Chronic Pulmonary Aspergillosis Following Nontuberculous Mycobacterial Infections: An Emerging Disease
Abstract
1. Introduction
2. Epidemiology
3. Pathogenesis
4. Risk Factors
5. NTM Species and Risk of CPA
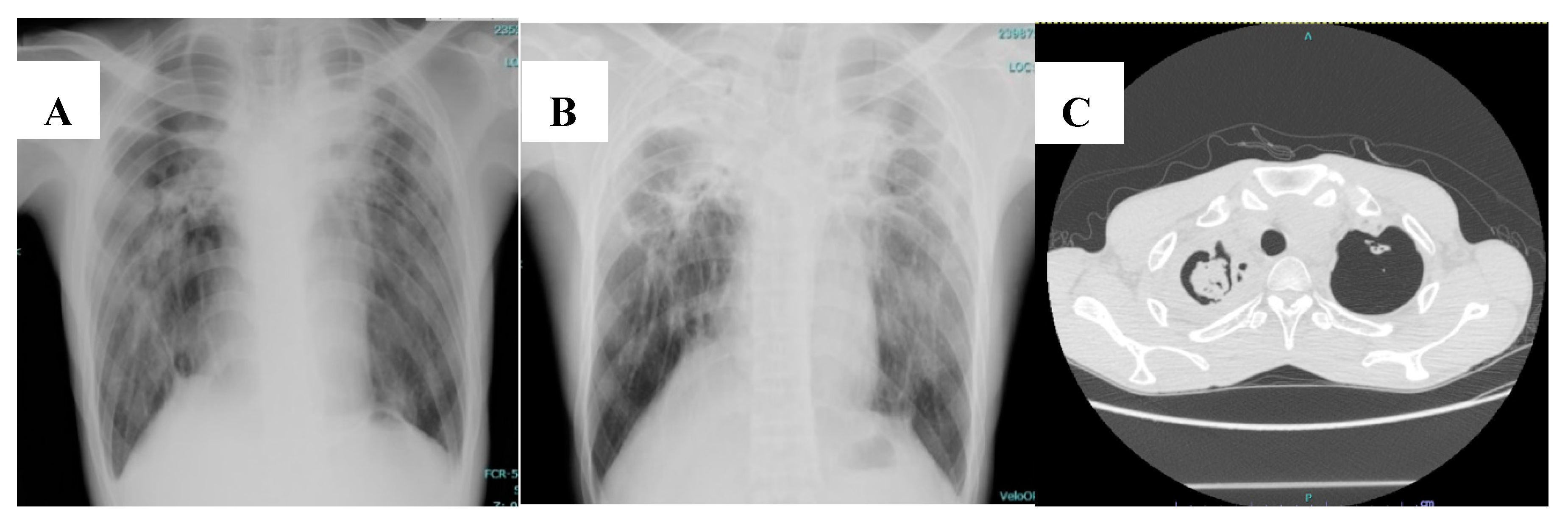
6. Clinical Manifestation and Diagnosis
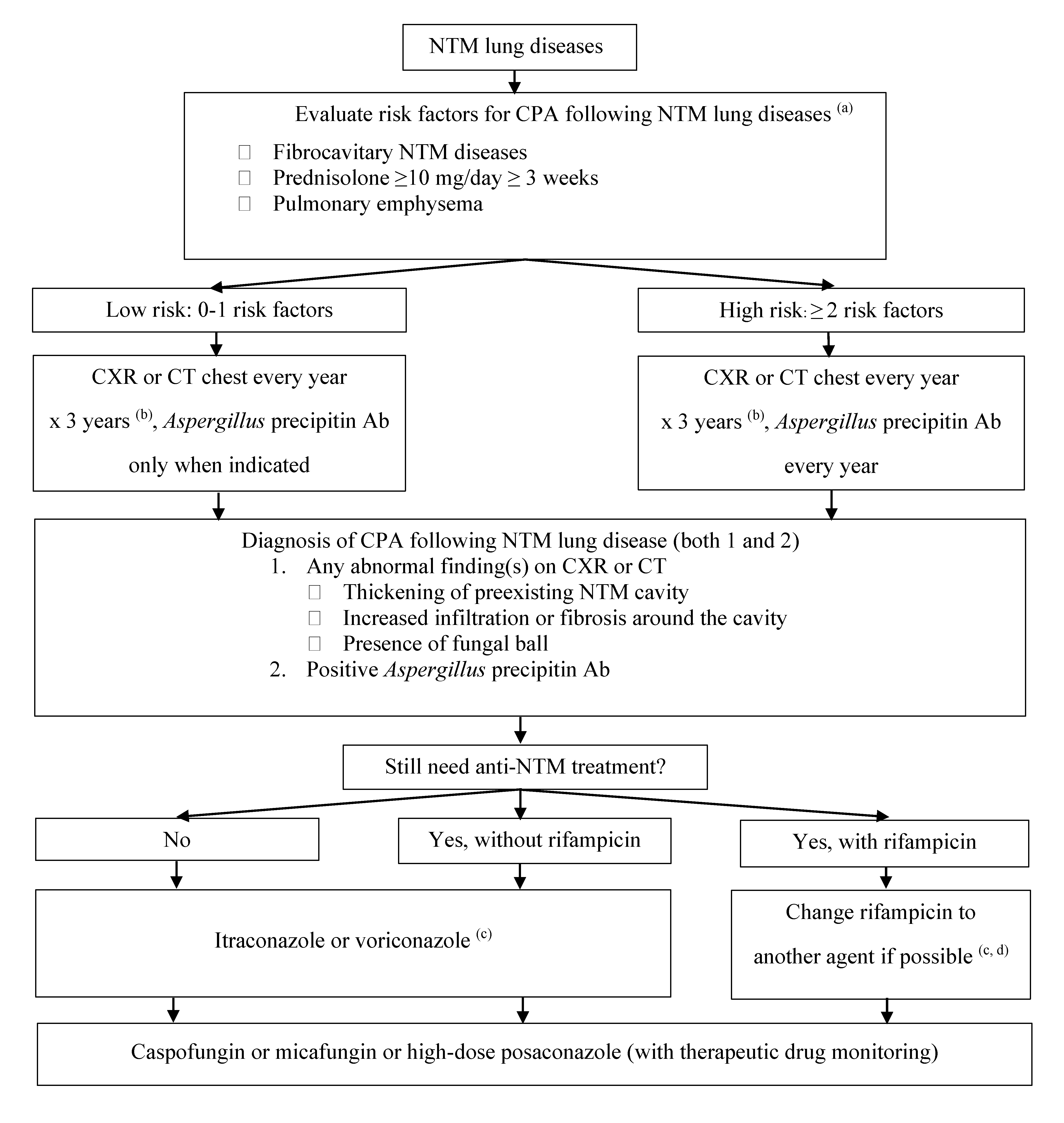
7. Treatment
8. Prognosis
9. Conclusions
10. Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kosmidis, C.; Newton, P.; Muldoon, E.G.; Denning, D.W. Chronic fibrosing pulmonary aspergillosis: A cause of ‘destroyed lung’ syndrome. Infect. Dis. (Lond.) 2017, 49, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.E.; Novak-Frazer, L. Chronic Pulmonary Aspergillosis-Where Are We? and Where Are We Going? J. Fungi 2016, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef]
- Page, I.D.; Byanyima, R.; Hosmane, S.; Onyachi, N.; Opira, C.; Richardson, M. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur. Respir. J. 2019, 53, 1801184. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Azimi, Y.; Droudinia, A.; Mousavi, B.; Khalilian, A.; Hedayati, N. Prevalence of chronic pulmonary aspergillosis in patients with tuberculosis from Iran. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1759–1765. [Google Scholar] [CrossRef]
- Bongomin, F. Post-tuberculosis chronic pulmonary aspergillosis: An emerging public health concern. PLoS Pathog. 2020, 16, e1008742. [Google Scholar] [CrossRef]
- Nam, H.S.; Jeon, K.; Um, S.W.; Suh, G.Y.; Chung, M.P.; Kim, H. Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: A review of 43 cases. Int. J. Infect. Dis. 2010, 14, e479–e482. [Google Scholar] [CrossRef]
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef]
- Ratnatunga, C.N.; Lutzky, V.P.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Field, M. The Rise of Non-Tuberculosis Mycobacterial Lung Disease. Front Immunol. 2020, 11, 303. [Google Scholar] [CrossRef]
- Zoumot, Z.; Boutou, A.K.; Gill, S.S.; van Zeller, M.; Hansell, D.M.; Wells, A.U. Mycobacterium avium complex infection in non-cystic fibrosis bronchiectasis. Respirology 2014, 19, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Yano, S.; Kadowaki, T.; Wakabayashi, K.; Kimura, M.; Kobayashi, K. [Clinical analysis of non-tuberculous mycobacteriosis cases complicated with pulmonary aspergillosis]. Kekkaku 2011, 86, 781–785. [Google Scholar] [PubMed]
