Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Peptide P10 Preparation
2.3. Preparation of Liposome
2.4. Determination of Diameter Size, Polydispersity and Zeta Potential of Liposomes
2.5. Adsorption Efficiency
2.6. Determination of Liposomes Morphology by Transmission Electronic Microscopy (TEM)
2.7. Experimental Infection
2.8. Vaccination Protocols
2.9. Determination of Fungal Burden
2.10. Quantification of Cytokines Levels of Homogenate Pulmonary for the ELISA Method
2.11. Histopathological Lungs Analysis
2.12. Statistical Analysis
3. Results
3.1. The Effect of Peptide P10 Adsorption on DDA/TDB Liposomes
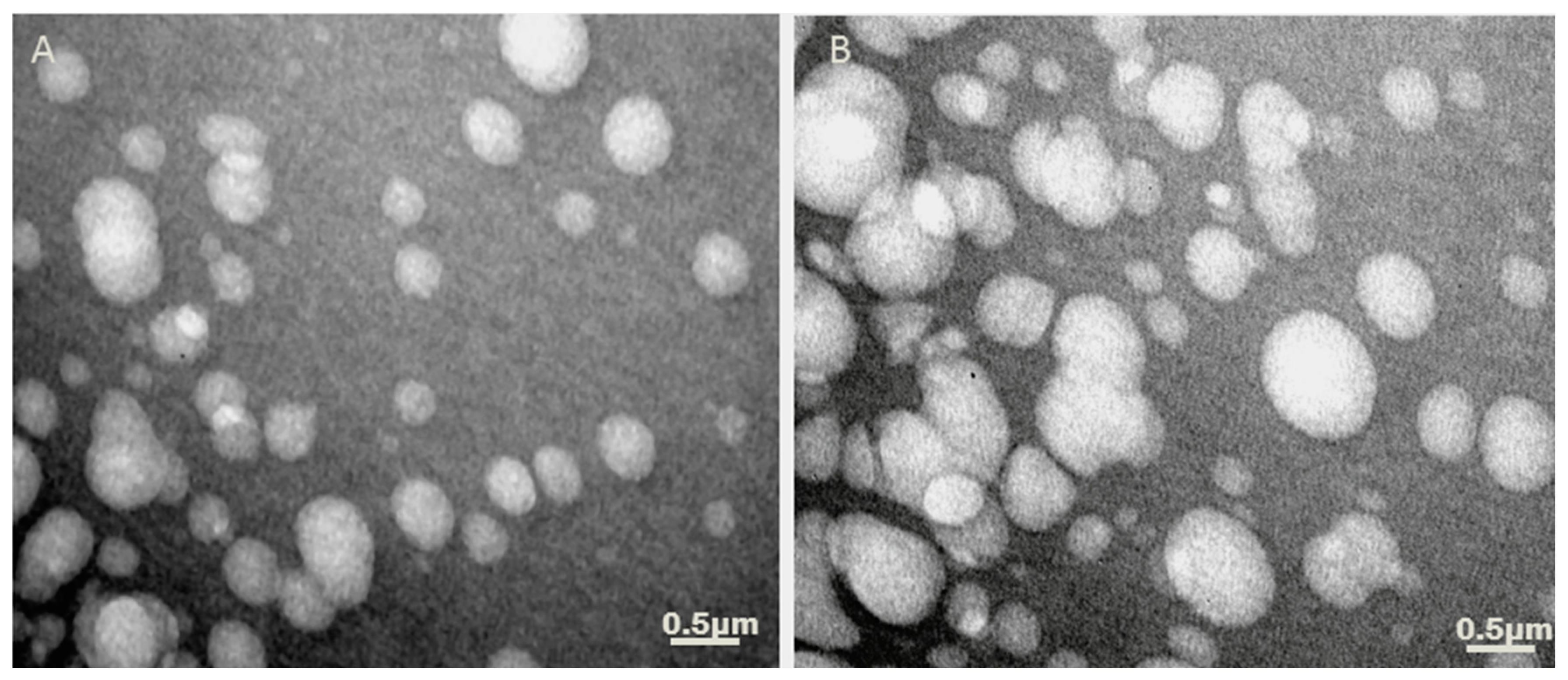
3.2. Determination of Liposomes Morphology after P10 Adsorption by TEM
3.3. Adsorption of Peptide to Adjuvant Liposome
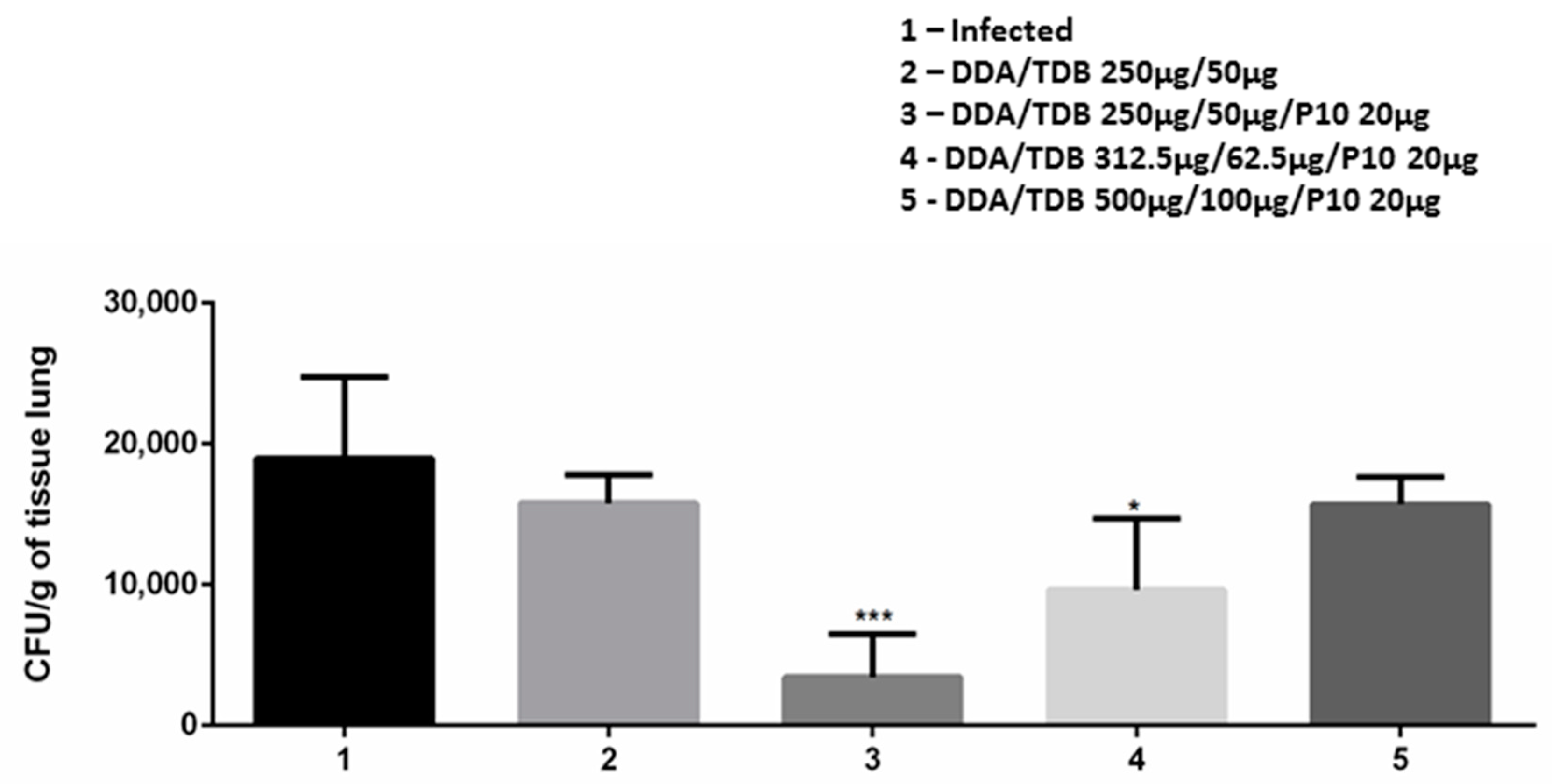
3.4. DDA/TDB/P10 Vaccination Controls Pulmonary Fungal Burden
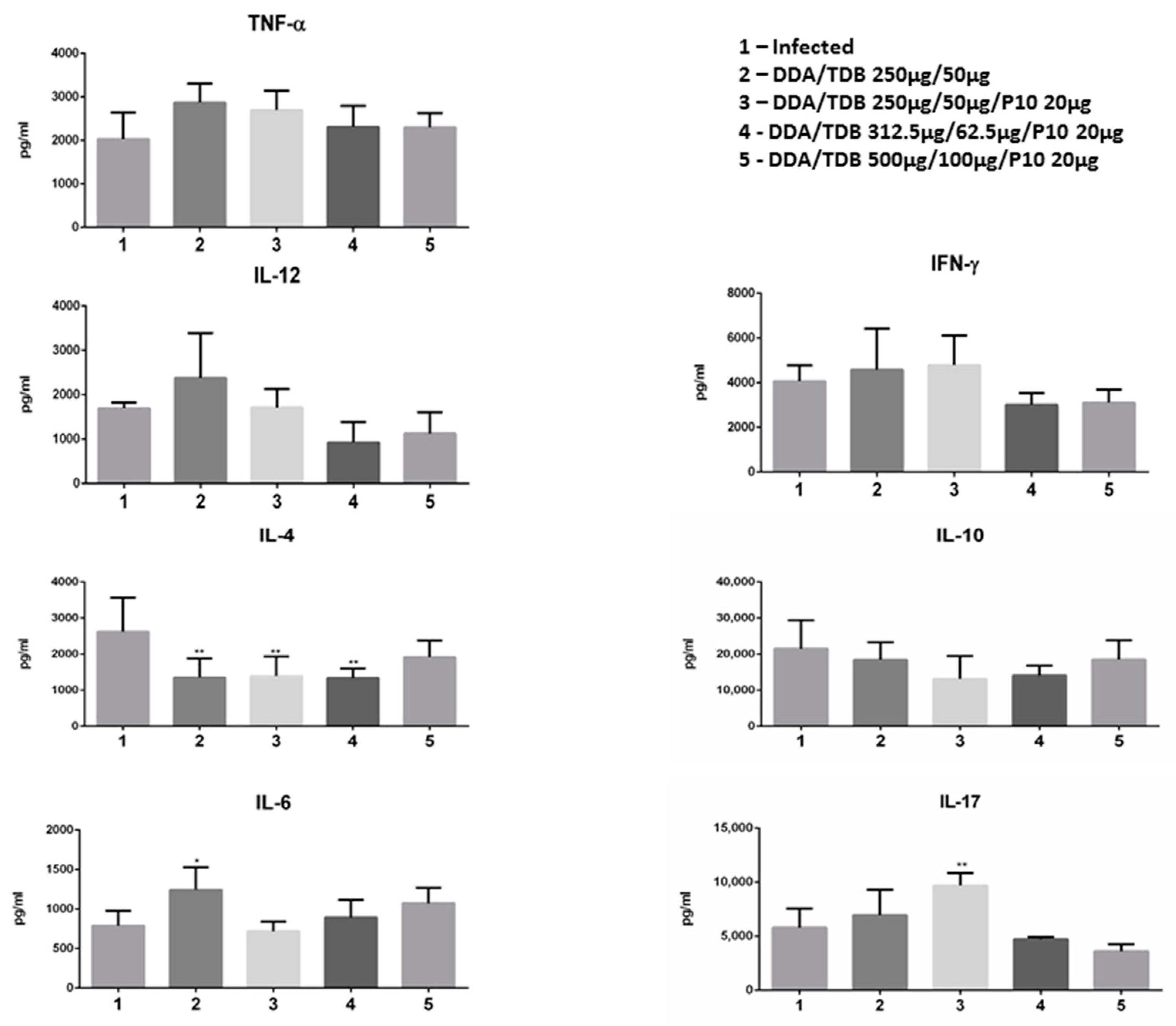
3.5. The Therapeutic Effect of DDA/TDB/P10 Vaccination Correlates with an IL-4/IL-17 Balance in the Lung Parenchyma
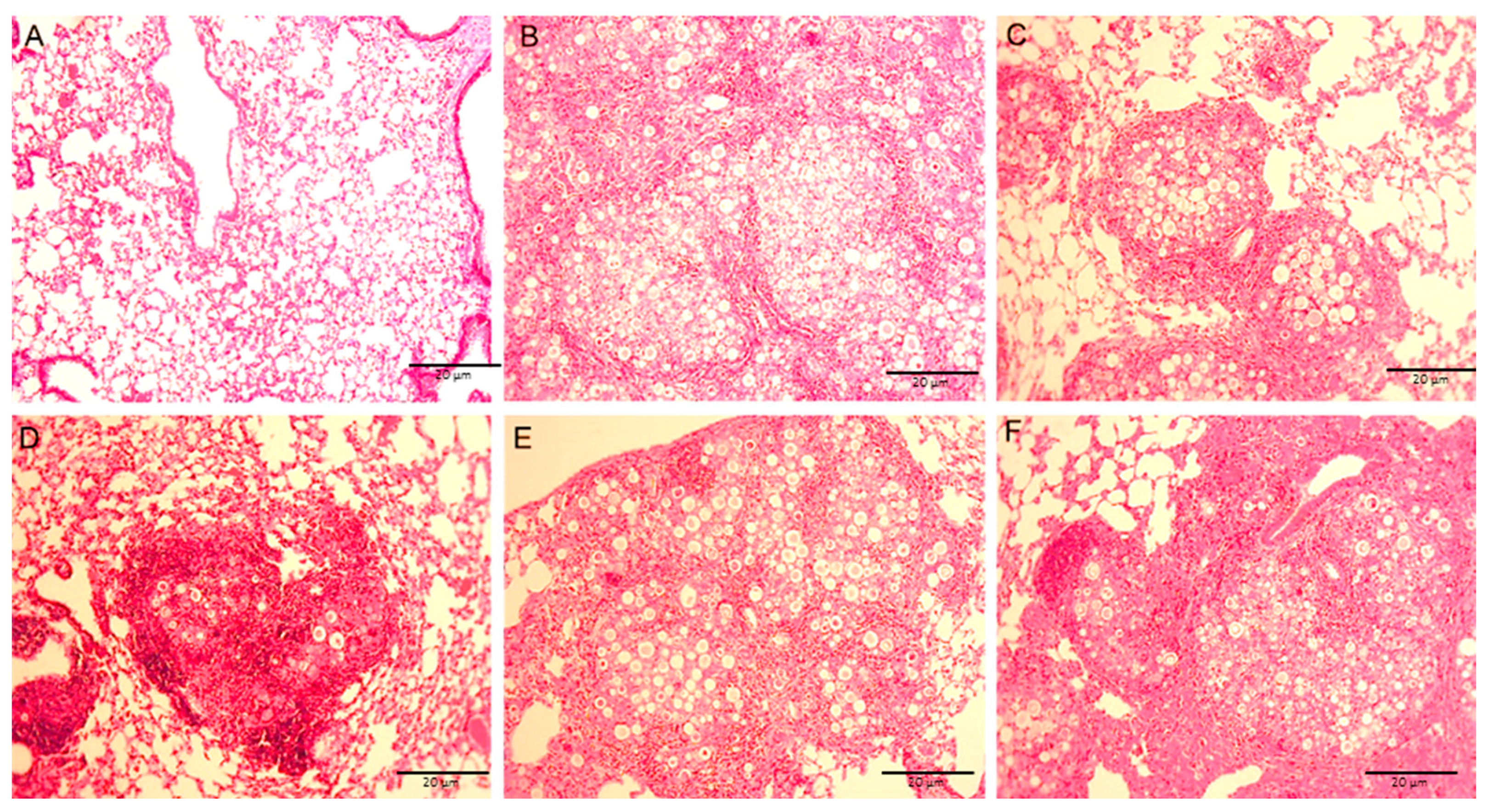
3.6. Lung Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Souza, A.C.O.; Taborda, C.P. Epidemiology of Dimorphic Fungi. In Reference Module in Life Sciences; Elsevier BV: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Shikanai-Yasuda, M.A.; Mendes, P.R.; Colombo, A.L.; De Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; Valle, A.C.F.D.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.D.M.; Theodoro, R.C.; De Oliveira, F.F.M.; Machado, G.C.; Hahn, R.C.; Bagagli, E.; San-Blas, G.; Felipe, M.S. Paracoccidioides lutziisp. nov.: Biological and clinical implications. Med. Mycol. 2013, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Matute, D.R.; McEwen, J.G.; Puccia, R.; Montes, B.A.; San-Blas, G.; Bagagli, E.; Rauscher, J.T.; Restrepo, A.; Morais, F.; Nino-Vega, G.; et al. Cryptic Speciation and Recombination in the Fungus Paracoccidioides brasiliensis as Revealed by Gene Genealogies. Mol. Biol. Evol. 2006, 23, 65–73. [Google Scholar] [CrossRef]
- Teixeira, M.D.M.; Theodoro, R.C.; De Carvalho, M.J.; Fernandes, L.; Paes, H.C.; Hahn, R.C.; Mendoza, L.; Bagagli, E.; San-Blas, G.; Felipe, M.S.S. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 2009, 52, 273–283. [Google Scholar] [CrossRef]
