Abstract
The relation between antifungal susceptibility and treatment outcomes is not well-characterized. There is paucity of surveillance data for cerebrospinal fluid (CSF) isolates of cryptococcus investigated with YEASTONE colorimetric broth microdilution susceptibility testing. A retrospective study of laboratory-confirmed cryptococcus meningitis (CM) patients was conducted. The antifungal susceptibility of CSF isolates was determined using YEASTONE colorimetric broth microdilution. Clinical parameters, CSF laboratory indices, and antifungal susceptibility results were analyzed to identify risk factors for mortality. High rates of resistance to fluconazole and flucytosine were observed in this cohort. Voriconazole had the lowest MIC (0.06 µg/mL) and lowest rate of resistance (3.8%). In a univariate analysis, hematological malignancy, concurrent cryptococcemia, high Sequential Organ Failure Assessment (SOFA) score, low Glasgow coma scale (GCS) score, low CSF glucose level, high CSF cryptococcal antigen titer, and high serum cryptococcal antigen burden were associated with mortality. In a multivariate analysis, meningitis with concurrent cryptococcemia, GCS score, and high CSF cryptococcus burden, were independent predictors of poor prognosis. Both early and late mortality rates were not significantly different between CM wild type and non-wild type species.
1. Introduction
Cryptococcosis is an infectious disease with worldwide distribution and a wide array of clinical presentations, including meningitis and disseminated disease [1]. Worldwide, nearly 220,000 new cases of cryptococcal meningitis (CM) occur each year, resulting in an estimated 181,000 deaths [2]. Pharmacological management of CM usually consists of primary therapy with amphotericin B, with or without flucytosine, followed by fluconazole maintenance therapy [3]. A regimen comprising amphotericin B or fluconazole is the preferred initial therapy for CM. There appears to be some correlation between minimal inhibitory concentration (MIC) and clinical resistance [4]. However, the guidelines of the Infectious Diseases Society of America do not suggest routine in vitro susceptibility testing of antifungal drugs in such cases [2]. Moreover, several reports have described the emergence of fluconazole-resistant cryptococcus and raised concerns regarding the widespread use of fluconazole in maintenance therapy for cryptococcal infection [5,6,7]. A reliable estimation of the antifungal susceptibility of CM isolates and an assessment of the correlation of the MIC of amphotericin B or fluconazole with the outcomes of CM are key objectives. Methods for the in vitro susceptibility testing of C. neoformans and C. gattii have been modified and standardized [8]. However, the value of MIC obtained by YEASTONE and its correlation with early and late outcomes of CM remain uncertain [9]. We utilized Thermo Fisher Scientific Sensititre YEASTONE colorimetric broth microdilution plates coupled with a Vizion Digital MIC Viewing System (a computer-assisted optical reading machine) to determine the in vitro susceptibility of cerebrospinal isolates of cryptococcus to amphotericin B, flucytosine, fluconazole, itraconazole, posaconazole, and voriconazole. This study aimed to assess the correlation between the antifungal susceptibility patterns of cerebrospinal cryptococcus isolates and to identify the risk factors for mortality in patients with CM.
2. Material and Methods
2.1. Study Population and Data Collection
We reviewed 53 patients with CM confirmed by CSF culture between 1 January 2010 and 31 December 2016. Of the 53 patients, 25 had concomitant cryptococcemia. The results of cryptococcus species identification, MICs of antifungal agents, and underlying comorbidities were analyzed. SOFA score was used to assess disease severity, and GCS score was used to evaluate the consciousness level. Laboratory results of CSF were collected. A cerebrospinal fluid cryptococcal antigen titer and serum antigen titer were used as indices of cryptococcus burden. Cryptococcus burden was defined as a logarithm of cryptococcus antigen to the base of 2. The treatment outcomes were 14-day mortality and overall in-hospital mortality.
