Abstract
Sesquiterpenes are a type of abundant natural product with widespread applications in several industries. They are biosynthesized by sesquiterpene synthases (STSs). As valuable and abundant biological resources, mushroom-forming fungi are rich in new sesquiterpenes and STSs, which remain largely unexploited. In the present study, we collected information on 172 STSs from mushroom-forming fungi with experimentally characterized products from the literature and sorted them to develop a dataset. Furthermore, we analyzed and discussed the phylogenetic tree, catalytic products, and conserved motifs of STSs. Phylogenetic analysis revealed that the STSs were clustered into four clades. Furthermore, their cyclization reaction mechanism was divided into four corresponding categories. This database was used to predict 12 putative STS genes from the edible fungi Flammulina velutipes. Finally, three FvSTSs were selected to experimentally characterize their functions. FvSTS03 predominantly produced Δ-cadinol and FvSTS08 synthesized β-barbatene as the main product; these findings were consistent with those of the functional prediction analysis. A product titer of 78.8 mg/L β-barbatene was achieved in Saccharomyces cerevisiae via metabolic engineering. Our study findings will help screen or design STSs from fungi with specific product profiles as functional elements for applications in synthetic biology.
1. Introduction
Sesquiterpenes are a major class of terpenoids. More than 300 types of basic skeletons have been discovered; these skeletons are widely present in plants, fungi, microorganisms, and insects [1]. Sesquiterpene structures present several acyclic, monocyclic, bicyclic, tricyclic, and tetracyclic systems [2]. Owing to their complex structures, inherent bioactivity, and aroma, sesquiterpenes have widespread applications in food [3], pharmaceutical [4], fragrance [5], fuel [6], and agricultural industries [7]. Mushroom-forming fungi are specific fungal groups with the most conspicuous fruiting bodies; for centuries, they have been used as food and traditional medicine [8]. Mushrooms tend to develop several protective strategies to protect the fruiting bodies from organism attack. For example, mushrooms can produce various structurally diverse sesquiterpenes, many of which exhibit antibacterial, antifungal, and cytotoxic activities [9,10]. These sesquiterpenes play a crucial role in inhibiting fungal growth, modifying bacterial motility, and defending against parasites [11,12]. For example, to protect against predators, Armillaria mellea can produce toxic protoilludane-type sesquiterpenes [13]. Furthermore, rufuslactone isolated from the fruiting bodies of Lactarius rufus exerts antifungal properties against some pathogenic fungi, including Alternaria alternata and Fusarium graminearum [14]. To date, various sesquiterpenoid natural products with an extensive repertoire of backbone structures have been isolated and characterized from mushrooms [15,16,17]. Elucidating their biosynthetic pathways has garnered considerable attention [18,19,20].
The biosynthesis of sesquiterpene natural products originates from the common precursor farnesyl pyrophosphate (FPP), which is derived from the C5 unit isopentenyl diphosphate and its isomer dimethylallyl diphosphate [21]. Subsequently, sesquiterpene synthases (STSs) catalyze linear FPP to generate sesquiterpene scaffolds, followed by a series of cyclization reactions and rearrangements, resulting in the synthesis of structurally diverse sesquiterpenoids [22]. To date, more than 150 STSs have been cloned, purified, and biochemically characterized from mushroom-forming fungi, including Postia placenta [18], Phanerochaete chrysosporium [23], Ganoderma lucidum [24], and Lactarius deliciosus [25]. Furthermore, the site-specific mutations and cyclization mechanisms of a few STSs have been investigated [26,27,28,29,30]. However, owing to these fungi’s complex life cycle and frequently poor growth under laboratory conditions, fungal STSs, particularly those in mushrooms (basidiomycetes), are not well studied compared with those in plants. In general, each basidiomycete contains, on average, more than 12 STS homologs [31]; this indicates that fungal STSs represent rich but largely unexploited natural resources. Over the past decade, continuous advances in sequencing technologies have led to the accumulation of a large amount of fungal genomic data, facilitating genome mining to discover new STSs.
Overall, our study aims to assemble a comprehensive dataset of experimentally characterized mushroom STSs to elucidate the relationship between STS sequences and their catalytic products, then we used the database as a reference to predict and exploit unidentified STSs from other species. Firstly, previously reported 172 STSs were collated and analyzed by phylogenetic, protein domain, and motif analyses. Then, based on the functional prediction of STSs, three new STS genes from Flammulina velutipes were experimentally characterized. Finally, we expressed β-barbatene in Saccharomyces cerevisiae and achieved the highest yield of 78.8 mg/L. On the one hand, this work offers rich functional elements to researchers for conducting synthetic biology research. On the other hand, it provides a reference for exploring new STSs with novel products, superior activity, and selectivity in nature.
2. Materials and Methods
2.1. Literature Search for Characterized STSs
The collected STSs were found by manually searching the articles published in PubMed that demonstrated the ability of STSs via in vivo or in vitro experimental characterization. The amino acid sequences and corresponding IDs were collected from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/), UniProtKB (http://www.uniprot.org/), and JGI (http://www.jgi.doe.gov/) collections (date last accessed on 27 July 2023).
2.2. Phylogenetic Tree Construction
Clustal W from MEGA7 was used to perform multiple sequence alignments of the proteins of the 172 STSs. Then, MEGA7 was used to develop a phylogenetic tree using the neighbor joining method. A bootstrap of 1000 replicates was performed [32].
2.3. Analysis of Protein Motifs and Domains
The specific domains of STS proteins were identified using Pfam (http://pfam-legacy.xfam.org/) (accessed on 15 August 2023). Furthermore, the conserved motifs in the identified STS proteins were predicted using the online tool Multiple Expectation Maximization for Motif (MEME) (https://meme-suite.org/meme/doc/meme.html) (accessed on 15 August 2023) using the default parameters.
2.4. Plasmids and Strain Construction
Table S1 and Table S2 present the plasmids and strains used and constructed in this study, respectively. Table S3 lists the primers used in this study. Table S4 lists the codon-optimized gene sequences. Phanta max super-fidelity DNA polymerase (Vazyme, P505, Nanjing, China) was used for PCR amplification. The recombinant strain was constructed using our previously described method [33]. The lithium acetate method was used for yeast transformation [34].
2.5. Heterologous Expression of STSs in Yeast
The pESC-URA plasmids containing the codon-optimized FvSTS genes were transformed into an engineered S. cerevisiae strain Sc027 [35] to produce sesquiterpenes. In addition, an empty pESC-URA vector was heterologously expressed in Sc027 as a control plasmid. The recombinant yeasts were cultured in 10 mL of synthetic complete (SC) drop-out medium (20 g/L glucose, 6.7 g/L yeast nitrogen base, and 2 g/L amino acid drop-out mix) at 220 rpm and 30 °C for 18 h. Subsequently, the culture solution was inoculated into 50 mL of SC medium with an initial OD600 of 0.05 and cultured at 30 °C and 200 rpm for 30 h. Then, galactose was added at a final concentration of 10 g/L for inducible protein expression. In situ product extraction into an organic phase is a widely used method to minimize the loss due to evaporation and improve the fermentative production of sesquiterpenes [36]. Thus, 5 mL of dodecane was added to the capture product in the fermentation medium after adding galactose. After culturing for 120 h, the fermentation broth was transferred into a 50 mL tube and centrifuged at 10,000 g for 10 min. The dodecane phase was collected and dehydrated by anhydrous sodium sulfate, then filtered using a 0.22 μm filter and analyzed via gas chromatography–mass spectroscopy (GC–MS).
