Abstract
During surveys of insect pathogenic fungi (IPF) in Thailand, fungi associated with scale insects and plants were found to represent five new species of the genus Ascopolyporus in Cordycipitaceae. Their macroscopic features resembled both Hyperdermium and Ascopolyporus. Morphological comparisons with the type and known Ascopolyporus and Hyperdermium species and phylogenetic evidence from a multigene dataset support the appointment of a new species of Ascopolyporus. Moreover, the data also revealed that the type species of Hyperdermium, H. caulium, is nested within Ascopolyporus, suggesting that Hyperdermium is congeneric with Ascopolyporus. The specimens investigated here differ from other Ascopolyporus species by phenotypic characters including size and color of stromata. Phylogenetic analyses of combined LSU, TEF1, RPB1 and RPB2 sequences strongly support the notion that these strains are distinct from known species of Ascopolyporus, and are proposed as Ascopolyporus albus, A. galloides, A. griseoperitheciatus, A. khaoyaiensis and A. purpuratus. Neohyperdermium gen. nov. is introduced for other species originally assigned to Hyperdermium and Cordyceps occurring on scale insects and host plants as epiphytes, accommodating two new combinations of Hyperdermium pulvinatum and Cordyceps piperis.
1. Introduction
Scale insects are a diverse group of sap-sucking insects in the superfamily Coccoidea of the order Hemiptera, associated with aphids (Aphidoidea) and whiteflies (Aleyrodoidea) [1,2]. These insects cause damage by sucking fluids from leaves, stems and other parts of host plants and excrete honeydew that favors sooty mold growth, which consequently decreases photosynthetic rates. They belong to seven families: Antennulariellaceae, Capnodiaceae, Chaetothyriaceae, Coccodiniaceae, Euantennariaceae, Metacapnodiaceae and Trichomeriaceae [3,4,5]. In addition, many groups of fungi are known to grow on various scale insects by covering the whole surface of the insect body and can be found in the phyla (a) Basidiomycota: Septobasidiales (Septobasidium and Uredinella), (b) Chytridiomycota: Blastocladiales (Myiophagus) and (c) Ascomycota: Myriangiales (Myriangium), Pleosporales (Podonectria), and especially in a large group of entomopathogens in the Hypocreales [6,7,8,9].
Hypocrealean fungi associated with armored (Diaspididae) and soft-scale insects (Coccidae) can be found in various genera within five families: (1) Bionectriaceae viz. Clonostachys Corda; (2) Nectriaceae viz. Microcera Desm. and Fusarium Link; (3) Cordycipitaceae viz. Ascopolyporus Möller, Cordyceps Fr. and Hyperdermium J.F. White, R.F. Sullivan, Bills and Hywel-Jones; (4) Ophiocordycipitaceae viz. Ophiocordyceps Petch; and (5) Clavicipitaceae viz. Aschersonia Mont., Conoideocrella D. Johnson, G.H. Sung, Hywel-Jones and Spatafora, Dussiella Pat., Helicocollum Luangsa-ard, Mongkols., Noisrip. and Thanakitp., Hypocrella Sacc., Regiocrella P. Chaverri and K.T. Hodge, Orbiocrella D. Johnson, G.H. Sung, Hywel-Jones and Spatafora and Samuelsia P. Chaverri and K.T. Hodge [9,10,11,12,13,14,15,16,17,18,19,20,21]. Among them, the most abundant and widespread members are found in Clavicipitaceae and Cordycipitaceae. The macromorphological characters of these genera in nature can be easily distinguished in each family. Scale insect pathogenic genera in Clavicipitaceae, such as Conoideocrella, Hypocrella, Moelleriella and Orbiocrella, possess diverse morphological characters, such as the formation of hard stromata, pulvinate, subglobose or hemispherical and ring-like stromata, as well as the presence of only superficial, cone-shaped perithecia, while in Cordycipitaceae, most have pulvinate, subglobose, hemispherical, soft stromata with crowded perithecia. Two different colors are found the upper and lower surface of stromata in some species of Ascopolyporus and Hyperdermium [11,12,14,16].
Ascopolyporus is an epiphytic fungal genus in Cordycipitaceae that produces stromata on the stems of living plants as biotrophs and infects scale insects as necrotrophs comprising only seven species [22]. Ascopolyporus species are commonly found in tropical forests where bamboo is present [23]. The type species of Ascopolyporus, A. polychrous, is a pathogen of bamboo scale insects that produces up to 4 cm large subglobose to polypore-like, bright rusty-red or white to yellow perithecial stromata, which are usually fertile only on the underside of the stroma [12,13,24]. In 2005, a new species of Ascopolyporus, A. philodendrus, was described by Bischoff et al. [14] on bamboo scale insects, and a new description for A. villosus was made. They considered that the morphology of perithecial stromata and the conidial states of Ascopolyporus resemble the scale insect pathogenic genus Hyperdermium, especially its type species, H. caulium [11,14]. Both of these species in the two genera share similar morphological characters, having large stromata, immersed perithecia, filiform ascospores and phialidic conidiogenous cells. The anamorph state is referred to as cylindrocarpon-like phialides, characterized by producing multiseptate conidia, a unique character in the Cordycipitaceae. Moreover, a species of Cordyceps, C. piperis, is also capable of parasitizing scale insects but differ by producing verticillium-like anamorph with aseptate conidia [11,12].
During our continuous survey of insect pathogenic fungi (IPF) in national parks and community forests in Thailand, we encountered hyperdermium-like specimens with differences in phenotypic characters including colors and sizes of stromata. These morphologically diverse specimens were preliminarily identified as members of the genus Hyperdermium and Ascopolyporus. The aims of this study are thus (1) to determine the phylogenetic relationship of these two genera and (2) to identify and describe new species of hyperdermium-like fungi on scale insects from Thailand by combining morphological characteristics and reconstructing their phylogeny based on sequence data of LSU, TEF1, RPB1 and RPB2 loci.
2. Materials and Methods
2.1. Collection and Isolation
The 63 epiphytic isolates in this study were collected from various localities in Thailand since June 1992, representing the first recorded collection from Khao Yai National Park, Nakhon Ratchasima Province. Thereafter, these specimens have been found throughout every region in Thailand, albeit not frequently, including the Ban Hua Thung community forest in Chiang Mai Province; Chiang Dao, Khao Soi Dao and Khlong Nakha wildlife sanctuaries; Kaeng Krachan and Khlong Lan national parks; and the Khao Chong wildlife development and conservation promotion station. Specimens were examined for fungal colonization from the stems and leaves of monocotyledonous and dicotyledonous plants. The specimens were collected and stored in plastic boxes before returning to the laboratory for isolation. Pure cultures were made from the isolation of the sexual morph following Luangsa-ard et al. [25]. The cultures and the voucher specimens were deposited in Thailand Bioresource Research Center (TBRC) and BIOTEC Bangkok Herbarium (BBH), Thailand, respectively.
2.2. Morphological Study
For obtaining morphological descriptions, all isolates were cultured on oatmeal agar (OA: oatmeal 60 g, agar 12.5 g, in 1 L distilled water, Difco) and potato dextrose agar (PDA: potato 200 g, dextrose 20 g, agar 15 g, in 1 L distilled water) for 14–20 days. Colony morphology was examined for color, size, shape and appearance. Fungal structures of teleomorph and anamorph states were mounted in lactophenol cotton blue solution, and their characters were investigated by light microscopy, as described by Mongkolsamrit et al. [26] and Khonsanit et al. [27]. Sections of the stroma on stems were prepared by using a freezing microtome (Slee Cryostat MEV, Mainz, Germany), and mounted in distilled water and in lactophenol cotton blue solution [28]. The Sixth Royal Horticultural Society (RHS) color chart was used to characterize the colors of fresh specimens and cultures [29]. Twenty to fifty individual length and width measurements were taken, and the amount of variability is provided as average ± standard deviation with absolute minima and maxima in parentheses.
