Arbuscular Mycorrhizal Fungi Promote Soil Respiration Primarily Through Mediating Microbial and Root Biomass in Rocky Desertification Habitat
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil, AM Fungi, and Plant Preparations
2.2. Experimental Design and Inoculation
2.3. Soil Respiration Measurement
2.4. Sampling and Determination
2.5. Data Analysis
3. Results
3.1. Temporal Variation in Soil Respiration Under AM Fungi Inoculation
3.2. Shift in Plant Growth, Microbial, and Soil Variables Under AM Fungi Inoculation
3.3. Linking Soil Respiration with Soil Variables, Microbial Biomass, and Root Biomass Under AM Fungi Inoculation
3.4. Pathways of Plant, Microbial, and Soil Properties Regulating Soil Respiration Under AM Fungi Inoculation
4. Discussion
4.1. Impact of AM Fungi Inoculation on Soil Respiration
4.2. Impact of Plant and Soil Variables on Soil Respiration
4.3. Impact of Microbial and Root Biomass on Soil Respiration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, P.; Ganjurjav, H.; Wan, Z.; Hu, G.; Gu, R.; Gao, Q. Changes in soil respiration after eight years of warming and increased precipitation in a semiarid temperate steppe. Agric. Ecosyst. Environ. 2024, 373, 109110. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, S.; Chen, S.P.; Ren, T.T.; Yang, Z.Q.; Zhao, H.L.; Liang, Y.; Han, X.G. Arbuscular mycorrhizal fungi regulate soil respiration and its response to precipitation change in a semiarid steppe. Sci. Rep. 2016, 6, 19990. [Google Scholar] [CrossRef]
- Laza, H.E.; Acosta-Martinez, V.; Cano, A.; Baker, J.; Mahan, J.; Gitz, D.; Emendack, Y.; Slaughter, L.; Lascano, R.; Tissue, D.; et al. Elevated [CO2] enhances soil respiration and AMF abundance in a semiarid peanut agroecosystem. Agric. Ecosyst. Environ. 2023, 355, 108592. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.J.; Yang, B.; Zuo, Q.Q.; Cao, Q.B.; Wang, P.; Zhang, L.L.; Zhang, K.F.; Fan, Y.X. Response of soil respiration to tropical forest secondary succession in Xishuangbanna. J. Nanjing For. Univ. 2022, 46, 12. [Google Scholar]