2.2. Antifungal Susceptibility Testing
The MICs of antifungal agents were determined by SENSITITRE YEASTONE®, a colorimetric broth microdilution method for in vitro susceptibility tests. The YEASTONE microdilution plates were set up following the manufacturer’s instructions [9]. As no thresholds have been established for cryptococcus species, the epidemiological cutoff values (ECVs) used were based on the CLSI guidelines.
2.3. Cryptococcus Species Identification
Cryptococcus species were identified using matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS) following the manufacturer’s recommendations [10,11,12].
2.4. Statistical Analysis
Statistical analysis was conducted using the SPSS software (IBM SPSS Statistics for Windows version 21.0; IBM Corp, NY, USA). Continuous variables were compared using the Mann–Whitney U-test for 2 groups. When the expected number of patients in any cell was less than 5, the categorical variables were compared using either the Chi-squared test or Fisher’s exact test. Correlations between continuous variables were assessed using the Pearson’s correlation coefficient. Risk factors associated with clinical outcomes were fitted in a logistic regression model. All statistical tests were 2-tailed, and p-values < 0.05 were considered indicative of statistical significance.
3. Results
3.1. Clinical Characteristics of the Study Population
A total of 53 patients with CM were enrolled in this study. The pathogenic species included 48 (90.6%) isolates of C. neoformans, 4 (7.5%) isolates of C. gattii, and 1 (1.9%) isolate of C. curvatus. The baseline demographic and clinical characteristics of the study population are summarized in Table 1. Of these, 12 (22.6%) patients were HIV-positive. Among the 53 meningitis patients, 25 (47.2%) had concurrent cryptococcemia. Three isolates (5.7%) of cryptococcus species were non-wild type isolates to amphotericin B (MIC > 0.5 µg/mL). Sixteen isolates (30.2%) were resistant to fluconazole (MIC > 8 µg/mL). The mortality rate during the 14-day induction therapy was 17%. The overall in-hospital mortality rate in this cohort was 50.9%.

Table 1.
Clinical characteristics, demographic data, and outcomes of patients with cryptococcal meningitis.
3.2. MIC of Antifungal Agents
The MIC50, MIC90, range, epidemiologic cutoff values, and wild type versus non-wild type rates are presented in Table 2. The widest range of MIC was found in flucytosine (0.5–128 µg/mL). Voriconazole had the lowest MIC (0.06 µg/mL) and lowest rate of resistance (3.8%). A high prevalence of non-wild type resistance was observed for both fluconazole and flucytosine (30.2% and 34%, respectively).

Table 2.
Distribution of the minimal inhibitory concentrations of antifungal agents among 53 cryptococcal meningitis CSF isolates.
3.3. Antifungal Susceptibility and Mortality Outcomes
Figure 1 depicts the correlation between MICs of amphotericin B and mortality outcomes. The 14-day mortality rates among amphotericin B MICs of 0.125 µg/mL, 0.25 µg/mL, 0.5 µg/mL, and 1 µg/mL were 0%, 25%, 14%, and 33%, respectively (Chi-squared test for trend p = 0.93). The overall in-hospital mortality rates associated with amphotericin B MICs of 0.125 µg/mL, 0.25 µg/mL, 0.5 µg/mL, and 1 µg/mL were 100%, 50%, 51%, and 33%, respectively (Chi-squared test for trend p = 0.37). The 14-day mortality rates and the overall in-hospital mortality rates among wild type and non-wild type were 16% vs. 33% (p = 0.47) and 52% vs. 33% (p = 0.7), respectively. There was no significant trend or statistical correlation between amphotericin B susceptibility and mortality outcomes. A similar result was observed for fluconazole (Figure 2). For fluconazole, the 14-day mortality rates and the overall in-hospital mortality rates among wild type vs non-wild type were 16% vs. 19% (p = 0.85) and 54% vs. 44% (p = 0.49), respectively.
