Effects of Mixed Adding Crude Extracts of β-Glucosidases from Three Different Non-Saccharomyces Yeast Strains on the Quality of Cabernet Sauvignon Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Medium
2.2. Preparing Crude Extracts of β-Glucosidase
2.3. Analyzing the β-Glucosidase Activity
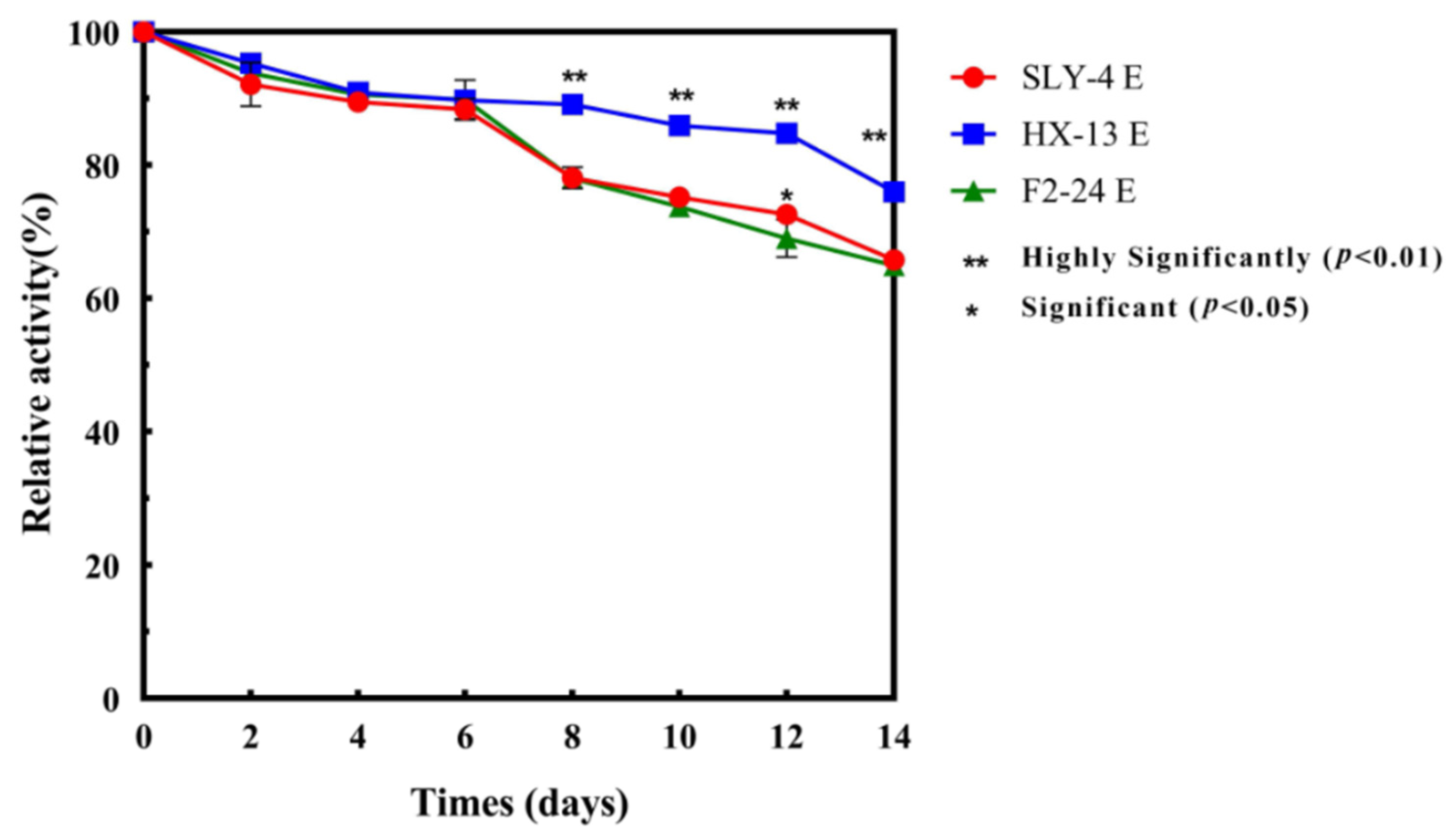
2.4. Stability of β-Glucosidase in Crude Extracts
2.5. Wine Fermentation
2.6. Analysis of Wines
2.7. Data Analyses
3. Results and Discussions
3.1. Stability of Crude Extracts of β-Glucosidase
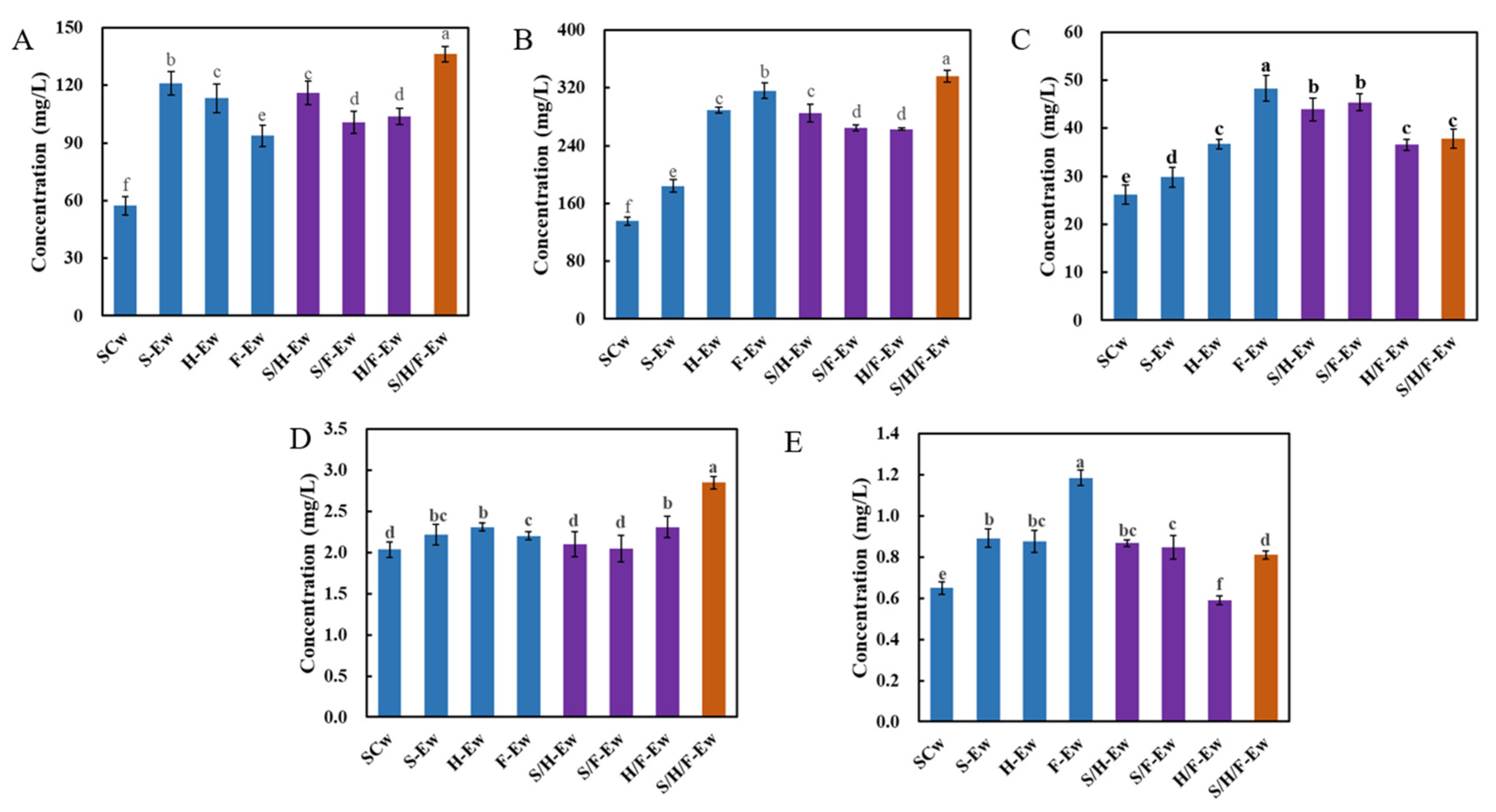
3.2. Physicochemical Characteristics of Wines
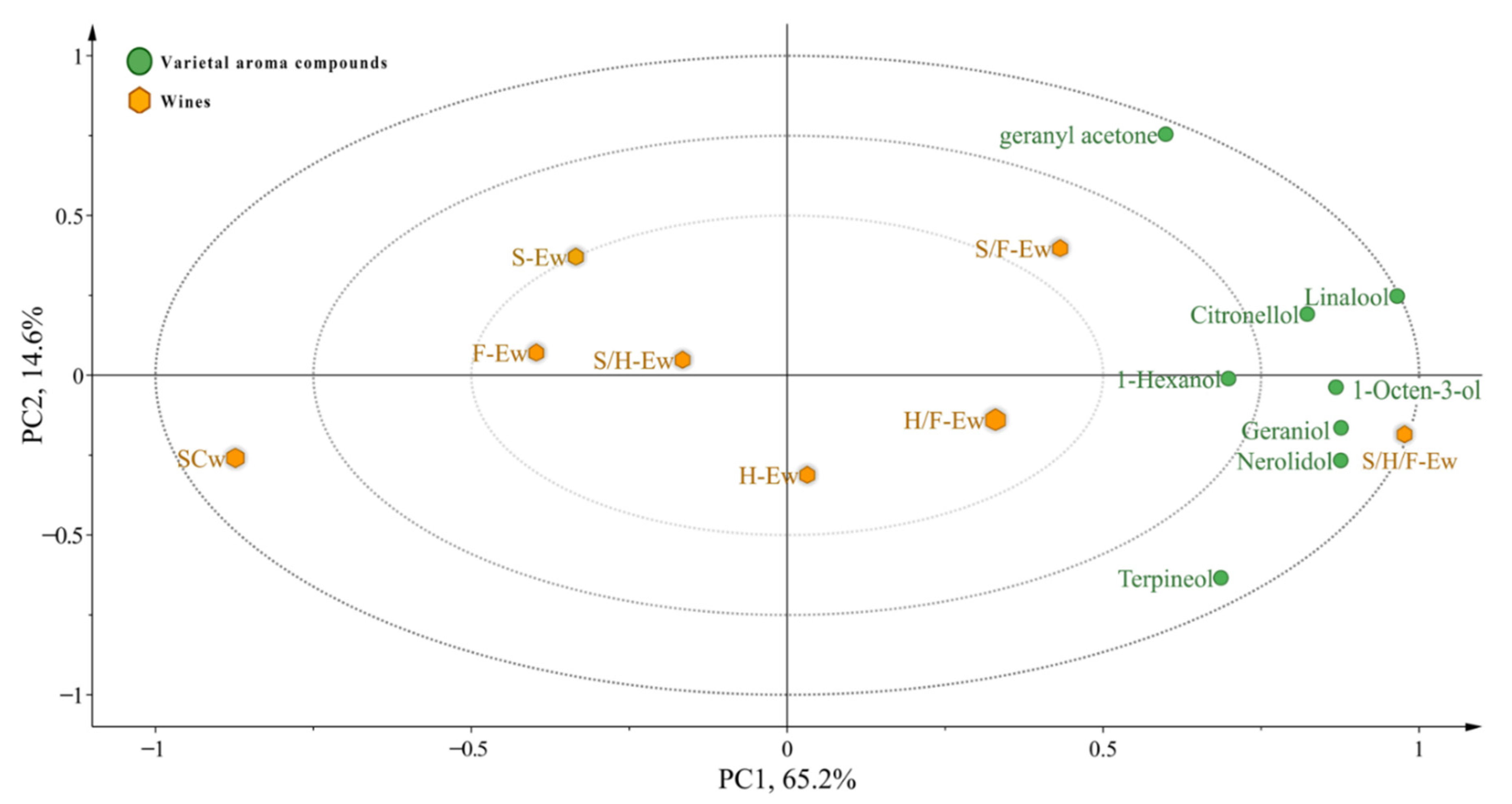
3.3. Varietal Aroma Compounds of Wines
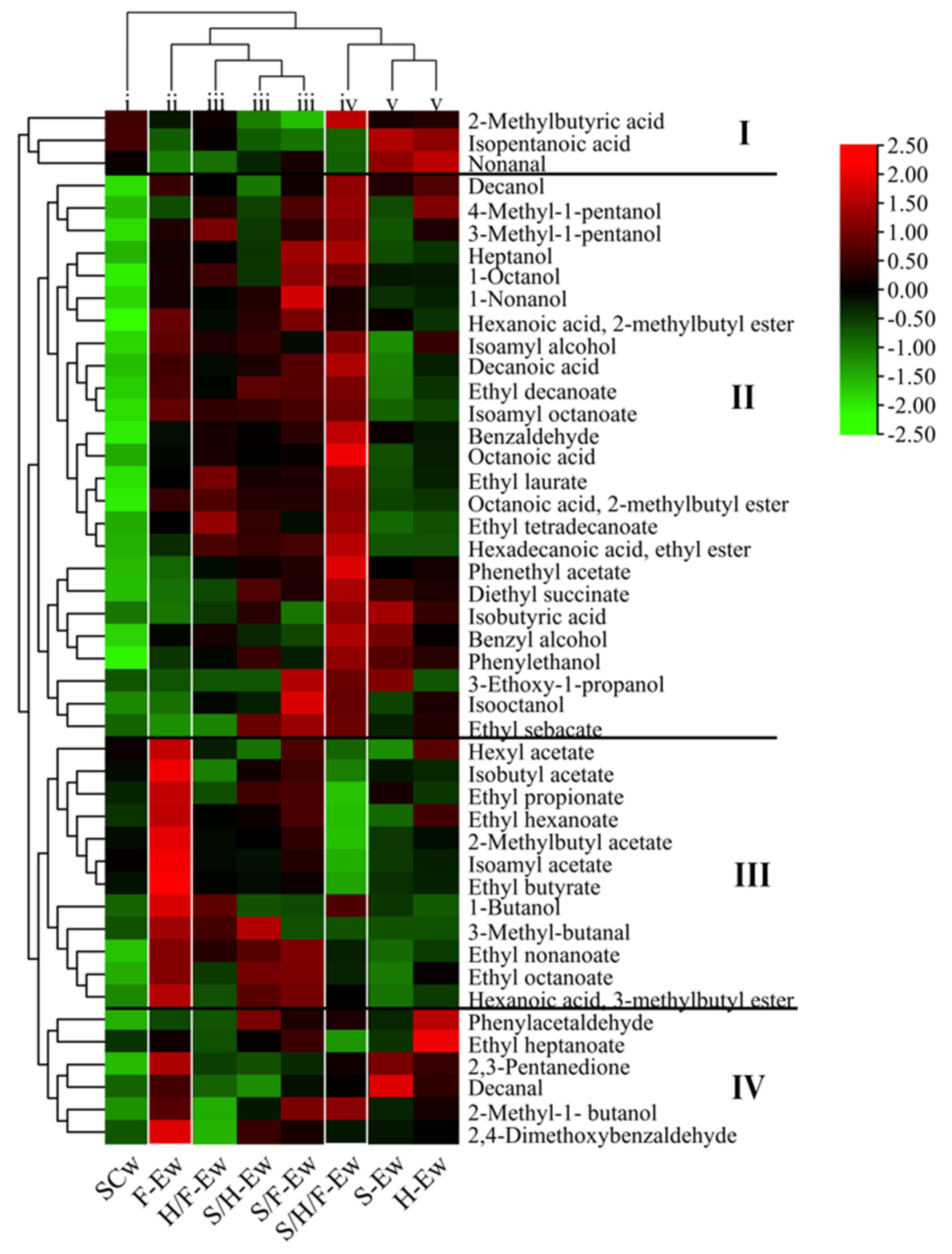
3.4. Fermentative Aroma Compounds
3.5. Sensory Analysis
4. Conclusions
| Compounds | Concentration (mg/L) | Threshold | OAV | Description | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCw | S-Ew | H-Ew | F-Ew | S/H-Ew | S/F-Ew | H/F-Ew | S/H/F-Ew | ||||
| volatile aroma | |||||||||||
| C6 compound | |||||||||||
| 1-Hexanol | 0.76 ± 0.01 b | 0.66 ± 0.01 de | 0.63 ± 0.03 e | 0.71 ± 0.01 c | 0.69 ± 0.02 cd | 0.93 ± 0.01 a | 0.96 ± 0.03 a | 0.96 ± 0.07 a | 8 [32] | <0.1 | Grass [44] |
| subtotal | 0.76 ± 0.01 b | 0.66 ± 0.01 de | 0.63 ± 0.03 e | 0.71 ± 0.01 c | 0.69 ± 0.02 cd | 0.93 ± 0.01 a | 0.96 ± 0.03 a | 0.96 ± 0.07 a | |||
| Terpenes | |||||||||||
| Linalool | 0.21 ± 0.02 e | 0.30 ± 0.01 c | 0.31 ± 0.01 c | 0.28 ± 0.01 d | 0.29 ± 0.02 cd | 0.38 ± 0.01 a | 0.33 ± 0.00 b | 0.40 ± 0.05 a | 0.1 [25] | >1 | Rose, fruit [45] |
| Citronellol | 0.06 ± 0.01 d | 0.09 ± 0.01 bc | 0.09 ± 0.02 bc | 0.06 ± 0.01 d | 0.09 ± 0.02 bc | 0.10 ± 0.02 b | 0.07 ± 0.02 cd | 0.12 ± 0.01 a | 0.1 [32] | 0.1–1 | Lime [44] |
| 1-Octen-3-ol | 0.07 ± 0.01 e | 0.11 ± 0.00 d | 0.15 ± 0.02 c | 0.17 ± 0.01 b | 0.16 ± 0.01 bc | 0.16 ± 0.01 bc | 0.17 ± 0.00 b | 0.23 ± 0.01 a | 0.02 [28] | >1 | Mushroom [46] |
| Geranyl acetone | Nd | 0.06 ± 0.00 b | 0.02 ± 0.00 f | 0.03 ± 0.01 e | 0.04 ± 0.00 d | 0.07 ± 0.00 a | 0.05 ± 0.00 c | 0.04 ± 0.01 d | 0.06 [32] | 0.1–1 | Flower [44] |
| Nerolidol | 0.03 ± 0.01 c | 0.04 ± 0.01 b | 0.05 ± 0.01 a | 0.03 ± 0.00 c | 0.04 ± 0.00 b | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.7 [32] | <0.1 | Rose, Apple, Orange [44] |
| Terpineol | Nd | Nd | 0.02 ± 0.00 b | Nd | 0.01 ± 0.00 c | Nd | 0.02 ± 0.00 b | 0.03 ± 0.00 a | 0.25 [32] | <0.1 | Flower, Pine [44] |
| Geraniol | Nd | Nd | 0.01 ± 0.00 b | 0.01 ± 0.00 b | Nd | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.02 ± 0.00 a | 0.03 [32] | <0.1 | Rose, Geranium [47] |
| subtotal | 0.37 ± 0.04 g | 0.60 ± 0.02 ef | 0.66 ± 0.06 d | 0.58 ± 0.04 f | 0.64 ± 0.05 de | 0.77 ± 0.04 b | 0.70 ± 0.04 c | 0.90 ± 0.09 a | |||
| total | 1.14 ± 0.02 d | 1.26 ± 0.01 c | 1.29 ± 0.04 c | 1.29 ± 0.02 c | 1.34 ± 0.03 c | 1.70 ± 0.02 b | 1.66 ± 0.03 b | 1.86 ± 0.06 a | |||
| Fermentation aroma | |||||||||||
| Benzene derivatives | |||||||||||
| Benzaldehyde | 0.20 ± 0.02 c | 0.38 ± 0.10 b | 0.35 ± 0.03 b | 0.36 ± 0.02 b | 0.38 ± 0.09 b | 0.41 ± 0.03 b | 0.39 ± 0.09 b | 0.52 ± 0.02 a | 2 [32] | 0.1–1 | Roasted almonds [44] |