- Matías, L.; Castro, J.; Zamora, R. Effect of simulated climate change on soil respiration in a Mediterranean-type ecosystem: Rainfall and habitat type are more important than temperature or the soil carbon pool. Ecosystems 2012, 15, 299–310. [Google Scholar] [CrossRef]
- Aganchich, B.; Wahbi, S.; Yaakoubi, A.; El-Aououad, H.; Bota, J. Effect of arbuscular mycorrhizal fungi inoculation on growth and physiology performance of olive tree under regulated deficit irrigation and partial rootzone drying. S. Afr. J. Bot. 2022, 148, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Ru, J.; Song, J.; Li, H.; Li, X.; Ma, Y.; Li, Z.; Hao, Y.; Chi, Z.; Hui, D.; et al. Increased precipitation and nitrogen addition accelerate the temporal increase in soil respiration during 8-year old-field grassland succession. Glob. Change Biol. 2022, 28, 3944–3959. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.C.; Xu, X.; Geng, Q.H.; Luo, Y.Q.; Ju, C.H.; Li, Q.; Zhou, Y. Global arbuscular mycorrhizal fungi diversity and abundance decreases with soil available phosphorus. Glob. Ecol. Biogeogr. 2023, 32, 1423–1434. [Google Scholar] [CrossRef]
- Xiao, D.; Gai, S.S.; He, X.Y.; Zhang, W.; Hu, P.L.; Andrey, V.S.; Yakov, K.; Wang, K.L. Habitat heterogeneity drives arbuscular mycorrhizal fungi and shrub communities in karst ecosystems. Catena 2023, 233, 107513. [Google Scholar] [CrossRef]
- Soundarya, R.; Bo, R.K.; Tae, K.L. Exploring the Roles of Arbuscular Mycorrhizal Fungi in Plant-Iron Homeostasis. Agriculture 2023, 13, 1918. [Google Scholar]
