomplete mixing of precursors. In contrast, the sol–gel method offers key advantages such as molecular-level mixing, lower crystallization temperatures, and improved control over particle morphology and stoichiometry [56]. These features contribute directly to enhanced dielectric response, reduced leakage, and more uniform grain boundary behavior. The present work highlights how the sol–gel route can yield BiBaO3 with improved microstructural uniformity and weak ferromagnetic response, offering potential benefits over more traditional synthesis approaches. The present study aims to synthesize BiBaO3 perovskite using the sol–gel method and perform a comprehensive investigation of its structural, microstructural, dielectric, electrical, and magnetic properties. Additionally, the results obtained will be compared with the existing literature on BaBiO3 and related perovskites to assess the influence of the synthesis method and compositional variation on the observed properties.
2. Synthesis and Characterization
2.1. Sample Preparation
The BiBaO3 perovskite material was synthesized using the sol–gel method, which is known for its ability to produce homogeneous and fine powders at low processing temperatures. High purity starting precursors were used, including bismuth nitrate pentahydrate [Bi(NO3)3·5H2O] and barium carbonate [BaCO3]. Initially, 20 mL of distilled water was used to dissolve the stoichiometric amount of Bi(NO3)3·5H2O. In a separate beaker, BaCO3 was dispersed in 40 mL of dilute nitric acid (HNO3) under continuous stirring. The acid facilitates the dissolution of BaCO3 by forming soluble Ba(NO3)2 and releasing CO2 gas. After full dissolution and clarification of the two solutions, they were mixed and stirred thoroughly. The mixed solution was maintained at approximately 70 °C for 5 h under continuous heating and stirring, during which a transparent solution formed. Once a uniform mixture is achieved, ethylene glycol and citric acid were added as complexing and gelling agents in a 1:1 molar ratio with respect to the total metal ions.
Ethylene glycol acts as a polymerization agent, while citric acid binds metal ions, ensuring a more homogeneous distribution. Stirring continued until a viscous and opaque gel formed, indicating the successful formation of a polymeric network containing the metal cations. The gel was then dried in an oven at 120 °C for 12 h to remove residual water and solvents, resulting in a dry foamy precursor. The dried gel was subjected to calcination in air at different temperatures of 500 °C, 600 °C, 700 °C, and 800 °C for 10 h each, to study the effect of temperature on phase formation and crystallinity. The resulting powders were then ground to obtain fine, homogeneous BiBaO3 powders for further characterization. To evaluate the sintering behavior and prepare the sample for electrical and magnetic measurements, pellets of the BiBaO3 powder were pressed using a uniaxial press and sintered at optimal temperatures identified through structural analysis.
After synthesis and calcination, the resulting BiBaO3 powders were divided into portions for various characterization techniques. For dielectric and electrical measurements, the powders were pressed into disk-shaped pellets and sintered at the optimal temperature. For magnetic measurements, a separate portion of the powder was used in loose form to evaluate the magnetic properties using SQUID magnetometry. Additional portions were allocated for structural and morphological analysis via XRD, SEM, and EDX. All measurements were performed on three independent synthesis batches to ensure reproducibility, and consistent trends were observed across all sample sets.
2.2. Sample Characterization
The structural characterization of the sample was conducted using several techniques. X-ray Diffraction was performed using an Empyrean diffractometer (Malvern Panalytical Ltd., Malvern, UK) (CuKα radiation, λ = 1.54060 Å) at 45 kV and 40 mA in the Bragg–Brentano prefocusing optics configuration. The step counting method was applied, with a step size of 0.02° and a time per step of 1 s, covering a 2θ angle range of 10° to 70°. Scanning electron microscopy and energy-dispersive X-ray spectroscopy measurements were performed using a Bruker Nano GmbH system, equipped with an XFlash 5010 silicon drift detector (Bruker, Billerica, MA, USA). The SEM analysis was conducted at a primary electron beam energy of 15 keV, providing detailed surface morphology. EDX was carried out in energy-dispersive mode using Esprit software version 2.3 (Bruker) for spectral acquisition and elemental mapping, with a take-off angle of 35° employed to optimize X-ray signal detection. A take-off angle of 35° was used during EDX analysis to optimize signal detection. To enhance the conductivity of the sample, a carbon coating was applied to its surface before observation. For electrical characterization, silver conducting paste was applied to the opposite sides of the pellets, and measurements were conducted in a helium atmosphere to reduce moisture and improve heat transfer. The electrical properties were analyzed using impedance spectroscopy (IS), with a frequency range of 100 Hz to 1 MHz and a temperature range of 200 to 380 K, using an Agilent 4294A precision impedance analyzer in the Cp-Rp configuration. The complex permittivity (ε′, ε″) and loss tangent (tan δ) were calculated using the following equations [57,58]:
where Cp and Rp represent the measured capacitance and resistance, ω is the angular frequency, d is the sample thickness, A is the electrode area, and ε0 is the vacuum permittivity (8.8542 × 10−12 F/m). The magnetic properties of the sample were investigated using a Quantum Design MPMS3 superconducting quantum interference device (SQUID) magnetometer (Quantum Design, San Diego, CA, USA). Magnetization curves were measured at temperatures of 5 K, 300 K, and 380 K, with applied fields ranging from −70 kOe to 70 kOe. To correct sample geometry and offset effects, the geometry-independent moment correction (GIMC) method was applied. Additionally, temperature-dependent magnetic measurements were conducted using a Field-Cooled (FC) method, with a magnetic field of 1000 Oe, and the sample was heated from 5 to 380 K. Although gas adsorption measurements such as BET or Langmuir surface area and micropore volume analysis are often valuable for understanding porosity in sol–gel-derived materials, our characterization focused on detailed SEM imaging and EDX analysis across multiple calcination temperatures to assess microstructural evolution. These methods effectively revealed grain connectivity, surface morphology, and compositional uniformity, providing insights directly relevant to the dielectric and electrical performance discussed in this work. A broader surface area-dependent study is currently under consideration as part of future investigations aimed at correlating porosity with functional behavior in BiBaO3.
3. Results and Discussion
3.1. X-Ray Diffraction Analysis
The XRD pattern of the synthesized BiBaO3 sample exhibits sharp and well-defined peaks, indicating a high degree of crystallinity. The prominent diffraction peaks are observed at 2θ values of approximately 22.3°, 27.6°, 31.7°, 40.1°, 46.5°, 52.6°, 57.3°, 63.0°, and 67.1°, with the most intense peak near 31.7°, suggesting a dominant crystallographic orientation. The XRD pattern of the prepared sample, as shown in Figure 1, exhibits well-defined peaks. The absence of additional peaks suggests phase purity, with no detectable secondary phases. To estimate the average crystallite size (D) of the sample, the Scherrer equation was employed [59]:
where DXRD is the average crystallite size, K is the shape factor (typically 0.9), λ is the X-ray wavelength (1.5406 Å for Cu Kα radiation), β is the full width at half maximum (FWHM) of the peak in radians, and θ is the Bragg angle (half of the 2θ value).
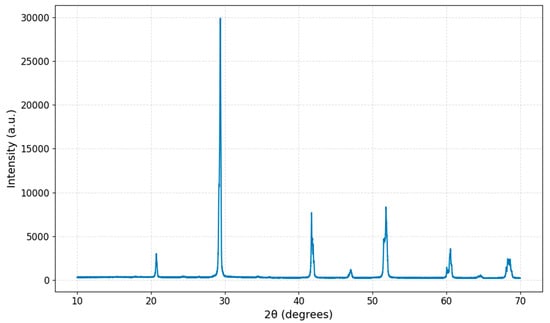
Figure 1.
X-ray diffraction (XRD) pattern of BiBaO3 synthesized at 700 °C, exhibiting sharp and well-defined peaks indicative of high crystallinity.