- Teixeira, M.D.M.; Theodoro, R.C.; Derengowski, L.D.S.; Nicola, A.M.; Bagagli, E.; Felipe, M.S. Molecular and Morphological Data Support the Existence of a Sexual Cycle in Species of the Genus Paracoccidioides. Eukaryot. Cell 2013, 12, 380–389. [Google Scholar] [CrossRef][Green Version]
- Theodoro, R.C.; Teixeira, M.D.M.; Felipe, M.S.S.; Paduan, K.D.S.; Ribolla, P.M.; San-Blas, G.; Bagagli, E. Genus Paracoccidioides: Species Recognition and Biogeographic Aspects. PLoS ONE 2012, 7, e37694. [Google Scholar] [CrossRef]
- Tobon, A.M.; Agudelo, C.A.; Osorio, M.L.; Alvarez, D.L.; Arango, M.; Cano, L.E.; Restrepo, A. Residual Pulmonary Abnormalities in Adult Patients with Chronic Paracoccidioidomycosis: Prolonged Follow-Up after Itraconazole Therapy. Clin. Infect. Dis. 2003, 37, 898–904. [Google Scholar] [CrossRef]
- Travassos, L.R.; Taborda, C.P. Linear Epitopes of Paracoccidioides brasiliensis and Other Fungal Agents of Human Systemic Mycoses as Vaccine Candidates. Front. Immunol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Rossi, S.A.; De Araujo, M.V.; Taira, C.L.; Travassos, L.R.; Taborda, C.P. Vaccine Development to Systemic Mycoses by Thermally Dimorphic Fungi. Curr. Trop. Med. Rep. 2019, 6, 64–75. [Google Scholar] [CrossRef]
- Taborda, C.P.; Juliano, M.A.; Puccia, R.; Franco, M.; Travassos, L.R. Mapping of the T-Cell Epitope in the Major 43-Kilodalton Glycoprotein of Paracoccidioides brasiliensisWhich Induces a Th-1 Response Protective against Fungal Infection in BALB/c Mice. Infect. Immun. 1998, 66, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Puccia, R.; Travassos, L.R. The 43-kDa glycoprotein from the human pathogen Paracoccidioides brasiliensis and its deglycosylated form: Excretion and susceptibility to proteolysis. Arch. Biochem. Biophys. 1991, 289, 298–302. [Google Scholar] [CrossRef]
- Da Silva, S.H.M.; Colombo, A.L.; Blotta, M.H.S.L.; Lopes, J.D.; Queiroz-Telles, F.; De Camargo, Z.P. Detection of Circulating gp43 Antigen in Serum, Cerebrospinal Fluid, and Bronchoalveolar Lavage Fluid of Patients with Paracoccidioidomycosis. J. Clin. Microbiol. 2003, 41, 3675–3680. [Google Scholar] [CrossRef]
- De Camargo, Z.; Unterkircher, C.; Campoy, S.P.; Travassos, L.R. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J. Clin. Microbiol. 1988, 26, 2147–2151. [Google Scholar] [CrossRef] [PubMed]
- Jr, N.P.L.; Vallejo, M.C.; Conceição, P.M.; Camargo, Z.P.; Hahn, R.; Puccia, R. Paracoccidioides lutzii Plp43 Is an Active Glucanase with Partial Antigenic Identity with P. brasiliensis gp43. PLoS Negl. Trop. Dis. 2014, 8, e3111. [Google Scholar] [CrossRef]