2.3. DNA Extraction, PCR and Sequencing
The mycelial mass of fungi was obtained from cultures grown on PDA for 7 days at 25 °C. A modified CTAB protocol used for DNA extraction using polyvinylpyrrolidone instead of β-mercaptoethanol in CTAB buffer and increasing temperature in the incubation process from 60 °C to 65 °C was previously described by Thanakitpipattana et al. [30]. PCR was used to amplify the nuclear ribosomal large subunits (LSU), the region of the elongation factor 1-α (TEF1), and the largest and second-largest subunits of RNA polymerase II (RPB1 and RPB2). The reaction mix was prepared in 25 μL volumes containing 1× Dream Taq Buffer (with included 20 mM MgCl2), 0.4 M betaine, 200 μM dNTP mix, 0.5 μM of each primer, 1 Unit Dream Taq DNA polymerase (Thermo Scientific, Waltham, MA, USA), 50 ng of DNA template and Milli-Q water. PCR amplifications of four loci were carried out with the following primers: LROR and LR5 for LSU [31,32], 983F and 2218R for TEF1 [33], CRPB1 and RPB1-Cr for RPB1 [34], and RPB2-5F2 and RPB2-7Cr for RPB2 [35,36]. The PCR conditions were performed as follows: 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at a suitable temperature for 1 min, extension at 72 °C for 1 min and a final extension of 72 °C for 10 min. The annealing temperature of each gene was 50 °C for RPB1 and RPB2, and 55 °C for TEF1 and LSU. PCR products were purified and subsequently sequenced with PCR amplification primers.
2.4. Sequence Alignment and Phylogenetic Analyses
The newly generated sequences from the twelve strains in this study were assembled using BioEdit v. 7.2.5 [37] and then deposited in the GenBank database under the accession numbers of TEF1 (OL322029-OL322040), LSU (OL322041-OL322052), RPB1 (OL322053-OL322059) and RPB2 (OL322060-OL322070) (Table 1). Sequences of each locus were aligned using MUSCLE 3.6 [38] together with other sequences of related taxa from previous studies for phylogenetic analyses (see Table 1), and manually refined to minimize gaps. The concatenated (LSU + TEF1 + RPB1 + RPB2) sequences were analyzed by maximum likelihood (ML) and Bayesian inference (BI), both on the CIPRES Science Gateway portal [39]. Maximum likelihood analysis was performed with RAxML-HPC2 on XSEDE v.8.2.12 with default parameters [40] using the GTRCAT substitution model with 1000 rapid bootstrap replicates. The program MrModeltest v.2.2 [41] was used to determine the model of evolution under the Akaike Information Criterion (AIC) implemented in PAUP v.4.0a169 [42], which selected SYM + G as the best nucleotide substitution model. The BI analysis was performed using MrBayes on XSEDE v.3.2.7a with default parameters [43]. The Markov Chain Monte Carlo (MCMC) searches were run for 5,000,000 generations with sampling every 1000 generations and a burn-in value of 10%. Nodes were considered as strongly supported with bootstrap and posterior probability values greater than 70% and 0.7, respectively.

Table 1.
List of species and GenBank accession numbers of sequences used in this study. Bold accession numbers were generated for this study. The symbol “–” denotes no available data.
3. Results
3.1. Molecular Phylogeny
The combined four-gene dataset of 54 taxa consisted of 3404 bp (LSU 861 bp, TEF1 954 bp, RPB1 730 bp, RPB2 859 bp). Flavocillium bifurcatum and Flavocillium sp. in Cordycipitaceae were used as an outgroups. Phylogenetic tree topology obtained from ML was similar to the BI analysis. Therefore, only the ML tree is shown (Figure 1). Multigene phylogenetic analyses revealed that the sequenced strains comprise five novel species and are nested with the type and other species of Ascopolyporus, A. polychrous and A. villosus, as well as type species of Hyperdermium, H. caulium, within the Ascopolyporus clade, with strong support (81% ML bootstrap (MLBS) and 0.99 BI posterior probability (BIPP)), as shown in Figure 1. The type species H. caulium is clustered within this clade, suggesting that Hyperdermium is congeneric with Ascopolyporus, although with low internal bootstrap support because only LSU sequence data are available (<50 MLBS and <0.5 BIPP, data not shown).
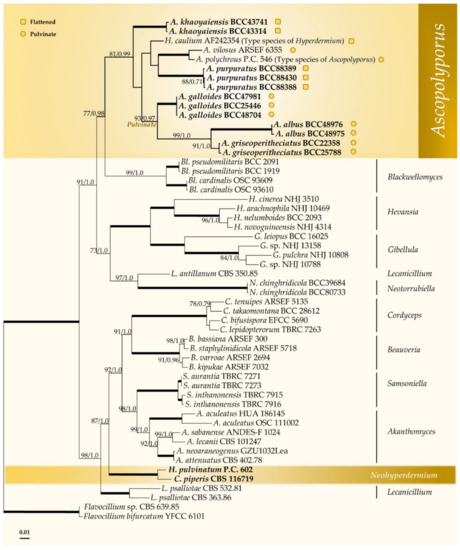
Figure 1.
Phylogenetic reconstruction of Ascopolyporus and related genera in the Cordycipitaceae obtained from the combined LSU, TEF1, RPB1 and RPB2 sequence dataset based on maximum likelihood (RAxML) and Bayesian inference. Numbers on the nodes are ML bootstrap and Bayesian posterior probability values above 70% (MLBS) or 0.7 (BIPP). Thickened lines mean support for the two analyses was 100% (MLBS) or 1.0 (BIPP).  represents species with pulvinate stromata while
represents species with pulvinate stromata while  represents species with flattened stromata.
represents species with flattened stromata.
 represents species with pulvinate stromata while
represents species with pulvinate stromata while  represents species with flattened stromata.
represents species with flattened stromata.
Three of our new species are found in the pulvinate subclade showing irregularly subglobose to globose stromata, namely, Ascopolyporus albus, A. galloides and A. griseoperitheciatus, with 93% MLBS and 0.97 BIPP. Another subclade comprises both flattened and pulvinate stromata of two new and known species, including Ascopolyporus khaoyaiensis, A. purpuratus, A. polychrous, A. villosus and H. caulium (Figure 1). The Ascopolyporus clade is sister to the Blackwellomyces clade, which produces similar types of phialides and conidial arrangement as well as acremonium-like or lecanicillium-like anamorphs.
The position of Hyperdermium pulvinatum and Cordyceps piperis, on the other hand, is clearly distant from the Ascopolyporus clade, and these two species always clustered together separate from the type species of Hyperdermium, H. caulium. These two species form a basal clade to Akanthomyces, Samsoniella, Beauveria and Cordyceps. Based on their multigene phylogenetic position presented in this study, we propose to transfer these two species to the genus Neohyperdermium.
3.2. Taxonomy
Ascopolyporus Möller emend. Thanakitpipattana and Luangsa-ard.
Stromatal mass exceeding scale insect host. Stroma bulbous (lumpy or tuberous) or ungulate, flattened or pulvinate, fleshy or gall-like, polypore-like, white, yellowish white, purple to orange; sterile surface and fertile underneath the stroma. Perithecia semi-immersed to immersed, ovate to obclavate or cone-shaped. Asci hyaline, filiform. Ascospores hyaline, whole with septation or aseptate. Conidiogenous cells phialidic, solitary, slightly curved. Conidia hyaline, fusiform to subcylindrical, acerose, aseptate or 1–5 septate when mature, in chains or in sticky heads.
Typification: Ascopolyporus polychrous.
Habit and type host: On dead culms of bamboo, stems or leaf midrib of monocotyledonous and dicotyledonous plants.
Distribution: Argentina, Bolivia, Brazil, Colombia, Costa Rica, Ecuador, Peru, Thailand [23].
Ascopolyporus albus Mongkolsamrit, Thanakitpipattana and Luangsa-ard sp. nov. Figure 2.

Figure 2.