| Benzyl alcohol | 0.03 ± 0.00 d | 0.08 ± 0.01 b | 0.06 ± 0.01 c | 0.06 ± 0.00 c | 0.05 ± 0.01 c | 0.05 ± 0.00 c | 0.06 ± 0.02 c | 0.09 ± 0.01 a | 200 [25] | <0.1 | Almond [45] |
| Phenylacetaldehyde | 0.05 ± 0.00 d | 0.08 ± 0.01 bc | 0.12 ± 0.02 a | 0.07 ± 0.00 c | 0.11 ± 0.01 a | 0.09 ± 0.02 b | 0.07 ± 0.01 c | 0.09 ± 0.01 b | 0.005 [36] | >1 | Flower, Rose, Honey [48] |
| Phenyl ethanol | 50.76 ± 4.45 f | 110.76 ± 5.32 b | 102.60 ± 6.32 c | 85.37 ± 4.53 e | 105.43 ± 4.96 bc | 89.79 ± 5.10 de | 93.80 ± 3.78 d | 121.64 ± 3.54 a | 7.5 [25] | >1 | Musk, Peach [45] |
| Phenethyl acetate | 6.28 ± 0.33 d | 9.75 ± 0.54 b | 10.18 ± 1.05 b | 7.84 ± 0.85 c | 10.08 ± 0.93 b | 10.42 ± 0.59 b | 9.56 ± 0.39 b | 13.92 ± 0.35 a | 0.65 [25] | >1 | Fruit, Flower [45] |
| subtotal | 57.31 ± 4.82 f | 121.05 ± 5.98 b | 113.32 ± 7.42 c | 93.70 ± 5.41 e | 116.06 ± 6.01 c | 100.76 ± 5.75 d | 103.88 ± 4.30 d | 136.26 ± 3.94 a | |||
| Higher alcohols | |||||||||||
| 1-Butanol | 0.75 ± 0.03 c | 0.81 ± 0.34 bc | 0.76 ± 0.01 c | 1.23 ± 0.09 a | 0.77 ± 0.04 bc | 0.79 ± 0.39 bc | 1.04 ± 0.14 ab | 1.02 ± 0.16 ab | 150 [25] | <0.1 | Fragrant [45] |
| Isoamyl alcohol | 90.98 ± 4.38 g | 129.74 ± 6.68 f | 230.17 ± 2.27 c | 251.61 ± 7.14 b | 230.11 ± 10.29 c | 196.84 ± 2.39 e | 219.07 ± 1.09 d | 267.10 ± 5.23 a | 30 [25] | >1 | Bitter almond [45] |
| 2-Methyl-1- butanol | 42.76 ± 1.28 e | 51.69 ± 1.50 d | 56.20 ± 1.29 c | 61.16 ± 3.60 b | 52.61 ± 1.72 d | 64.23 ± 1.06 a | 40.58 ± 0.32 e | 65.55 ± 2.54 a | 65 [26] | 0.1–1 | |
| 4-Methyl-1-pentanol | 0.12 ± 0.02 c | 0.20 ± 0.05 bc | 0.36 ± 0.22 a | 0.20 ± 0.04 bc | 0.21 ± 0.01 bc | 0.32 ± 0.12 ab | 0.29 ± 0.02 ab | 0.38 ± 0.20 a | 50 [29] | <0.1 | Almond [49] |
| 3-Ethoxy-1-propanol | Nd | 0.38 ± 0.01 b | Nd | Nd | Nd | 0.46 ± 0.00 a | Nd | 0.34 ± 0.03 c | |||
| 3-Methyl-1-pentanol | 0.54 ± 0.01 e | 0.81 ± 0.08 d | 1.04 ± 0.01 b | 1.03 ± 0.04 b | 0.86 ± 0.00 c | 1.05 ± 0.02 b | 1.21 ± 0.05 a | 1.24 ± 0.02 a | 0.5 [34] | >1 | Earthy, Mushroom [50] |
| Heptanol | 0.08 ± 0.00 e | 0.12 ± 0.00 d | 0.13 ± 0.01 c | 0.16 ± 0.00 b | 0.13 ± 0.00 c | 0.22 ± 0.02 a | 0.15 ± 0.00 b | 0.23 ± 0.01 a | 0.2–0.3 [34] | 0.1–1 | Lemon, Orange [50] |
| Isooctanol | 0.03 ± 0.00 d | 0.03 ± 0.00 d | 0.04 ± 0.01 c | 0.03 ± 0.00 d | 0.04 ± 0.00 c | 0.06 ± 0.00 a | 0.04 ± 0.00 c | 0.05 ± 0.00 b | Sweet, Flower, Rose [51] | ||
| 1-Octanol | 0.16 ± 0.01 f | 0.25 ± 0.01 de | 0.25 ± 0.04 de | 0.27 ± 0.01 cd | 0.23 ± 0.00 e | 0.31 ± 0.00 a | 0.28 ± 0.00 bc | 0.30 ± 0.01 ab | 0.9 [25] | 0.1–1 | Orange, Vanilla [45] |
| 1-Nonanol | 0.06 ± 0.00 d | 0.12 ± 0.03 c | 0.13 ± 0.01 c | 0.15 ± 0.02 b | 0.15 ± 0.00 b | 0.22 ± 0.01 a | 0.14 ± 0.00 bc | 0.15 ± 0.00 b | 0.015 [25] | >1 | Orange [45] |
| Decanol | 0.03 ± 0.00 e | 0.07 ± 0.01 b | 0.07 ± 0.00 b | 0.07 ± 0.01 b | 0.05 ± 0.01 d | 0.06 ± 0.01 c | 0.06 ± 0.00 c | 0.08 ± 0.00 a | 0.4 [25] | 0.1–1 | Flower [45] |
| subtotal | 135.51 ± 5.74 f | 184.22 ± 8.71 e | 289.15 ± 3.86 c | 315.89 ± 10.96 b | 285.17 ± 12.08 c | 264.56 ± 4.02 d | 262.86 ± 1.63 d | 336.42 ± 8.21 a | |||
| Acetate esters | |||||||||||
| Isobutyl acetate | 0.03 ± 0.00 d | 0.03 ± 0.00 d | 0.02 ± 0.00 e | 0.09 ± 0.00 a | 0.04 ± 0.00 c | 0.05 ± 0.00 b | Nd | Nd | 1.6 [32] | <0.1 | Banana [52] |