- Emayorga, O.; Muñoz, J.E.; Lincopan, N.; Teixeira, A.F.; Ferreira, L.C.D.S.; Travassos, L.R.; Taborda, C.P. The role of adjuvants in therapeutic protection against paracoccidioidomycosis after immunization with the P10 peptide. Front. Microbiol. 2012, 3, 154. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Barnier-Quer, C.; Elsharkawy, A.; Romeijn, S.; Kros, A.; Jiskoot, W. Adjuvant Effect of Cationic Liposomes for Subunit Influenza Vaccine: Influence of Antigen Loading Method, Cholesterol and Immune Modulators. Pharmaceutics 2013, 5, 392–410. [Google Scholar] [CrossRef]
- Souza, A.C.O.; Amaral, A.C. Antifungal Therapy for Systemic Mycosis and the Nanobiotechnology Era: Improving Efficacy, Biodistribution and Toxicity. Front. Microbiol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Christensen, D.; Korsholm, K.S.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 513–521. [Google Scholar] [CrossRef]
- Hunter, R.; Olsen, M.; Jagannath, C.; Actor, J.K. Trehalose 6,6′-Dimycolate and Lipid in the Pathogenesis of Caseating Granulomas of Tuberculosis in Mice. Am. J. Pathol. 2006, 168, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L.; Olsen, M.R.; Jagannath, C.; Actor, J.K. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann. Clin. Lab. Sci. 2006, 36, 371–386. [Google Scholar] [PubMed]
- Hunter, R.L.; Venkataprasad, N.; Olsen, M.R. The role of trehalose dimycolate (cord factor) on morphology of virulent M. tuberculosis in vitro. Tuberculosis 2006, 86, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D. Development and Evaluation of CAF01. In Immunopotentiators in Modern Vaccines; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 333–345. [Google Scholar]
- Henriksen-Lacey, M.; Devitt, A.; Perrie, Y. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J. Control. Release 2011, 154, 131–137. [Google Scholar] [CrossRef]
- Sakurai, T.; Saiki, I.; Ishida, H.; Takeda, K.; Azuma, I. Lethal toxicity and adjuvant activities of synthetic TDM and its related compounds in mice. Vaccine 1989, 7, 269–274. [Google Scholar] [CrossRef]
- Desel, C.; Werninghaus, K.; Ritter, M.; Jozefowski, K.; Wenzel, J.; Russkamp, N.; Schleicher, U.; Christensen, D.; Wirtz, S.; Kirschning, C.; et al. The Mincle-Activating Adjuvant TDB Induces MyD88-Dependent Th1 and Th17 Responses through IL-1R Signaling. PLoS ONE 2013, 8, e53531. [Google Scholar] [CrossRef]