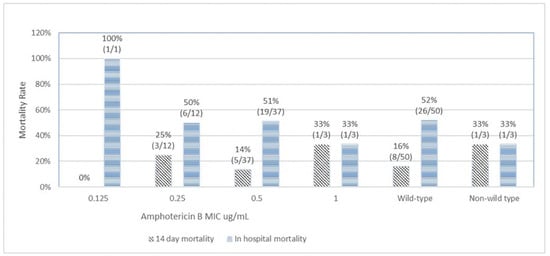
Figure 1.
Correlation of amphotericin B minimal inhibitory concentration (MIC) with 14-day and in-hospital mortality.
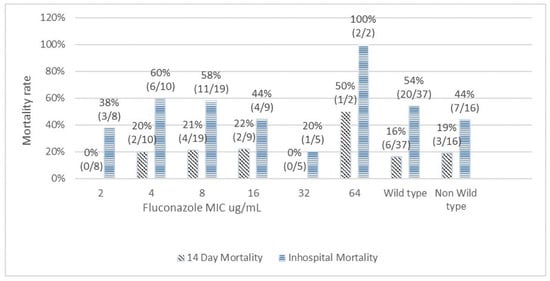
Figure 2.
Correlation of fluconazole minimal inhibitory concentration with 14-day and in-hospital mortality.
3.4. Poor Prognostic Factors for 14-Day Mortality
On univariate analysis (Table 3), CSF glucose level <5 mg/dL (11.3% vs. 55.6%, p = 0.008), higher CSF cryptococcal antigen burden (10 vs. 12, p = 0.002), and higher serum cryptococcal antigen burden (9 vs. 12, p = 0.007) were found to contribute to 14-day mortality (Table 3). Disease severity, as assessed by SOFA score (1.5 vs. 3, p = 0.13) or GCS score (15 vs. 13, p = 0.43), and delayed amphotericin B induction (6.8% vs. 0%, p = 0.42) were not found to have contributed to the prognosis of initial 2-week therapy.

Table 3.
Factors associated with 14-day mortality of patients with cryptococcal meningitis.
3.5. Poor Prognostic Factors for Overall in-Hospital Mortality
The risk factors associated with in-hospital mortality (Table 4) in the univariate analysis included hematology malignancy (4 vs. 0, p = 0.03), concurrent cryptococcemia (23.1% vs. 70.4%, p = 0.001), higher SOFA score (1 vs. 4, p = 0.001), lower GCS score (15 vs. 13, p = 0.001), lower CSF glucose level <5 mg/dL (7.7% vs. 29.6%, p = 0.04), higher CSF cryptococcal antigen burden (9 vs. 11, p = 0.003), and higher serum cryptococcal antigen burden (8.5 vs. 11, p = 0.023).

Table 4.
Factors associated with in-hospital mortality.
3.6. Multivariate Analysis for Poor Prognostic Factors
For multiple logistic regression, the candidate risk factors associated with p < 0.05 in univariate analyzes were selected. Along with SOFA score, GCS score, and cryptococcemia were set as covariates. On multivariate analysis, low CSF glucose level < 5 mg/dL (p = 0.034, odds ratio (OR) = 0.075, 95% confidence interval (CI) 0.007–0.85), high CSF cryptococcus burden (p = 0.023, OR = 2.588, 1.141–5.87), and high serum cryptococcus burden (p = 0.031, OR = 1.791, 95% CI 1.053–3.04) were identified as independent risk factors for 14-day mortality. For overall in-hospital mortality, meningitis with concurrent cryptococcemia (p = 0.013, OR = 0.034, 95% CI 0.002–0.48), GCS score (p = 0.028, OR = 0.262, 95% CI 0.079–0.86), and high CSF cryptococcus burden (p = 0.032, OR = 2.145, 95% CI 1.069–4.3) were identified as independent risk factors for a poor prognosis (Table 5).

Table 5.
Multivariate analysis of factors associated with unfavorable outcomes.
