Contrasting Effects of Grazing in Shaping the Seasonal Trajectory of Foliar Fungal Endophyte Communities on Two Semiarid Grassland Species
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Impact of Grazing in Shaping the Seasonal Trajectory of the Foliar Fungal Community
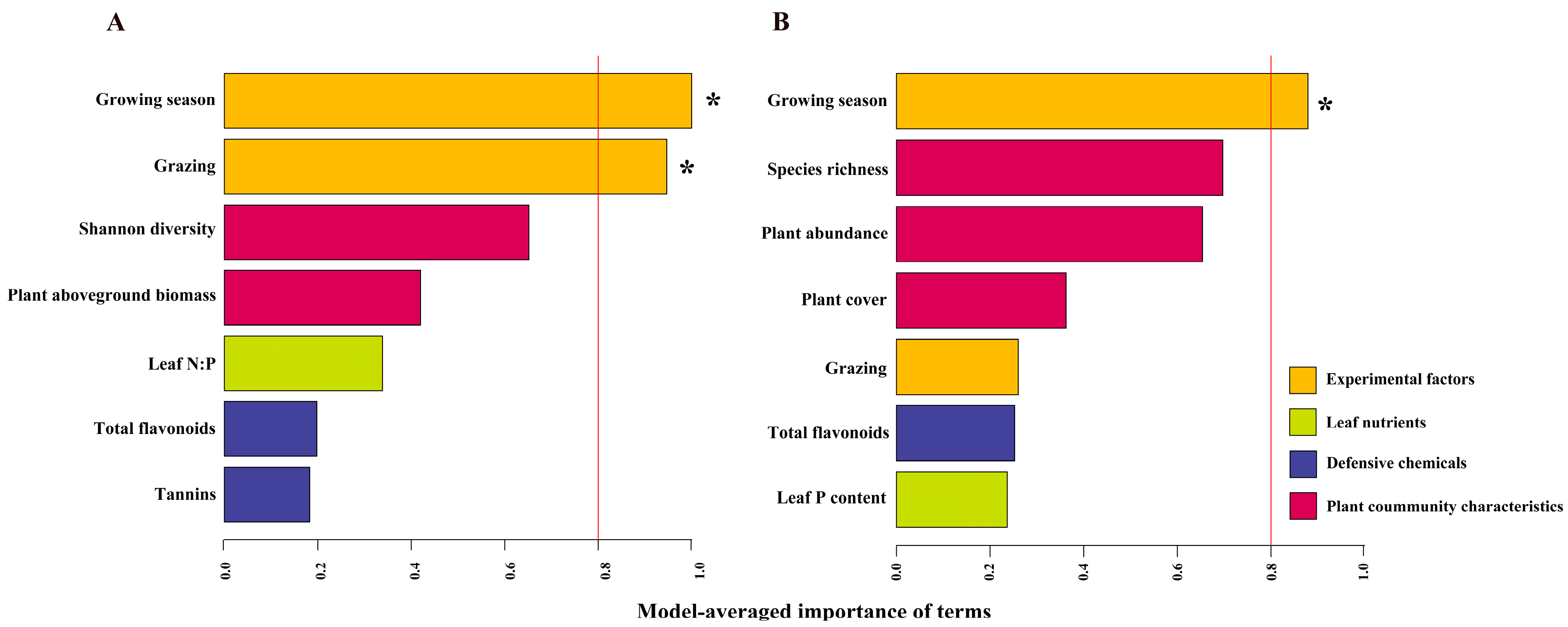
3.2. Relationships between Foliar Fungal Community and Plant Community Characteristics, Leaf Nutrients and Defensive Chemicals
4. Materials and Methods
4.1. Study System and Experimental Design
4.2. Plant Sampling
4.3. Molecular Methods and Bioinformatics
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, C.E.; Kinkel, L.L. Fifty years of phyllosphere microbiology: Significant contributions to research in related fields. In Phyllosphere Microbiology; American Phytopathological Society: St. Paul, MN, USA, 2002; pp. 365–375. [Google Scholar]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef]
- Vacher, C.; Hampe, A.; Porté, A.J.; Sauer, U.; Compant, S.; Morris, C.E. The phyllosphere: Microbial jungle at the plant–climate interface. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Peay, K.G.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Santini, A.; Liebhold, A.; Migliorini, D.; Woodward, S. Tracing the role of human civilization in the globalization of plant pathogens. ISME J. 2018, 12, 647–652. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Penuelas, J. Changes in the environmental microbiome in the Anthropocene. Glob. Chang. Biol. 2020, 26, 3175–3177. [Google Scholar] [CrossRef]
- Bálint, M.; Bartha, L.; O’Hara, R.B.; Olson, M.S.; Otte, J.; Pfenninger, M.; Robertson, A.L.; Tiffin, P.; Schmitt, I. Relocation, high-latitude warming and host genetic identity shape the foliar fungal microbiome of poplars. Mol. Ecol. 2015, 24, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Peay, K.G.; Bidartondo, M.I.; Arnold, A.E. Not every fungus is everywhere: Scaling to the biogeography of fungal–plant interactions across roots, shoots and ecosystems. New Phytol. 2010, 185, 878–882. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Condon, B.; Kinkel, L.; Komatsu, K.J.; Lumibao, C.Y.; May, G.; McCulley, R.L.; Borer, E.T. Effects of nutrient supply, herbivory, and host community on fungal endophyte diversity. Ecology 2019, 100, e02758. [Google Scholar] [CrossRef]
- Larkin, B.G.; Hunt, L.S.; Ramsey, P.W. Foliar nutrients shape fungal endophyte communities in Western white pine (Pinus monticola) with implications for white-tailed deer herbivory. Fungal Ecol. 2012, 5, 252–260. [Google Scholar] [CrossRef]
- Saunders, M.; Kohn, L.M. Evidence for alteration of fungal endophyte community assembly by host defense compounds. New Phytol. 2009, 182, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, J.; Wang, G.; Chen, J. Host identity and phylogeny shape the foliar endophytic fungal assemblages of Ficus. Ecol. Evol. 2019, 9, 10472–10482. [Google Scholar] [CrossRef]
- Peñuelas, J.; Terradas, J. The foliar microbiome. Trends Plant Sci. 2014, 19, 278–280. [Google Scholar] [CrossRef]
- Faticov, M.; Abdelfattah, A.; Roslin, T.; Vacher, C.; Hambäck, P.; Blanchet, F.G.; Lindahl, B.D.; Tack, A.J.M. Climate warming dominates over plant genotype in shaping the seasonal trajectory of foliar fungal communities on oak. New Phytol. 2021, 231, 1770–1783. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Xiong, C.; Wei, Z.; Chen, Q.L.; Ma, B.; Zhou, S.Y.D.; Tan, J.; Zhang, L.M.; Cui, H.L.; Duan, G.L. Impacts of global change on the phyllosphere microbiome. New Phytol. 2022, 234, 1977–1986. [Google Scholar] [CrossRef]