- Chen, J.C.; Chang, Y.L.; Luh, S.P.; Lee, J.M.; Lee, Y.C. Surgical treatment for pulmonary aspergilloma: A 28 year experience. Thorax 1997, 52, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Vaideeswar, P.; Pandit, S.P. Pathology of pulmonary aspergillomas. Indian J. Pathol. Microbiol. 2008, 51, 342–345. [Google Scholar]
- Maliwan, N.; Zvetina, J.R. Pulmonary mycetoma following Mycobacterium kansasii infection. Report of seven cases. Arch. Intern. Med. 1985, 145, 2180–2183. [Google Scholar] [CrossRef]
- Bollert, F.G.; Sime, P.J.; MacNee, W.; Crompton, G.K. Pulmonary Mycobacterium malmoense and aspergillus infection: A fatal combination? Thorax 1994, 49, 521–522. [Google Scholar] [CrossRef][Green Version]
- Johnston, I.D. Mycobacterium xenopi infection and aspergilloma. Tubercle 1988, 69, 139–143. [Google Scholar] [CrossRef]
- Hafeez, I.; Muers, M.F.; Murphy, S.A.; Evans, E.G.; Barton, R.C.; McWhinney, P. Non-tuberculous mycobacterial lung infection complicated by chronic necrotising pulmonary aspergillosis. Thorax 2000, 55, 717–719. [Google Scholar] [CrossRef]
- Kunst, H.; Wickremasinghe, M.; Wells, A.; Wilson, R. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur. Respir. J. 2006, 28, 352–357. [Google Scholar] [CrossRef]
- Henkle, E.; Winthrop, K.L. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin. Chest Med. 2015, 36, 91–99. [Google Scholar] [CrossRef]
- Fowler, S.J.; French, J.; Screaton, N.J.; Foweraker, J.; Condliffe, A.; Haworth, C.S. Nontuberculous mycobacteria in bronchiectasis: Prevalence and patient characteristics. Eur. Respir. J. 2006, 28, 1204–1210. [Google Scholar] [CrossRef]
- Damaraju, D.; Jamieson, F.; Chedore, P.; Marras, T.K. Isolation of non-tuberculous mycobacteria among patients with pulmonary tuberculosis in Ontario, Canada. Int. J. Tuberc. Lung. Dis. 2013, 17, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Imamura, Y.; Takazono, T.; Yoshida, M.; Ide, S.; Hirano, K. The risk factors for developing of chronic pulmonary aspergillosis in nontuberculous mycobacteria patients and clinical characteristics and outcomes in chronic pulmonary aspergillosis patients coinfected with nontuberculous mycobacteria. Med. Mycol. 2016, 54, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Furuuchi, K.; Ito, A.; Hashimoto, T.; Kumagai, S.; Ishida, T. Risk stratification for the development of chronic pulmonary aspergillosis in patients with Mycobacterium avium complex lung disease. J. Infect. Chemother. 2018, 24, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Furuuchi, K.; Fujiwara, K.; Nakamoto, K.; Tanaka, Y.; Ishii, H. Impact of Aspergillus precipitating antibody test results on clinical outcomes of patients with Mycobacterium avium complex lung disease. Respir. Med. 2020, 166, 105955. [Google Scholar] [CrossRef] [PubMed]