4. Discussion
Cryptococcus neoformans and cryptococcus gattii are encapsulated, heterobasidiomycetous fungi first identified from an environmental source in 1894 [13]. These were initially considered as rare opportunistic pathogens in immunocompromised human populations. However, cases of advanced cryptococcosis have remarkably increased during the past two decades. Most patients with invasive CM had underlying conditions, including HIV, prolonged corticosteroid usage, organ transplantation, hematology malignancy, and diabetes [14]. However, an estimated 20% cryptococcosis patients without HIV infection have no apparent underlying disease or risk factors [15]. In our study, only 22.6% of patients with CM were HIV-positive. Furthermore, only 28% patients in our study had diabetic mellitus, and 18.9% had systemic lupus erythematous. There were still 14.2% of CM patients with no underlying conditions, which is consistent with previous studies [16]. The most common species causing CM in our cohort was Cryptococcus neoformans, accounting for 90.6% patients.
The methods for in vitro susceptibility testing of cryptococcus species have been modified and the ECVs are well-established [10,17]. The purpose of ECVs for antifungal agents is to enable the early detection of emerging resistance. A global antifungal surveillance study, conducted between 1997 and 2007, documented a progressive increase in resistance to fluconazole among C. neoformans isolates (resistant rates 7.3–11.1%) [18]. The increasing trend of fluconazole resistance is more noticeable in Asia. In our study, we noticed an ominously high percentage of non-wild type strains toward fluconazole and flucytosine. In our cohort, the resistance rate to fluconazole was 30.2%, and the resistant rate to flucytosine was 34%. Another study conducted by Yi-Chun Chen et al. also reported similar rates of non-susceptibility in Southern Taiwan [6]. The reported resistance rate of flucytosine among cryptococcus isolates from Africa and Cambodia was approximately 1–2%, but ranged up to 7% [19]. Our study reported a 34% resistance rate of flucytosine. We believe this is the first case-series from Taiwan to report the prevalence of flucytosine resistance. The practice of flucytosine monotherapy in the treatment of invasive cryptococcosis should be particularly discouraged due to a high resistance.
The reports of in vitro susceptibility were reported as wild type and non-wild type based on the ECV. No clinical threshold is currently available for any antifungal agent. The role of the susceptibility test result as a predictor for early or late clinical outcomes remains unclear. A previous study showed some correlation between fluconazole MICs and the poor prognosis of CM [4,11,20]. However, we did not observe such correlation in our study. We analyzed the 14-day mortality as early outcome and overall in-hospital mortality as a late outcome and assessed its correlation with the individual MIC range of antifungal agents. With escalating fluconazole MIC range, we found no significant trend of increasing mortality. The mortality rates between wild type and non-wild type did not show any significant difference, both for amphotericin B and fluconazole. Likewise, in the multivariate analysis, antifungal susceptibility was not found to be an independent predictor of 14-day or in-hospital mortality. However, we did find an extremely high rate of mortality (50% for 14-day and 100% for in-hospital) for pathogens with fluconazole MIC ≥64 µg/mL. When treating CM patients with initial MICs of fluconazole >64 µg/mL, treatment failure may possibly be directly related to drug resistance.
The clinical manifestations of CM are nonspecific and difficult to distinguish from those of meningitis due to other causes. The most important prognostic factor for successful treatment of cryptococcosis is the ability to control a patient’s underlying disease. Several studies have examined the prognostic factors of CM; however, the correlation between clinical manifestations and the prognosis remains unclear [20,21,22]. In our study, a low CSF glucose level (<5 mg/dL), high CSF cryptococcal antigen titers, and high serum cryptococcus antigen burden were independent risk factors for poor early prognosis. Patients’ underlying conditions such as DM, SLE, or malignancy and treatment modalities such as early amphotericin B induction were not related to early treatment outcomes. For late outcomes, we identified meningitis concurrent with cryptococcemia, a low GCS score, and high CSF cryptococcus burden as independent risk factors for poor prognosis.