- Nordly, P.; Rose, F.; Christensen, D.; Nielsen, H.M.; Andersen, P.; Agger, E.M.; Foged, C. Immunity by formulation design: Induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J. Control. Release 2011, 150, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Werninghaus, K.; Babiak, A.; Groß, O.; Hölscher, C.; Dietrich, H.; Agger, E.M. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ–Syk–Card9–dependent innate immune activation. J. Exp. Med. 2009, 206, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, G.K.; Andersen, P.; Christensen, D. Immunocorrelates of CAF family adjuvants. Semin. Immunol. 2018, 39, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.J.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef]
- Lincopan, N.; Espíndola, N.M.; Vaz, A.J.; Da Costa, M.H.B.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Novel immunoadjuvants based on cationic lipid: Preparation, characterization and activity in vivo. Vaccine 2009, 27, 5760–5771. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, E.; Barreleiro, P.; Olofsson, G. Phase transition in dioctadecyldimethylammonium bromide and chloride vesicles prepared by different methods. Chem. Phys. Lipids 2000, 105, 201–213. [Google Scholar] [CrossRef]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—A novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta Biomembr. 2005, 1718, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.J.; Wilkinson, A.; Bramwell, V.W.; Christensen, D.; Perrie, Y. Th1 immune responses can be modulated by varying dimethyldioctadecylammonium and distearoyl-sn-glycero-3-phosphocholine content in liposomal adjuvants. J. Pharm. Pharmacol. 2014, 66, 358–366. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Qu, W.; Li, N.; Yu, R.; Zuo, W.; Fu, T.; Fei, W.; Hou, Y.; Liu, Y.; Yang, J. Cationic DDA/TDB liposome as a mucosal vaccine adjuvant for uptake by dendritic cells in vitro induces potent humoural immunity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 852–860. [Google Scholar] [CrossRef]
- Singer-Vermes, L.M.; Burger, E.; Franco, M.F.; Di-Bacchi, M.M.; Mendes-Giannini, M.J.; Calich, V.L. Evaluation of the pathogenicity and immunogenicity of seven Paracoccidioides brasiliensis isolates in susceptible inbred mice. J. Med. Veter. Mycol. 1989, 27, 71–82. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Luft, V.D.; Amorim, J.; Magalhães, A.; Thomaz, L.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Immunization with P10 Peptide Increases Specific Immunity and Protects Immunosuppressed BALB/c Mice Infected with Virulent Yeasts of Paracoccidioides brasiliensis. Mycopathologia 2014, 178, 177–188. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef]