- Lumibao, C.Y.; Borer, E.T.; Condon, B.; Kinkel, L.; May, G.; Seabloom, E.W. Site-specific responses of foliar fungal microbiomes to nutrient addition and herbivory at different spatial scales. Ecol. Evol. 2019, 9, 12231–12244. [Google Scholar] [CrossRef]
- Barker, H.L.; Holeski, L.M.; Lindroth, R.L. Genotypic variation in plant traits shapes herbivorous insect and ant communities on a foundation tree species. PLoS ONE 2018, 13, e0200954. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, N.B.; Vitousek, P.M. Fungal endophyte communities reflect environmental structuring across a Hawaiian landscape. Proc. Natl. Acad. Sci. USA 2012, 109, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.-C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef] [PubMed]
- Collado, J.; Platas, G.; González, I.; Peláez, F. Geographical and seasonal influences on the distribution of fungal endophytes in Quercus ilex. New Phytol. 1999, 144, 525–532. [Google Scholar] [CrossRef]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Rei, F.; do Rosário Félix, M. Spatial and temporal variation of fungal endophytic richness and diversity associated to the phyllosphere of olive cultivars. Fungal Biol. 2019, 123, 66–76. [Google Scholar] [CrossRef]
- Jumpponen, A.; Jones, K.L. Seasonally dynamic fungal communities in the Quercus macrocarpa phyllosphere differ between urban and nonurban environments. New Phytol. 2010, 186, 496–513. [Google Scholar] [CrossRef]
- Oita, S.; Ibáñez, A.; Lutzoni, F.; Miadlikowska, J.; Geml, J.; Lewis, L.A.; Hom, E.F.Y.; Carbone, I.; U’Ren, J.M.; Arnold, A.E. Climate and seasonality drive the richness and composition of tropical fungal endophytes at a landscape scale. Commun. Biol. 2021, 4, 313. [Google Scholar] [CrossRef]
- Copeland, J.K.; Yuan, L.; Layeghifard, M.; Wang, P.W.; Guttman, D.S. Seasonal community succession of the phyllosphere microbiome. Mol. Plant-Microbe Interact. 2015, 28, 274–285. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.-T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Griffin, E.A.; Wright, S.J.; Morin, P.J.; Carson, W.P. Pervasive interactions between foliar microbes and soil nutrients mediate leaf production and herbivore damage in a tropical forest. New Phytol. 2017, 216, 99–112. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, D.; Jiang, F.; Tian, Z.; Feng, X.; Wu, N.; Hou, F.; Kardol, P.; Nan, Z.; Chen, T. Long-term heavy grazing increases community-level foliar fungal diseases by shifting plant composition. J. Appl. Ecol. 2021, 59, 791–800. [Google Scholar] [CrossRef]
- Bosso, L.; Lacatena, F.; Varlese, R.; Nocerino, S.; Cristinzio, G.; Russo, D. Plant pathogens but not antagonists change in soil fungal communities across a land abandonment gradient in a Mediterrane-an landscape. Acta Oecol. 2017, 78, 1–6. [Google Scholar] [CrossRef]
- Wu, J.; Buckley, H.L.; Curry, L.; Stevenson, B.A.; Schipper, L.A.; Lear, G. Livestock exclusion re-duces the spillover effects of pastoral agriculture on soil bacterial communities in adjacent forest fragments. Environ. Microbiol. 2021, 23, 2919–2936. [Google Scholar] [CrossRef]
- De Vos, M.; Van Zaanen, W.; Koornneef, A.; Korzelius, J.P.; Dicke, M.; Van Loon, L.; Pieterse, C.M. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol. 2006, 142, 352–363. [Google Scholar] [CrossRef]