5. Conclusions
In summary, CM may also occur in non-high-risk groups, such as patients without HIV infection or predisposing underlying conditions. The overall mortality rate in this cohort was high. Risk factors associated with mortality included concomitant cryptococcemia, low CSF glucose level, high CSF and serum cryptococcus antigen burden. Both early and late mortality rates were not significantly different between wild type and non-wild type species.
Author Contributions
Conceptualization, T.-S.W. and J.-H.Y.; methodology, P.-Y.H.; software, P.-Y.H.; validation, C.-W.C.; formal analysis, J.-F.L.; investigation, J.-F.L.; resources, C.-W.C.; data curation, J.-F.L.; writing—original draft preparation, J.-H.Y.; writing—review and editing, T.-S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by Chang Gung Medication Foundation grant number CMRPG3M0201.
Institutional Review Board Statement
This study was approved by the institutional review board of Chang Gung Medical Foundation (IRB document number: 201701804B0). The requirement for written informed consent of participants was waived, and all data were analyzed anonymously.
Informed Consent Statement
Patient consent was waived due to all personal information were de-identified.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perfect, J.R.; Durack, D.T.; Gallis, H.A. Cryptococcemia. Medicine 1983, 62, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O.; European Society of Clinical Microbiology and in Efectious Diseases Fungal Infection Study Group. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Chang, T.-Y.; Liu, J.-W.; Chen-Hsiang, L.; Chien, C.-C.; Tang, Y.-F.; Lu, C.-H. Correlation of anti-fungal susceptibility with clinical outcomes in patients with cryptococcal meningitis. BMC Infect. Dis. 2012, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Boyken, L.; Rice, C.; Tendolkar, S.; Hollis, R.J.; Doern, G.V.; Diekema, D.J. Global Trends in the Antifungal Susceptibility of Cryptococcus neoformans (1990 to 2004). J. Clin. Microbiol. 2005, 43, 2163–2167. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, T.-Y.; Liu, J.-W.; Chen, F.-J.; Chien, C.-C.; Lee, C.-H.; Lu, C.-H. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: A 12-year longitudinal study. BMC Infect. Dis. 2015, 15, 277. [Google Scholar] [CrossRef]
- Govender, N.P.; Patel, J.; van Wyk, M.; Chiller, T.M.; Lockhart, S.R. Trends in Antifungal Drug Susceptibility of Cryptococcus neoformans Isolates Obtained through Population-Based Surveillance in South Africa in 2002-2003 and 2007-2008. Antimicrob. Agents Chemother. 2011, 55, 2606–2611. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef]
- Yang, J.-H.; Huang, P.-Y.; Cheng, C.-W.; Shie, S.-S.; Lin, Z.-F.; Yang, L.-Y.; Lee, C.-H.; Wu, T.-S. Antifungal susceptibility testing with YeastONE™ is not predictive of clinical outcomes of Cryptococcus neoformans var. grubii fungemia. Med. Mycol. 2021, 59, 1114–1121. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Aller, A.I.; Canton, E.; Castañón-Olivares, L.R.; Chowdhary, A.; Cordoba, S.; Cuenca-Estrella, M.; Fothergill, A.; Fuller, J.; Govender, N.; et al. Cryptococcus neoformans-Cryptococcus gattii Species Complex: An International Study of Wild-Type Susceptibility Endpoint Distributions and Epidemiological Cutoff Values for Fluconazole, Itraconazole, Posaconazole, and Voriconazole. Antimicrob. Agents Chemother. 2012, 56, 5898–5906. [Google Scholar] [CrossRef]