| Isoamyl acetate | 4.39 ± 0.78 bc | 3.45 ± 0.53 d | 3.85 ± 0.03 cd | 8.01 ± 0.43 a | 4.03 ± 0.27 c | 4.82 ± 0.53 b | 4.16 ± 0.36 c | 1.66 ± 0.03 e | 0.2 [25] | >1 | Banana [45], Green apple [46] |
| 2-Methylbutyl acetate | 0.42 ± 0.07 c | 0.36 ± 0.01 d | 0.42 ± 0.01 c | 0.76 ± 0.04 a | 0.44 ± 0.04 c | 0.50 ± 0.02 b | 0.42 ± 0.02 c | 0.16 ± 0.01 e | 0.16 [26] | >1 | |
| Hexyl acetate | 0.06 ± 0.00 bc | 0.04 ± 0.01 d | 0.07 ± 0.00 b | 0.08 ± 0.00 a | 0.04 ± 0.01 d | 0.06 ± 0.01 b | 0.05 ± 0.01 c | 0.04 ± 0.00 d | 1.5 [32] | <0.1 | Fruit, Pear, Cherry [44] |
| subtotal | 4.90 ± 0.85 c | 3.87 ± 0.56 e | 4.36 ± 0.04 d | 8.94 ± 0.47 a | 4.55 ± 0.32 cd | 5.43 ± 0.55 b | 4.64 ± 0.39 cd | 1.86 ± 0.04 f | |||
| Fatty acid ethyl esters | |||||||||||
| Ethyl propionate | 0.11 ± 0.01 d | 0.14 ± 0.00 c | 0.11 ± 0.00 d | 0.20 ± 0.00 a | 0.15 ± 0.01 b | 0.16 ± 0.01 b | 0.10 ± 0.00 e | 0.05 ± 0.00 f | 1.8 [32] | <0.1 | Pineapple, Banana, Apple [46] |
| Ethyl butyrate | 0.20 ± 0.01 bcd | 0.18 ± 0.01 d | 0.19 ± 0.01 cd | 0.41 ± 0.05 a | 0.20 ± 0.01 bc | 0.22 ± 0.00 b | 0.21 ± 0.01 bc | 0.08 ± 0.00 e | 0.02 [32] | >1 | Strawberry, Apple, Banana [44] |
| Ethyl hexanoate | 4.00 ± 0.14 de | 3.72 ± 0.28 e | 4.60 ± 0.12 bc | 5.25 ± 0.60 a | 4.33 ± 0.29 bcd | 4.65 ± 0.07 b | 4.25 ± 0.05 cd | 3.27 ± 0.40 f | 0.014 [25] | >1 | Green apple, Fennel [45] |
| Ethyl heptanoate | 0.04 ± 0.01 b | 0.04 ± 0.00 b | 0.06 ± 0.00 a | 0.04 ± 0.00 b | 0.04 ± 0.01 b | 0.04 ± 0.00 b | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.002 [28] | >1 | Sweet, Strawberry, Banana [53] |
| Ethyl octanoate | 9.65 ± 0.41 e | 10.93 ± 0.59 d | 14.2 ± 0.26 b | 17.35 ± 0.80 a | 16.92 ± 1.17 a | 17.18 ± 0.79 a | 12.57 ± 0.34 c | 13.16 ± 0.84 c | 0.25 [25] | >1 | Fruit [45] |
| Ethyl nonanoate | 0.09 ± 0.00 g | 0.13 ± 0.00 f | 0.15 ± 0.00 e | 0.25 ± 0.00 a | 0.22 ± 0.00 b | 0.24 ± 0.01 a | 0.20 ± 0.01 c | 0.18 ± 0.01 d | 1.3 [32] | 0.1–1 | Flower, Fruit [52] |
| Ethyl sebacate | 0.49 ± 0.00 e | 0.72 ± 0.07 d | 0.95 ± 0.00 c | 0.34 ± 0.00 f | 1.17 ± 0.07 b | 1.35 ± 0.05 a | 0.38 ± 0.05 f | 1.19 ± 0.08 b | |||
| Ethyl decanoate | 5.10 ± 0.52 f | 7.33 ± 0.54 e | 9.13 ± 0.46 d | 12.32 ± 0.66 b | 12.81 ± 0.42 b | 12.61 ± 0.19 b | 10.29 ± 0.25 c | 13.55 ± 0.54 a | 0.2 [25] | >1 | Apple, Flower [45] |
| Ethyl laurate | 0.87 ± 0.02 g | 1.61 ± 0.00 f | 1.83 ± 0.01 e | 1.98 ± 0.02 d | 2.10 ± 0.02 c | 2.13 ± 0.06 c | 2.57 ± 0.04 b | 2.75 ± 0.06 a | 1.5 [29] | >1 | Fruit, Fatty [49] |
| Ethyl tetradecanoate | 0.07 ± 0.01 e | 0.09 ± 0.01 d | 0.10 ± 0.00 d | 0.12 ± 0.00 c | 0.13 ± 0.00 b | 0.11 ± 0.00 c | 0.15 ± 0.00 a | 0.16 ± 0.00 a | 2 [34] | <0.1 | Sweet fruit, Butter, Fatty [50] |
| Hexadecanoic acid, ethyl ester | 0.16 ± 0.00 f | 0.22 ± 0.00 e | 0.22 ± 0.00 e | 0.24 ± 0.00 d | 0.30 ± 0.01 c | 0.31 ± 0.00 b | 0.31 ± 0.00 b | 0.38 ± 0.01 a | 1.5 [30] | 0.1–1 | Fruit, Sweet, Fatty [51] |
| Diethyl succinate | 0.32 ± 0.01 f | 0.57 ± 0.00 bc | 0.54 ± 0.06 c | 0.40 ± 0.01 e | 0.59 ± 0.05 b | 0.54 ± 0.02 c | 0.44 ± 0.02 d | 0.67 ± 0.02 a | 200 [25] | <0.1 | Fruit, Melon [52] |
| subtotal | 21.08 ± 1.12 e | 25.66 ± 1.50 d | 32.07 ± 0.93 c | 38.89 ± 2.15 a | 38.96 ± 2.07 a | 39.55 ± 1.20 a | 31.50 ± 0.78 c | 35.46 ± 1.96 b | |||
| Other esters | |||||||||||