2.6. GC–MS Analysis
The assay products were analyzed using an 8890–7000D GC–MS system equipped with a 7693A automatic liquid sampler and flame ionization detector (Agilent Technologies, Santa Clara, CA, USA). GC analysis was performed on an HP-5MS capillary column (30 m × 0.25 mm × 0.25 µm). One microliter of each dodecane sample was injected into the system at a split ratio of 1:10. Helium was used as the carrier gas at a constant flow rate of 1 mL/min. The temperature of the inlet and detector were set to 280 °C and 200 °C, respectively. The oven temperature was maintained at 70 °C for 2 min and then gradually increased to 300 °C at a rate of 10 °C/min. The MS scan range (m/z) was 35–350 [37]. The fermentation products were identified by comparing their MS spectra and retention times with the NIST17 library. The standard β-caryophyllene was dissolved in dodecane and used to construct the standard curves for quantification.
3. Results
3.1. Database of Characterized STSs
To obtain a comprehensive dataset of functional STSs in mushrooms, the public databases NCBI, UniProt, and JGI were searched and studies on enzymes with characteristic STS domains in mushrooms were manually reviewed. In total, 174 STSs identified from 35 basidiomycetes have been experimentally characterized in previous studies. Table 1 and Table S5 present the information on these 174 STSs, including gene name, species origin, GenBank or JGI protein ID, major and minor products, and type of cyclization reaction. Among these, the amino acid sequence information of 172 STSs was available (File S1). Apart from these functionally characterized STSs, thousands of putative STSs have been identified in sequenced mushroom genomes and transcriptomes; however, their product specificities remain unknown [17].

Table 1.
Sesquiterpene synthases cloned in mushroom-forming fungi to date.
To understand the relationship between the STS sequences and their product structures, a protein sequence-based phylogenetic tree of the 172 STSs was constructed using the neighbor joining method with the Jones–Taylor–Thornton model and pairwise deletion with 1000 bootstrap replicates using MEGA7 software. The phylogenetic tree revealed that the STS proteins were clustered into four distinct clades: clades I, II, III, and IV (Figure 1); this finding is consistent with previous studies [16,61,62]. Clustering via sequence conservation suggested that the STSs within one specific clade catalyze the same or a related cyclization reaction. The STSs in clade I produce terpenes such as α-muurolene, germacrene A, and cadinoles via the 1,10-cyclization of (2E,6E)-FPP with the (E,E)-germacradienyl cation as an intermediate. The STSs in clade II catalyze the 1,10-ring closure of the FPP stereoisomer (3R)-nerolidyl diphosphate ((3R)-NPP) to produce the intermediate (Z,E)-germacradienyl cation. The STSs in clade III are characterized by the 1,11-cyclization of (2E,6E)-FPP via a trans-humulyl cation intermediate. Lastly, the STSs in clade IV initiate 1,6-ring closure using (3R)-NPP as a substrate to yield the intermediate (6R)-β-bisabolol cation (Figure 2) [31,38,42].
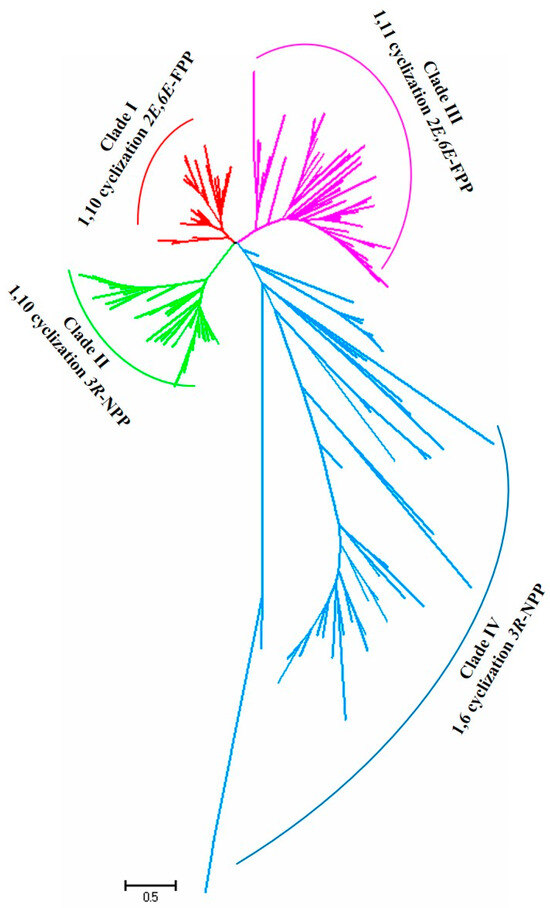
Figure 1.
Phylogenetic analysis of the functional STSs in mushroom-forming fungi. Red lines represent clade I STSs, green lines represent clade II STSs, purple lines represent clade III STSs, and blue lines represent clade IV STSs.
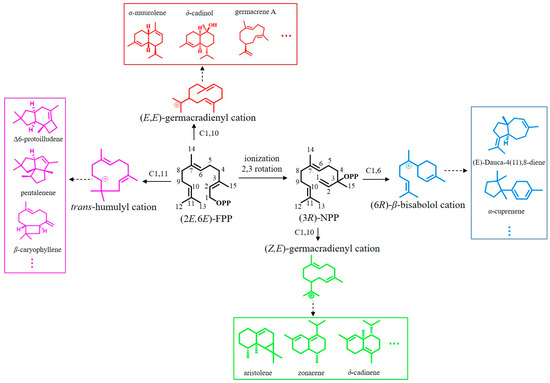
Figure 2.
Reaction mechanisms underlying sesquiterpene production starting from FPP in basidiomycetes.