The FWHM values were corrected for instrumental broadening using the following relation [60]:
Assuming negligible instrumental broadening, the crystallite sizes for the prominent peaks were calculated as follows (Table 1):

Table 1.
Crystallite size estimation using Scherrer equation.
These results indicate that the crystallite sizes range from approximately 45.9 nm to 125.8 nm, with larger crystallites corresponding to higher-angle reflections. To further analyze the microstructural properties, the Williamson–Hall (W-H) method applied. This method considers both size-induced and strain-induced broadening of XRD peaks. The W-H equation is as follows [61]:
where β is the FWHM in radians, θ is the Bragg angle, and ε is the Var epsilon is the microstrain. By plotting β cosθ against 4 sinθ, a linear fit yields the crystallite size from the intercept and the microstrain from the slope. Figure 2 illustrates the Williamson–Hall plot for the BiBaO3 sample. The linear fit to the data points yields a crystallite size of approximately 65.3 nm and a microstrain of 0.0012 (or 0.12%). These values suggest that the sample possesses relatively large crystalline strain with minimal lattice strain, indicative of a well-ordered crystalline structure.
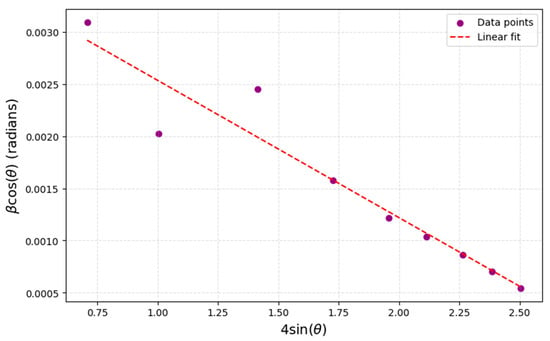
Figure 2.
Williamson–Hall plot for BiBaO3 sample.
The crystallite sizes of the BiBaO3 sample were analyzed using both the Scherrer equation and the Williamson–Hall method. The Scherrer equation estimated the crystallite size to be in the range of 45.9 to 125.8 nm, whereas the Williamson–Hall method provided an average size of 65.3 nm. Unlike the Scherrer equation, which considers only size-induced broadening of diffraction peaks, the Williamson–Hall approach considers both crystallite size and microstrain effects, offering a more comprehensive understanding of the sample’s microstructure. The slight difference between the two results highlights the presence of microstrain in the material; however, the low microstrain value indicates minimal lattice distortions. This low strain is beneficial for the material’s structural stability and potential applications. The consistency between the crystallite sizes obtained from both methods further confirms the reliability of the measurements and demonstrates the effectiveness of the Williamson–Hall analysis in capturing the material’s microstructural properties.
To determine the crystal structure and refine the lattice parameters of the BiBaO3 sample, Rietveld refinement was performed using the FullProf Suite. This refinement involved fitting the entire XRD pattern to a structural model, allowing precise extraction of crystallographic information. The results reveal that BiBaO3 crystallizes in a monoclinic structure with the space group P21/n. The refined lattice parameters were found to be a = 6.183 Å, b = 6.137 Å, c = 8.648 Å, and β = 90.0°, confirming the monoclinic symmetry of the material. Based on these parameters, interatomic distances were calculated, yielding Ba–O and Bi–O distances of 2.75 Å and 2.60 Å, respectively, which align well with the expected coordination environments in BiBaO3. These structural parameters are consistent with previously reported values for BiBaO3 synthesized under similar conditions, such as at temperatures around 700 °C, reinforcing the reliability of the refinement and confirming the phase identification.
Studies on the related compound BaBiO3 have reported similar structural characteristics, including monoclinic symmetry and comparable lattice parameters, reinforcing the reliability of our results. For instance, Korotin et al. [62] investigated electronic correlations and crystal structure distortions in BaBiO3 and successfully reproduced the monoclinic phase, emphasizing the role of breathing distortions and octahedral tilting in stabilizing this structure. Their theoretically derived lattice parameters closely match our experimental findings, further supporting the validity of our structural model. Regarding interatomic distances, our calculated Ba–O and Bi–O bond lengths of 2.75 Å and 2.60 Å, respectively, are in good agreement with values reported in the Materials Project database, which lists Ba–O distances ranging from 2.60 to 2.93 Å and Bi–O distances from 2.24 to 2.66 Å for BaBiO3. This agreement further substantiates the accuracy of our structural analysis. Additionally, Plumb et al. [63] conducted angle-resolved photoemission spectroscopy on BaBiO3 thin films and observed Brillouin zone folding consistent with BiO6 breathing distortions, supporting the existence of a monoclinic structure. Their findings corroborate ours, highlighting the structural stability of BiBaO3 in the monoclinic phase.
3.2. Morphological Study
The surface morphology of the prepared BiBaO3 ceramic was examined using scanning electron microscopy, and the elemental composition was analyzed through energy-dispersive X-ray spectroscopy. As shown in Figure 3, SEM images of the sample synthesized at 700 °C reveal significant microstructural evolution characteristic of sol–gel-derived oxide ceramics. In Figure 3a (scale: 2 μm), the microstructure appears heterogeneous, with a high degree of grain agglomeration and irregularly shaped particles. The surface shows pronounced porosity and uneven grain distribution, indicating incomplete densification. These features are commonly attributed to the evolution of gases during the thermal decomposition of organic precursors in sol–gel synthesis. Figure 3b (scale: 5 μm), the ceramic surface becomes relatively smoother, and some degree of grain growth is evident. The porosity is less prominent, and the grain contacts are more developed, suggesting that the sintering process has initiated neck formation between grains and Figure 3c (scale: 10 μm) shows a more interconnected grain structure with reduced porosity and clearer grain boundaries. Larger grain clusters are visible, implying enhanced grain coalescence and densification at this stage. This interconnected network is favorable for improving the electrical conductivity and dielectric response of perovskite-based ceramics such as BiBaO3.
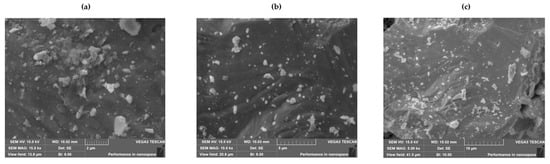
Figure 3.
Scanning electron microscopy (SEM) images of the BiBaO3 ceramic, showing (a) a microstructure captured at 15,000× magnification with a 2 µm scale bar, displaying agglomerated and irregular grains with high porosity; (b) an image taken at 10,000× magnification with a 5 µm scale bar, showing smoother surfaces and moderate grain growth with partial interconnection; and (c) an image at 5000× magnification with a 10 µm scale bar, illustrating a denser grain structure with more continuous boundaries and reduced porosity.
To further investigate the chemical composition, EDX analysis was conducted on the same area, and the resulting spectrum is presented in Figure 4. The spectrum confirms the presence of the main constituent elements, bismuth (Bi), barium (Ba), and oxygen (O), in proportions that closely align with the expected stoichiometry of BiBaO3. The semi-quantitative data shows a predominant concentration of bismuth (50.70 wt%) and barium (34.57 wt%), with oxygen comprising 8.98 wt%. A small amount of carbon (5.74 wt%) was also detected, which is attributed to either residual organics from the synthesis or the carbon tape used for mounting the sample. The absence of any impurity peaks in the EDX spectrum indicates the successful formation of a chemically pure single-phase material. The relatively high bismuth content supports the structural distortion observed in XRD results, suggesting the incorporation of Bi3+ ions into the perovskite lattice and possibly contributing to oxygen vacancy formation. These morphological and compositional findings not only confirm the successful synthesis of BiBaO3 but also provide insight into how the microstructure may influence the sample’s functional properties, such as its dielectric and magnetic behaviors.