- Badran, M. Formulation and in vitro evaluation of flufenamic acid loaded deformable liposomes for improved skin delivery. Dig. J. Nanomater. Biostructures 2014, 9, 83–91. [Google Scholar]
- Chen, M.; Liu, X.; Fahr, A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: In vitro study with finite and infinite dosage application. Int. J. Pharm. 2011, 408, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Putri, D.C.A.; Dwiastuti, R.; Marchaban, M.; Nugroho, A.K. Optimization of mixing temperature and sonication duration in liposome preparation. J. Pharm. Sci. Commun. 2017, 14, 79–85. [Google Scholar] [CrossRef]
- Marques, A.F.; Da Silva, M.B.; Juliano, M.A.P.; Travassos, L.R.; Taborda, C.P. Peptide Immunization as an Adjuvant to Chemotherapy in Mice Challenged Intratracheally with Virulent Yeast Cells of Paracoccidioides brasiliensis. Antimicrob. Agents Chemother. 2006, 50, 2814–2819. [Google Scholar] [CrossRef] [PubMed]
- Abeyratne, E.; Tharmarajah, K.; Freitas, J.R.; Mostafavi, H.; Mahalingam, S.; Zaid, A.; Zaman, M.; Taylor, A. Liposomal Delivery of the RNA Genome of a Live-Attenuated Chikungunya Virus Vaccine Candidate Provides Local, but Not Systemic Protection After One Dose. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Rosenkrands, I.; Vingsbo-Lundberg, C.; Bundgaard, T.J.; Lindenstrøm, T.; Enouf, V.; Van Der Werf, S.; Andersen, P.; Agger, E.M. Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine 2011, 29, 6283–6291. [Google Scholar] [CrossRef]
- Nguyen, N.D.N.T.; Olsen, A.W.; Lorenzen, E.; Andersen, P.; Hvid, M.; Follmann, F.; Dietrich, J. Parenteral vaccination protects against transcervical infection with Chlamydia trachomatis and generate tissue-resident T cells post-challenge. NPJ Vaccines 2020, 5, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Riccomi, A.; Piccaro, G.; Christensen, D.; Palma, C.; Andersen, P.; Vendetti, S. Parenteral Vaccination with a Tuberculosis Subunit Vaccine in Presence of Retinoic Acid Provides Early but Transient Protection to M. Tuberculosis Infection. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Woodworth, J.S.; Christensen, D.; Cassidy, J.P.; Agger, E.M.; Mortensen, R.; Andersen, P. Mucosal boosting of H56:CAF01 immunization promotes lung-localized T cells and an accelerated pulmonary response to Mycobacterium tuberculosis infection without enhancing vaccine protection. Mucosal Immunol. 2019, 12, 816–826. [Google Scholar] [CrossRef]