3.2. Phylogenetic Analysis
To better understand the evolutionary relationships of the STSs in each clade, phylogenetic analysis was performed separately (Figure 3). The first clade (clade I) comprised 33 representative STSs from 18 different basidiomycetes, with STSs from Craterellus cinereus (Cop1–3) and Omphalotus olearius (Omp1 and Omp3), which are known to use the 1,10-cyclization of (2E,6E)-FPP to produce sesquiterpenes derived from the (E,E)-germacradienyl cation. Cop1 and Cop2 can synthesize germacrene A as the major product, whereas Cop3, Omp1, and Omp3 can synthesize α-muurolene as the main product [31,38]. Most STSs in clade I were identified as promiscuous enzymes that produce multiple sesquiterpene scaffolds. Of these STSs, 12 STSs could synthesize α-muurolene as the main product, whereas 8 STSs could synthesize α-muurolene as a side product. Furthermore, nine STSs that can produce cadinols as the main product were identified (Figure 3a). The second clade (clade II) comprised 41 STSs from 22 species. Most of the STSs (Cop4, Omp4, Omp5a, Omp5b, etc.) in clade II could transform FPP to generate β-copaene or cadinene via its isomer (3R)-NPP. Similarly, most STSs found in this clade also generated multiple products. Furthermore, clade II was divided into three clusters, with 17 of the 18 STSs that could produce cadinene as the major product distributed in a larger cluster and 6 STSs that could synthesize viridiflorol as the main product being grouped in the other cluster. Notably, LbSTS4a could produce the acyclic sesquiterpene (E)-nerolidol and form a separate cluster within clade II (Figure 3b).
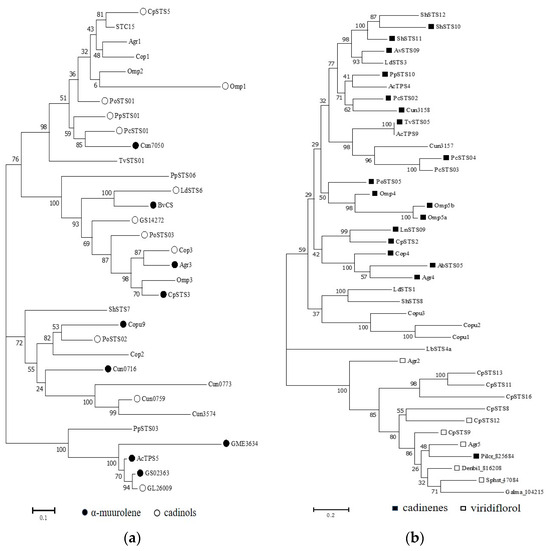
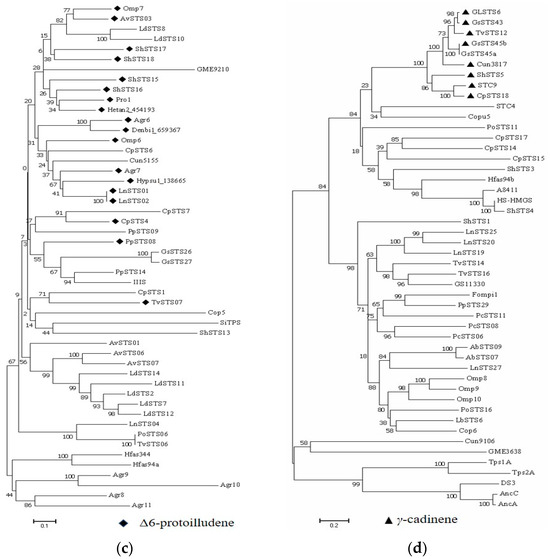
Figure 3.
The phylogeny of STSs in each clade. (a) Clade I; (b) Clade II; (c) Clade III; (d) Clade IV.
The third clade (clade III) comprised 50 STSs from 21 different species. Nearly 50% (23) of the STSs grouped in this clade could produce tricyclic sesquiterpenes, including Δ6-protoilludene, pentalenene, and aromadendrene, which are derived from the common transhumulyl cation intermediate (Figure 3c). Furthermore, most of the STSs identified in this clade could generate a single major product. For example, AvSTS01 can synthesize a sesquiterpene alcohol as a single major product, whereas PoSTS06 can synthesize a sesquiterpene hydrocarbon as a single major product [56]. Moreover, PpSTS08 can specifically produce Δ6-protoilludene [18]. The last clade (clade IV) comprised 48 representative STSs from 22 different mushroom species. The STSs found in this clade could synthesize cadinane-type sesquiterpenoids. Eight STSs appeared in the same small branch and were all specific enzymes coding a single product, γ-cadinene (Figure 3d). Interestingly, the STSs grouped in this clade could also synthesize acyclic (E)-nerolidol, α-farnesene and β-farnesene, monocyclic (Z)-α-bisabolene, bicyclic α-cuprenene, tricyclic α-santalene, α-barbatene, and β-barbatene. Therefore, the STSs that appear in this clade may be functionally diverse to synthesize different types of sesquiterpene skeletons. A simple phylogenetic analysis can offer a predictive framework for discovering more STSs from underexploited mushroom-forming fungi in nature.
3.3. Analysis of Protein Conserved Motifs
STSs have well-known conserved domains that contain aspartate-rich and NSE/DTE motifs, which play important roles in coordinating Mg2+ to facilitate the ionization of FPP/NPP in the active site [63]. First, Pfam analysis was performed to identify the specific protein domains of the 172 STSs. In total, 144 STSs were identified to contain the following conserved domains (Table 2 and Table S5): PF19086 (Terpene_syn_C_2) or PF03936 (Terpene_synth_C), which correspond to the C-terminal domain of terpene synthase. Twenty-five STSs had the domain with the Pfam ID PF06330 (TRI5), described as trichodiene synthase, which is a terpenoid cyclase domain that catalyzes the FPP cyclization to form the bicyclic sesquiterpene hydrocarbon trichodiene [64]. Two STSs from Antrodia cinnamomea, namely Tps1A and Tps2A, were characterized by the UbiA prenyltransferase domain (Pfam ID: PF01040). The other three TPSs, namely, AncA, AncC, and DS3, were characterized by the HAD_2 domain (Pfam ID: PF13419), described as a haloacid dehalogenase-like hydrolase domain. Five STSs (Tps1A, Tps2A, AncA, AncC, and DS3) appeared in the same small branch and formed a separate cluster within clade IV (Figure 3d). Furthermore, the conserved motifs of the 172 STS proteins were analyzed using MEME software. The NDxxSxxxE (NSE) motif was identified as the most conserved motif, covering 161 of the 172 STS protein sequences; in contrast, the asparagine-rich regions [D(D/E/N)xx(D/E)] had various sequences among the 172 STSs. The DExxD sequence was often observed in an appropriate position in 87 STSs. Furthermore, the DD(N)xxD sequence was identified in the aspartate-rich region of 53 STSs. Sequence conservation analysis of these STSs supports more effective site-directed mutagenesis to modulate enzyme activity and specificity and helps us to understand the cyclization mechanisms.

Table 2.
Conserved motifs and domains of STSs.