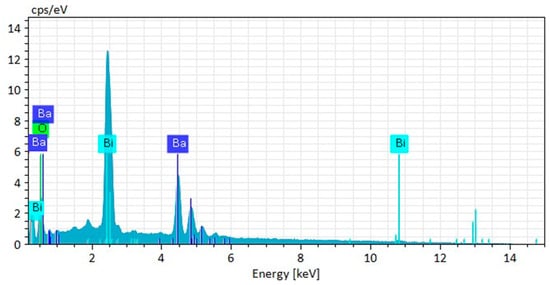
Figure 4.
Energy-dispersive X-ray spectrum of the BiBaO3 sample confirming the elemental composition, with prominent peaks corresponding to bismuth (Bi), barium (Ba), and oxygen (O), consistent with the expected stoichiometry of the compound.
The combination of SEM and EDX analysis provides a comprehensive view of the sample’s morphology and elemental composition, validating the effectiveness of the synthesis method and offering insights into the structural integrity and chemical homogeneity of the material.
3.3. Dielectric Properties
The dielectric properties of the synthesized material were investigated over a wide frequency range (100 Hz to 1 MHz) and temperatures between 200 K and 400 K to understand its polarization mechanisms and energy dissipation behavior. Figure 5a illustrates the real part of the dielectric permittivity (ε′), and Figure 5b presents the dielectric loss tangent (tan δ) under these varying conditions. As shown in Figure 5a, ε′ exhibits a pronounced frequency-dependent dispersion across all temperatures. At low frequencies (~100 Hz), ε′ reaches its maximum value, especially at higher temperatures. As the frequency increases toward 1 MHz, the permittivity steadily decreases, showing typical relaxation behavior. Moreover, ε′ decreases with increasing frequency due to limited dipole mobility and the suppression of interfacial polarization and it increases with temperature, especially at lower frequencies, indicating thermally assisted polarization processes.
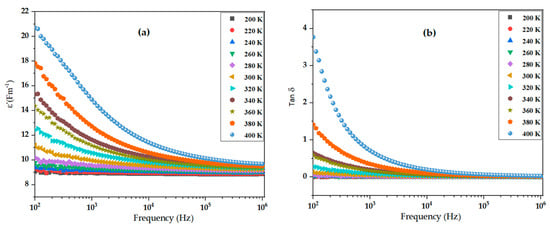
Figure 5.
(a) Frequency and temperature dependence of the real part of dielectric permittivity (ε′) of BiBaO3. (b) Frequency and temperature dependence of dielectric loss tangent (tan δ) of BiBaO3.
This trend can be attributed to the Maxwell–Wagner interfacial polarization mechanism, which dominates at low frequencies due to the presence of grain boundaries and interfaces within the polycrystalline material [64]. At lower temperatures (200 K), the ε′ values are relatively low across all frequencies, while at higher temperatures (400 K), the values increase significantly at low frequencies, indicating thermally activated polarization processes. This enhancement with temperature suggests the involvement of space charge accumulation at interfaces that become more mobile at elevated temperatures. The sharp decrease in ε′ with frequency at all temperatures suggests that dipolar or interfacial polarization mechanisms are strongly suppressed as the external field oscillates faster than the charge carriers or dipoles can respond [65].
Figure 5b shows the dielectric loss tangent (tan δ) as a function of frequency and temperature. Notably, tan δ remains relatively low across the entire frequency range, especially at higher frequencies. At low frequencies (~100 Hz) and higher temperatures (400 K), a small increase in tan δ is observed, likely due to enhanced conduction losses and charge carrier mobility. The low values of tan δ (below 0.1) even at elevated temperatures highlight the low dielectric loss behavior of the material, which is desirable for high-frequency applications such as capacitors, where minimal energy dissipation is critical. Also, tan δ slightly decreases with increasing frequency for all temperature conditions, which is consistent with reduced hopping conduction and energy loss as the field oscillates faster than the response time of charge carriers [66]. It remains low overall, showing only a slight increase at low frequencies and high temperatures, confirming the material’s low-loss dielectric nature. These behaviors reflect a typical non-Debye-type dielectric relaxation, associated with complex microstructural features and space charge dynamics. The stability of both ε′ and tan δ at high frequencies indicates the potential of the material for use in miniaturized electronic devices and high-frequency capacitor applications.
3.4. Electrical Conductivity and Modulus Analysis
The electrical behavior of the BiBaO3 perovskite was thoroughly investigated by analyzing its AC conductivity and complex electric modulus over a frequency range from 100 Hz to 100 kHz and a temperature range of 200 K to 400 K. This specific temperature interval was selected to probe both low-temperature charge localization and high-temperature thermal activation effects. The evolution of electrical response with temperature reveals a rich transport mechanism involving both grain and grain boundary contributions. The variation in AC conductivity (σac) with frequency and temperature is depicted in Figure 6. At lower frequencies, σac remains relatively constant, corresponding to DC-like behavior where mobile charge carriers contribute to long-range conduction. As the frequency increases, σac increases significantly due to the short-range hopping of localized charge carriers. This behavior conforms to Jonscher’s universal power law [67,68]:
where is the DC conductivity, A is a temperature-dependent pre-factor, ω = 2πf is the angular frequency, and s is an exponent (0 < s < 1) that characterizes the conduction mechanism. The value of s generally decreases with increasing temperature, indicating a thermally activated hopping process where carriers gain sufficient energy to overcome potential barriers between localized states.
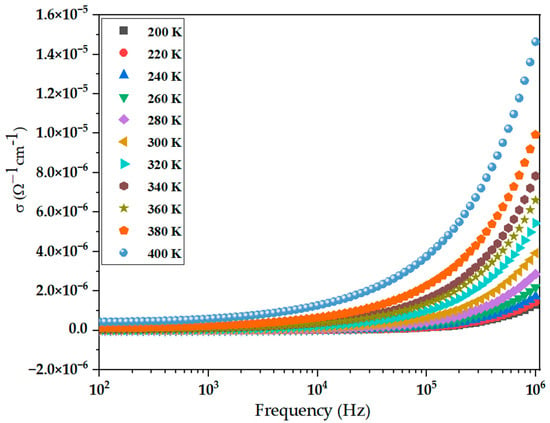
Figure 6.
Frequency dependence of AC conductivity (σac) of BiBaO3 at various temperatures.
To further elucidate the conduction mechanism, the temperature dependence of σac was analyzed using an Arrhenius plot, as shown in Figure 7. This plot, constructed by graphing ln (σac) versus 1000/T, allows the extraction of energy activation (Ea) for charge transport. The data reveal two distinct linear regions, each corresponding to a different thermal regime. The relationship follows the Arrhenius equation [69,70,71]:
where σ0 is the pre-exponential factor, Ea is the activation energy, KB is Boltzmann’s constant, and T is the absolute temperature. Using linear fitting in Origin Pro 2025 software, the slopes of these two regions yield activation energy values of approximately 0.36 eV for the high-temperature regime and 0.18 eV for the low-temperature regime. The presence of two activation energies strongly suggests two predominant conduction processes: the lower activation energy is typically associated with grain boundary conduction or short-range hopping, while the higher energy corresponds to long-range bulk conduction within grains.
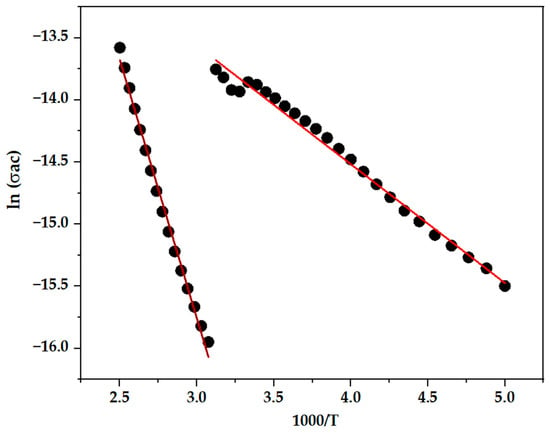
Figure 7.