- Dejon-Agobe, J.C.; Ateba-Ngoa, U.; Lalremruata, A.; Homoet, A.; Engelhorn, J.; Nouatin, O.P.; Edoa, J.R.; Fernandes, J.F.; Esen, M.; Mouwenda, Y.D.; et al. Controlled Human Malaria Infection of Healthy Adults with Lifelong Malaria Exposure to Assess Safety, Immunogenicity, and Efficacy of the Asexual Blood Stage Malaria Vaccine Candidate GMZ2. Clin. Infect. Dis. 2019, 69, 1377–1384. [Google Scholar] [CrossRef]
- Mortensen, R.; Christensen, D.; Hansen, L.B.; Christensen, J.P.; Andersen, P.; Dietrich, J. Local Th17/IgA immunity correlate with protection against intranasal infection with Streptococcus pyogenes. PLoS ONE 2017, 12, e0175707. [Google Scholar] [CrossRef]
- Travassos, L.R.; Taborda, C.P.; Colombo, A.L. Treatment options for paracoccidioidomycosis and new strategies investigated. Expert Rev. Anti-Infect. Ther. 2008, 6, 251–262. [Google Scholar] [CrossRef]
- Brewer, J.M.; Tetley, L.; Richmond, J.; Liew, F.Y.; Alexander, J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J. Immunol. 1998, 161, 4000–4007. [Google Scholar] [PubMed]
- Mann, J.F.S.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 2009, 27, 3643–3649. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, F. Targeting to macrophages: Role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J. Control. Release 2002, 79, 29–40. [Google Scholar] [CrossRef]
- Allen, T.; Austin, G.; Chonn, A.; Lin, L.; Lee, K. Uptake of liposomes by cultured mouse bone marrow macrophages: Influence of liposome composition and size. Biochim. Biophys. Acta Biomembr. 1991, 1061, 56–64. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.-H.; Ahn, J.C.; Rajendiran, J.C.B.; Arai, Y. Influence of cationic lipid concentration on properties of lipid–polymer hybrid nanospheres for gene delivery. Int. J. Nanomed. 2015, 10, 5367. [Google Scholar] [CrossRef]
- Hamborg, M.; Jorgensen, L.; Bojsen, A.R.; Christensen, D.; Foged, C. Protein Antigen Adsorption to the DDA/TDB Liposomal Adjuvant: Effect on Protein Structure, Stability, and Liposome Physicochemical Characteristics. Pharm. Res. 2013, 30, 140–155. [Google Scholar] [CrossRef]