3.4. Characterization of FvSTSs
Previous studies have reported that F. velutipes is rich in bioactive sesquiterpenes, including sixteen cuparene-type sesquiterpenes, enokipodins with antimicrobial activity, and flammulinolides with antitumor and anticancer activities [65,66,67,68]. To better understand sesquiterpene biosynthesis in F. velutipes, candidate STS sequences in the genomic database of F. velutipes (ASM1180015v1) were searched using BLAST and the 172 STSs in our database. Twelve putative STS genes were identified, which were named FvSTS01–12 (Table S6). The twelve FvSTSs were widely distributed in the phylogenetic tree, and four STSs belonged to clade III, six to clade IV, one to clade I, and one to clade II (Figure 4). To verify the results of our bioinformatics analysis, FvSTS03, FvSTS08, and FvSTS11 were selected for experimental characterization. The codon optimization is the most critical determinant of increasing heterologous protein expression [69]. Thus, three STSs were codon-optimized for expression in S. cerevisiae and synthesized into pESC-URA vectors by GENEWIZ (Suzhou, China). Then, three plasmids were transformed into the engineered S. cerevisiae strain Sc027. GC–MS analysis (Figure 5a) revealed that FvSTS03 abundantly produced Δ-cadinol, with small amounts of minor products, including γ-muurolene, α-muurolene, β-cadinene, and α-cadinol; this is consistent with the findings of the functional evolutionary analysis. Furthermore, FvSTS08 produced β-barbatene as the main product and α-barbatene, dauca-4(11),8-diene, and α-cuprenene as side products (Figure 5b), which were also produced by the other STSs in clade IV of the phylogenetic tree. However, no products were detected in FvSTS11-expressing S. cerevisiae cultures. These results confirm the prediction capability of our method; however, the method still has some limitations.
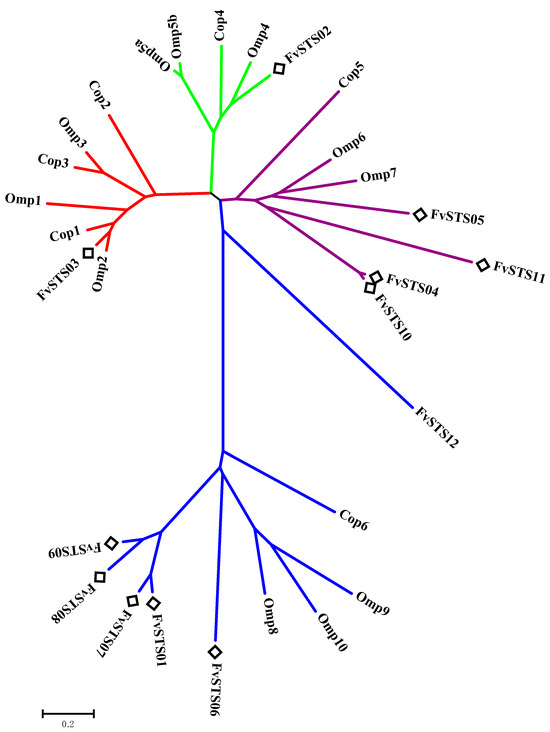
Figure 4.
The phylogeny of candidate STSs in F. velutipes.
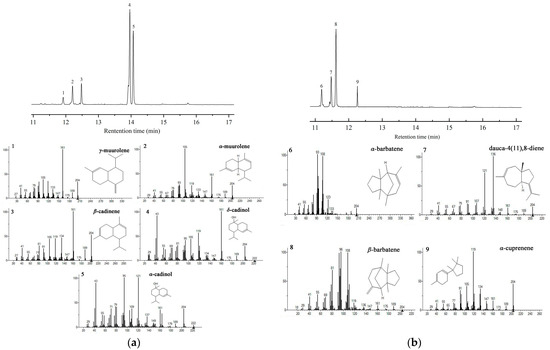
Figure 5.
GC–MS analysis of the assay products of FvSTSs in engineered yeast: (a) FvSTS03 and (b) FvSTS08. The produced sesquiterpenes were putatively annotated as 1, γ-muurolene; 2, α-muurolene; 3, β-cadinene; 4, Δ-cadinol; 5, α-cadinol; 6, α-barbatene; 7, dauca-4(11),8-diene; 8, β-barbatene; and 9, α-cuprenene.
3.5. Heterologous Production of β-Barbatene in S. cerevisiae
Studies have reported that β-barbatene can attract insects for spore dispersal and respond to herbivore infestation [70,71,72]. In this work, we obtained FvSTS08 by a bioinformatics approach, and then experimentally demonstrated that FvSTS08 can synthesize β-barbatene. To improve the heterologous production of β-barbatene in yeast, an effective strategy was developed to increase substrate FPP supply by enhancing the mevalonate (MVA) pathway and inhibiting the branch pathways (Figure S1). In a previous study, Sc027 was engineered to increase FPP supplementation by enhancing MVA. The genes dpp1 (encoding phosphatidate phosphatase) and lpp1 (encoding phosphatidate phosphatase) were reported to be responsible for converting FPP to farnesol; their knockout can enhance FPP supplementation [73]. Therefore, the genes dpp1 and lpp1 were knocked out in Sc027, generating the strain WSL01 (Figure S2). Furthermore, the plasmid pESC-FvSTS08 was transformed into strain WSL01, resulting in the strain WSL01-FvSTS08. GC−MS analysis revealed that WSL01-FvSTS08 produced higher levels of β-barbatene, with a titer of 78.8 mg/L; this was higher than that achieved using the strain Sc027-FvSTS08 (43.2 mg/L) (Figure 6a). Barbatene has two isomers: α-barbatene and β-barbatene. Interestingly, we observed a rearrangement of β-barbatene to the better-described α-isomer under different strong acid conditions (Figure 6b–d). α-Barbatene possesses considerable potential as a high-energy aviation fuel [74]. Overall, our study provides an alternative approach for producing α-barbatene.
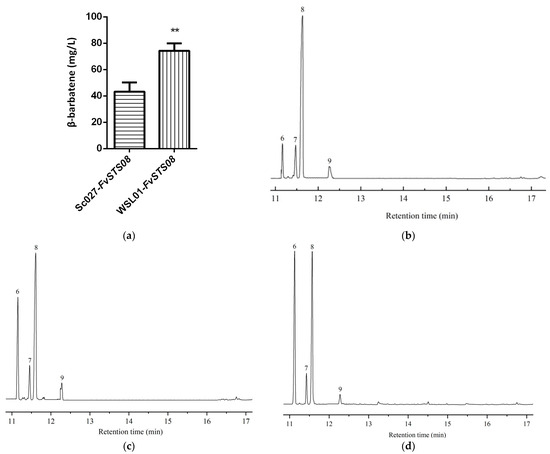
Figure 6.
Heterologous production of β-barbatene in S. cerevisiae and gas chromatograms of the WSL01-FvSTS08 strain under different acid treatment conditions. (a) β-Barbatene production; (b) 3 M HCl treatment; (c) 3 M H2SO4 treatment; and (d) 3 M HNO3 treatment. The produced sesquiterpenes were putatively annotated as 6, α-barbatene; 7, dauca-4(11),8-diene; 8, β-barbatene; and 9, α-cuprenene. The value is the mean of three independent experiments. (** represents p < 0.01).