Arrhenius plot of ln(σac) vs. 1000/T for BiBaO3: extraction of activation energies.
This dual activation behavior reflects the microstructural heterogeneity of the BiBaO3 material. Grain boundaries generally exhibit higher resistivity and dominate conduction at lower temperatures due to their barrier potential. As temperature increases, the grains become more conductive due to enhanced carrier mobility, and bulk conduction becomes the primary mechanism [72]. To deepen the understanding of dielectric and relaxation phenomena in BiBaO3, the electric modulus formalism was applied. The complex electric modulus is defined as follows [73]:
The equation for the modulus technique’s general relation was derived and is presented as follows [74]:
In the equation, A represents the ratio of capacitance (C) in vacuum to dielectric (Co/C), R stands for resistance, ω denotes the angular frequency of the applied AC field, ε∗(ω) is the complex dielectric permittivity, and M′ and M″ are the real and imaginary parts of the electric modulus, respectively. The real part of the electric modulus, M′, as presented in Figure 8a, provides critical insights into the long-range conductivity and dielectric behavior of BiBaO3. At low frequencies, M′ approaches zero, which suggests that the material allows for significant long-range charge transport without strong impedance from interfacial polarization (i.e., electrode effects) [75]. This behavior confirms that the response in this region is dominated by DC conductivity and indicates the existence of mobile charge carriers that traverse larger distances. As the frequency increases, M′ begins to rise, showing a dispersion behavior. This increase is linked to the onset of relaxation phenomena, where the electric field begins to interact with more localized charge dynamics, such as trapped charges or hopping between localized states [76]. The shift in the M′ curve to higher frequencies with increasing temperature suggests a thermally activated process, where increased thermal energy allows charges to respond more rapidly to the alternating field [77]. Such behavior is characteristic of dielectric relaxation due to localized charge motion becoming more significant at elevated temperatures.
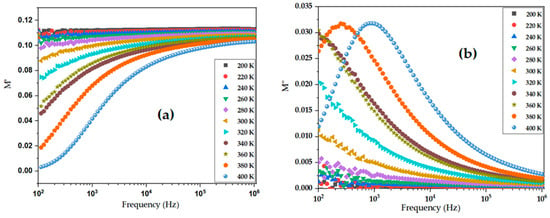
Figure 8.
Frequency dependence of electric modulus components of BiBaO3 at various temperatures: (a) real part (M′), showing dispersion with frequency; (b) imaginary part (M″), indicating thermally activated dielectric relaxation behavior.
In Figure 8b, the imaginary part of the modulus, M″, is plotted against frequency and shows well-defined peaks for each temperature. These peaks represent the characteristic relaxation frequency fmax of the material, where the imaginary part reaches a maximum, indicating the most efficient energy loss from the system due to relaxation processes. Notably, the M″ peak shifts towards higher frequencies with increasing temperature, a hallmark of thermally activated relaxation mechanisms [78]. This shift indicates that as temperature increases, the relaxation time τ of the system decreases, allowing the system to relax faster in response to the applied field. The relationship between the relaxation time τ and peak frequency fmax is given by the following equation:
Furthermore, the temperature dependence of τ can be described by an Arrhenius-type equation, which allows the extraction of the activation energy associated with the relaxation process:
where is the pre-exponential factor, Ea is the activation energy for dielectric relaxation, KB is the Boltzmann constant, and T is the absolute temperature in Kelvin. This model explains how increased thermal energy facilitates faster charge mobility and shorter relaxation times. The well-defined and thermally shifting peaks in M′′ support the existence of localized ionic or polaronic hopping mechanisms, particularly relevant in complex perovskite structures like BiBaO3. The analysis of M′ and M′′ provides robust evidence for the coexistence of long-range conductivity and localized relaxation phenomena in BiBaO3. These behaviors, together with the dual activation energies observed in the conductivity data, emphasize the significant role of both grain and grain boundary processes in shaping the dielectric and conductive properties of this material.
3.5. Impedance Spectroscopy Analysis
Impedance spectroscopy is a powerful technique to investigate electrical properties and charge transport mechanisms in perovskite materials. In this study, the impedance response of the BiBaO3 sample was analyzed over a range of frequencies, with results presented in Figure 9a,b, showing the real (Z′) and imaginary (Z″) parts of the impedance, respectively. Figure 9a illustrates the frequency dependence of the real part of impedance. At low frequencies, a higher Z′ value is observed, indicating a dominant resistive behavior attributed to grain boundary and electrode effects [79]. As the frequency increases, Z′ decreases, demonstrating enhanced charge carrier mobility within the material’s bulk. This trend suggests the presence of a frequency-dependent conduction mechanism typically seen in perovskite oxides [80].
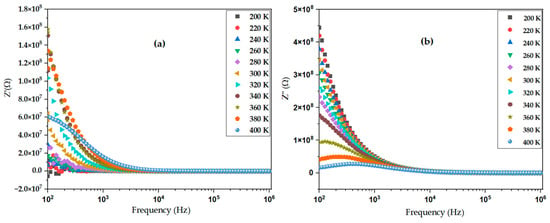
Figure 9.
Frequency dependence of impedance spectroscopy of BiBaO3 at various temperatures: (a) real part (Z′), showing frequency-dependent dispersion; (b) imaginary part (Z).
Figure 9b presents the imaginary part of impedance, which exhibits a peak corresponding to a relaxation process in the material. The peak position shifts toward higher frequencies with increasing temperature, indicating thermally activated relaxation phenomena [81]. This peak corresponds to the characteristic relaxation time of charge carriers or dipolar species in the BiBaO3 lattice. Together, these impedance spectra provide insights into the electrical conduction and polarization processes in the prepared sample. The semicircular arcs typically reflect the bulk and grain boundary contributions to impedance, allowing for equivalent circuit modeling to extract resistance and capacitance parameters. Overall, the impedance spectroscopy analysis confirms that the electrical behavior of BiBaO3 is governed by both bulk and interfacial effects, crucial for optimizing its performance in electronic and energy devices [82].
3.6. Magnetic Properties
The magnetic behavior of BiBaO3 was investigated at room temperature using vibrating sample magnetometry, with the corresponding hysteresis loop shown in Figure 10. The loop exhibits a narrow, symmetric S-shaped curve centered near the origin, indicative of weak ferromagnetic or soft magnetic characteristics. Quantitative analysis of the curve yields a saturation magnetization (Ms) of approximately 0.0058 emu/g, a remanent magnetization (Mr) near 0.0004 emu/g, and an extremely low coercive field (Hc) around 0.7 Oe. These values clearly demonstrate that BiBaO3 exhibits minimal magnetic ordering with very low hysteresis losses, typical of materials that possess soft magnetic behavior or weak ferromagnetism rather than strong or hard ferromagnetic ordering. The origin of this magnetic response in BiBaO3 is intriguing, given that both Bi3+ and Ba2+ ions are nominally non-magnetic [83]. However, subtle effects arising from the complex crystal chemistry of perovskite oxides can lead to emergent magnetism. One crucial factor is the possibility of mixed valence states such as Bi3+/Bi4+ and Ba2+/Ba3+, which may develop due to oxygen vacancies or synthesis conditions that alter stoichiometry. These mixed valence ions enable electron hopping via double exchange mechanisms, which facilitate the alignment of neighboring spins and generate ferromagnetic coupling despite the absence of traditional magnetic ions [84,85]. In addition, super exchange interactions mediated through oxygen ions may contribute further complexity to the magnetic structure, with the nature of coupling (ferromagnetic or antiferromagnetic) depending strongly on local bonding geometries and orbital overlaps.