Ascopolyporus albus. (a) Stromata on living stem of bamboo (Bambusae); (b) cross-section through stroma showing perithecia (BBH30734); (c) perithecia; (d,e) asci. (f) ascospores; (g) colony obverse on OA; (h) colony reverse on OA; (i,j) phialide apex with conidial head on OA; (k) conidia on OA; (l) colony obverse on PDA; (m) colony reverse on PDA; (n,o) phialide and conidia on PDA; (p) conidia on PDA. Scale bars: (g,h,l,m) = 10 mm; (a) = 5 mm; (b) = 200 μm; (c) = 100 μm; (d,f,j,n,o) = 20 μm; (e,k,p) = 10 μm.
MycoBank: MB 841855.
Etymology: From the Latin “albus”, referring to the white color of the fresh stromata.
Typification: Thailand, Chiang Mai Province, Chiang Dao Wildlife Sanctuary, Doi Chiang Dao Wildlife Research Station; 19°23′10.70″ N, 98°50′28.50″ E, on scale insects (Coccidae; Hemiptera), on living stem of bamboo (Bambusae), 17 August 2011, K. Tasanathai (K.T.), P. Srikitikulchai (P.S.), S. Mongkolsamrit (S.M.), A. Khonsanit (A.K.) (holotype BBH30734, ex-holotype culture BCC48975). GenBank: ITS = OL331502, LSU = OL322048, TEF1 = OL322035, RPB1 = OL322056, RPB2 = OL322065.
Description: Stromata epibiotic, pulvinate, subglobose, globose, white (NN155C) to pinkish white (N155B), becoming dark brown when old, 3–6 mm wide, 2–3 mm thick. Perithecia semi-immersed, with slightly protruding orifices, obpyriform, 250–320 × 100–120 µm. Asci cylindrical up to 250 µm long and 3–4 µm wide, Asci caps 2–3 × 3–4 μm. Ascospores hyaline, whole, filiform, multiseptate, 95–135 × 1 µm.
Culture characteristics: Colonies on OA attaining a diameter of 3.5–4 cm in 14 days, slightly convex to the agar surface, white, reverse moderate orange yellow (164C). Phialides arising from aerial hyphae, solitary, cylindrical, slightly curved, up to 80 µm long, 1–2 µm wide. Conidia hyaline, enteroblastic, fusiform to acerose, early in development aseptate, becoming 1–4 septa, aggregated at the apex of the phialides, (8–)10–23(–28) × (2–)2.5–3 µm. Colonies on PDA attaining a diameter of 3.5–4 cm in 14 days, slightly convex to the agar surface, white, reverse light yellow (162C). Phialides arising from aerial hyphae, solitary, cylindrical, slightly curved, up to 120 µm long, 1–2 µm wide. Conidia hyaline, enteroblastic, fusiform to acerose, early in development aseptate, developing 1–4 septa, aggregated at the apex of the phialides, (8–)10.5–21(–30) × (2–)2.5–3(–3.5) µm.
Habitat: On scale insects (Coccidae; Hemiptera), found on living stems of bamboo (Bambusae).
Additional specimen examined: Thailand, Chiang Mai Province, Chiang Dao Wildlife Sanctuary, Doi Chiang Dao Wildlife Research Station; 19°23′10.70″ N, 98°50′28.50″ E, on scale insects (Coccidae; Hemiptera), on the living stems, 17 August 2011, K.T., P.S., S.M., A.K. (BBH30734, BCC48976). GenBank: ITS = OL331503, LSU = OL322049, TEF1 = OL322036, RPB1 = OL322057, RPB2 = OL322066.
Notes: Ascopolyporus albus significantly differs from other species in Ascopolyporus herein. The difference is in the color of stromata. Ascopolyporus albus produces white to pinkish white stromata (Figure 2), whereas other species produce very pale violet (91D) to yellowish white (158) with strong orange (25A) stromata. Based on Ascopolyporus species from Thailand, the perithecia of A. albus are semi-immersed, similar to those in A. galloides, A. griseoperitheciatus and A. purpuratus. The perithecial shape of A. albus differs from A. galloides, A. griseoperitheciatus and A. purpuratus by having an obpyriform shape, whereas perithecia in A. galloides, A. griseoperitheciatus and A. purpuratus are obclavate, obovoid and ovoid, respectively.
Ascopolyporus caulium (Berk. and M.A. Curtis) Thanakitp. and Luangsa-ard, comb. nov.
MycoBank: MB 842779.
≡ Corticium caulium Berk. and M.A. Curtis, J. Acad. nat. Sci. Philad. 2: 279. 1854.
≡ Hypocrella caulium (Berk. and M.A. Curtis) Pat., Bull. Soc. Mycol. France 30: 346. 1915.
≡ Hyperdermium caulium (Berk. and M.A. Curtis) P. Chaverri and K.T. Hodge, 2008.
= Hypocrella camerunensis Henn., Engler’s Bot. Jahrb. 23: 540. 1897.
= Hypocrella brasiliana (Henn.) Mains, Mycopath. Myc. Appl. 11: 311. 1959.
≡ Stigmatea brasiliana Henn., Hedwigia 36: 230. 1897.
≡ Hypocrella camerunensis var. brasiliana Henn., Hedwigia 43: 85. 1904.
= Hyperdermium bertonii J.F. White, R.F. Sullivan, Bills and Hywel-Jones, Mycologia 92: 910. 2000.
≡ Epichloë bertonii Speg., An. Mus. Nac. Hist. Nat. Buenos Aires 31: 416. 1922.
Ascopolyporus galloides Khonsanit, Thanakitpipattana and Luangsa-ard sp. nov. Figure 3.

Figure 3.
Ascopolyporus galloides. (a,b) Stromata on living stem of dicotyledonous plant (BBH48704); (c) cross-section through stroma showing perithecia; (d) perithecia; (e) asci; (f) asci-caps; (g) ascospores; (h) colony obverse on OA; (i) colony reverse on OA; (j–l) phialide apex with conidial head on OA; (m) conidia on OA; (n) colony obverse on PDA; (o) colony reverse on PDA; (p–r) phialide apex with conidial head on PDA; (s) conidia on PDA. Scale bars: (h,i,n,o) = 10 mm; (a) = 5 mm; (b) = 1 mm; (c) = 200 μm; (d,g) = 100 μm; (j,k,l,q,r) = 20 μm; (e,f,m,p,s) = 10 μm.
MycoBank: MB 841853.
Etymology: Refers to the character of the stromata, which look similar to plant galls.
Typification: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, Mo Singto Nature Trail; 14°26′21.46″ N, 101°22′20.20 ″E, on scale insects (Coccidae; Hemiptera), on the living stems of dicotyledonous plant, 5 July 2011, A.K., K.T., K. Sansatchanon (K.S.), P.S., S.M., W. Noisripoom (W.N.) (holotype BBH30629, ex-holotype culture BCC48704). GenBank: ITS = OL331509, LSU = OL322044, TEF1 = OL322031, RPB1 = OL322055, RPB2 = OL322062.
Description: Stromata epibiotic, pulvinate, subglobose, hemispherical, upper surface white (NN155B) to yellowish white (158); lower surface strong orange (N25C), 1–7 mm wide. Perithecia semi-immersed, crowded, obclavate, 170–340 × 60–110 μm. Asci cylindrical, (129–)133–153.5(–175) × (3–)3.5–5(–6) μm. Asci caps 1–2 × 2.5–4 μm. Ascospores hyaline, whole, filiform, aseptate, (131–) 154.5–211.5(–216) × 0.5 μm.
Culture characteristics: Colony on OA attaining a diameter of 3.5–4 cm in 20 days, flat, slightly convex to the agar surface, white (158), reverse pale greenish yellow (2D). Phialides arising from aerial hyphae, solitary, cylindrical, slightly curved, 35–161 × 1–2 μm. Conidia hyaline, enteroblastic, fusiform to acerose, early in development aseptate, becoming 1–3 septa, aggregated at the apex of the phialides, (4–)6–22(–34) × (1.5–)2–2.5(–3) µm.