4. Discussion
Mushroom-forming fungi are particularly well known for their ability to synthesize several structurally diverse sesquiterpenoids, many of which are used as lead compounds for new drugs owing to their diverse pharmacological activities, including anticancer, antifungal, and antibiotic effects [15]. A comprehensive understanding of fungal STSs can help elucidate the biosynthetic mechanism of sesquiterpenoids, which has gradually become the focus of attention. In the present study, we collected 174 functionally characterized fungal STSs from previous studies. Phylogenetic analysis was performed and 172 STSs were divided into four distinct clades. Similar studies have reported that basidiomycetous STSs can be divided into four distinct clades (clades I–IV) [16,41,50,61]. However, some other studies have revealed that basidiomycetous STSs can be grouped into five distinct clades (clades I–V) [18,19,31,39]. The STSs in clade V possess significant sequence similarity to those in clade IV and probably prefer to catalyze the 1,6-cyclization of NPP to generate the bisabolyl cation intermediate, which is similar to the cyclization mechanism observed in the STSs in clade IV [31,39,75].
In general, the phylogenetic tree-based classification of STS protein sequences is consistent with the classification based on the mechanisms of the cyclization reaction of their products. However, some discrepancies remain; therefore, phylogenetic analysis cannot be an accurate predictor of the product specificities of STSs. First, acyclic sesquiterpenes do not undergo cyclization; however, STSs synthesizing acyclic sesquiterpenes appeared in clades II, III, and IV. This may be because acyclic sesquiterpenes (farnesene and nerolidol) are derived from primary cations via proton loss or a reaction with water molecules in the early stage of FPP conversion, which is shared by the biosynthetic pathways of multiple sesquiterpenes. Second, sesquiterpene biosynthesis may occur via different cyclization reactions. For example, in a previous study, researchers proposed a mechanism for viridiflorol formation based on quantum chemical calculations, starting with the 1,10-cyclization of (2E,6E)-FPP [76]. Phylogenetic analysis revealed that Agr5 belongs to clade II (Figure 3b); in contrast, SiTPS was placed in Clade III (Figure 3c). This result indicates that viridiflorol biosynthesis can occur via both routes. In addition, (−)-germacrene D can be synthesized from farnesyl cations via both routes: 1,10 or 1,11-cyclization. Although each enzyme may only follow one cyclization route to form (−)-germacrene D, to date, this route remains unelucidated [77]. Third, differences are often observed between the product profiles of STSs encoded by homologous genes from the same or related species in the same clade. For example, PcSTS03 and PcSTS04 from P. chrysosporium are the closest on the evolutionary tree (Figure 3a); however, PcSTS03 predominantly produces epicubenol, whereas PcSTS04 synthesizes Δ-cadinene as the main product [23]. This may be because some STSs are promiscuous enzymes that may convert the substrate FPP into various side products via cascade reactions of hydroxylation, elimination, cyclization, and rearrangement [78,79]. Furthermore, a single substitution of an amino acid residue may significantly alter the product profiles of STSs [26,55].
Many STSs have been characterized in plants and fungi; however, information on bacterial STSs is scarce. Typical STSs comprise two conserved metal-binding motifs. The first conserved motif is the aspartate-rich region. In a previous study, the canonical form of the aspartate-rich region DDxx(D/E) was identified in 247 of 249 spermatophyte enzymes in plants [77]. However, in our database, the aspartate-rich regions of fungal STSs had various sequences (D(D/E/N)xx(D/E)). The DExxD sequence was often observed in an appropriate position in basidiomycetous STSs. The second conserved motif is called the NSE/DTE motif. The consensus sequence NDxxSxxxE was identified in 161 of the 172 fungal STSs; however, the NSE/DTE motif of plant spermatophyte STSs has various sequences ((N/D)Dxx(S/T/G)xxxE) [57]. In addition to the two conserved motifs, other characteristic conserved motifs, including DxDTT, DDxDTT, and QDxxDxxxD, are present in fungal STS sequences.
In a previous study, 30 sesquiterpenes were isolated from the solid and liquid cultures of F. velutipes, including β-cadinene and α-muurolene [46]. Furthermore, six oxygenated cuprenene derivatives were isolated from a solid culture of F. velutipes growing on cooked rice [80]. In our study, we found that FvSTS03 of F. velutipes can produce small amounts of α-muurolene and β-cadinene and that FvSTS08 of F. velutipes can produce cuprenene as a minor product. Other products such as β-barbatene and Δ-cadinol identified in this study have not been discovered from F. velutipes. This may be because the contents of these products are very low in vivo or because these products are only released under specific stress conditions.
5. Conclusions
In the present study, we collected the information of mushroom STSs with experimentally identified functions and constructed a phylogenetic tree of mushroom functional STSs based on the amino acid sequences. The catalytic products, and conserved domains and motifs of these STSs were analyzed and discussed to explore the sequence–structure–function relationships. Then, our database was applied to predict 12 putative FvSTS genes from F. velutipes and 3 FvSTS genes were experimentally verified. Finally, the product titer of 78.8 mg/L β-barbatene in S. cerevisiae was achieved through expressing FvSTS08. The approach can also be valuable for exploring other new STSs and sesquiterpenes from mushroom-forming fungi in nature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9101017/s1, Table S1: Overview of plasmids used in this study; Table S2: Overview of strains used in this study; Table S3: Primers used in this study; Table S4: The coding sequences of yeast codon-optimized genes; Table S5: Information and bioinformatic analysis of STSs cloned from mushroom-forming fungi; Table S6: Predicted sequence details for putative F. velutipes STSs; Figure S1: Scheme for the biosynthesis pathway of β-barbatene in S. cerevisiae; Figure S2. Preparation of DNA donor expression box of genes to be knock out; File S1: Amino acid sequence information of 172 STSs.