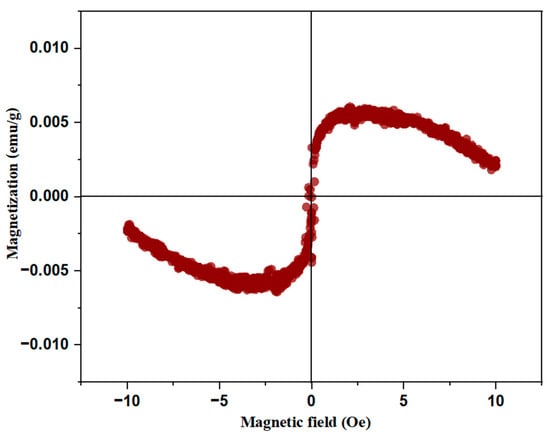
Figure 10.
Magnetic hysteresis loop of BiBaO3 at room temperature.
Moreover, the presence of the stereochemical active 6 s2 lone pair on Bi3+ ions introduce lattice distortions that disrupt the ideal symmetry of the perovskite framework. These distortions can cause spin canting, where the magnetic moments tilt away from perfect antiparallel alignment, resulting in a small net magnetization and weak ferromagnetism. Oxygen vacancies exacerbate this effect by creating localized states and modifying the electronic environment, which enhances the likelihood of unpaired spins and mixed valence formations. These factors collectively give rise to defect-driven magnetism in the otherwise non-magnetic BiBaO3 lattice. The nearly linear increase in magnetization at higher applied fields and the lack of a well-defined saturation plateau suggest that the material does not achieve full magnetic saturation, likely due to competing interactions such as antiferromagnetic coupling coexisting with weak ferromagnetic domains [86].
Taken together, the magnetic properties of BiBaO3 highlight the subtle interplay between electronic structure, lattice distortions, and defect chemistry in determining its magnetic ground state. The weak ferromagnetic signature observed, despite the absence of conventional magnetic ions, underscores the potential of the sample as a multifunctional perovskite oxide where magnetism can be tuned through controlled doping, defect engineering, or external stimuli. Such tunability opens prospects for applications in spintronics, sensors, and magnetoelectric devices, where a controlled weak magnetic response is desirable. Further investigations, including temperature-dependent magnetic measurements and theoretical modeling, will be essential to fully elucidate the microscopic mechanisms governing the emergent magnetism in the prepared sample. In this study, magnetic characterization was carried out at room temperature, where BiBaO3 exhibited clear signatures of weak ferromagnetic behavior. These findings are significant, as they confirm the presence of defect-driven magnetism in the material, despite the absence of conventional magnetic ions. The results are consistent with structural distortions and mixed valence effects that emerge during sol–gel synthesis. While our primary emphasis was on analyzing the dielectric, electrical, and impedance properties over a broad temperature range (200–400 K), the magnetic measurements at ambient conditions provided valuable initial insight into the magnetic nature of BiBaO3. In future work, we aim to extend the magnetic analysis to cover a wider temperature range and incorporate theoretical modeling approaches such as the Jiles–Atherton model. Such studies will further enhance understanding of the material’s magnetic domain dynamics and magneto-thermal behavior, building upon the solid foundation established in the present work.
Beyond the mechanisms previously discussed, it is worth noting that weak ferromagnetic signatures in materials like BiBaO3 may also stem from nanoscale phase inhomogeneities or interface effects between structurally distorted regions. In perovskites synthesized via sol–gel routes, the local strain fields, precursor reactivity, and calcination kinetics can result in subtle spatial variations that are not easily detected by bulk structural analysis yet have a strong influence on magnetic behavior. Techniques such as magnetic force microscopy (MFM) or Mössbauer spectroscopy could provide further insight into domain-level magnetic features and hyperfine interactions. Additionally, the presence of a low-field magnetic response may offer functionality in magnetic sensing or low-power spintronic applications where controlled weak magnetism is advantageous. These directions warrant future exploration to fully establish the link among synthesis conditions, local structure, and functional magnetic behavior in BiBaO3 and related systems.
4. Conclusions
In this study, BiBaO3 perovskite was successfully synthesized via the sol–gel method and subjected to a detailed investigation of its structural, electrical, dielectric, and magnetic properties. The structural characterization confirmed the formation of a single-phase perovskite structure with high crystalline, as evidenced by X-ray diffraction. SEM analysis further revealed a uniform microstructure, while EDX validated the stoichiometric composition of the synthesized material. Dielectric and impedance spectroscopy revealed significant frequency and temperature dependence, highlighting the presence of space charge polarization and grain boundary effects. AC conductivity analysis, modeled by Jonscher’s power law, confirmed thermally activated charge transport, with two distinct activation energies extracted from the Arrhenius plots. These two activation energies, approximately 0.18 eV and 0.37 eV, correspond to grain boundary and bulk conduction mechanisms, respectively, demonstrating the complexity of charge transport in BiBaO3.
The electric modulus formalism provided further insight into the dielectric relaxation processes, where the imaginary part of the modulus showed a thermally activated relaxation peak shifting toward higher frequencies with increasing temperature. This behavior confirms that the relaxation mechanisms in BiBaO3 are governed by localized hopping and interfacial phenomena. Magnetic measurements uncovered weak ferromagnetic behavior at room temperature, which is unusual for undoped BaBiO3 systems. The observed magnetism may be attributed to subtle structural distortions, oxygen non-stoichiometry, or mixed valence states of bismuth ions introduced during the sol–gel synthesis. The comprehensive analysis of BiBaO3 demonstrates its multifunctional nature, combining semiconducting electrical behavior with dielectric response and unexpected magnetic ordering. These results underscore the potential of sol–gel-derived BiBaO3 for applications in multifunctional electronic, sensing, and spintronic devices. The ability to tailor electrical and magnetic properties through synthesis parameters opens new pathways for optimizing performance in advanced material systems.
Author Contributions
F.T. contributed to the sol–gel synthesis, sample preparation, and preliminary structural analysis. K.I.N. conceived the study, supervised the project, and contributed to the interpretation of the structural and magnetic data. S.S.T. assisted with magnetic characterization and data interpretation. I.H. and J.P.C. contributed to XRD data refinement and phase identification. S.R.G. performed complementary structural and microstructural analyses. M.P.F.G. provided guidance on electrical characterization techniques and contributed to data interpretation. M.A.V. supervised the overall experimental design and provided critical revisions to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created.