- Hamborg, M.; Rose, F.; Jorgensen, L.; Bjorklund, K.; Pedersen, H.B.; Christensen, D.; Foged, C. Elucidating the mechanisms of protein antigen adsorption to the CAF/NAF liposomal vaccine adjuvant systems: Effect of charge, fluidity and antigen-to-lipid ratio. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2001–2010. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Foged, C.; Korsholm, K.S.; Rades, T.; Christensen, D. Liposome-Based Adjuvants for Subunit Vaccines: Formulation Strategies for Subunit Antigens and Immunostimulators. Pharmaceutics 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.R.; Liu, J.S.; Pociask, D.A.; Zheng, M.; Mietzner, T.A.; Berger, T.; Mak, T.W.; Clifton, M.C.; Strong, R.K.; Ray, P.; et al. Lipocalin 2 Is Required for Pulmonary Host Defense against Klebsiella Infection. J. Immunol. 2009, 182, 4947–4956. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Whibley, N.; Mamo, A.J.; Siebenlist, U.; Chan, Y.R.; Gaffen, S.L. Interleukin-17-Induced Protein Lipocalin 2 Is Dispensable for Immunity to Oral Candidiasis. Infect. Immun. 2013, 82, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S. Immunity against Fungal Infections. Immunol. Immunogenet. Insights 2016, 8, S38707. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Hernández-Santos, N.; Gaffen, S.L. Th17 Cells in Immunity to Candida albicans. Cell Host Microbe 2012, 11, 425–435. [Google Scholar] [CrossRef]
- Perez-Nazario, N.; Rangel-Moreno, J.; O’Reilly, M.A.; Pasparakis, M.; Gigliotti, F.; Wright, T.W. Selective Ablation of Lung Epithelial IKK2 Impairs Pulmonary Th17 Responses and Delays the Clearance ofPneumocystis. J. Immunol. 2013, 191, 4720–4730. [Google Scholar] [CrossRef]
- Wüthrich, M.; Gern, B.; Hung, C.Y.; Ersland, K.; Rocco, N.; Pick-Jacobs, J. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Investig. 2011, 121, 554–568. [Google Scholar] [CrossRef]
- Kim, J.S.; Jordan, M.S. Diversity of IL-17-producing T lymphocytes. Cell. Mol. Life Sci. 2012, 70, 2271–2290. [Google Scholar] [CrossRef]
- Taga, T.; Kishimoto, T. Gp 130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997, 15, 797–819. [Google Scholar] [CrossRef]
- Yang, X.O.; Pappu, B.P.; Nurieva, R.; Akimzhanov, A.; Kang, H.S.; Chung, Y.; Ma, L.; Shah, B.; Panopoulos, A.D.; Schluns, K.S.; et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORα and ROR & gamma. Immunity 2008, 28, 29–39. [Google Scholar] [CrossRef]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 Regulates Cytokine-mediated Generation of Inflammatory Helper T Cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef] [PubMed]