Author Contributions
J.Q., S.W. and R.C. conceived and designed the experiments; S.W. and R.C. performed most of the experiments; L.Y., C.Z. and D.L. provided assistance; and J.Q., S.W. and R.C. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Shaoxing “MingShiZhiXiang” Meritocrat Project (CXCQ2402376) and National Key Research and Development Project of China (2020YFA0907900).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2012, 29, 133. [Google Scholar]
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar]
- Bartikova, H.; Hanusova, V.; Skalova, L.; Ambroz, M.; Bousova, I. Antioxidant, pro-oxidant and other biological activities of ssesquiterpenes. Curr. Top. Med. Chem. 2014, 14, 2478–2494. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar]
- Chen, X.; Zhang, C.; Lindley, N.D. Metabolic engineering strategies for sustainable terpenoid flavor and fragrance synthesis. J. Agric. Food Chem. 2020, 68, 10252–11026. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, C.; Chen, R.; Li, W.; Song, H.; Zhao, G.; Wen, M.; Liang, D.; Qiao, J. Achievements and perspectives of synthetic biology in botanical insecticides. J. Cell Physiol. 2022. [Google Scholar] [CrossRef]
- Lee, K.H.; Morris-Natschke, S.L.; Yang, X.; Huang, R.; Zhou, T.; Wu, S.F.; Shi, Q.; Itokawa, H. Recent progress of research on medicinal mushrooms, foods, and other herbal products used in traditional chinese medicine. J. Tradit. Complement. Med. 2012, 2, 1–12. [Google Scholar]
- Alves, M.; Ferreira, I.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antifungal activity of mushroom (basidiomycetes) extracts and isolated compounds. Curr. Top. Med. Chem. 2013, 13, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [PubMed]
- Fukushima-Sakuno, E. Bioactive small secondary metabolites from the mushrooms Lentinula edodes and Flammulina velutipes. J. Antibiot. 2020, 73, 687–696. [Google Scholar]
- Wirth, S.; Krause, K.; Kunert, M.; Broska, S.; Paetz, C.; Boland, W.; Kothe, E. Function of sesquiterpenes from Schizophyllum commune in interspecific interactions. PLoS ONE 2021, 16, e0245623. [Google Scholar]
- Abraham, W.R. Bioactive sesquiterpenes produced by fungi are they useful for humans as well? Curr. Med. Chem. 2001, 8, 583–606. [Google Scholar] [CrossRef]
- Luo, D.Q.; Wang, F.; Bian, X.Y.; Liu, J.K. Rufuslactone, a new antifungal sesquiterpene from the fruiting bodies of the basidiomycete Lactarius rufus. J. Antibiot. 2005, 58, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Liu, J.K. Secondary metabolites from higher fungi. Prog. Chem. Org. Nat. Prod. 2017, 106, 1–201. [Google Scholar]
- Wu, J.; Yang, X.; Duan, Y.; Wang, P.; Qi, J.; Gao, J.M.; Liu, C. Biosynthesis of sesquiterpenes in basidiomycetes: A review. J. Fungi 2022, 8, 913. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Orban, A.; Shukal, S.; Birk, F.; Too, H.P.; Rühl, M. Agrocybe aegerita serves as a gateway for identifying sesquiterpene biosynthetic enzymes in higher fungi. ACS Chem. Biol. 2020, 15, 1268–1277. [Google Scholar]
- Ichinose, H.; Kitaoka, T. Insight into metabolic diversity of the brown-rot basidiomycete Postia placenta responsible for sesquiterpene biosynthesis: Semi-comprehensive screening of cytochrome P450 monooxygenase involved in protoilludene metabolism. Microb. Biotechnol. 2018, 11, 952–996. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.K.; Zhao, Q.; He, Q.L. Mechanistic investigations of hirsutene biosynthesis catalyzed by a chimeric sesquiterpene synthase from Steccherinum ochraceum. Fungal Genet. Biol. 2022, 161, 10370. [Google Scholar] [CrossRef]
- Asai, S.; Tsunematsu, Y.; Masuya, T.; Otaka, J.; Osada, H.; Watanabe, K. Uncovering hidden sesquiterpene biosynthetic pathway through expression boost area-mediated productivity enhancement in basidiomycete. J. Antibiot. 2020, 73, 721–728. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [PubMed]
- Yuan, Y.; Litzenburger, M.; Cheng, S.; Bian, G.; Hu, B.; Yan, P.; Cai, Y.; Deng, Z.; Bernhardt, R.; Liu, T. Sesquiterpenoids produced by combining two sesquiterpene cyclases with promiscuous myxobacterial CYP260B1. ChemBioChem 2019, 20, 677–682. [Google Scholar] [CrossRef]
- Ichinose, H.; Ukeba, S.; Kitaoka, T. Latent potentials of the white-rot basidiomycete Phanerochaete chrysosporium responsible for sesquiterpene metabolism: CYP5158A1 and CYP5144C8 decorate (E)-α-Bisabolene. Enzyme Microb. Technol. 2022, 158, 110037. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wu, X.; Wang, Q.; Qi, P.; Zhang, Y.; Wang, L.; Sun, C. Characterization of γ-cadinene enzymes in Ganoderma lucidum and Ganoderma sinensis from basidiomycetes provides insight into the identification of terpenoid synthases. ACS Omega 2022, 7, 7229–7723. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.B.; Peh, G.; Wei, Y.; T, R.; Ang, E.L.; Zhao, H.; Zhang, C.; Lim, Y.H. A spirobicyclo [3.1.0]terpene from the investigation of sesquiterpene synthases from Lactarius deliciosus. ACS Chem. Biol. 2023, 18, 134–140. [Google Scholar] [CrossRef] [PubMed]
- López-Gallego, F.; Wawrzyn, G.T.; Schmidt-Dannert, C. Selectivity of fungal sesquiterpene synthases: Role of the active site’s H-1α Loop in catalysis. Appl. Environ. Microbiol. 2010, 76, 7723–7773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lauchli, R.; Pitzer, J.; Kitto, R.Z.; Kalbarczyk, K.Z.; Rabe, K.S. Improved selectivity of an engineered multi-product terpene synthase. Org. Biomol. Chem. 2014, 12, 4013–4402. [Google Scholar]
- Quin, M.B.; Michel, S.N.; Schmidt-Dannert, C. Moonlighting metals: Insights into regulation of cyclization pathways in fungal Δ6 -protoilludene sesquiterpene synthases. ChemBioChem 2015, 16, 2191–2219. [Google Scholar] [CrossRef]
- Quin, M.B.; Wawrzyn, G.; Schmidt-Dannert, C. Purification, crystallization and preliminary X-ray diffraction analysis of Omp6, a protoilludene synthase from Omphalotus olearius. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 574–577. [Google Scholar] [CrossRef]
- Ringel, M.; Dimos, N.; Himpich, S.; Haack, M.; Huber, C.; Eisenreich, W.; Schenk, G.; Loll, B.; Brück, T. Biotechnological potential and initial characterization of two novel sesquiterpene synthases from basidiomycota Coniophora puteana for heterologous production of Δ-Cadinol. Microb. Cell Fact. 2022, 21, 6. [Google Scholar]