- Humphrey, P.T.; Nguyen, T.T.; Villalobos, M.M.; Whiteman, N.K. Diversity and abundance of phyllosphere bacteria are linked to insect herbivory. Mol. Ecol. 2014, 23, 1497–1515. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; White, J., Jr.; Arnold, A.E.; Redman, A.R.A. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- David, A.S.; Quiram, G.L.; Sirota, J.I.; Seabloom, E.W. Quantifying the associations between fungal endophytes and biocontrol-induced herbivory of invasive purple loosestrife (Lythrum salicaria L.). Mycologia 2016, 108, 625–637. [Google Scholar] [CrossRef]
- Quadt-Hallmann, A.; Kloepper, J.; Benhamou, N. Bacterial endophytes in cotton: Mechanisms of entering the plant. Can. J. Microbiol. 1997, 43, 577–582. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Tilman, D.; Groth, J.V. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology 2002, 83, 1713–1726. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Godsey, M.S.; King, R.J.; Guptill, S.C. Avian diversity and West Nile virus: Testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B Biol. Sci. 2006, 273, 109–117. [Google Scholar] [CrossRef]
- Begon, M. Effects of host diversity on disease dynamics. In Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems; Princeton University Press: Princeton, NJ, USA, 2008; pp. 12–29. [Google Scholar]
- Albrectsen, B.R.; Björkén, L.; Varad, A.; Hagner, Å.; Wedin, M.; Karlsson, J.; Jansson, S. Endophytic fungi in European aspen (Populus tremula) leaves—Diversity, detection, and a suggested correlation with herbivory resistance. Fungal Divers. 2010, 41, 17–28. [Google Scholar] [CrossRef]
- Thomas Clark, A.; Lehman, C.; Tilman, D. Identifying mechanisms that structure ecological communities by snapping model parameters to empirically observed tradeoffs. Ecol. Lett. 2018, 21, 494–505. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Borer, E.T.; Kinkel, L.L. No evidence for trade-offs in plant responses to consumer food web manipulations. Ecology 2018, 99, 1953–1963. [Google Scholar] [CrossRef]
- Trlica, M.J.; Rittenhouse, L.R. Grazing and plant performance. Ecol. Appl. 1993, 3, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Matches, A.G. Plant response to grazing: A review. J. Prod. Agric. 1992, 5, 1–7. [Google Scholar] [CrossRef]
- Agrawal, A.A. Macroevolution of plant defense strategies. Trends Ecol. Evol. 2007, 22, 103–109. [Google Scholar] [CrossRef]
- De Coninck, B.; Timmermans, P.; Vos, C.; Cammue, B.P.; Kazan, K. What lies beneath: Belowground defense strategies in plants. Trends Plant Sci. 2015, 20, 91–101. [Google Scholar] [CrossRef]
- Gómez, J.M.; Zamora, R. Thorns as induced mechanical defense in a long-lived shrub (Hormathophylla spinosa, Cruciferae). Ecology 2002, 83, 885–890. [Google Scholar] [CrossRef]
- Christensen, L.; Coughenour, M.B.; Ellis, J.E.; Chen, Z.Z. Vulnerability of the Asian typical steppe to grazing and climate change. Clim. Chang. 2004, 63, 351–368. [Google Scholar] [CrossRef]
- Chen, T.; Christensen, M.; Nan, Z.; Hou, F. The effects of different intensities of long-term grazing on the direction and strength of plant–soil feedback in a semiarid grassland of Northwest China. Plant Soil 2017, 413, 303–317. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Wu, X.; Syed, S.I.; Syed, I.U.S.; Huang, B.; Guan, P.; Wang, D. Grazing affects bacterial and fungal diversities and communities in the rhizosphere and endosphere compartments of Leymus chinensis through regulating nutrient and ion distribution. Microorganisms 2021, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Fort, T.; Robin, C.; Capdevielle, X.; Delière, L.; Vacher, C. Foliar fungal communities strongly differ between habitat patches in a landscape mosaic. PeerJ 2016, 4, e2656. [Google Scholar] [CrossRef]