- De Moura, J.B.; Ramos, M.L.G.; de Freitas Konrad, M.L.; Júnior, O.J.S.; Silva, S.D. Mycorrhizal fungi arbuscular in organic and conventional sugarcane systems. Sci. Rep. 2024, 14, 14322. [Google Scholar] [CrossRef]
- Silva, D.F.D.; Moreira, J.V.; Sousa, L.I.S.D.; Santana, M.C.; Mota, J.C.A.; Queiroz, A.D.S.; Nascimento, Í.V.D.; Silva, A.M.M.; Araújo, A.S.F.D.; Melo, V.M.M.; et al. Arbuscular mycorrhizal fungi community in soils under desertification and restoration in the Brazilian semiarid. Microbiol. Res. 2022, 264, 127161. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Cheng, J.H.; Zhang, H.; Wang, X.L.; Shi, D.W. Response of preferential flow to soil–root–rock fragment system in karst rocky desertification areas. Ecol. Indic. 2024, 165, 112234. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Oliveira, C.M.; Silva, A.J.; Junior, L.F.G.O.; Fontes, P.T.N.; Fernandes, M.R.D.M.; Oliveira, D.D.S.; Santos, C.D.; Filho, R.N.D.A.; Cunha Filho, R.R.G.; et al. The inoculation with arbuscular mycorrhizal fungi improved ecophysiological and growth parameters of Schinus terebinthifolius and Caesalpinia ferrea in degraded mining sites. Environ. Chall. 2021, 4, 100181. [Google Scholar] [CrossRef]
- Kuyper, T.W.; Jansa, J. Arbuscular mycorrhiza: Advances and retreats in our understanding of the ecological functioning of the mother of all root symbioses. Plant Soil 2023, 489, 41–88. [Google Scholar] [CrossRef]
- Xia, J.H.; Wang, S.J.; Luo, S.; Li, R.; Yang, S.Q.; Lan, M.J.; Guo, X.F. Effects of earthworm and arbuscular mycorrhizal fungal inoculation on carbon component accumulation and allocation in rocky desertification soils. Chin. J. Appl. Ecol./Yingyong Shengtai Xuebao 2024, 35. [Google Scholar]