| Hexanoic acid, 3-methylbutyl ester | 0.04 ± 0.00 e | 0.05 ± 0.00 d | 0.06 ± 0.00 c | 0.09 ± 0.01 a | 0.08 ± 0.00 b | 0.08 ± 0.00 b | 0.05 ± 0.00 d | 0.06 ± 0.00 c | 1 [35] | <0.1 | Apple, Pineapple [49] |
| Hexanoic acid, 2-methylbutyl ester | Nd | 0.03 ± 0.00 b | 0.02 ± 0.00 c | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.04 ± 0.00 a | 0.02 ± 0.00 c | 0.03 ± 0.00 b | |||
| Isoamyl octanoate | 0.09 ± 0.01 f | 0.14 ± 0.00 e | 0.16 ± 0.01 d | 0.23 ± 0.01 a | 0.21 ± 0.01 c | 0.22 ± 0.01 b | 0.21 ± 0.01 c | 0.24 ± 0.00 a | 0.125 [29] | >1 | Fruit, Cheese [49] |
| Octanoic acid, 2-methylbutyl ester | 0.03 ± 0.01 e | 0.08 ± 0.01 d | 0.09 ± 0.00 d | 0.12 ± 0.01 c | 0.11 ± 0.01 c | 0.11 ± 0.00 c | 0.13 ± 0.00 b | 0.15 ± 0.00 a | |||
| subtotal | 0.17 ± 0.01 d | 0.30 ± 0.01 c | 0.32 ± 0.02 c | 0.48 ± 0.02 a | 0.43 ± 0.02 b | 0.45 ± 0.02 b | 0.41 ± 0.01 b | 0.47 ± 0.01 a | |||
| total esters | 26.15 ± 1.99 e | 29.84 ± 2.07 d | 36.75 ± 0.98 c | 48.31 ± 2.65 a | 43.95 ± 2.40 b | 45.43 ± 1.77 b | 36.55 ± 1.18 c | 37.80 ± 2.01 c | |||
| Fatty acids | |||||||||||
| Isobutyric acid | Nd | 0.02 ± 0.00 a | 0.01 ± 0.00 b | Nd | 0.01 ± 0.00 b | Nd | Nd | 0.02 ± 0.00 a | 2.3 [26] | <0.1 | Sour, Cheese [49] |
| 2-Methylbutyric acid | 0.65 ± 0.04 b | 0.60 ± 0.06 c | 0.62 ± 0.01 bc | 0.55 ± 0.00 d | 0.44 ± 0.06 e | 0.37 ± 0.01 f | 0.59 ± 0.00 cd | 0.78 ± 0.02 a | 0.05 [25] | >1 | |
| Isopentanoic acid | 0.33 ± 0.02 ab | 0.38 ± 0.01 a | 0.36 ± 0.03 a | 0.27 ± 0.04 c | 0.27 ± 0.06 c | 0.26 ± 0.04 c | 0.31 ± 0.06 bc | 0.27 ± 0.02 c | 3 [29] | <0.1 | Fatty [49] |
| Octanoic acid | 0.92 ± 0.03 e | 1.06 ± 0.05 d | 1.13 ± 0.00 c | 1.16 ± 0.01 bc | 1.17 ± 0.02 bc | 1.19 ± 0.10 bc | 1.21 ± 0.06 b | 1.52 ± 0.03 a | 15 [25] | <0.1 | Sour, Cheese [45] |
| Decanoic acid | 0.13 ± 0.00 g | 0.15 ± 0.00 f | 0.19 ± 0.00 e | 0.22 ± 0.00 c | 0.21 ± 0.00 d | 0.23 ± 0.00 b | 0.19 ± 0.00 e | 0.26 ± 0.01 a | 8 [25] | <0.1 | An unpleasant fatty [45] |
| subtotal | 2.03 ± 0.09 d | 2.21 ± 0.12 bc | 2.31 ± 0.05 b | 2.20 ± 0.05 c | 2.10 ± 0.15 d | 2.05 ± 0.16 d | 2.31 ± 0.13 b | 2.85 ± 0.08 a | |||
| Carbonyl compounds | |||||||||||
| 3-Methyl-butanal | Nd | Nd | Nd | 0.01 ± 0.00 a | 0.01 ± 0.00 a | Nd | 0.01 ± 0.00 b | Nd | |||
| 2,3-Pentanedione | 0.20 ± 0.01 h | 0.33 ± 0.00 b | 0.3 ± 0.00 c | 0.35 ± 0.01 a | 0.24 ± 0.00 g | 0.26 ± 0.00 e | 0.25 ± 0.00 f | 0.28 ± 0.00 d | 2 [35] | 0.1–1 | Pecan [46] |
| Nonanal | 0.04 ± 0.00 c | 0.05 ± 0.00 b | 0.06 ± 0.00 a | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.05 ± 0.00 b | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.015 [32] | >1 | Herb, Slightly spicy [44] |
| Decanal | 0.05 ± 0.00 c | 0.08 ± 0.01 a | 0.06 ± 0.00 b | 0.06 ± 0.00 b | 0.04 ± 0.00 d | 0.06 ± 0.01 b | 0.05 ± 0.00 c | 0.06 ± 0.01 b | 0.001 [30] | >1 | Flower [51] |
| 2,4-Dimethoxybenzaldehyde | 0.36 ± 0.02 e | 0.43 ± 0.03 d | 0.46 ± 0.05 cd | 0.72 ± 0.03 a | 0.53 ± 0.01 b | 0.49 ± 0.05 c | 0.25 ± 0.01 f | 0.43 ± 0.01 d | |||
| subtotal | 0.65 ± 0.03 e | 0.89 ± 0.04 b | 0.87 ± 0.05 bc | 1.18 ± 0.04 a | 0.87 ± 0.02 bc | 0.85 ± 0.06 c | 0.59 ± 0.02 f | 0.81 ± 0.02 d | |||
| total | 221.66 ± 5.17 f | 338.21 ± 6.91 e | 442.40 ± 5.05 c | 461.30 ± 7.80 b | 448.15 ± 8.44 c | 413.65 ± 4.80 d | 406.20 ± 2.96 d | 514.14 ± 5.82 a | |||