- Smith, V.H.; Holt, R.D. Resource competition and within-host disease dynamics. Trends Ecol. Evol. 1996, 11, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Sheik, C.S.; Beasley, W.H.; Elshahed, M.S.; Zhou, X.; Luo, Y.; Krumholz, L.R. Effect of warming and drought on grassland microbial communities. ISME J. 2011, 5, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.C.; Street, N.R.; Sjödin, A.; Lee, N.M.; Högberg, M.N.; Näsholm, T.; Hurry, V. Microbial community response to growing season and plant nutrient optimisation in a boreal Norway spruce forest. Soil Biol. Biochem. 2018, 125, 197–209. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, S.-P.; Han, X.-G.; Lin, G.-H. Effects of long-term grazing on the morphological and functional traits of Leymus chinensis in the semiarid grassland of Inner Mongolia, China. Ecol. Res. 2009, 24, 99–108. [Google Scholar] [CrossRef]
- Xun, W.; Yan, R.; Ren, Y.; Jin, D.; Xiong, W.; Zhang, G.; Cui, Z.; Xin, X.; Zhang, R. Grazing-induced microbiome alterations drive soil organic carbon turnover and productivity in meadow steppe. Microbiome 2018, 6, 170. [Google Scholar] [CrossRef]
- Wang, L.; Delgado-Baquerizo, M.; Wang, D.; Isbell, F.; Liu, J.; Feng, C.; Liu, J.; Zhong, Z.; Zhu, H.; Yuan, X. Diversifying livestock promotes multidiversity and multifunctionality in managed grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 6187–6192. [Google Scholar] [CrossRef]
- Wilson, C.H.; Strickland, M.S.; Hutchings, J.A.; Bianchi, T.S.; Flory, S.L. Grazing enhances belowground carbon allocation, microbial biomass, and soil carbon in a subtropical grassland. Glob. Chang. Biol. 2018, 24, 2997–3009. [Google Scholar] [CrossRef]
- Petit Bon, M.; Gunnarsdotter Inga, K.; Jónsdóttir, I.S.; Utsi, T.A.; Soininen, E.M.; Bråthen, K.A. Interactions between winter and summer herbivory affect spatial and temporal plant nutrient dynamics in tundra grassland communities. Oikos 2020, 129, 1229–1242. [Google Scholar] [CrossRef]
- Dong, S.; Shang, Z.; Gao, J.; Boone, R.B. Enhancing sustainability of grassland ecosystems through ecological restoration and grazing management in an era of climate change on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2020, 287, 106684. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.G.; Wang, J.T.; Singh, B.; Han, L.L.; Shen, J.P.; Li, P.P.; Wang, G.B.; Wu, C.F.; Ge, A.H.; et al. Host selection shapes crop microbiome assembly and network complexity. New Phytol. 2021, 229, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Peay, K.G.; Baraloto, C.; Fine, P.V. Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Perreault, R.; Laforest-Lapointe, I. Plant-microbe interactions in the phyllosphere: Facing challenges of the anthropocene. ISME J. 2022, 16, 339–345. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Öpik, M.; Ainsaar, L.; Ducousso, M.; Hiiesalu, I.; Jairus, T.; Johnson, N.; Jourand, P.; Kalamees, R.; et al. Microbial island biogeography: Isolation shapes the life history characteristics but not diversity of root-symbiotic fungal communities. ISME J. 2018, 12, 2211–2224. [Google Scholar] [CrossRef]
- Kinkel, L.L.; Andrews, J.H.; Berbee, F.M.; Nordheim, E.V. Leaves as islands for microbes. Oecologia 1987, 71, 405–408. [Google Scholar] [CrossRef]
- Kuris, A.M.; Blaustein, A.R.; Alio, J.J. Hosts as islands. Am. Nat. 1980, 116, 570–586. [Google Scholar] [CrossRef]