- Jhun, B.W.; Jung, W.J.; Hwang, N.Y.; Park, H.Y.; Jeon, K.; Kang, E.S. Risk factors for the development of chronic pulmonary aspergillosis in patients with nontuberculous mycobacterial lung disease. PLoS ONE 2017, 12, e0188716. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Kontoyiannis, D.P. Glucocorticoids and invasive fungal infections. Lancet 2003, 362, 1828–1838. [Google Scholar] [CrossRef]
- Ng, T.T.; Robson, G.D.; Denning, D.W. Hydrocortisone-enhanced growth of Aspergillus spp.: Implications for pathogenesis. Microbiology (Read.) 1994, 140 Pt 9, 2475–2479. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Amaya-Villar, R.; Ortiz-Leyba, C.; Leon, C.; Alvarez-Lerma, F.; Nolla-Salas, J. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: Risk factors, clinical presentation and outcome. Crit. Care 2005, 9, R191–R199. [Google Scholar] [CrossRef]
- Kobashi, Y.; Fukuda, M.; Yoshida, K.; Miyashita, N.; Niki, Y.; Oka, M. Chronic necrotizing pulmonary aspergillosis as a complication of pulmonary Mycobacterium avium complex disease. Respirology 2006, 11, 809–813. [Google Scholar] [CrossRef]
- Furuuchi, K.; Ito, A.; Hashimoto, T.; Kumagai, S.; Ishida, T. Clinical significance of Aspergillus species isolated from respiratory specimens in patients with Mycobacterium avium complex lung disease. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Cheng, M.H.; Lu, P.L.; Shu, C.C.; Wang, J.Y.; Wang, J.T. Epidemiology and Predictors of NTM Pulmonary Infection in Taiwan-a Retrospective, Five-Year Multicenter Study. Sci. Rep. 2017, 7, 16300. [Google Scholar] [CrossRef] [PubMed]
- Furuuchi, K.; Morimoto, K.; Yoshiyama, T.; Tanaka, Y.; Fujiwara, K.; Okumura, M. Interrelational changes in the epidemiology and clinical features of nontuberculous mycobacterial pulmonary disease and tuberculosis in a referral hospital in Japan. Respir. Med. 2019, 152, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.Y.H.; Chotirmall, S.H.; Fok, E.T.K.; Verma, A.; De, P.P.; Goh, S.K. Profiling non-tuberculous mycobacteria in an Asian setting: Characteristics and clinical outcomes of hospitalized patients in Singapore. BMC Pulm. Med. 2018, 18, 85. [Google Scholar] [CrossRef]