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of Non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Zhu, X.L.; Mu, H.; Ma, Y.; Ullah, N.; Tao, Y.S. A novel extracellular glycosidase activity from Rhodotorula mucilaginosa: Its application potential in wine aroma enhancement. Lett. Appl. Microbiol. 2016, 62, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Baffi, M.A.; Tobal, T.; Henrique, J.; Lago, G.; Leite, R.S.R.; Boscolo, M.; Gomes, E.; Da-Silva, R. A Novel β-Glucosidase from Sporidiobolus pararoseus: Characterization and Application in Winemaking. J. Food Sci. 2011, 76, C997–C1002. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Liao, J.; Zheng, Y.; Zhang, W.; Zhang, X. Oenological characteristics of four Non-Saccharomyces yeast strains with β-Glycosidase activity. Front. Microbiol. 2021, 12, 626920. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Yuan, Y.; Dai, L.; Yue, T. Characteristic fruit wine production via reciprocal selection of juice and non-Saccharomyces species. Food Microbiol. 2019, 79, 66–74. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.; Loira, I.; Del Fresno, J.; González, C.; Suárez-Lepe, J. Contribution of Non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011, 46, 385–389. [Google Scholar] [CrossRef]
- Ma, D.; Yan, X.; Wang, Q.; Zhang, Y.; Tao, Y. Performance of selected P. fermentans and its excellular enzyme in co-inoculation with S. cerevisiae for wine aroma enhancement. LWT 2017, 86, 361–370. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuo, X.; Hu, L.; Zhang, X. Effects of Crude β-Glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the Flavor Complexity and Characteristics of Wines. Microorganisms 2020, 8, 953. [Google Scholar] [CrossRef]
- Shi, W.; Wang, J.; Chen, F.; Zhang, X. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT 2019, 116, 108477. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. Nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, R.R.; Da Conceição, P.J.P.; De Menezes, C.L.A.; De Oliveira Nascimento, C.E.; Machado Bertelli, M.; Pessoa Júnior, A.; De Souza, G.M.; Da Silva, R.; Gomes, E. Biochemical characteristics and potential application of a novel ethanol and glucose-tolerant β-glucosidase secreted by Pichia guilliermondii G1.2. J. Biotechnol. 2019, 294, 73–80. [Google Scholar] [CrossRef]
- Sarry, J.; Gunata, Z. Plant and microbial glycoside hydrolases: Volatile release from glycosidic aroma precursors. Food Chem. 2004, 87, 509–521. [Google Scholar] [CrossRef]
- Belancic, A.; Gunata, Z.; Vallier, M.; Agosin, E. β-Glucosidase from the Grape Native Yeast Debaryomyces vanrijiae: Purification, Characterization, and its Effect on Monoterpene Content of a Muscat Grape Juice. J. Agric. Food Chem. 2003, 51, 1453–1459. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, J.; Xu, Y. Different influences of β-glucosidases on volatile compounds and anthocyanins of Cabernet Gernischt and possible reason. Food Chem. 2013, 140, 245–254. [Google Scholar] [CrossRef]
- Sabel, A.; Martens, S.; Petri, A.; König, H.; Claus, H. Wickerhamomyces anomalus AS1: A new strain with potential to improve wine aroma. Ann. Microbiol. 2014, 64, 483–491. [Google Scholar] [CrossRef]