Acknowledgments
The authors acknowledge the support of FCT-Fundação para a Ciência e a Tecnologia, I.P., within the scope of the projects LA/P/0037/2020, UIDP/50025/2020, and UIDB/50025/2020 of the Associate Laboratory Institute of Nanostructures, Nanomodeling and Nanofabrication-i3N.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, M.; Qin, T.; Gao, G.; Zu, K.; Zhang, D.; Chen, N.; Zhu, J. Multiple defects renovation and phase reconstruction of reduced-dimensional perovskites via in situ chlorination for efficient deep-blue (454 nm) light-emitting diodes. Light Sci. Appl. 2025, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Wang, B.; He, Q.; Cao, J.; Zhu, Y.; Shao, J. Ultra-Bandwidth Microwave Absorption and Low Angle Sensitivity in Dual-Network Aerogels with Dual-Scale Pores. Small 2025, 2412744. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, Z.; Han, S.; Hou, G.; Chen, Q.; Jing, C. Fingerprint visualization and anti-counterfeiting applications using lead-free Cs3Cu2Cl5 perovskite via a facile green synthesis. J. Alloys Compd. 2025, 1014, 178816. [Google Scholar] [CrossRef]
- Yin, X.; Lai, Y.; Zhang, X.; Zhang, T.; Tian, J.; Du, Y.; Gao, J. Targeted sonodynamic therapy platform for holistic integrative Helicobacter pylori therapy. Adv. Sci. 2025, 12, 2408583. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Yang, H. A meshless symplectic algorithm for nonlinear wave equation using highly accurate RBFs quasi-interpolation. Appl. Math. Comput. 2017, 314, 110–120. [Google Scholar] [CrossRef]
- Peng, T.; Ning, Z.; Yang, H. Embodied CO2 in China’s trade of harvested wood products based on an MRIO model. Ecol. Indic. 2022, 137, 108742. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Yang, H. Estimating the opportunity costs of avoiding oil palm-based deforestation in Indonesia: Implications for REDD+. Chin. J. Popul. Resour. Environ. 2020, 18, 9–15. [Google Scholar] [CrossRef]
- Bai, B.; Xu, T.; Nie, Q.; Li, P. Temperature-driven migration of heavy metal Pb2+ along with moisture movement in unsaturated soils. Int. J. Heat Mass Transf. 2020, 153, 119573. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Nie, Q.; Jia, X. A high-strength red mud–fly ash geopolymer and the implications of curing temperature. Powder Technol. 2023, 416, 118242. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Chen, J.; Bai, F.; Nie, Q.; Jia, X. Corrosion effect of acid/alkali on cementitious red mud-fly ash materials containing heavy metal residues. Environ. Technol. Innov. 2024, 33, 103485. [Google Scholar]
- Zhang, B.; Chen, H.; Chen, P. A novel thermodynamic constitutive model of coarse-grained soils considering the particle breakage. Transp. Geotech. 2025, 50, 101462. [Google Scholar]
- Wu, H.; Nie, Q.; Liu, J.; Jia, X. Granular thermodynamic migration model suitable for high-alkalinity red mud filtrates and test verification. Int. J. Numer. Anal. Methods Geomech. 2025, 49, 1530–1543. [Google Scholar]
- Chen, J.; Zhang, B. Flowing-water remediation simulation experiments of lead-contaminated soil using UCB technology. Int. J. Phytoremediat. 2025, 27, 761–770. [Google Scholar]
- Wang, C.; Yang, L.; Hu, M.; Wang, Y.; Zhao, Z. On-demand airport slot management: Tree-structured capacity profile and coadapted fire-break setting and slot allocation. Transp. A Transp. Sci. 2024, 1–35. [Google Scholar] [CrossRef]
- Bai, B.; Zhang, B.; Ji, Y.; Zong, Y. A thermodynamic multi-field model for unsaturated sulfate-saline soils considering crystallization process. Comput. Geotech. 2025, 184, 107251. [Google Scholar] [CrossRef]
- Yang, H.; Li, X. Potential variation in opportunity cost estimates for REDD+ and its causes. For. Policy Econ. 2018, 95, 138–146. [Google Scholar] [CrossRef]
- Qin, X.; Yang, W.; Zhang, Z.; Wangari, V.W. Simulation and design of T-shaped barrier tops including periodic split ring resonator arrays for increased noise reduction. Appl. Acoust. 2025, 236, 110751. [Google Scholar] [CrossRef]
- Zhu, B.P.; Wu, D.W.; Zhou, Q.F.; Shi, J.; Shung, K.K. Lead zirconate titanate thick film with enhanced electrical properties for high frequency transducer applications. Appl. Phys. Lett. 2008, 93, 012905. [Google Scholar] [CrossRef]
- Zhu, B.; Wei, W.; Li, Y.; Yang, X.; Zhou, Q.; Shung, K.K. KNN-based single crystal high frequency transducer for intravascular photoacoustic imaging. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; pp. 1–4. [Google Scholar]
- Ni, Z.L.; Ma, J.S.; Liu, Y.; Li, B.H.; Nazarov, A.A.; Li, H.; Wang, X.X. Numerical Analysis of Ultrasonic Spot Welding of Cu/Cu Joints. J. Mater. Eng. Perform 2025, 1–12. [Google Scholar] [CrossRef]
- Zhu, B.P.; Li, W.; Lu, Y.; Yan, H.; Zhang, Y. Structure and electrical properties of (111)-oriented Pb(Mg1/3Nb2/3)O3–PbZrO3–PbTiO3 thin film for ultra-high-frequency transducer applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, L.; Zhao, Q.; Chen, Y.; Sun, Q. Perovskite/organic tandem device to realize light detection and emission dual function. Chem. Eng. J. 2024, 490, 151573. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Y.; Liu, H.; Yang, X.; Chen, W. Realization of p-type MA-based perovskite solar cells based on exposure of the (002) facet. Appl. Phys. Lett. 2025, 126, 021104. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Ji, X.A. Changes of neuronal nitric oxide synthase in relevant cerebral regions in spontaneous senile dementia model and regulation of Tiantai. Chin. J. Tissue Eng. Res. 2005, 244–247. [Google Scholar]
- Gao, C.; Jia, S.; Yin, X.; Li, Z.; Yang, G.; Chen, J.; Li, Z.; An, X.-T. Enhancing open-circuit voltage in FAPbI3 perovskite solar cells via self-formation of coherent buried interface FAPbIxCl3−x. Chem. Commun. 2025, 61, 2758–2761. [Google Scholar] [CrossRef]
- Zhu, B.P.; Li, W.; Guo, R.; Yan, H. Low temperature fabrication of the giant dielectric material CaCu3Ti4O12 by oxalate coprecipitation method. Mater. Chem. Phys. 2009, 113, 746–748. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, W.; Kuang, H.; Chen, Z.; Yang, J.; Chen, Z.; Chen, F. Dynamic model and vibration of rack vehicle on curve line. Vehicle System Dynamics. 2025, 1–19. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Howard, C.J.; Knight, K.S.; Zhang, Z.; Zhou, Q. Structures and phase transitions in the ordered double perovskites Ba2BiIIIBiVO6 and Ba2BiIIISbVO6. Struct. Sci. 2006, 62, 537–546. [Google Scholar] [CrossRef]
- Moskvin, A.S. Disproportionation and electronic phase separation in parent manganite LaMnO3. Phys. Rev. B 2009, 79, 115102. [Google Scholar] [CrossRef]