- Taborda, C.P.; Travassos, L.R. Peptide Vaccine Against Paracoccidioidomycosis. In Methods in Molecular Biology; Springer Science, Business Media, LLC: Berlin/Heidelberg, Germany, 2017; Volume 1625, pp. 113–128. [Google Scholar]
- Woodworth, J.S.; Aagaard, C.S.; Hansen, P.R.; Cassidy, J.P.; Agger, E.M.; Andersen, P. Protective CD4 T Cells Targeting Cryptic Epitopes of Mycobacterium tuberculosis Resist Infection-Driven Terminal Differentiation. J. Immunol. 2014, 192, 3247–3258. [Google Scholar] [CrossRef]
- Tristão, F.S.M.; Rocha, F.A.; Carlos, D.; Ketelut-Carneiro, N.; Souza, C.O.S.; Milanezi, C.M.; Silva, J.S. Th17-Inducing Cytokines IL-6 and IL-23 Are Crucial for Granuloma Formation during Experimental Paracoccidioidomycosis. Front. Immunol. 2017, 8, 949. [Google Scholar] [CrossRef]
- Petersen, H.J.; Smith, A.M. The Role of the Innate Immune System in Granulomatous Disorders. Front. Immunol. 2013, 4, 120. [Google Scholar] [CrossRef]
| Description | Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| DDA /TDB 250/50 μg | 471.5 ± 2 | 0.528 | 44.3 ± 2 |
| DDA/TDB 250/50 μg/P10 20 μg | 667.9 ± 3 | 0.302 | 39 ± 3 |
| DDA/TDB 312.5/62.5 μg/P10 20 μg | 650.8 ± 5 | 0.369 | 44.7 ± 3 |
| DDA/TDB 100/500 μg/P10 20 μg | 639.2 ± 3 | 0.411 | 45.7 ± 4 |
| Description | Adsorption Efficiency % |
|---|---|
| DDA/TDB 250/50 μg/P10 20 μg | 84.5% (*) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, M.V.d.; Santos Júnior, S.R.D.; Nosanchuk, J.D.; Taborda, C.P. Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis. J. Fungi 2020, 6, 347. https://doi.org/10.3390/jof6040347
Araújo MVd, Santos Júnior SRD, Nosanchuk JD, Taborda CP. Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis. Journal of Fungi. 2020; 6(4):347. https://doi.org/10.3390/jof6040347
Chicago/Turabian StyleAraújo, Marcelo Valdemir de, Samuel Rodrigues Dos Santos Júnior, Joshua D. Nosanchuk, and Carlos Pelleschi Taborda. 2020. "Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis" Journal of Fungi 6, no. 4: 347. https://doi.org/10.3390/jof6040347
APA StyleAraújo, M. V. d., Santos Júnior, S. R. D., Nosanchuk, J. D., & Taborda, C. P. (2020). Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis. Journal of Fungi, 6(4), 347. https://doi.org/10.3390/jof6040347