Colony on PDA attaining a diameter of 4 cm in 20 days, fluffy in the middle, flat to umbonate, white in the middle, pale orange yellow (23D), light yellow (11B), brilliant yellow (11A), reverse moderate orange (173C) in the middle, strong orange yellow (N163D), light yellow (14D). Phialides arising from aerial hyphae, solitary, cylindrical, slightly curved, 30–294 × 1–2 μm. Conidia hyaline, enteroblastic, fusiform to acerose, cylindrical, early in development aseptate, becoming 1–4 septa, aggregated at the apex of the phialides, (5–)8–16(–27) × (2–)2.5–3.5(–4) µm.
Habitat: On scale insects (Coccidae, Hemiptera), found on living stems of dicotyledonous plant.
Additional specimens examined: Thailand, Ranong Province, Khlong Nakha Wildlife Sanctuary, Khlong Nakha Nature Trail; 9°27′33″ N, 98°30′16″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 5 October 2004, B. Thongnuch (B.T.), D. Johnson (D.J.), K.T., S.M., W. Chaygate (W.C.) (BBH10163, BCC16408; BBH10176, BCC16419; BBH10177, BCC16420; BBH10178, BCC16421; BBH10179, BCC16422; BBH10180, BCC16423); Nakhon Nayok Province, Khao Yai National Park, Tat Ta Phu Waterfall Nature Trail; 14°26′21.46” N, 101°22′20.20” E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 24 August 2005, K.T. (BBH14835, BCC18980); Phetchaburi Province, Kaeng Krachan National Park, Ban Krang Camp Nature Trail; 12°54′05″ N, 99°37′48″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 14 November 2005, B.T., K.T., R. Ridkaew (R.R.), W.C. (BBH15034, BCC19720); Ranong Province, Khlong Nakha Wildlife Sanctuary, Khlong Nakha Nature Trail; 9°27′33″ N, 98°30′16″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 10 January 2006, B.T., K.T., L.N. Yen (L.N.Y.), L.T. Huyen (L.T.H.), PS, SM, WC (BBH16500, BCC20115); 12 January 2006, B.T., K.T., L.N.Y., L.T.H., P.S., S.M., W.C. (BBH16554, BCC20123); Nakhon Ratchasima Province, Khao Yai National Park, Bueng Phai Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 5 July 2006, B.T., J. Luangsa-ard (J.J.L.), K.T., P.S., S.M., W.C. (BBH18631, BCC22237; BBH18632, BCC22238); Chanthaburi Province, Khao Soi Dao Wildlife Sanctuary, Withiphrai Nature Trail; 13°06′13″ N, 102°11′39″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 1 May 2007, B.T., K.T., R.R., S.M., W.C. (BBH19873, BCC25446; BCC25447, BCC25448); Nakhon Ratchasima Province, Khao Yai National Park, km. 33 Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 8 August 2007, B.T., P. Puyngain (P.P.), W.C. (BBH22627, BCC26680), Trang Province, Khao Chong Wildlife Development and Conservation Promotion Station, 1.8 km. Nature Trail; 7°32′57″ N, 99°47′11″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 18 September 2007, B.T., K.T. (BBH23089, BCC27812); Nakhon Ratchasima Province, Khao Yai National Park, Mo Singto Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 18 June 2009, K.T., P.S., R.R., S.M., T. Chohmee (T.C.) (BBH30139, BCC36656); 20 July 2009, K.T., P.S., R.R., S.M., T.C. (BBH27634, BCC37668); 23 July 2009, K.T., P.S., R.R., S.M. (BCC37879); Khao Yai National Park, km. 29 Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 2 June 2011, A.K., K.T., K.S., P.S., S.M., W.N. (BBH30577, BCC47981); GenBank: ITS = OL331511, LSU = OL322043, TEF1 = OL322030, RPB1 = OL322054, RPB2 = OL322061; Khao Yai National Park, Mo Singto Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 3 August 2011, A.K., K.T., K.S., P.S., S.M., W.N. (BBH30683, BCC48951).
Notes: Based on the macromorphologies of the natural samples, the lower surface of stromata of A. galloides and A. griseoperitheciatus are orange and their perithecial layers are white and pale violet to light purplish gray, respectively. The perithecia in these two species are semi-immersed, but perithecia in A. galloides are obclavate, whereas those of A. griseoperitheciatus are obovoid. The colony color of A. galloides on PDA is pale orange yellow, light yellow and brilliant yellow, whereas A. griseoperitheciatus is white and produces a pale purplish pink pigment diffusing in the medium.
Ascopolyporus griseoperitheciatus Khonsanit, Thanakitpipattana and Luangsa-ard sp. nov. Figure 4.

Figure 4.
Ascopolyporus griseoperitheciatus. (a,b) Stromata on living stem of dicotyledonous plants (a BBH18679, b BBH30155); (c,d) perithecia; (e) asci; (f) asci-caps; (g) colony obverse on OA; (h) colony reverse on OA; (i) microsclerotium-like structure on OA; (j) phialide apex with conidial head; (k) conidium germination; (l) phialide and conidia on OA; (m) conidia on OA; (n) colony obverse on PDA; (o) colony reverse on PDA; (p) conidia germination; (q–s) phialide and conidia on PDA; (t) conidia on PDA. Scale bars: (a,g,h,n,o) = 10 mm; (b) = 5 mm; (c) = 100 μm; (d,e,l) = 50 μm; (p,q,r,s) = 20 μm; (i,j,k,m,t) = 10 μm; (f) = 5 μm.
MycoBank: MB 841854.
Etymology: From the Latin “griseo”, referring to the gray color of the fresh stromata.
Typification: Thailand, Kamphaeng Phet Province, Khlong Lan National Park, Khlong Lan Waterfall; 16°07′50.20″ N, 99°16′36.30″ E, on scale insects (Coccidae; Hemiptera), on the living stems of dicotyledonous plant, 19 June 2006, B.T., J.J.L., K.T., P.S., R.R., S.M., W.C. (holotype BBH18679, ex-holotype culture BCC22358). GenBank: ITS = OL331507, LSU = OL322050, TEF1 = OL322037, RPB1 = RPB2 = OL322067.
Description: Stromata epibiotic, irregularly pulvinate or subglobose, upper surface very pale violet (91D) to light purplish gray (N187D); lower surface vivid yellow (14C) to strong orange (25A), 3–7 mm wide. Perithecia semi-immersed, crowded, obovoid, 150–320 × 80–140 μm. Asci cylindrical, (150–)154–179(–193) × 4–5 μm. Asci caps 1.5–2 × 3–3.5 μm. Ascospores hyaline, whole, filiform, aseptate, extending the length of ascus.
Culture characteristics: Colony on OA attaining a diameter of 4 cm in 20 days, flat, slightly convex to the agar surface, white with light yellow green (150D), reverse pale orange yellow (23D). Phialides arising from aerial hyphae, solitary, cylindrical or acremonium-like, slightly curved, 50–250 × 1–2 μm. Conidia hyaline, enteroblastic, fusiform to acerose, early in development aseptate, becoming 1–2 septa, aggregated at the apex of the phialides, (6–)7–14(–19) × (1.5–)2–3 µm.
Colony on PDA attaining a diameter of 3.5–4 cm in 20 days, compact mycelium, slightly convex to the agar surface, white (158), pale purplish pink (56A) pigment diffusing in medium, reverse strong yellowish pink (31C). Phialides arising from aerial hyphae, solitary, cylindrical or acremonium-like, slightly curved, 43–265 × 1–2 μm. Conidia hyaline, enteroblastic, fusiform to acerose, early in development aseptate, becoming 1–2 septa, aggregated at the apex of the phialides, (4–)6–11(–17) × 2–3.5(–4) µm.
Habitat: On scale insects (Coccidae; Hemiptera), found on living stems of dicotyledonous plants.
Additional specimens examined: Thailand, Chanthaburi Province, Khao Soi Dao Wildlife Sanctuary, Withiphrai Nature Trail; 13°06′13″ N, 102°11′39″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 1 May 2007, B.T., K.T., R.R., S.M., W.C. (BBH19872, BCC25788); Nakhon Ratchasima Province, Khao Yai National Park, Mo Singto Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 30 June 2010, A.K., K.T., K.S., P.S., R. Somnuk (R.S.), S.M. (BBH30155, BCC43315).