- Wawrzyn, G.T.; Quin, M.B.; Choudhary, S.; López-Gallego, F.; Schmidt-Dannert, C. Draft genome of Omphalotus olearius provides a predictive framework for sesquiterpenoid natural product biosynthesis in basidiomycota. Chem. Biol. 2012, 19, 772–778. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, X.; Zhang, Y.; Liang, D.; Caiyin, Q.; Qiao, J. Characterization of trans-nerolidol synthase from Celastrus angulatus maxim and production of trans-nerolidol in engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 2236–2244. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 31–33. [Google Scholar] [CrossRef]
- Zeng, B.X.; Yao, M.D.; Wang, Y.; Xiao, W.H.; Yuan, Y.J. Metabolic engineering of Saccharomyces cerevisiae for enhanced dihydroartemisinic acid production. Front. Bioeng. Biotechnol. 2020, 8, 15. [Google Scholar] [CrossRef]
- Rodriguez, S.; Kirby, J.; Denby, C.M.; Keasling, J.D. Production and quantification of sesquiterpenes in Saccharomyces cerevisiae, including extraction, detection and quantification of terpene products and key related metabolites. Nat. Protoc. 2014, 9, 1980–1996. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, J.; Ge, J.; Li, W.; Liang, D.; Singh, W.; Black, G.; Nie, S.; Liu, J.; Sun, M.; et al. Computer-informed engineering: A new class I sesquiterpene synthase JeSTS4 for the synthesis of an unusual C10-(S)-bicyclogermacrene. ACS Catal. 2022, 12, 4037–4404. [Google Scholar] [CrossRef]
- Agger, S.; Lopez-Gallego, F.; Schmidt-Dannert, C. Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus. Mol. Microbiol. 2009, 72, 1181–1195. [Google Scholar] [CrossRef]
- Quin, M.B.; Flynn, C.M.; Wawrzyn, G.T.; Choudhary, S.; Schmidt-Dannert, C. Mushroom hunting by using bioinformatics: Application of a predictive framework facilitates the selective identification of sesquiterpene synthases in basidiomycota. ChemBioChem 2013, 14, 2480–2491. [Google Scholar] [CrossRef]
- Flynn, C.M.; Schmidt-Dannert, C. Sesquiterpene synthase–3-hydroxy-3-methylglutaryl coenzyme A synthase fusion protein responsible for hirsutene biosynthesis in Stereum hirsutum. Appl. Environ. Microbiol. 2018, 84, e00036-1. [Google Scholar] [CrossRef]
- Nagamine, S.; Liu, C.; Nishishita, J.; Kozaki, T.; Sogahata, K.; Sato, Y.; Minami, A.; Ozaki, T.; Schmidt-Dannert, C.; Maruyama, J.; et al. Ascomycete Aspergillus oryzae is an efficient expression host for production of basidiomycete terpenes by using genomic DNA sequences. Appl. Environ. Microbiol. 2019, 85, e00409-1. [Google Scholar] [PubMed]
- Lin, Y.L.; Ma, L.T.; Lee, Y.R.; Shaw, J.F.; Wang, S.Y.; Chu, F.H. Differential gene expression network in terpenoid synthesis of Antrodia cinnamomea in mycelia and fruiting bodies. J. Agric. Food Chem. 2017, 65, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Hewage, R.T.; Tseng, C.C.; Liang, S.Y.; Lin, H.C. Genome mining of cryptic bisabolenes that were biosynthesized by intramembrane terpene synthases from Antrodia cinnamomea. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220033. [Google Scholar] [CrossRef]
- Chen, T.; Chen, C.; Lee, C.; Huang, R.; Chen, K.; Lu, Y.; Liang, S.; Pham, M.; Rao, Y.K.; Wu, S.; et al. The biosynthetic gene cluster of mushroom-derived antrocin encodes two dual-functional haloacid dehalogenase-like terpene cyclases. Angew. Chem. Int. Ed. 2023, 62, e202215566. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Y.L.; Zeng, J.; Zhang, L.; Ding, Z.H.; Zeng, Y. Identification and characterization of a Δ-cadinol synthase potentially involved in the formation of boreovibrins in Boreostereum vibrans of basidiomycota. Nat. Prod. Bioprospect. 2016, 6, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.Y.Y.; Muria-Gonzalez, M.J.; Kong, B.H.; Stubbs, K.A.; Tan, C.S.; Ng, S.T.; Tan, N.H.; Solomon, P.S.; Fung, S.Y.; Chooi, Y.H. Heterologous expression of cytotoxic sesquiterpenoids from the medicinal mushroom Lignosus rhinocerotis in Yeast. Microb. Cell Fact. 2017, 16, 10. [Google Scholar] [CrossRef]
- Mischko, W.; Hirte, M.; Fuchs, M.; Mehlmer, N.; Brück, T.B. Identification of sesquiterpene synthases from the basidiomycota Coniophora puteana for the efficient and highly selective β-copaene and cubebol production in E. coli. Microb. Cell Fact. 2018, 17, 164. [Google Scholar] [CrossRef]
- Engels, B.; Heinig, U.; Grothe, T.; Stadler, M.; Jennewein, S. Cloning and characterization of an Armillaria gallica cDNA encoding protoilludene synthase, which catalyzes the first committed step in the synthesis of antimicrobial melleolides. J. Biol. Chem. 2011, 286, 6871–6878. [Google Scholar] [CrossRef]
- Al-Salihi, S.A.A.; Dao, T.T.; Williams, K.; Bailey, A.M.; Foster, G.D. The biogenetic origin of the biologically active naematolin of Hypholoma species involves an unusual sesquiterpene synthase. Mol. Biotechnol. 2019, 61, 754–776. [Google Scholar] [CrossRef]
- Püth, N.; Ersoy, F.; Krings, U.; Berger, R.G. Sesquiterpene cyclases from the basidiomycete Cerrena unicolor. Catalysts 2021, 11, 136. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, P.; Zhao, C.; Zhang, Y.; Wang, L.; Yu, H. Tandem expression of Ganoderma sinense sesquiterpene synthase and IDI promotes the production of gleenol in E. coli. Appl. Microbiol. Biotechnol. 2022, 106, 7779–7791. [Google Scholar] [PubMed]
- Chu, L.H.; Wang, L.Z.; Chen, S.L.; Zeng, X.Y.; Xu, J.; Li, Y.; Sun, C. Functional identification of a multi-product sesquiterpene synthase from Ganoderma sinense. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 151–157. [Google Scholar]
- Wei, J.; Pu, X.; Wang, L.; Sun, S.; Sun, C.; Wang, H. Cloning and characterization of sesquiterpene synthase genes from the Ganoderma sinense genome. Sci. Sin. Vitae 2018, 48, 447–454. [Google Scholar] [CrossRef]
- Lizhi, W.; Xiangdong, P.; Shiqiang, T.; Sijie, S.; Yutong, B.; Chao, S.; Shilin, C.; Haiying, W. Cloning and expression of the sesquiterpene synthase gene from Ganoderma lucidum. J. Agric. Univ. Hebei 2017, 40, 67–72. [Google Scholar]
- Burkhardt, I.; Kreuzenbeck, N.B.; Beemelmanns, C.; Dickschat, J.S. Mechanistic characterization of three sesquiterpene synthases from the termite-associated fungus Termitomyces. Org. Biomol. Chem. 2019, 17, 3348–3355. [Google Scholar] [CrossRef]