- Glassman, S.I.; Lubetkin, K.C.; Chung, J.A.; Bruns, T.D. The theory of island biogeography applies to ectomycorrhizal fungi in subalpine tree “islands” at a fine scale. Ecosphere 2017, 8, e01677. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The theory of island biogeography. In The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Kapas, R.E.; Dalton, R.M.; Graae, B.J.; Heiling, J.M.; Opedal, Ø.H. Microenvironment and functional-trait context dependence predict alpine plant community dynamics. J. Ecol. 2018, 106, 1323–1337. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Q.; Shi, G.; Liu, Y.; Du, G.; Feng, H. Can nitrogen supersede host identity in shaping the community composition of foliar endophytic fungi in an alpine meadow ecosystem? Front. Microbiol. 2022, 13, 895533. [Google Scholar] [CrossRef]
- Wu, D.; Ma, Y.; Yang, T.; Gao, G.; Wang, D.; Guo, X.; Chu, H. Phosphorus and Zinc Are Strongly Associated with Belowground Fungal Communities in Wheat Field under Long-Term Fertilization. Microbiol. Spectr. 2022, 10, e00110-22. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Weisenhorn, P.; Gilbert, J.A.; Ni, Y.; Sun, R.; Shi, Y.; Chu, H. Carbon constrains fungal endophyte assemblages along the timberline. Environ. Microbiol. 2016, 18, 2455–2469. [Google Scholar] [CrossRef]
- Chen, T.; Nan, Z.; Kardol, P.; Duan, T.; Song, H.; Wang, J.; Li, C.; Hou, F. Effects of interspecific competition on plant-soil feedbacks generated by long-term grazing. Soil Biol. Biochem. 2018, 126, 133–143. [Google Scholar] [CrossRef]
- Li, J. Flora of China. Harv. Pap. Bot. 2007, 13, 301–302. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Cosoveanu, A.; Cabrera, R. Endophytic fungi in species of Artemisia. J. Fungi 2018, 4, 53. [Google Scholar] [CrossRef]
- Cosoveanu, A.; Rodriguez Sabina, S.; Cabrera, R. Fungi as endophytes in Artemisia thuscula: Juxtaposed elements of diversity and phylogeny. J. Fungi 2018, 4, 17. [Google Scholar] [CrossRef]
- Jie, C.; Tian-Ming, H.; Ji-Min, C.; Gao-Lin, W. Distribution of biomass and diversity of Stipa bungeana community to climatic factors in the Loess Plateau of northwestern China. Afr. J. Biotechnol. 2010, 9, 6733–6739. [Google Scholar]
- Hechmi, N.; Bosso, L.; El-Bassi, L.; Scelza, R.; Testa, A.; Jedidi, N.; Rao, M.A. Depletion of pentachlorophenol in soil microcosms with Byssochlamys nivea and Scopulariopsis brumptii as detoxification agents. Chemosphere 2016, 165, 547–554. [Google Scholar] [CrossRef]
- Purahong, W.; Mapook, A.; Wu, Y.-T.; Chen, C.-T. Characterization of the Castanopsis carlesii deadwood mycobiome by Pacbio sequencing of the full-length fungal nuclear ribosomal internal transcribed spacer (ITS). Front. Microbiol. 2019, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Liu, Y.-X.; Qin, Y.; Chen, T.; Lu, M.; Qian, X.; Guo, X.; Bai, Y. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell 2021, 12, 315–330. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Edgar, R.C.; Flyvbjerg, H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 2015, 31, 3476–3482. [Google Scholar] [CrossRef] [PubMed]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Chase, J.M.; Knight, T.M. Scale-dependent effect sizes of ecological drivers on biodiversity: Why standardised sampling is not enough. Ecol. Lett. 2013, 16, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 0.13.17. 2010. Available online: http://cran.r-project.org/package=mumin (accessed on 21 April 2022).