- Phoompoung, P.; Ankasekwinai, N.; Pithukpakorn, M.; Foongladda, S.; Umrod, P.; Suktitipat, B. Factors associated with acquired Anti IFN- gamma autoantibody in patients with nontuberculous mycobacterial infection. PLoS ONE 2017, 12, e0176342. [Google Scholar] [CrossRef]
- Delliere, S.; Angebault, C.; Fihman, V.; Foulet, F.; Lepeule, R.; Maitre, B. Concomitant Presence of Aspergillus Species and Mycobacterium Species in the Respiratory Tract of Patients: Underestimated Co-occurrence? Front Microbiol. 2019, 10, 2980. [Google Scholar] [CrossRef]
- Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef]
- Drayton, J.; Dickinson, G.; Rinaldi, M.G. Coadministration of rifampin and itraconazole leads to undetectable levels of serum itraconazole. Clin. Infect. Dis. 1994, 18, 266. [Google Scholar] [CrossRef]
- Maghrabi, F.; Denning, D.W. The Management of Chronic Pulmonary Aspergillosis: The UK National Aspergillosis Centre Approach. Curr. Fungal Infect. Rep. 2017, 11, 242–251. [Google Scholar] [CrossRef]
- Kohno, S.; Izumikawa, K.; Ogawa, K.; Kurashima, A.; Okimoto, N.; Amitani, R. Intravenous micafungin versus voriconazole for chronic pulmonary aspergillosis: A multicenter trial in Japan. J. Infect. 2010, 61, 410–418. [Google Scholar] [CrossRef]
- Kohno, S.; Izumikawa, K.; Yoshida, M.; Takesue, Y.; Oka, S.; Kamei, K. A double-blind comparative study of the safety and efficacy of caspofungin versus micafungin in the treatment of candidiasis and aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 387–397. [Google Scholar] [CrossRef]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R., 3rd. Aspiring Antifungals: Review of Current Antifungal Pipeline Developments. J. Fungi 2020, 6, 28. [Google Scholar] [CrossRef]
- Moon, S.M.; Park, H.Y.; Jeong, B.H.; Jeon, K.; Lee, S.Y.; Koh, W.J. Effect of rifampin and rifabutin on serum itraconazole levels in patients with chronic pulmonary aspergillosis and coexisting nontuberculous mycobacterial infection. Antimicrob. Agents Chemother. 2015, 59, 663–665. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline: Executive Summary. Clin. Infect. Dis. 2020, 71, e1–e36. [Google Scholar] [CrossRef]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72 (Suppl. 2), ii1–ii64. [Google Scholar] [CrossRef]
- Debieuvre, D. Pulmonary Mycobacterium malmoense and Aspergillus infection. Thorax 1995, 50, 216. [Google Scholar] [CrossRef][Green Version]