- Lu, Y.; Klein, J.; Herbstritt, F.; Philipp, J.B.; Marx, A.; Gross, R. Effect of strain and tetragonal lattice distortions in doped perovskite manganites. Phys. Rev. B 2006, 73, 184406. [Google Scholar] [CrossRef]
- Gu, G.; Liu, N.; Chen, Q.; Sui, X. Response model of resistance-type microbolometer. Opt. Rev. 2010, 17, 525–531. [Google Scholar]
- Kim, M.; McNally, G.M.; Kim, H.-H.; Oudah, M.; Gibbs, A.S.; Manuel, P.; Green, R.J.; Sutarto, R.; Takayama, T.; Yaresko, A. Superconductivity in (Ba, K) SbO3. Nat. Mater. 2022, 21, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Imaduddin, A.; Herbirowo, S.; Nugraha, H.; Hendrik, H.; Aisatun, A.; Giovanni, A.R.; Effendi, M.; Sari, K.; Pramono, A.W.; Yuwono, A.H. Evolution of morphological, crystal structure, and electrical properties of Ba-Pb-Bi-O superconducting materials. S. Afr. J. Chem. Eng. 2023, 46, 112–121. [Google Scholar] [CrossRef]
- Albertini, R.; Macis, S.; Ivanov, A.A.; Menushenkov, A.P.; Puri, A.; Monteseguro Padrón, V.; Campi, G. Tensile microstrain fluctuations in the BaPbO units in superconducting BaPb1−xBxO3 by scanning dispersive micro-XANES. Condens. Matter 2023, 8, 57. [Google Scholar] [CrossRef]
- Sun, L.; Han, J.; Zhu, X.; Zhang, J.F.; Cai, S.; Guo, J.; Xiang, T. Superconducting-insulating quantum phase transition associated with valence change in compressed perovskite bismuth-oxides. arXiv 2023, arXiv:2305.08406. [Google Scholar]
- Menushenkov, A.P.; Ivanov, A.; Neverov, V.; Lukyanov, A.; Krasavin, A.; Yastrebtsev, A.A.; Kovalev, I.A.; Zhumagulov, Y.; Kuznetsov, A.V.; Popov, V. Direct evidence of real-space pairing in BaBiO3. Phys. Rev. Res. 2024, 6, 023307. [Google Scholar] [CrossRef]
- Boumaza, S.; Boudjellal, L.; Brahimi, R.; Belhadi, A.; Trari, M. Synthesis by citrates sol-gel method and characterization of the perovskite LaFeO3: Application to oxygen photo-production. J. Sol-Gel Sci. Technol. 2020, 94, 486–492. [Google Scholar] [CrossRef]
- Çoban Özkan, D.; Türk, A.; Celik, E. Synthesis and characterizations of sol–gel derived LaFeO3 perovskite powders. J. Mater. Sci. Mater. Electron. 2020, 31, 22789–22809. [Google Scholar] [CrossRef]
- Soni, R.; Soni, V.; Lokhande, P.E.; Kumar, D.; Mubarak, N.M.; Kumar, S.P.; Krishnamoorthy, S. Recent advances in lead-free carbon supported perovskites based on Z-scheme and S-scheme for photocatalytic energy conversion. Mater. Horiz. 2025, 12, 3234–3266. [Google Scholar] [CrossRef]
- Acero, G.; Flores, E.M.; Ramirez, M.A.; Moreno, H.; Ortega, P.P.; Aguiar, E.C.; Simões, A.Z. Fatigue endurance and leakage characteristics of ferroelectric BaBiO3 thin films obtained by the polymeric precursor method. J. Alloys Compd. 2025, 1011, 178341. [Google Scholar] [CrossRef]
- Vasala, S. Properties and Applications of A2B’B″O6 Perovskites: From Fuel Cells to Quasi-Low-Dimensional Magnetism. Ph.D. Thesis, Aalto University, Espoo, Finland, 2014. [Google Scholar]
- Li, F.; Wang, L.; Jin, L.; Lin, D.; Li, J.; Li, Z.; Zhang, S. Piezoelectric activity in perovskite ferroelectric crystals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Iben Nassar, K.; Rammeh, N.; Teixeira, S.S.; Graça, M.P.F. Physical properties, complex impedance, and electrical conductivity of double perovskite LaBa0.5Ag0.5FeMnO6. J. Electron. Mater. 2022, 51, 370–377. [Google Scholar] [CrossRef]
- Singh, R.; Luthra, V.; Rawat, R.S.; Tandon, R.P. Structural, dielectric and piezoelectric properties of SrBi2Nb2O9 and Sr0.8Bi2.2 Nb2O9 ceramics. Ceram. Int. 2015, 41, 4468–4478. [Google Scholar] [CrossRef]
- Mojumdar, P.; Shaily, R.; Bokolia, R. Structural properties of strontium bismuth niobate (SrBi2Nb2O9) ferroelectric ceramics. Mater. Today Proc. 2021, 47, 4661–4665. [Google Scholar] [CrossRef]
- Garlapati, V.L.; Jaladi, N.K.; Kotikala, S.B. Investigation on the structure-optimized magnetic properties of hydro-thermally synthesized SrBi2−X(CF)XNb2O9 multiferroic nanocomposites. Phys. Scr. 2023, 99, 015954. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, J.; Ma, J.; Ma, J.; Nan, C.W. Polarization control of photoconductivity in BiFeO3/La1-xSrxMnO3 (x = 0.33, 0.5) heterostructures. Ceram. Int. 2019, 45, 19550–19553. [Google Scholar] [CrossRef]
- Ma, J.; Tian, Y.; Chen, M.; Wang, J.; Ma, J.; Zhang, J.; Nan, C.W. Acidic aqueous solution switching of magnetism in BiFeO3/La1-xSrxMnO3 heterostructures. J. Appl. Phys. 2019, 126, 7. [Google Scholar] [CrossRef]
- Shilna, K.V.; Sahoo, S.C.; Thomas, K.J. Novel ferromagnetism and negative magnetoresistance in BaBiO3 nanoparticles. Appl. Mater. Today 2022, 27, 101427. [Google Scholar] [CrossRef]
- Foyevtsov, O.; Balandeh, S.; Chi, S.; Sawatzky, G. Structural electronic and magnetic properties of BaBiO3 single crystals. Physica B Condens. Matter. 2019, 570, 328–333. [Google Scholar] [CrossRef]
- Shilna, K.V.; Thomas, K.J. Tuning the Magnetic Behavior of BaBiO3 Nanoparticles by Pb Doping. J. Supercond. Nov. Magn. 2025, 38, 62. [Google Scholar] [CrossRef]
- Feng, N.; Han, J.; Lin, C.; Ai, Z.; Lan, C.; Bi, K.; Xu, B. Anti-Jahn-Teller effect induced ultrafast insulator to metal transition in perovskite BaBiO3. npj Comput. Mater. 2022, 8, 226. [Google Scholar] [CrossRef]
- Ning, L.; Xiu-Bao, S.; Guo-Hua, G.; Qian, C. Research on the response model of microbolometer. Chin. Phys. B 2010, 19, 108702. [Google Scholar]
- Rojas-Cervantes, M.L.; Castillejos, E. Perovskites as catalysts in advanced oxidation processes for wastewater treatment. Catalysts 2019, 9, 230. [Google Scholar] [CrossRef]
- Beuerlein, M.A.; Kumar, N.; Usher, T.M.; Brown-Shaklee, H.J.; Raengthon, N.; Reaney, I.M.; Brennecka, G.L. Current understanding of structure–processing–property relationships in BaTiO3-Bi(M)O3 dielectrics. J. Am. Ceram. Soc. 2016, 99, 2849–2870. [Google Scholar] [CrossRef]
- Nassar, K.I.; Rammeh, N.; Teixeira, S.S.; Graça, M.P.F. Effect of Pr substitution in the A site on the structural, dielectric and magnetic properties of double perovskite La2NiMnO6. Appl. Phys. A 2022, 128, 373. [Google Scholar] [CrossRef]
- Iben Nassar, K.; Slimi, M.; Rammeh, N.; Teixeira, S.S.; Graça, M.P.F. Structural and electrical properties of double perovskite oxide LaPbFeTiO6 synthesized by a sol–gel process. Appl. Phys. A 2021, 127, 940. [Google Scholar] [CrossRef]
- Mohamed, M.; Nassar, K.I.; Mohamed, M.; Rammeh, N.; Graça, M.P.F. Effects of partial Li-substitution on structural, electrical and dielectric properties in La1−xLixSrMn2O5+δ (x = 0.05, 0.10 and 0.15) brownmillerite oxides. J. Mol. Struct. 2022, 1258, 132658. [Google Scholar] [CrossRef]