Notes: Our molecular phylogenetic study has shown that A. griseoperitheciatus is closely related to A. albus. However, A. griseoperitheciatus significantly differs from A. albus in having a perithecial layer on the upper surface of stromata that is very pale violet to light purplish gray, the lower surface of stromata is vivid yellow to strong orange, while in A. albus, the stromata are only white. Additionally, A. griseoperitheciatus produces a pale purplish pink pigment diffusing in PDA plates, whereas A. albus does not produce any pigment.
Ascopolyporus gollmerianus Henn., Hedwigia 41: 8. 1902.
Ascopolyporus khaoyaiensis Mongkolsamrit, Thanakitpipattana and Luangsa-ard sp. nov. Figure 5.
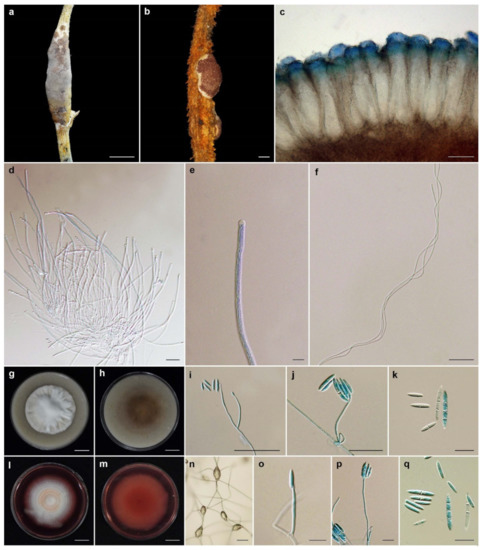
Figure 5.
Ascopolyporus khaoyaiensis. (a,b) Stromata on living stem of dicotyledonous plants (a BBH30157, b BBH30154); (c) perithecia; (d) asci; (e) asci-caps; (f) ascospores; (g) colony obverse on OA; (h) colony reverse on OA; (i,j) phialide apex with conidial head on OA; (k) conidia on OA; (l) colony obverse on PDA; (m) colony reverse on PDA; (n–p) phialide apex with conidial head on PDA; (q) conidia on PDA. Scale bars: (b,g,h,l,m) = 10 mm; (a) = 5 mm; (c) = 100 μm; (i) = 50 μm; (d,f) = 20 μm; (g,j,k,n,o,p,q) = 10 μm; (e) = 5 μm.
MycoBank: MB 841856.
Etymology: Named after Khao Yai National Park, where the type specimen was found.
Typification: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, Mo Singto Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on the living stems of dicotyledonous plant, 5 A0ugust 2010, K.T., P.S., S.M., A.K., R.S., K.S. (holotype BBH30157, ex-holotype culture BCC43741). GenBank: ITS = OL331513, LSU = OL322041, TEF1 = OL322040, RPB2 = OL322070.
Description: Stromata epibiotic, flattened to convex, cylindrical to irregularly shaped, upper surface very pale violet (91D) to dark purple (59A); lower surface white to pale orange (20A), 3–25 mm wide, 1–3 mm thick. Perithecia semi-immersed, slightly protruding apices, narrow flask shaped, slightly protruding, obclavate, 300–360 × 100–120 µm. Asci cylindrical, up to 215 µm long, 3–4 µm wide, Asci caps 2–4 × 3–4 μm. Ascospores hyaline, whole, filiform, aseptate, 175–200 × 1 µm.
Culture characteristics: Colonies on OA attaining a diameter of 3.5 cm in 14 days, cottony, white, reverse moderate brown (165A). Phialides arising from aerial hyphae, solitary, cylindrical or acremonium-like, slightly curved, up to 60 µm long, 1–2 µm wide. Conidia hyaline, enteroblastic, fusiform to acerose, early in development aseptate, mostly becoming 1 septum, occasionally 2–3 septa, aggregated at the apex of the phialides, (5–)8–16(–20) × (1.5–)2–3 µm.
Colonies on PDA attaining a diameter of 3–4 cm in 14 days, cottony, white, pale orang in the middle of colony, reverse dark red (59A) in the middle, bright strong purplish red (60D) pigment diffusing in medium. Phialides arising from aerial hyphae, solitary, cylindrical or acremonium-like, up to 50 µm long, 1–2 µm wide. Conidia hyaline, enteroblastic, fusiform to acerose, early in development aseptate, mostly becoming 1 septum, occasionally 2–3 septa, aggregated at the apex of the phialides, (7–)9–16.5(–22) × (1.5–)2–3 µm.
Habitat: On scale insects (Coccidae; Hemiptera), found on living stems of dicotyledonous plant.
Additional specimen examined: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, Mo Singto Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on living stems of dicotyledonous plant, 30 June 2010, K.T., P.S., S.M., A.K., R.S., K.S. (BBH30154, BCC43314). GenBank: ITS = OL331512, LSU = OL322052, TEF1 = OL322039, RPB2 = OL322069.
Notes: The stromatal color of the natural samples of A. khaoyaiensis is similar to the purple stromata of A. purpuratus. However, perithecia in A. khaoyaiensis are immersed and obclavate, whereas perithecia in A. purpuratus are semi-immersed and ovoid. Asci of A. khaoyaiensis are shorter than those of A. purpuratus (up to 215 × 3–4 vs. 200–240 × 4–5 µm). Additionally, A. khaoyaiensis and A. purpuratus produce bright strong purplish red pigment diffusing in PDA plates.
Ascopolyporus möellerianus (Henn.) Möller, Phycomyc. Ascomyc. Bras.: 301. 1901.
Ascopolyporus philodendri J.F. Bisch. (as “philodendrus”), Mycologia 97(3): 711. 2005.
Ascopolyporus polychrous Möller, Bot. Mitt. Trop. 9: 300. 1901.
Ascopolyporus polyporoïdes Möller, Bot. Mitt. Trop. 9: 301. 1901.
Ascopolyporus purpuratus Mongkolsamrit, Thanakitpipattana, Himaman and Luangsa-ard sp. nov. Figure 6.

Figure 6.
Ascopolyporus purpuratus. (a,b) Stromata on living midrib of leaves of dicotyledonous plant (BBH44511); (c) cross-section through stroma showing perithecia; (d) ovoid perithecia; (e) asci; (f) asci-caps; (g) ascospores; (h) colony obverse on OA; (i) colony reverse on OA; (j,k) phialide apex with conidial head on OA; (l) conidia on OA; (m) colony obverse on PDA; (n) colony reverse on PDA; (o,p) phialide apex with conidial head on PDA; (q) conidia on PDA. Scale bars: (h,i,m,n) = 10 mm; (a,b,c) = 1 mm; (d,e) = 100 μm; (g,j,o) = 20 μm; (f,k,l,p,q) = 10 μm.
MycoBank: MB 841857.
Etymology: Referring to the purple color of the fresh stroma.
Typification: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, Pong Chang Chomrom Phoen (Nong Phakchi); 14°27’04.0″ N, 101°22’03.60″ E, on scale insects (Coccidae; Hemiptera), on the living stems and midrib of dicotyledonous leaves, 19 September 2018, J.J.L., K.T., D. Thanakitpipattana (D.T.), B. Sakolrak (B.S.), R.S., S.M., W.N., W. Himaman (W.H.), P.S. (holotype BBH44511, ex-holotype culture BCC88430). GenBank: ITS = OL331506, LSU = OL322045, TEF1 = OL322032, RPB1 = OL322059.
Description: Stromata epibiotic, flattened to convex, consisting of dense white mycelial mat, upper surface yellow (18D) to very pale purple-violet (75D), 5–12 mm long, 3–8 mm wide. Perithecia semi-immersed, crowded, ovoid, (300–)335–414(–420) × (100–)110–142(–150) µm. Asci cylindrical, up to 240 × 2–4 µm. Asci caps 2–4 × 3–4 μm. Ascospores hyaline, whole, filiform, aseptate, (100–)131–190(–220) × 1–1.5 µm.