- Mesas, J.M.; Rodríguez, M.C.; Alegre, M.T. Basic characterization and partial purification of β-glucosidase from cell-free extracts of Oenococcus oeni ST81. Lett. Appl. Microbiol. 2012, 55, 247–255. [Google Scholar] [CrossRef]
- Garbin, A.P.; Garcia, N.F.L.; Cavalheiro, G.F.; Silvestre, M.A.; Rodrigues, A.; Paz, M.F.D.; Fonseca, G.G.; Leite, R.S.R. β-glucosidase from thermophilic fungus Thermoascus crustaceus: Production and industrial potential. An. Acad. Bras. Cienc. 2021, 93. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Yu, Z.; Liu, X.; Hardie, W.J.; Huang, M. Screening and characterisation of β-glucosidase production strains from Rosa roxburghii Tratt. Int. J. Food Eng. 2021, 17, 1–9. [Google Scholar] [CrossRef]
- Vernocchi, P.; Ndagijimana, M.; Serrazanetti, D.I.; López, C.C.; Fabiani, A.; Gardini, F.; Elisabetta Guerzoni, M.; Lanciotti, R. Use of Saccharomyces cerevisiae strains endowed with β-glucosidase activity for the production of Sangiovese wine. World J. Microbiol. Biotechnol. 2011, 27, 1423–1433. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Effects of acetic acid, ethanol, and SO2 on the removal of volatile acidity from acidic wines by two Saccharomyces cerevisiae commercial strains. Appl. Microbiol. Biot. 2010, 87, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.E.; Lopes, C.A.; Van Broock, M.; Valles, S.; Ramón, D.; Caballero, A.C. Screening and typing of Patagonian wine yeasts for glycosidase activities. J. Appl. Microbiol. 2004, 96, 84–95. [Google Scholar] [CrossRef]
- Manzanares, P.; Rojas, V.; Genovés, S.; Vallés, S. A preliminary search for anthocyanin-β-D-glucosidase activity in non-Saccharomyces wine yeasts. Int. J. Food Sci. Technol. 2000, 35, 95–103. [Google Scholar] [CrossRef]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and Formation of Varietal Aroma Compounds during Alcoholic Fermentation from Nonfloral Grape Odorless Flavor Precursors Fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef]
- Park, S.K.; Morrison, J.C.; Adams, D.O.; Noble, A.C. Distribution of free and glycosidically bound monoterpenes in the skin and mesocarp of Muscat of Alexandria grapes during development. J. Agric. Food Chem. 1991, 39, 514–518. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biot. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
- Zietsman, A.J.J.; De Klerk, D.; Van Rensburg, P. Coexpression of α-l-arabinofuranosidase and β-glucosidase in Saccharomyces cerevisiae. FEMS Yeast Res. 2011, 11, 88–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, C.; Li, A.; Jin, G.; Zhu, X.; Tao, Y. Evolution of volatile compounds treated with selected non-Saccharomyces extracellular extract during Pinot noir winemaking in monsoon climate. Food Res. Int. 2019, 119, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Hu, K.; Zhang, J.; Zhu, X.; Tao, Y. Aroma modulation of Cabernet Gernischt dry red wine by optimal enzyme treatment strategy in winemaking. Food Chem. 2018, 245, 1248–1256. [Google Scholar] [CrossRef]