- Masunaga, N.; Kitaoka, T.; Ichinose, H. Biocatalyst collection and heterologous expression of sesquiterpene synthases from basidiomycetous fungi: Discovery of a novel sesquiterpene hydrocarbon. Microb. Biotechnol. 2023, 16, 632–664. [Google Scholar] [CrossRef]
- Chen, R.; Feng, T.; Li, M.; Zhang, X.; He, J.; Hu, B.; Deng, Z.; Liu, T.; Liu, J.K.; Wang, X.; et al. Characterization of tremulane sesquiterpene synthase from the basidiomycete Irpex lacteus. Org. Lett. 2022, 24, 5669–5673. [Google Scholar] [CrossRef]
- Ntana, F.; Bhat, W.W.; Johnson, S.R.; Jørgensen, H.J.L.; Collinge, D.B.; Jensen, B.; Hamberger, B. A sesquiterpene synthase from the endophytic fungus serendipita indica catalyzes formation of viridiflorol. Biomolecules 2021, 11, 89. [Google Scholar] [CrossRef]
- Kreuzenbeck, N.B.; Dhiman, S.; Roman, D.; Burkhardt, I.; Conlon, B.H.; Fricke, J.; Guo, H.; Blume, J.; Görls, H.; Poulsen, M.; et al. Isolation, (bio)synthetic studies and evaluation of antimicrobial properties of drimenol-type sesquiterpenes of Termitomyces fungi. Commun. Chem. 2023, 6, 7. [Google Scholar]
- Nosenko, T.; Zimmer, I.; Ghirardo, A.; Köllner, T.G.; Weber, B.; Polle, A.; Rosenkranz, M.; Schnitzler, J.P. Predicting functions of putative fungal sesquiterpene synthase genes based on multiomics data analysis. Fungal Genet. Biol. 2023, 165, 103779. [Google Scholar] [CrossRef]
- Gressler, M.; Löhr, N.A.; Schäfer, T.; Lawrinowitz, S.; Seibold, P.S.; Hoffmeister, D. Mind the mushroom: Natural product biosynthetic genes and enzymes of basidiomycota. Nat. Prod. Rep. 2021, 38, 702–772. [Google Scholar] [PubMed]
- Quin, M.B.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the fungal terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [PubMed]
- Aaron, J.A.; Christianson, D.W. Trinuclear metal clusters in catalysis by terpenoid synthases. Pure Appl. Chem. 2010, 82, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, M.J.; Cane, D.E.; Christianson, D.W. Structure of trichodiene synthase from Fusarium sporotrichioides provides mechanistic inferences on the terpene cyclization cascade. Proc. Natl. Acad. Sci. USA 2001, 98, 13543–13548. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, A.; Fukushima-Sakuno, E.; Osaki-Oka, K.; Futamura, Y.; Motoyama, T.; Osada, H.; Ishikawa, N.K.; Nagasawa, E.; Tokimoto, K. Productivity and bioactivity of enokipodins A–D of Flammulina rossica and Flammulina velutipes. Biosci. Biotechnol. Biochem. 2020, 84, 876–888. [Google Scholar] [CrossRef]
- Ishikawa, N.K.; Yamaji, K.; Tahara, S.; Fukushi, Y.; Takahashi, K. Highly oxidized cuparene-type sesquiterpenes from a mycelial culture of Flammulina velutipes. Phytochemistry 2000, 54, 777–778. [Google Scholar] [CrossRef]
- Ishikawa, N.K.; Fukushi, Y.; Yamaji, K.; Tahara, S.; Takahashi, K. Antimicrobial cuparene-type sesquiterpenes, enokipodins C and D, from a mycelial culture of Flammulina velutipes. J. Nat. Prod. 2001, 64, 932–993. [Google Scholar] [CrossRef]
- Li, H.P.; Yang, W.J.; Qu, S.X.; Pei, F.; Luo, X.; Mariga, A.M.; Ma, L. Variation of volatile terpenes in the edible fungi mycelia Flammulina velutipes and communications in fungus-mite interactions. Food Res. Int. 2018, 103, 150–155. [Google Scholar] [CrossRef]
- Fu, H.; Liang, Y.; Zhong, X.; Pan, Z.; Huang, L.; Zhang, H.; Xu, Y.; Zhou, W.; Liu, Z. Codon optimization with deep learning to enhance protein expression. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Chen, X.; Köllner, T.G.; Jia, Q.; Norris, A.; Santhanam, B.; Rabe, P.; Dickschat, J.S.; Shaulsky, G.; Gershenzon, J.; Chen, F. Terpene synthase genes in eukaryotes beyond plants and fungi: Occurrence in social amoebae. Proc. Natl. Acad. Sci. USA 2016, 113, 12132–12137. [Google Scholar] [CrossRef]
- Petek, M.; Rotter, A.; Kogovšek, P.; Baebler, Š.; Mithöfer, A.; Gruden, K. Potato virus Y infection hinders potato defence response and renders plants more vulnerable to colorado potato beetle attack. Mol. Ecol. 2014, 23, 5378–5539. [Google Scholar] [CrossRef] [PubMed]
- Faldt, J.; Jonsell, M.; Nordlander, G.; Borg-Karlson, A.K. Volatiles of bracket fungi fomitopsis pinicola and fomes fomentarius and their functions as insect attractants. J Chem Ecol 1999, 25, 567–590. [Google Scholar] [CrossRef]
- Faulkner, A.; Chen, X.; Rush, J.; Horazdovsky, B.; Waechter, C.J.; Carman, G.M.; Sternweis, P.C. The LPP1 and DPP1 gene products account for most of the isoprenoid phosphate phosphatase activities in Saccharomyces Cerevisiae. J. Biol. Chem. 1999, 274, 14831–14837. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ye, Z.; Wan, X.; Yao, G.; Duan, J.; Liu, J.; Yao, M.; Sun, X.; Deng, Z.; Shen, K.; et al. Systematic mining and evaluation of the sesquiterpene skeletons as high energy aviation fuel molecules. Adv. Sci. 2023, 10, 2300889. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Tantillo, D.J. Branching out from the bisabolyl cation. Unifying mechanistic pathways to barbatene, bazzanene, chamigrene, chamipinene, cumacrene, cuprenene, dunniene, isobazzanene, iso-γ-Bisabolene, isochamigrene, laurene, microbiotene, sesquithujene, sesquisabinene, thujopsene, trichodiene, and widdradiene sesquiterpenes. J. Am. Chem. Soc. 2014, 136, 2450–2463. [Google Scholar]
- Hong, Y.J.; Tantillo, D.J. Is a 1,4-alkyl shift involved in the biosynthesis of ledol and viridiflorol? J. Org. Chem. 2017, 82, 3957–3959. [Google Scholar] [CrossRef]
- Durairaj, J.; Di Girolamo, A.; Bouwmeester, H.J.; de Ridder, D.; Beekwilder, J.; van Dijk, A.D.J. An analysis of characterized plant sesquiterpene synthases. Phytochemistry 2019, 158, 157–165. [Google Scholar] [CrossRef]
- Yoshikuni, Y.; Ferrin, T.E.; Keasling, J.D. Designed divergent evolution of enzyme function. Nature 2006, 440, 1078–1082. [Google Scholar] [CrossRef]
- Huang, J.Q.; Li, D.M.; Li, J.X.; Lin, J.L.; Tian, X.; Wang, L.J.; Chen, X.Y.; Fang, X. 1,10/1,11-Cyclization catalyzed by diverged plant sesquiterpene synthases is dependent on a single residue. Org. Biomol. Chem. 2021, 19, 6650–6656. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, L.; Yang, X.; Li, L.; Li, S.; Gao, H.; Yao, X.S.; Wen, H.; Liu, H.W. Bioactive sesquiterpenoids from the solid culture of the edible mushroom Flammulina velutipes growing on cooked rice. Food Chem. 2012, 132, 1346–1353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).