- Wang, S.J.; Zuo, Q.Q.; Cao, Q.B.; Wang, P.; Yang, B.; Zhao, S.; Chen, M.K. Response of readily oxidized carbon to arbuscular mycorrhizal (AM) fungi inoculations in rocky desert soil, Xundian, Yunnan Province. J. Nanjing For. Univ. 2022, 46, 7–14. [Google Scholar] [CrossRef]
- Li, Y.M.; Wang, S.J.; Lu, M.; Zhang, Z.; Chen, M.K.; Li, S.H.; Cao, R. Rhizosphere interactions between earthworms and arbuscular mycorrhizal fungi increase nutrient availability and plant growth in the desertification soils. Soil Tillage Res. 2019, 186, 146–151. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Wang, S.J.; Wang, H. Response of soil respiration to a severe drought in Chinese Eucalyptus plantations. J. For. Res. 2017, 28, 841–847. [Google Scholar] [CrossRef]
- Bekku, Y.; Koizumi, H.; Oikawa, T.; Iwaki, H. Examination of four methods for measuring soil respiration. Appl. Soil Ecol. 1997, 5, 247–254. [Google Scholar] [CrossRef]
- Gui, W.; Ren, H.; Liu, N.; Zhang, Y.; Cobb, A.B.; Wilson, G.W.; Sun, X.; Hu, J.; Xiao, Y.; Zhang, F.; et al. Plant functional group influences arbuscular mycorrhizal fungi abundance and hyphal contribution to soil CO2 efflux in temperate grasslands. Plant Soil 2018, 432, 157–170. [Google Scholar] [CrossRef]
- Kawakami, E.; Ataka, M.; Kume, T.; Shimono, K.; Harada, M.; Hishi, T.; Katayama, A. Root exudation in a sloping Moso bamboo forest in relation to fine root biomass and traits. PLoS ONE 2022, 17, e0266131. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.K. Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; Volume 107, pp. 147–150. [Google Scholar]