- Thongekkaew, J.; Fujii, T.; Masaki, K.; Koyama, K. Evaluation of Candida easanensis JK8 beta-glucosidase with potentially hydrolyse non-volatile glycosides of wine aroma precursors. Nat. Prod. Res. 2019, 33, 3563–3567. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Ullah, N.; Tao, Y. Aroma glycosides in grapes and wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Noguerol-Pato, R.; González-álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Evolution of the aromatic profile in Garnacha Tintorera grapes during raisining and comparison with that of the naturally sweet wine obtained. Food Chem. 2013, 139, 1052–1061. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Santos, S.A.O.; Silvestre, A.J.D.; Barros, A.S.; Ferreira, A.C.S.; Silva, A.M.S. Quinones as Strecker degradation reagents in wine oxidation processes. Food Chem. 2017, 228, 618–624. [Google Scholar] [CrossRef]
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Higher alcohols concentration and its relation with the biological aging evolution. Eur. Food Res. Technol. 2006, 222, 629–635. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Clímaco, M.C.; Mendes Faia, A. The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components-a preliminary study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef] [Green Version]
- López-Rituerto, E.; Avenoza, A.; Busto, J.H.; Peregrina, J.M. Evidence of Metabolic Transformations of Amino Acids into Higher Alcohols through 13C NMR Studies of Wine Alcoholic Fermentation. J. Agric. Food Chem. 2010, 58, 4923–4927. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Swangkeaw, J.; Vichitphan, S.; Butzke, C.E.; Vichitphan, K. Characterization of β-glucosidases from Hanseniaspora sp. and Pichia anomala with potentially aroma-enhancing capabilities in juice and wine. World J. Microbiol. Biotechnol. 2011, 27, 423–430. [Google Scholar] [CrossRef]
- Rensburg, P.; Stidwell, T.; Lambrechts, M.; Cordero-Otero, R.; Pretorius, I. Development and assessment of a recombinant Saccharomyces cerevisiae wine yeast producing two aroma-enhancing β-glucosidase encoded by the Saccharomycopsis fibuligera BGL1 and BGL2 genes. Ann. Microbiol. 2005, 55, 33–42. [Google Scholar]
- Cheynier, V.; Sarni-Manchado, P. 2—Wine taste and mouthfeel. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 29–72. [Google Scholar]
- Chidi, B.S.; Bauer, F.; Rossouw, D. Organic acid metabolism and the impact of fermentation practices on wine acidity: A review. S. Afr. J. Enol. Vitic. 2018, 39, 1–15. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Liang, N.; Mu, L.; Pan, Q.; Wang, J.; Reeves, M.J.; Duan, C. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Wen, Y.; Tao, Y.; Lan, Y. Modulating the formation of Meili wine aroma by prefermentative freezing process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, K.; Yang, X.; Li, J.; Li, X. Comparative study of the key aromatic compounds of Cabernet Sauvignon wine from the Xinjiang region of China. J. Food Sci. Technol. 2021, 58, 2109–2120. [Google Scholar] [CrossRef]
- Tang, K.; Tian, X.; Ma, Y.; Sun, Y.; Qi, X.; Miu, C.; Xu, Y. Aroma characteristics of Cabernet Sauvignon wines from Loess Plateau in China by QDA®, Napping® and GC–O analysis. Eur. Food Res. Technol. 2020, 246, 821–832. [Google Scholar] [CrossRef]
- Villamor, R.R.; Ross, C.F. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef]