Culture characteristics: Colonies on OA attaining a diameter of 3–4 cm in 14 days, flat, white, reverse white. Phialides arising from aerial hyphae, solitary, cylindrical or acremonium-like, slightly curved, up to 40 µm long, 1–2 µm wide. Conidia hyaline, enteroblastic, fusiform to acerose, aseptate, aggregated at the apex of the phialides, (5–)6.5–18(–25) × (1.5–)2–2.5(–3) µm.
Colonies on PDA attaining a diameter of 2.5–3.5 cm in 14 days, slightly convex to the agar surface, vivid orange (28B) with white in the middle of colony, reverse strong reddish orange (34C), bright moderate reddish orange (N34D) pigment diffusing in medium. Phialides arising from aerial hyphae, solitary, cylindrical or acremonium-like, slightly curved, up to 55 µm long, 1–2 µm wide. Conidia hyaline, enteroblastic, fusiform to acerose, aseptate, aggregated at the apex of the phialides, (5–)7–13.5(–18) × 1.5–2.5(–3) µm.
Habitat: On scale insects (Coccidae; Hemiptera), found on living stem and midrib of leaves of dicotyledonous plants.
Additional specimen examined: Thailand, Phetchaburi Province, Kaeng Krachan National Park, Ban Krang Camp Nature Trail; 12°54′05″ N, 99°37′48″ E, on scale insects (Coccidae; Hemiptera), on the midrib of leaves, 14 November 2005, K.T., W.C., R.R., B.T. (BBH 15035, BCC 19721); Nakhon Ratchasima Province, Khao Yai National Park, Mo Singto Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on the twigs of tree, 23 July 2009, K.T., P.S., R.R., S.M. (BBH26373, BCC37880); Nakhon Ratchasima Province, Khao Yai National Park, Bueng Phai Nature Trail; 14°26′21.46″ N, 101°22′20.20″ E, on scale insects (Coccidae; Hemiptera), on the midrib of leaves and twigs of tree, 18 September 2018, J.J.L., K.T., D.T., B.S., R.S., S.M., W.N., W.H., P.S. (BBH44547, BCC88388); GenBank: ITS = OL331505, LSU = OL322046, TEF1 = OL322033, RPB2 = OL322064, (BBH44551, BCC88389); GenBank: ITS = OL331504, LSU = OL322047, TEF1 = OL322034.
Notes: Ascopolyporus purpuratus can be found on the midrib of leaves and living stems of dicotyledonous plants. In natural samples, the perithecia are pale yellow and/or very pale purple-violet. Additionally, A. purpuratus produces red pigment diffusing in PDA plates the same as A. griseoperitheciatus and A. khaoyaiensis. However, the mycelia of A. purpuratus are white in the middle of the colony with orange edges, whereas A. griseoperitheciatus produces only white mycelia and A. khaoyaiensis produces white mycelia that turn pale orange in the center of colony.
Ascopolyporus villosus Möller, Bot. Mitt. Trop. 9: 301. 1901.
Neohyperdermium Thanakitpipattana and Luangsa-ard, gen. nov.
MycoBank: MB 842780.
Etymology: Referring to the phenotypic similarity of the stromatal formation to Hyperdermium.
Typification: Neohyperdermium piperis (J.F. Bisch. and J.F. White) Thanakitpipattana and Luangsa-ard.
Description: Stroma epibiotic, flattened to pulvinate, white to yellow. Hosts are scale insects (Coccoidea, Hemiptera). Perithecia immersed, obpyriform, cymbiform to cone-shaped. Asci cylindrical, linear with enlarged refractive tip. Asexual morph verticillium-like.
Notes: This genus is a phylogenetically separate lineage from other scale insect pathogens in Cordycipitaceae, as shown in Figure 1. Two species are recognized in this genus that produce white to yellow stromata, immersed perithecia and a verticillium-like anamorph.
Neohyperdermium piperis (J.F. Bisch. and J.F. White) Thanakitpipattana and Luangsa-ard, comb. nov.
MycoBank MB 842782.
≡ Torrubiella piperis J.F. Bisch. and J.F. White, Studies in Mycology 50: 89–94. 2004.
≡ Cordyceps piperis (J.F. Bisch. and J.F. White) D. Johnson, G.H. Sung, J.F. Bisch. and Spatafora, Mycol. Res. 113(3): 284. 2009.
Description and illustration: See J.F. Bisch. and J.F. White (2004).
Typification: Panama, Barro Colorado Island, Lutz Creek, scale insect (Coccoidea, Hemiptera) on Piper carrilloanum (Piperaceae) August 2003, J.F. Bischoff and J.F. White, Jr., New York Botanical Garden (NY), culture ex-type CBS 116719.
Habitat: Scale insects.
Known distribution: Panama.
Note: Neohyperdermium piperis is closely related to N. pulvinatum and can be distinguished from N. pulvinatum in producing part-ascospores, whereas in N. piperis, the ascospores are whole with multiple septations and the conidia are aseptate.
Neohyperdermium pulvinatum (J.F. White et al.) Thanakitpipattana and Luangsa-ard, comb. nov.
MycoBank: MB 842783.
≡ Hyperdermium pulvinatum J.F. White et al., Mycologia 92(5): 908–918. 2000.
Description and illustration: See J.F. White et al. (2000).
Typification: Costa Rica, Guanacaste, Parque Nacional Guanacaste, Sector El Hacha, Puesto Los Almendros, on Asteraceae, 6 October 1998, J.F. White, G. Bills and S. Salas, RUTPP, culture ex-type ATCC MYA-69.
Habitat: Scale insects.
Known distribution: Costa Rica.
Note: Neohyperdermium pulvinatum is closely related to N. piperis, which can be distinguished by the type of ascospores and the presence of multiseptate conidia.
4. Discussion
The results of our multigene phylogenetic analyses show that our specimens were closely related to Ascopolyporus polychorus, A. villosus and Hyperdermium caulium (Figure 1). Importantly, the specimens in this study are clearly distinct species in Ascopolyporus because of the differences in the sizes, color, perithecial position and features of the stromata, which also overlap with morphological characters of some species previously treated as belonging to the genera Hyperdermium (H. caulium, H. bertonii, H. pulvinatum) and Cordyceps (C. piperis) in Cordycipitaceae, by producing flattened to pulvinate stromata and producing unique cylindrocarpon-like anamorph with multiseptate conidia [11,12,14,16]. The two genera, Ascopolyporus and Hyperdermium, differ only in the sizes and characters of ascomata [11,13,14]; Hyperdermium stromata are either flattened or pulvinate, whereas in Ascopolyporus sensu Möller, the stromata are subglobose to polypore-like. Based on these results, since the type species of Hyperdermium, H. caulium, is nested within Ascopolyporus, Hyperdermium is synonymized with Ascopolyporus and a new combination is proposed for H. caulium. The generic description of Ascopolyporus is therefore emended to include flattened to pulvinate stromata.
Our new species in Ascopolyporus are characterized by possessing flattened and pulvinate stromata, two groups that are supported as separate clades in phylogenetic analyses (Figure 1). The three pulvinate species (Ascopolyporus albus, A. galloides and A. griseoperitheciatus) have smaller stromata than previously described by Möller [24] and Bischoff et al. [14]. The two new species of Ascopolyporus khaoyaiensis and A. purpuratus have flattened stromata, and their sizes are in the same range as A. caulium (Table 2). All new Ascopolyporus species in this study possess semi-immersed perithecia with ostioles slightly protruding on the surface of the fertile cushion, whereas A. polychrous and A. philodendrus have completely immersed perithecia; A. vilosus does not produce perithecia on stromata [14].

Table 2.
Morphological comparisons of Ascopolyporus and related species. NA, not applicable.
Another species in Hyperdermium found in Cordycipitaceae, H. pulvinatum, did not cluster with type species H. caulium, which was congruent with Sung et al. [45], Kepler et al. [44] and Wang et al. [54], and is grouped together with Cordyceps piperis possessing pulvinate stromata that are white to yellow, producing aseptate, subcylindrical conidia on cultures (Table 2). These two species are proposed as new combinations in a new genus Neohyperdermium, as Neohyperdermium pulvinatum and N. piperis, which were described as epiphytes on scale insect pathogens in Cordycipitaceae.