- Fukushima, K.; Kitada, S.; Abe, Y.; Yamamoto, Y.; Matsuki, T.; Kagawa, H. Long-Term Treatment Outcome of Progressive Mycobacterium Avium Complex Pulmonary Disease. J. Clin. Med. 2020, 9, 1315. [Google Scholar] [CrossRef]
- Lowes, D.; Al-Shair, K.; Newton, P.J.; Morris, J.; Harris, C.; Rautemaa-Richardson, R. Predictors of mortality in chronic pulmonary aspergillosis. Eur. Respir. J. 2017, 49, 1601062. [Google Scholar] [CrossRef]
- Naito, M.; Kurahara, Y.; Yoshida, S.; Ikegami, N.; Kobayashi, T.; Minomo, S. Prognosis of chronic pulmonary aspergillosis in patients with pulmonary non-tuberculous mycobacterial disease. Respir. Investig. 2018, 56, 326–331. [Google Scholar] [CrossRef]
| Parameter | CPA Following Pulmonary TB | CPA Following NTM Lung Disease |
|---|---|---|
| Incidence | Higher | Lower |
| Host | Mostly immunocompetent | Immunocompetent and immunocompromised |
| Risk factors | Residual cavitation | Fibrocavitary disease Prednisolone ≥ 10 mg/day ≥ 3 weeks Pulmonary emphysema |
| Microbiological diagnosis | Aspergillus precipitin or fungal culture | Aspergillus precipitin is preferred Fungal culture had lower specificity due to Aspergillus colonization |
| Treatment | Drug interaction is less concerning since most CPA cases occurred after anti-mycobacterial agent discontinuation | Drug interaction is of more concern since most CPA cases occurred while receiving anti-mycobacterial agents |
| Prognosis | Better | Poorer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoompoung, P.; Chayakulkeeree, M. Chronic Pulmonary Aspergillosis Following Nontuberculous Mycobacterial Infections: An Emerging Disease. J. Fungi 2020, 6, 346. https://doi.org/10.3390/jof6040346
Phoompoung P, Chayakulkeeree M. Chronic Pulmonary Aspergillosis Following Nontuberculous Mycobacterial Infections: An Emerging Disease. Journal of Fungi. 2020; 6(4):346. https://doi.org/10.3390/jof6040346
Chicago/Turabian StylePhoompoung, Pakpoom, and Methee Chayakulkeeree. 2020. "Chronic Pulmonary Aspergillosis Following Nontuberculous Mycobacterial Infections: An Emerging Disease" Journal of Fungi 6, no. 4: 346. https://doi.org/10.3390/jof6040346
APA StylePhoompoung, P., & Chayakulkeeree, M. (2020). Chronic Pulmonary Aspergillosis Following Nontuberculous Mycobacterial Infections: An Emerging Disease. Journal of Fungi, 6(4), 346. https://doi.org/10.3390/jof6040346