- Xie, S.; Tang, Y.; Wang, P.; Song, C.; Duan, B.; Zhang, Z.; Meng, J. Influence of natural variation in berry size on the volatile profiles of Vitis vinifera L. Cv. Merlot and Cabernet Gernischt grapes. PLoS ONE 2018, 13, e201374. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.; Wang, Y.; Lu, L.; Lan, Y.; Reeves, M.J.; Duan, C. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, Y.; Li, H. Sensory characters of Cabernet Sauvignon dry red wine from Changli County (China). Food Chem. 2009, 114, 565–569. [Google Scholar] [CrossRef]
- Celik, Z.D.; Cabaroglu, T.; Krieger-Weber, S. Impact of malolactic fermentation on the volatile composition of Turkish Kalecik karası red wines. J. Inst. Brew. 2019, 125, 92–99. [Google Scholar] [CrossRef]
- Soares, R.D.; Welke, J.E.; Nicolli, K.P.; Zanus, M.; Caramão, E.B.; Manfroi, V.; Zini, C.A. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chem. 2015, 183, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, G.; Aihaiti, A. Combined indigenous yeast strains produced local wine from over ripen Cabernet Sauvignon grape in Xinjiang. World J. Microbiol. Biotechnol. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]

| Wines | Add the Crude Extracts of β-Glucosidase From | ||
|---|---|---|---|
| SLY-4E | HX-13E | F2-24E | |
| SCw | |||
| S-Ew | yes | ||
| H-Ew | yes | ||
| F-Ew | yes | ||
| S/H-Ew | yes | yes | |
| S/F-Ew | yes | yes | |
| H/F-Ew | yes | yes | |
| S/H/F-Ew | yes | yes | yes |
| Wines | Alcohol (%, v/v) | Total Acids (g/L) | Volatile Acids (g/L) | Anthocyanin (mg/L) |
|---|---|---|---|---|
| SCw | 12.56 ± 0.53 a | 5.81 ± 0.22 a | 0.68 ± 0.00 a | 187.79 ± 1.25 a |
| S-Ew | 12.07 ± 0.14 b | 5.81 ± 0.22 a | 0.61 ± 0.01 c | 182.83 ± 0.42 c |
| H-Ew | 12.13 ± 0.17 ab | 5.53 ± 0.19 ab | 0.68 ± 0.04 a | 163.30 ± 0.84 f |
| F-Ew | 12.21 ± 0.13 ab | 5.20 ± 0.32 b | 0.49 ± 0.02 d | 176.08 ± 0.84 d |
| S/H-Ew | 12.26 ± 0.23 ab | 5.39 ± 0.28 ab | 0.61 ± 0.04 c | 173.24 ± 0.05 e |
| S/F-Ew | 12.51 ± 0.11 ab | 5.44 ± 0.22 ab | 0.65 ± 0.07 b | 184.13 ± 2.93 bc |
| H/F-Ew | 12.25 ± 0.23 ab | 5.20 ± 0.49 b | 0.49 ± 0.01 d | 172.17 ± 3.76 e |
| S/H/F-Ew | 12.33 ± 0.09 ab | 5.72 ± 0.19 a | 0.61 ± 0.03 c | 184.96 ± 2.09 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Zhang, S.; Zhang, X. Effects of Mixed Adding Crude Extracts of β-Glucosidases from Three Different Non-Saccharomyces Yeast Strains on the Quality of Cabernet Sauvignon Wines. J. Fungi 2022, 8, 710. https://doi.org/10.3390/jof8070710
Liao J, Zhang S, Zhang X. Effects of Mixed Adding Crude Extracts of β-Glucosidases from Three Different Non-Saccharomyces Yeast Strains on the Quality of Cabernet Sauvignon Wines. Journal of Fungi. 2022; 8(7):710. https://doi.org/10.3390/jof8070710
Chicago/Turabian StyleLiao, Jing, Shuangmei Zhang, and Xiuyan Zhang. 2022. "Effects of Mixed Adding Crude Extracts of β-Glucosidases from Three Different Non-Saccharomyces Yeast Strains on the Quality of Cabernet Sauvignon Wines" Journal of Fungi 8, no. 7: 710. https://doi.org/10.3390/jof8070710
APA StyleLiao, J., Zhang, S., & Zhang, X. (2022). Effects of Mixed Adding Crude Extracts of β-Glucosidases from Three Different Non-Saccharomyces Yeast Strains on the Quality of Cabernet Sauvignon Wines. Journal of Fungi, 8(7), 710. https://doi.org/10.3390/jof8070710