The evolution and ecology of insect pathogenic fungi using insects and plants as the main source of nutrients remain not fully understood. Humber [55] suggested that the interaction between higher fungi and plants range from virulent pathogens to decomposer to mutualistic symbiosis. In Hypocreales, the genera Aschersonia, Ascopolyporus, Conoideocrella, Dussiella, Hyperdermium, Hypocrella, Moelleriella, Regiocrella and Samuelsia also utilize nutrients from the phloem of host plants through scale insects and white flies (Coccidae and Aleyrodidae) to continue their growth on plants [11,13,14,15]. Our new Ascopolyporus species are also found in this position, in which the scale insect attached to host plants was parasitized until it was consumed, but the fungus continues to utilize the nutrients that are being released through the stylet apparatus. The interactions occurred on the underside of fungal stroma, which is where the bridge for the exchange of nutrients between the fungus and plant exists (Figure 7).

Figure 7.
Photographs showing the interaction between fungus, plant and scale insect. (a) Fungal stroma on the stem of a dicotyledonous plant. (b) Underside of fungal stroma removed from the stem. (c,d) Underside of fungal stromata with ventage (arrow) from which the scale insect stylet entered the plant host. Scale bars = (a,b,c) = 1 mm; (d) = 0.05 mm.
Hypocrealean fungi are excellent producers of secondary metabolites which can be used to reduce the damage from insect fungi herbivores and phytopathogenic fungi [13,56]. However, no report has been made on the secondary metabolites produced from any of the reported species in Ascopolyporus, which should be a focus of future studies.
Residual Species of Ascopolyporus.
The remaining taxon could not be accommodated in the genus Ascopolyporus because its morphological description resembles other genera in Clavicipitaceae by producing paraphyses, which are not found in Ascopolyporus (Cordycipitaceae; Hypocreales), and molecular phylogenetic data are not available.
Ascopolyporus puttemansii Henn., Hedwigia 48: 6. 1908.
Key to Ascopolyporus species:
1a. Conidia aseptate……………………………………………………………………………………………2
1b. Conidia aseptate to multiseptate…………………………………………………………………………3
2a. Conidia oval………………………………………………………………………………………………..A. möllerianus
2b. Conidia fusiform to acerose………………………………………………………………………………A. purpuratus
3a. Conidia 1–5 septate, cylindrical to fusiform, 5–30 × 1–3 µm………………………………………….A. caulium
3b. Conidia 1–4 septate………………………………………………………………………………………..4
4a. Conidia subcylindrical…………………………………………………………………………………….5
4b. Conidia fusiform to acerose………………………………………………………………………………6
5a. Conidia subcylindrical, 7–25 × 3–4 µm………………………………………………………………….A. philodendrous
5b. Conidia subcylindrical, guttulate, 10–22 × 2–5 µm……………………………………………………A. villosus
6a. Ascospores hyaline, disarticulate into part-spores…………………………………………………….7
6b. Ascospores hyaline, whole……………………………………………………………………………….8
7a. Ascospores filiform to spiroid, 6 × 1 µm……………………………………………………………….A. polychrous
7b. Ascospores filiform, 8–15 µm……………………………………………………………………………A. polyporoïdes
8a. Ascospores multiseptate, 95–135 × 1 µm……………………………………………………………….A. albus
8b. Ascospores aseptate……………………………………………………………………………………….9
9a. Perithecia semi-immersed, obovoid…………………………………………………………………….A. griseoperithciatus
9b. Perithecia semi-immersed, obclavate…………………………………………………………………..10
10a. Stromata pulvinate, hemispherical, 1–7 mm…………………………………………………………A. galloides
10b. Stromata flattened to convex, cylindrical to irregular shaped, 3–25 mm…………………………A. khaoyaiensis
Key to Neohyperdermium species
1a. Ascospores filiform, multiseptate, whole………………………………………………………………N. pulvinatum
1b. Ascospores filiform, disarticulating into part-spores…………………………………………………N. piperis
Author Contributions
Conceptualization, D.T., A.K., J.J.L.-a. and N.P.; Data curation, W.H. and N.P.; Formal analysis, D.T. and A.K.; Funding acquisition, D.T., S.M., J.J.L.-a. and N.P.; Investigation, D.T., S.M., A.K., W.H., J.J.L.-a. and N.P.; Methodology, D.T., S.M., A.K., W.H., J.J.L.-a. and N.P.; Resources, W.H. and N.P.; Supervision, D.T., S.M., J.J.L.-a. and N.P.; Writing—original draft, D.T., S.M., A.K., W.H. and J.J.L.-a.; Writing—review and editing, D.T., S.M., A.K., J.J.L.-a. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Graduate School Thesis Grant, Chulalongkorn University and Platform Technology Management Section, National Center for Genetic Engineering and Biotechnology (BIOTEC), grant no. P19-50231.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found in: Genbank, https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 9 November 2021; MycoBank, https://www.mycobank.org/ (accessed on 9 November 2021); Index Fungorum, http://www.indexfungorum.org/Names/Names.asp (accessed on 5 December 2021).
Acknowledgments
The authors would like to thank Philip Shaw for editing the manuscript. We are also grateful to the anonymous reviewers for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kondo, T.; Gullan, P.J.; Williams, D.J. Coccidology. The study of scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Cienc. Tecnol. Agropecuaria 2008, 9, 55–61. [Google Scholar] [CrossRef]
- Mansour, R.; Grissa-Lebdi, K.; Suma, P.; Mazzeo, G.; Russo, A. Key scale insects (Hemiptera: Coccoidea) of high economic importance in a Mediterranean area: Host plants, bio-ecological characteristics, natural enemies and pest management strategies—A review. Plant Prot. Sci. 2017, 53, 1–14. [Google Scholar]
- Dhami, M.K.; Weir, B.S.; Taylor, M.W.; Beggs, J.R. Diverse honeydew-consuming fungal communities associated with scale insects. PLoS ONE 2013, 8, e70316. [Google Scholar]
- Chomnunti, P.; Hongsanan, S.; Aguirre-Hudson, B.; Tian, Q.; Peršoh, D.; Dhami, M.K.; Alias, A.S.; Xu, J.; Liu, X.; Stadler, M.; et al. The sooty moulds. Fungal Divers. 2014, 66, 1–36. [Google Scholar] [CrossRef]
- Hongsanan, S.; Tian, Q.; Hyde, K.; Chomnunti, P. Two new species of sooty moulds, Capnodium coffeicola and Conidiocarpus plumeriae in Capnodiaceae. Mycosp 2015, 6, 814–824. [Google Scholar] [CrossRef]
- Henk, D.A.; Vilgalys, R. Molecular phylogeny suggests a single origin of insect symbiosis in the Pucciniomycetes with support for some relationships within the genus Septobasidium. Am. J. Bot. 2007, 94, 1515–1526. [Google Scholar] [CrossRef]
- Dao, H.T.; Beattie, G.A.C.; Rossman, A.Y.; Burgess, L.W.; Holford, P. Four putative entomopathogenic fungi of armoured scale insects on Citrus in Australia. Mycol. Prog. 2016, 15, 47. [Google Scholar] [CrossRef]
- Araújo, J.P.; Hughes, D.P. Diversity of entomopathogenic fungi: Which groups conquered the insect body? Adv. Genet. 2016, 94, 1–39. [Google Scholar]
- Xu, X.-L.; Zeng, Q.; Lv, Y.-C.; Jeewon, R.; Maharachchikumbura, S.S.; Wanasinghe, D.N.; Hyde, K.D.; Xiao, Q.-G.; Liu, Y.-G.; Yang, C.-L. Insight into the systematics of novel entomopathogenic fungi associated with armored scale insect, kuwanaspis howardi (Hemiptera: Diaspididae) in China. J. Fungi 2021, 7, 628. [Google Scholar] [CrossRef]
- Hywel-Jones, N.L.; Samuels, G.J. Three species of Hypocrella with large stromata pathogenic on scale insects. Mycologia 1998, 90, 36–46. [Google Scholar] [CrossRef]