- Bai, J.; Chen, Y.; Ning, Z.; Liu, S.; Xu, C.; Yan, J.K. Proteoglycan isolated from Corbicula fluminea exerts hepato-protective effects against alcohol-induced liver injury in mice. Int. J. Biol. Macromol. 2020, 142, 1–10. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Q.L.; Ma, Z.T.; Jin, H.J.; Chag, X.L.; Marchenko, S.S.; Spektor, V.V. Seasonal variations in temperature sensitivity of soil respiration in a larch forest in the Northern Daxing’an Mountains in Northeast China. J. For. Res. 2021, 33, 1061–1070. [Google Scholar] [CrossRef]
- Wang, J.N.; Han, S.J.; Wang, C.G.; Li, M.H. Long-term nitrogen-addition-induced shifts in the ectomycorrhizal fungi community are associated with changes in fine root traits and soil properties in a mixed Pinus koraiensis forest. Eur. J. Soil Biol. 2022, 112, 103431. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Du, Y.; Zhang, D.; Tang, Z. Nutrients regulate the effects of arbuscular mycorrhizal fungi on the growth and reproduction of cherry tomato. Front. Microbiol. 2022, 13, 843010. [Google Scholar] [CrossRef]
- Mitra, D.; Nayeri, F.D.; Sansinenea, E.; Ortiz, A.; Bhatta, B.B.; Adeyemi, N.O.; Janeeshma, E.; Tawfeeq Al-Ani, L.K.; Sharma, S.B.; Boutaj, H.; et al. Unraveling arbuscular mycorrhizal fungi interaction in rice for plant growth development and enhancing phosphorus use efficiency through recent development of regulatory genes. J. Plant Nutr. 2023, 46, 3184–3220. [Google Scholar] [CrossRef]
- Dickson, S.; Smith, F.A.; Smith, S.E. Structural differences in arbuscular mycorrhizal symbioses: More than 100 years after Gallaud, where next? Mycorrhiza 2007, 17, 375–393. [Google Scholar] [CrossRef]
- Burke, D.J.; Weintraub, M.N.; Hewins, C.R.; Kalisz, S. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biol. Biochem. 2011, 43, 795–803. [Google Scholar] [CrossRef]
- Noceto, P.A.; Bettenfeld, P.; Boussageon, R.; Hériché, M.; Sportes, A.; van Tuinen, D.; Courty, P.E.; Wipf, D. Arbuscular mycorrhizal fungi, a key symbiosis in the development of quality traits in crop production, alone or combined with plant growth-promoting bacteria. Mycorrhiza 2021, 31, 655–669. [Google Scholar] [CrossRef]