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘Vegan’. Community Ecology Package, Version 2.0-10. 2013. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 30 April 2022).
- Legendre, P.; Oksanen, J.; ter Braak, C.J.F. Testing the significance of canonical axes in redundancy analysis. Methods Ecol. Evol. 2011, 2, 269–277. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]

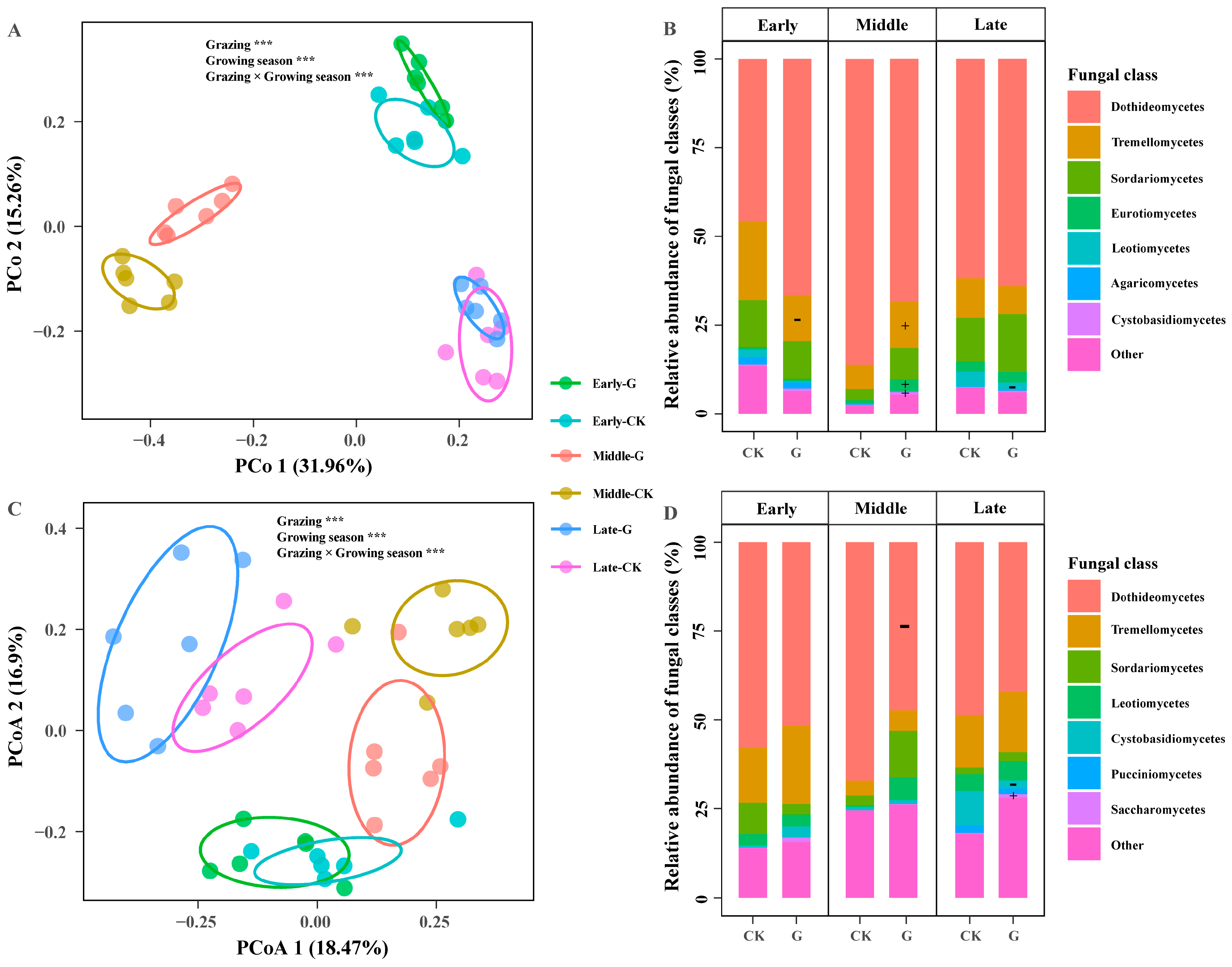

| Sources | Grazing (G) | Season (S) | G × S | |||
|---|---|---|---|---|---|---|
| p | R2 | p | R2 | p | R2 | |
| Artemisia capillaris | ||||||
| ENSPIE | <0.001 | 0.174 | <0.001 | 0.566 | 0.243 | 0.752 |
| Community composition | 0.001 | 0.094 | 0.001 | 0.446 | 0.001 | 0.118 |
| Stipa bungeana | ||||||
| ENSPIE | 0.468 | 0.010 | 0.003 | 0.213 | 0.004 | 0.400 |
| Community composition | 0.001 | 0.058 | 0.001 | 0.272 | 0.001 | 0.091 |
| Predictors | Artemisia capillaris | Stipa bungeana | ||||
|---|---|---|---|---|---|---|
| Estimate | R2 | p | Estimate | R2 | p | |
| Plant abundance | −0.015 | 0.025 | 0.355 | −0.120 | 0.144 | 0.022 |
| Plant cover/log | −2.937 | 0.090 | 0.075 | −0.227 | 0.112 | 0.047 |
| Plant aboveground biomass | −0.164 | 0.299 | <0.001 | −0.305 | 0.012 | 0.518 |
| Species richness | −0.652 | 0.026 | 0.343 | −1.199 | 0.206 | 0.005 |
| Shannon diversity | 8.273 | 0.120 | 0.038 | −2.795 | 0.033 | 0.292 |
| Leaf C content | 0.002 | 0.001 | 0.942 | 0.009 | 0.013 | 0.507 |
| Leaf N content | 0.242 | 0.062 | 0.143 | 0.024 | 0.001 | 0.839 |
| Leaf P content | −4.241 | 0.064 | 0.135 | 5.066 | 0.150 | 0.019 |
| Leaf C:N | −0.227 | 0.082 | 0.090 | −0.051 | 0.006 | 0.653 |