- Sullivan, R.F.; Bills, G.F.; Hywel-Jones, N.L.; White, J.F. Hyperdermium: A new clavicipitalean genus for some tropical epibionts of dicotyledonous plants. Mycologia 2000, 92, 908–918. [Google Scholar] [CrossRef]
- Bischoff, J.F.; White, J.F. Torrubiella piperis sp. nov. (Clavicipitaceae, Hypocreales), a new teleomorph of the Lecanicillium complex. Stud. Mycol. 2004, 50, 89–94. [Google Scholar]
- Koroch, A.; Juliani, H.; Bischoff, J.; Lewis, E.; Bills, G.; Simon, J.; White, J.F. Examination of plant biotrophy in the scale insect parasitizing fungus Dussiella tuberiformis. Symbiosis 2004, 37, 267–280. [Google Scholar]
- Bischoff, J.F.; Chaverri, P.; White, J.F. Clarification of the host substrate of Ascopolyporus and description of Ascopolyporus philodendrus sp. nov. Mycologia 2005, 97, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Bischoff, J.F.; Evans, H.C.; Hodge, K.T. Regiocrella, a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 2005, 97, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Liu, M.; Hodge, K. A monograph of the entomopathogenic genera Hypocrella, Moelleriella, and Samuelsia gen. nov. (Ascomycota, Hypocreales, Clavicipitaceae), and their aschersonia-like anamorphs in the Neotropics. Stud. Mycol. 2008, 60, 1–66. [Google Scholar] [CrossRef]
- Johnson, D.; Sung, G.H.; Hywel-Jones, N.L.; Luangsa-Ard, J.J.; Bischoff, J.F.; Kepler, R.M.; Spatafora, J.W. Systematics and evolution of the genus Torrubiella (Hypocreales, Ascomycota). Mycol. Res. 2009, 113, 279–289. [Google Scholar] [CrossRef]
- Fan, J.-H.; Xie, Y.-P.; Xue, J.-L.; Xiong, Q.; Jiang, W.-J.; Zhang, Y.-J.; Ren, Z.-M. The strain HEB01 of Fusarium sp., a new pathogen that infects brown soft scale. Ann. Microbiol. 2014, 64, 333–341. [Google Scholar] [CrossRef]
- Luangsa-ard, J.J.; Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Khonsanit, A.; Wutikhun, T. Helicocollum, a new clavicipitalean genus pathogenic to scale insects (Hemiptera) in Thailand. Mycol. Prog. 2017, 16, 419–431. [Google Scholar] [CrossRef]
- Deng, J.; Yu, Y.; Wang, X.; Liu, Q.; Huang, X. The ubiquity and development-related abundance dynamics of Ophiocordyceps fungi in soft scale insects. Microorganisms 2021, 9, 404. [Google Scholar] [CrossRef]
- Szklarzewicz, T.; Michalik, K.; Grzywacz, B.; Kalandyk-Kołodziejczyk, M.; Michalik, A. Fungal Associates of Soft Scale Insects (Coccomorpha: Coccidae). Cells 2021, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Index Fungorum Database. Available online: http://www.indexfungorum.org/ (accessed on 5 December 2021).
- Wood, M. Webwatch: Observing Mushrooms. Fungi Magazine 1, 2. Available online: https://mushroomobserver.org/ (accessed on 27 December 2021).
- Möller, A. Phycomyceten und Ascomyceten. Untersuchungen aus Brasilien. Botanische Mittheilungen aus den Tropen 9; Fischer: Jena, Germany, 1901. [Google Scholar]
- Luangsa-ard, J.J.; Tasanathai, K.; Thanakitpipattana, D.; Khonsanit, A.; Stadler, M. Novel and interesting Ophiocordyceps spp. (Ophiocordycipitaceae, Hypocreales) with superficial perithecia from Thailand. Stud. Mycol. 2018, 89, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Khonsanit, A.; Lamlertthon, S.; Luangsa-ard, J.J. New species in Aciculosporium, Shimizuomyces and a new genus Morakotia associated with plants in Clavicipitaceae from Thailand. FUSE 2021, 8, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Khonsanit, A.; Noisripoom, W.; Mongkolsamrit, S.; Phosrithong, N.; Luangsa-ard, J.J. Five new species of Moelleriella infecting scale insects (Coccidae) in Thailand. Mycol. Prog. 2021, 20, 847–867. [Google Scholar] [CrossRef]
- Heritage, J.; Evans, E.G.V.; Killington, R. Introductory Microbiology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- The Royal Horticultural Society. The Fifth Edition Published by The Royal Horticultural Society; Vincent Square: London, UK, 2007. [Google Scholar]
- Thanakitpipattana, D.; Tasanathai, K.; Mongkolsamrit, S.; Khonsanit, A.; Lamlertthon, S.; Luangsa-Ard, J.J. Fungal pathogens occurring on Orthopterida in Thailand. Persoonia 2020, 44, 140. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cyptococcus species. J. Bacteriol. Res. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.H.; Hyten, A.; Spatafora, J.W. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sarver, B.A.; Brandt, M.; Chang, D.; Noble-Wang, J.; Park, B.J.; Sutton, D.A.; Benjamin, L.; Lindsley, M.; Padhye, A.; et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007, 45, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: A community resource for phylogenetic analyses. In Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery; Association for Computing Machinery: New York, NY, USA, 2011; pp. 1–8. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J. MrModeltest v2. Program Distributed by the Author; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Wilgenbusch, J.C.; Swofford, D. Inferring evolutionary trees with PAUP. Curr. Protoc. Bioinform. 2003, 1, 6.4.1–6.4.28. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Chen, W.; Han, Y.; Liang, Z.; Jin, D. Lecanicillium araneogenum sp. nov., a new araneogenous fungus. Phytotaxa 2017, 305, 29–34. [Google Scholar] [CrossRef][Green Version]
- Chiriví-Salomón, J.S.; Danies, G.; Restrepo, S.; Sanjuan, T. Lecanicillium sabanense sp. nov. (Cordycipitaceae) a new fungal entomopathogen of coccids. Phytotaxa 2015, 234, 63–74. [Google Scholar] [CrossRef]
- Rehner, S.A.; Minnis, A.M.; Sung, G.H.; Luangsa-ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; White, J.F. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol. Ecol. 2007, 16, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Spatafora, J.W. Cordyceps cardinalis sp. nov., a new species of Cordyceps with an east Asian-eastern North American distribution. Mycologia 2004, 96, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Wutikhun, T.; Spatafora, J.W.; Luangsa-Ard, J.J. Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 2018, 110, 230–257. [Google Scholar] [CrossRef]
- Tasanathai, K.; Thanakitpipattana, D.; Noisripoom, W.; Khonsanit, A.; Kumsao, J.; Luangsa-ard, J.J. Two new Cordyceps species from a community forest in Thailand. Mycol. Prog. 2016, 15, 28. [Google Scholar] [CrossRef]
- Kepler, R.M.; Sung, G.H.; Ban, S.; Nakagiri, A.; Chen, M.J.; Huang, B.; Li, Z.; Spatafora, J.W. New teleomorph combinations in the entomopathogenic genus Metacordyceps. Mycologia 2012, 104, 182–197. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, Y.; Fan, Q.; Duan, D.E.; Zhang, G.D.; Dai, R.Q.; Dai, Y.D.; Zeng, W.B.; Chen, Z.H.; Li, D.D. Multigene phylogeny of the family Cordycipitaceae (Hypocreales): New taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Divers. 2020, 103, 1–46. [Google Scholar] [CrossRef]
- Humber, R.A. Evolution of entomopathogenicity in fungi. J. Invertebr. Pathol. 2008, 98, 262–266. [Google Scholar] [CrossRef]
- Torres, M.S.; Singh, A.P.; Vorsa, N.; White, J.F. An analysis of ergot alkaloids in the Clavicipitaceae (Hypocreales, Ascomycota) and ecological implication. Symbiosis 2008, 46, 11–19. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).