- Niemann, R.G.; Kontos, A.G.; Palles, D.; Kamitsos, E.I.; Kaltzoglou, A.; Brivio, F.; Cameron, P.J. Halogen effects on ordering and bonding of CH3NH3+ in CH3NH3PbX3 (X = Cl, Br, I) hybrid perovskites: A vibrational spectroscopic study. J. Phys. Chem. C 2016, 120, 2509–2519. [Google Scholar] [CrossRef]
- Mishra, S.; Choudhary, R.N.P.; Parida, S.K. Structural, dielectric, electrical and optical properties of a double perovskite: BaNaFeWO6 for some device applications. J. Mol. Struct. 2022, 1265, 133353. [Google Scholar] [CrossRef]
- Korotin, D.; Kukolev, V.; Kozhevnikov, A.V.; Novoselov, D.; Anisimov, V.I. Electronic correlations and crystal structure distortions in BaBiO3. J. Phys. Condens. Matter 2012, 24, 415603. [Google Scholar] [CrossRef]
- Plumb, N.C.; Gawryluk, D.J.; Wang, Y.; Ristić, Z.; Park, J.; Lv, B.Q.; Radović, M. Momentum-resolved electronic structure of the high-Tc superconductor parent compound BaBiO3. Phys. Rev. Lett. 2016, 117, 037002. [Google Scholar] [CrossRef] [PubMed]
- Tayari, F.; Nassar, K.I.; Benamara, M.; Ben Moussa, S.; Alzahrani, A.Y.A.; Teixeira, S.S.; Graça, M.P.F. Insights into dielectric and electrical conductivity dynamics in sol–gel synthesized Ba0.75Ni0.25Tc0.88Mn0.12O3 perovskite ceramic. J. Sol-Gel Sci. Technol. 2024, 111, 132–143. [Google Scholar] [CrossRef]
- Wu, J. Lead-Free Perovskite Piezoelectric Materials—Part Two. Piezoelectric Mater. 2024, 1, 85–113. [Google Scholar]
- Huamán, J.L.C.; Riverab, V.A.G.; Pinto, A.H. Multiferroic perovskite ceramics: Properties and applications. In Perovskite Ceramics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 339–381. [Google Scholar]
- Rao, S.; Sau, S.; Venkatakrishnan, K.; Vaitheeswaran, G.; Nagarajan, R. Implications of magnetic dilution of PrFeO3 with Bi3+ on its dielectric and magnetic properties. Phys. Chem. 2025, 27, 11628–11639. [Google Scholar] [CrossRef]
- Ndeugueu, J.L.; Aniya, M. On the power law behavior of the AC conductivity in Li ion conducting perovskites. J. Phys. Soc. Jpn. 2010, 79, 72–75. [Google Scholar] [CrossRef]
- Moualhi, Y.; Smari, M.; Rahmouni, H.; Khirouni, K.; Dhahri, E. Superlinear dependence of the conductivity, double/single Jonscher variations and the contribution of various conduction mechanisms in transport properties of La0.5Ca0.2Ag0.3MnO3 manganite. J. Alloys Compd. 2022, 898, 162866. [Google Scholar] [CrossRef]
- Hcini, S.; Khadhraoui, S.; Triki, A.; Zemni, S.; Boudard, M.; Oumezzine, M. Impedance spectroscopy properties of Pr0.67A0.33MnO3 (A = Ba or Sr) perovskites. J. Supercond. Nov. Magn. 2014, 27, 195–201. [Google Scholar] [CrossRef]
- Sui, X.; Chen, Q.; Gu, G.; Liu, N. Multi-sampling and filtering technology of IRFPA. Optik 2011, 122, 1037–1041. [Google Scholar] [CrossRef]
- Khalid, M.; Mallick, T.K.; Sundaram, S. Recent advances in perovskite-containing tandem structures. In Photovoltaics Beyond Silicon; Elsevier: Amsterdam, The Netherlands, 2024; pp. 545–581. [Google Scholar]
- Aljaafari, A. Effect of metal and non-metal doping on the photocatalytic performance of titanium dioxide (TiO2): A review. Curr. Nanosci. 2022, 18, 499–519. [Google Scholar] [CrossRef]
- Molak, A.; Paluch, M.; Pawlus, S.; Klimontko, J.; Ujma, Z.; Gruszka, I. Electric modulus approach to the analysis of electric relaxation in highly conducting (Na0.75Bi0.25)(Mn0.25Nb0.75)O3 ceramics. J. Phys. D Appl. Phys. 2005, 38, 1450. [Google Scholar] [CrossRef]
- El Hasnaoui, M.; Graça, M.P.F.; Achour, M.E.; Costa, L.C. Electric modulus analysis of carbon black/copolymer composite materials. Mater. Sci. Appl. 2011, 2, 1421–1426. [Google Scholar] [CrossRef]
- Guerrero, A.; Bisquert, J.; Garcia-Belmonte, G. Impedance spectroscopy of metal halide perovskite solar cells from the perspective of equivalent circuits. Chem. Rev. 2021, 121, 14430–14484. [Google Scholar] [CrossRef]
- Swain, S.; Samal, H.B.; Satpathy, S.; Jena, B.R.; Pattnaik, G.; Bashar, S.; Barad, S. The Prospective Applications of Arising Nanostructured Dielectric Materials in Storage of Energy: A Comprehensive Review. Micro Nanosyst. 2024, 16, 2–20. [Google Scholar] [CrossRef]
- Pal, A.; Kuo, T.W.; Hsu, C.H.; Kakarla, D.C.; Tiwari, A.; Chou, M.C.; Yang, H.D. Interplay of lattice, spin, and dipolar properties in CoTeMo6: Emergence of Griffiths-like phase, metamagnetic transition, and magneto-dielectric effect. Phys. Rev. B 2022, 105, 024420. [Google Scholar] [CrossRef]
- Mishra, S.; Choudhary, R.N.P.; Parida, S.K. Structural, Optical, Relaxor and Transport Properties of a Nanocrystalline Double Perovskite: Ba2(FeMo)O6. Spin 2024, 14, 2350030. [Google Scholar] [CrossRef]
- Ray, A.; Behera, B.; Basu, T.; Vajandar, S.; Satpathy, S.K.; Nayak, P. Modification of structural and dielectric properties of polycrystalline Gd-doped BFO–PZO. J. Adv. Dielectr. 2018, 8, 1850031. [Google Scholar] [CrossRef]
- Venkataraman, B.H.; Varma, K.B.R. Microstructural, dielectric, impedance and electric modulus studies on vanadium doped and pure strontium bismuth niobate (SrBi2Nb2O9) ceramics. J. Mater. Sci. Mater. Electron. 2005, 16, 335–344. [Google Scholar] [CrossRef]
- Kumari, P.; Rai, R.; Sharma, S.; Valente, M.A. Dielectric, electrical conduction and magnetic properties of multiferroic Bi0.8Tb0.1Ba0.1Fe0.9Ti0.1O3 perovskite compound. J. Adv. Dielectr. 2017, 7, 1750034. [Google Scholar] [CrossRef]
- Cherasse, M.; Heshmati, N.; Urban, J.M.; Ünlü, F.; Spencer, M.S.; Frenzel, M.; Maehrlein, S.F. Enhanced Lattice Coherences and Improved Structural Stability in Quadruple A-Site Substituted Lead Bromide Perovskites. Small 2025, 21, 2500977. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.M.; McGuire, M.A.; Cho, Y.; Downer, M.C.; Wan, Y.; Zhou, J.S. Spin freezing into a disordered state in CaFeTi2O6 synthesized under high pressure. Phys. Rev. B 2018, 98, 064201. [Google Scholar] [CrossRef]
- Dey, P.; Nath, T.K.; Manna, P.K.; Yusuf, S.M. Enhanced grain surface effect on magnetic properties of nanometric La0.7Ca0.3MnO3 manganite: Evidence of surface spin freezing of manganite nanoparticles. J. Appl. Phys. 2008, 104, 104101. [Google Scholar] [CrossRef]
- Pal, A.; Rao, A.; Kekuda, D.; Nagaraja, B.S.; Mondal, R.; Biswas, D. Investigation of cationic disorder effects on the transport and magnetic properties of perovskite Pr0.7−xRExSr0.3MnO3 (x = 0.0, 0.2; RE = Nd, Sm, & Gd). J. Magn. Magn. Mater. 2020, 512, 167011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).