- An, X.; Liu, J.; Liu, X.; Ma, C.; Zhang, Q. Optimizing phosphorus application rate and the mixed inoculation of arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria can improve the phosphatase activity and organic acid content in alfalfa soil. Sustainability 2022, 14, 11342. [Google Scholar] [CrossRef]
- Qu, M.; Li, S.; Yu, Y.; Zhang, J. Effects of arbuscular mycorrhizal fungi on the root morphology of Zenia insignis in karst soil habitat. Sci. Soil Water Conserv. 2021, 19, 106–114. [Google Scholar]
- Liu, H.F.; Wang, X.K.; Liang, C.T.; Ai, Z.; Wu, Y.; Xu, H.W.; Xue, S. Glomalin-related soil protein affects soil aggregation and recovery of soil nutrient following natural revegetation on the Loess Plateau. Geoderma 2020, 357, 113921. [Google Scholar] [CrossRef]
- Cavagnaro, T.R. Soil moisture legacy effects: Impacts on soil nutrients, plants and mycorrhizal responsiveness. Soil Biol. Biochem. 2016, 95, 173–179. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.J.; Yang, B.; Zhang, K.F.; Zhang, L.L.; Fan, Y.X. Effects of arbuscular mycorrhiza fungi inoculations on soil respiration in Yunnan rocky desertification habitat. Acta Ecol. Sin. 2022, 4, 8830–8838. [Google Scholar] [CrossRef]
- Herms, C.H.; Hennessy, R.C.; Bak, F.; Dresbøll, D.B.; Nicolaisen, M.H. Back to our roots: Exploring the role of root morphology as a mediator of beneficial plant-microbe interactions. Environ. Microbiol. 2022, 24, 3264–3272. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, F.; Liu, Y. Response of soil respiration under different mycorrhizal strategies to precipitation and temperature. J. Soil Sci. Plant Nutr. 2012, 12, 411–420. [Google Scholar] [CrossRef][Green Version]