- Araújo, M.R.B.; Santos, E.G.D.M.; Wolf, V.; Seabra, L.F. Identification of Cryptococcus neoformans by MALDI-TOF mass spectrometry in blood culture. Clin. Biomed. Res. 2018, 38, 200–202. [Google Scholar] [CrossRef]
- Tarumoto, N.; Sakai, J.; Kodana, M.; Kawamura, T.; Ohno, H.; Maesaki, S. Identification of Disseminated Cryptococcosis Using MALDI-TOF MS and Clinical Evaluation. Med. Mycol. J. 2016, 57, E41–E46. [Google Scholar] [CrossRef]
- Perfect, J.R.; Casadevall, A. Cryptococcosis. Infect. Dis. Clin. 2002, 16, 837–874, v–vi. [Google Scholar] [CrossRef]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 2009, 23, 525–530. [Google Scholar] [CrossRef]
- Pappas, P.G.; Perfect, J.R.; Cloud, G.A.; Larsen, R.A.; Pankey, G.A.; Lancaster, D.J.; Henderson, H.; Kauffman, C.A.; Haas, D.W.; Saccente, M.; et al. Cryptococcosis in Human Immunodeficiency Virus–Negative Patients in the Era of Effective Azole Therapy. Clin. Infect. Dis. 2001, 33, 690–699. [Google Scholar] [CrossRef]
- Yang, H.; Yin, F.; Xiao, T.; Gan, S.; Pan, Z.; Peng, J.; Wu, L. A correlation analysis between clinical manifestations, therapeutic strategies, and the prognosis of children with cryptococcal meningitis in China. Int. J. Infect. Dis. 2020, 95, 241–245. [Google Scholar] [CrossRef]
- Lozano-Chiu, M.; Paetznick, V.L.; Ghannoum, M.A.; Rex, J.H. Detection of Resistance to Amphotericin B among Cryptococcus neoformans Clinical Isolates: Performances of Three Different Media Assessed by Using E-Test and National Committee for Clinical Laboratory Standards M27-A Methodologies. J. Clin. Microbiol. 1998, 36, 2817–2822. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.; Gibbs, D.L.; Newell, V.A.; Bijie, H.; Dzierzanowska, D.; Klimko, N.; Letscher-Bru, V.; Lisalova, M.; Muehlethaler, K.; et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5-Year Analysis of Susceptibilities of Noncandidal Yeast Species to Fluconazole and Voriconazole Determined by CLSI Standardized Disk Diffusion Testing. J. Clin. Microbiol. 2009, 47, 117–123. [Google Scholar] [CrossRef]
- Chandenier, J.; Adou-Bryn, K.D.; Douchet, C.; Sar, B.; Kombila, M.; Swinne, D.; Buisson, Y.; Richard-Lenoble, D. In vitro activity of amphotericin B, fluconazole and voriconazole against 162 Cryptococcus neoformans isolates from Africa and Cambodia. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 506–508. [Google Scholar] [CrossRef]
- Aller, A.I.; Martin-Mazuelos, E.; Lozano, F.; Gomez-Mateos, J.; Steele-Moore, L.; Holloway, W.J.; Gutiérrez, M.J.; Recio, F.J.; Espinel-Ingroff, A. Correlation of Fluconazole MICs with Clinical Outcome in Cryptococcal Infection. Antimicrob. Agents Chemother. 2000, 44, 1544–1548. [Google Scholar] [CrossRef]
- Bennett, J.E.; Dismukes, W.E.; Duma, R.J.; Medoff, G.; Sande, M.A.; Gallis, H.; Leonard, J.; Fields, B.T.; Bradshaw, M.; Haywood, H.; et al. A Comparison of Amphotericin B Alone and Combined with Flucytosine in the Treatment of Cryptoccal Meningitis. N. Engl. J. Med. 1979, 301, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, A.C.; Bittencourt Severo, C.; de Mattos Oliveira, F.; Severo, L.C. An analysis of 28 cases with emphasis on the clinical out-come and its etiologic agent. Rev. Iberoam. Micol. 2004, 21, 143–146. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).