| Leaf C:P | 0.020 | 0.044 | 0.222 | −0.021 | 0.090 | 0.075 |
| Leaf N:P | 0.573 | 0.221 | 0.004 | −0.135 | 0.020 | 0.416 |
| Tannins | 10.398 | 0.121 | 0.038 | 0.894 | 0.002 | 0.806 |
| Total flavonoids | 4.517 | 0.334 | <0.001 | −2.267 | 0.104 | 0.055 |
| Total phenols | 2.008 | 0.059 | 0.153 | −1.009 | 0.023 | 0.377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Jiang, F.; Duan, D.; Tian, Z.; Liu, H.; Zhang, Y.; Hou, F.; Nan, Z.; Chen, T. Contrasting Effects of Grazing in Shaping the Seasonal Trajectory of Foliar Fungal Endophyte Communities on Two Semiarid Grassland Species. J. Fungi 2023, 9, 1016. https://doi.org/10.3390/jof9101016
Dong X, Jiang F, Duan D, Tian Z, Liu H, Zhang Y, Hou F, Nan Z, Chen T. Contrasting Effects of Grazing in Shaping the Seasonal Trajectory of Foliar Fungal Endophyte Communities on Two Semiarid Grassland Species. Journal of Fungi. 2023; 9(10):1016. https://doi.org/10.3390/jof9101016
Chicago/Turabian StyleDong, Xin, Feifei Jiang, Dongdong Duan, Zhen Tian, Huining Liu, Yinan Zhang, Fujiang Hou, Zhibiao Nan, and Tao Chen. 2023. "Contrasting Effects of Grazing in Shaping the Seasonal Trajectory of Foliar Fungal Endophyte Communities on Two Semiarid Grassland Species" Journal of Fungi 9, no. 10: 1016. https://doi.org/10.3390/jof9101016
APA StyleDong, X., Jiang, F., Duan, D., Tian, Z., Liu, H., Zhang, Y., Hou, F., Nan, Z., & Chen, T. (2023). Contrasting Effects of Grazing in Shaping the Seasonal Trajectory of Foliar Fungal Endophyte Communities on Two Semiarid Grassland Species. Journal of Fungi, 9(10), 1016. https://doi.org/10.3390/jof9101016