- Dang, H.L.; Zhang, T.; Wang, Z.K.; Li, G.F.; Zhao, W.Q.; Lv, X.; Zhuang, L. Succession of endophytic fungi and arbuscular mycorrhizal fungi associated with the growth of plant and their correlation with secondary metabolites in the roots of plants. BMC Plant Biol. 2021, 21, 165. [Google Scholar] [CrossRef]
- Thilagar, G.; Bagyaraj, D.J. Influence of different arbuscular mycorrhizal fungi on growth and yield of chilly. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 71–75. [Google Scholar] [CrossRef]
- Khan, Y.; Shah, S.; Tian, H. The roles of arbuscular mycorrhizal fungi in influencing plant nutrients, photosynthesis, and metabolites of cereal crops—A review. Agronomy 2022, 12, 2191. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef]
- Wang, F.Y.; Lin, X.G.; Yin, R.; Wu, L.H. Effects of arbuscular mycorrhizal inoculation on the growth of Elsholtzia splendens and Zea mays and the activities of phosphatase and urease in a multi-metal-contaminated soil under unsterilized conditions. Appl. Soil Ecol. 2006, 31, 110–119. [Google Scholar] [CrossRef]
- Wu, Q.S.; Lou, Y.G.; Li, Y. Plant growth and tissue sucrose metabolism in the system of trifoliate orange and arbuscular mycorrhizal fungi. Sci. Hortic. 2015, 181, 189–193. [Google Scholar] [CrossRef]
- Alguacil, M.M.; Torrecillas, E.; Lozano, Z.; Roldán, A. Arbuscular mycorrhizal fungi communities in a coral cay system (Morrocoy, Venezuela) and their relationships with environmental variables. Sci. Total Environ. 2015, 505, 805–813. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; He, J.Z.; Zhu, Y.G.; Qiao, N.H.; Ge, Y. Arbuscular mycorrhizal fungi and plant diversity drive restoration of nitrogen-cycling microbial communities. Mol. Ecol. 2021, 30, 4133–4146. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sarkar, S. Arbuscular mycorrhizal fungal contribution towards plant resilience to drought conditions. Front. Fungal Biol. 2024, 5, 1355999. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Feng, G.; Limpens, E.; Bonfante, P.; Xie, X.N.; Zhang, L. Cross-kingdom nutrient exchange in the plant–arbuscular mycorrhizal fungus–bacterium continuum. Nat. Rev. Microbiol. 2024, 22, 773–790. [Google Scholar] [CrossRef]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.N.; Fu, S. Effects of arbuscular mycorrhizal fungi and biochar on growth, nutrient absorption, and physiological properties of maize (Zea mays L). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, B.; Honrubia, M.; Morte, A. How root structure defines the arbuscular mycorrhizal symbiosis and what we can learn from it? In Root Engineering: Basic and Applied Concepts; Springer: Berlin/Heidelberg, Germany, 2014; pp. 145–169. [Google Scholar]
- Zhou, X.; Zhang, J.; Khashi, U.; Rahman, M.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plants 2023, 16, 849–864. [Google Scholar] [CrossRef]
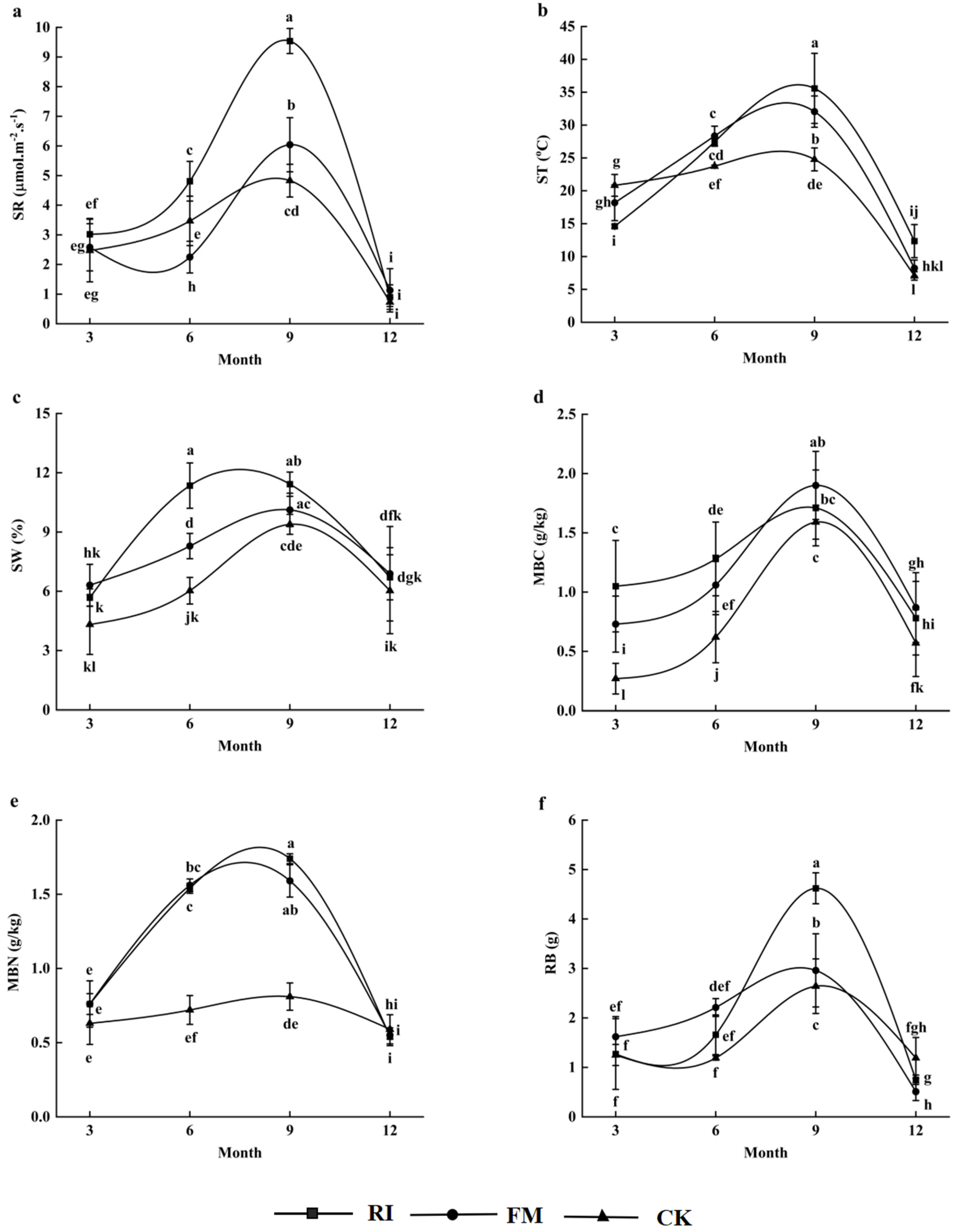
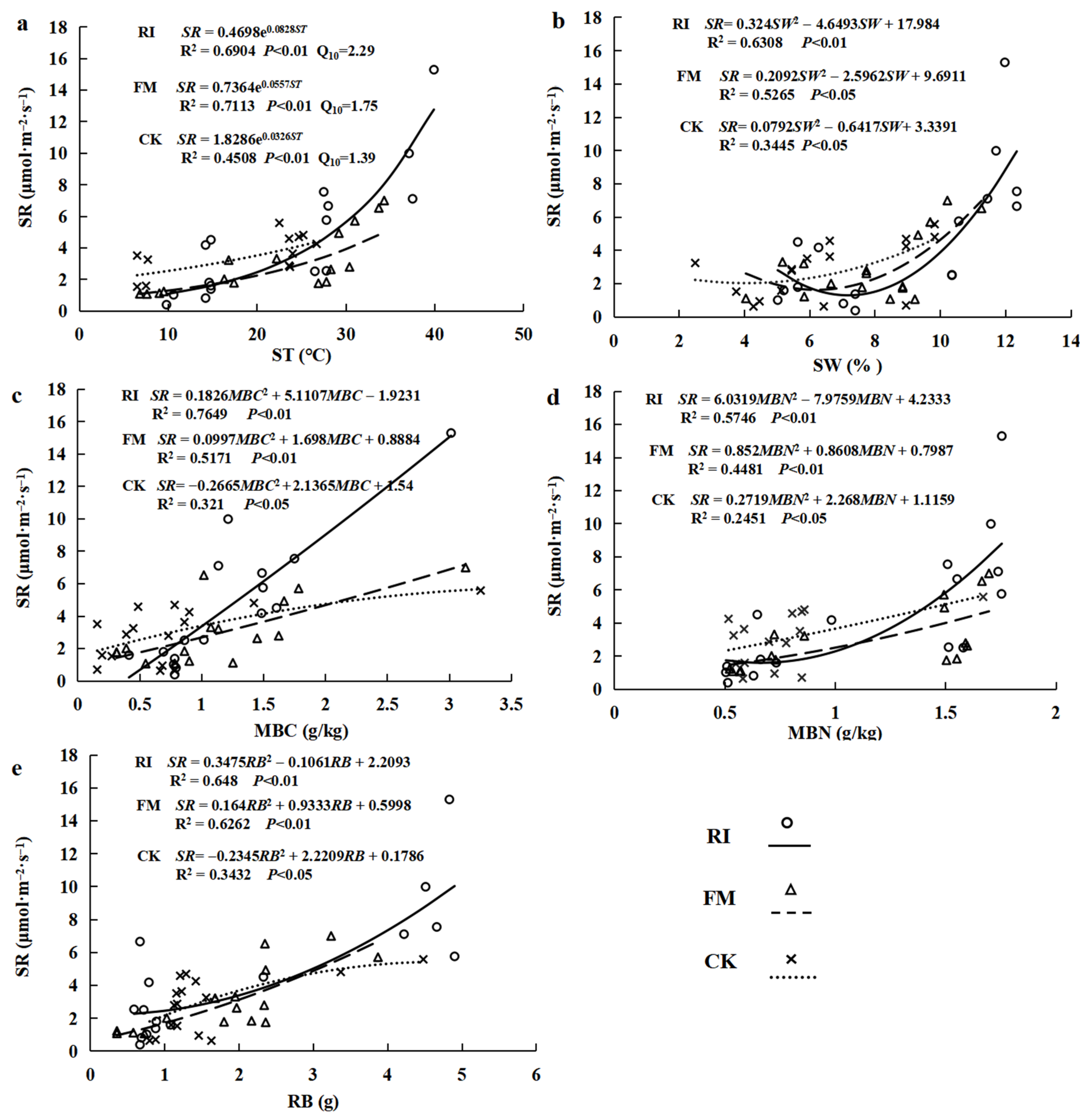
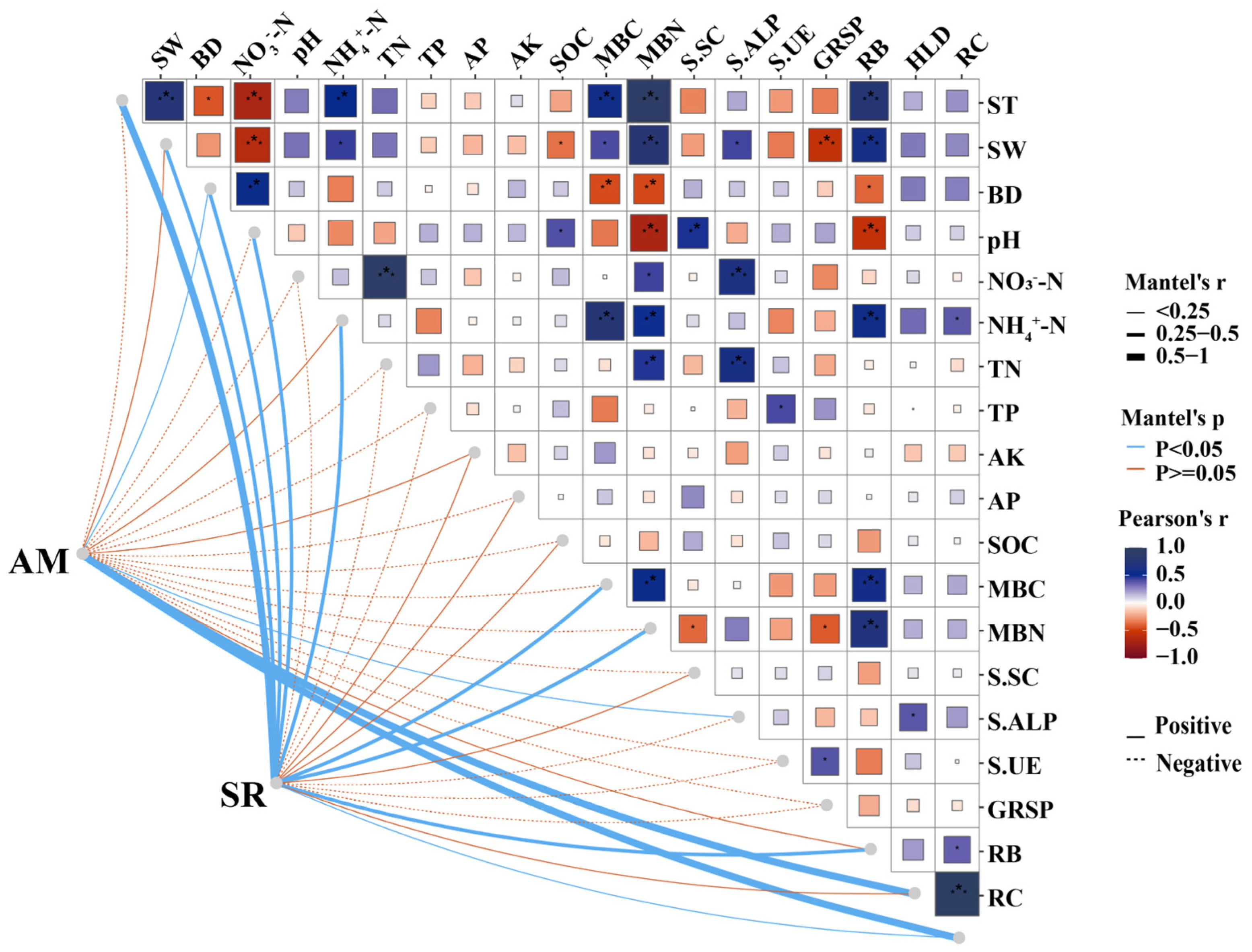
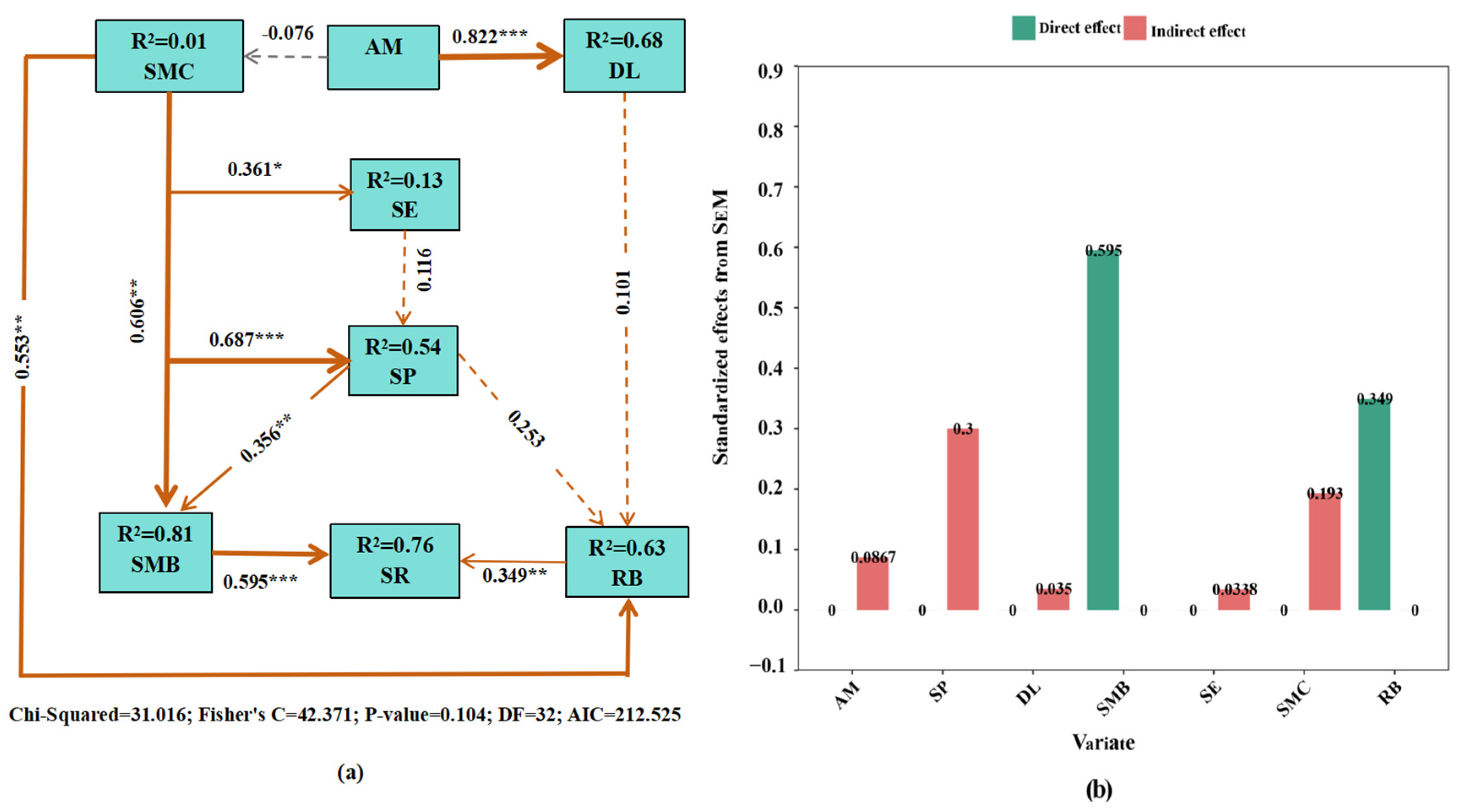
| Source | df | SR | ST | SW | MBC | MBN | RB |
|---|---|---|---|---|---|---|---|
| E | 2 | 5.45 ** | 10.42 ** | 14.30 ** | 2.65 ** | 130.83 ** | 1.55 |
| S | 3 | 27.88 ** | 226.64 ** | 35.60 ** | 8.11 ** | 244.96 ** | 22.29 ** |
| E × S | 6 | 2.13 | 11.31 ** | 3.22 | 0.33 | 37.12 ** | 2.58 |
| Treatment | RI | FM | CK |
|---|---|---|---|
| ST (°C) | 22.49 ± 10.16 a | 21.70 ± 9.77 a | 19.09 ± 7.42 a |
| SW (%) | 8.79 ± 2.82 a | 7.90 ± 1.97 ab | 6.44 ± 2.27 b |
| BD | 1.58 ± 0.18 a | 1.44 ± 0.13 ab | 1.74 ± 0.88 b |
| pH | 7.68 ± 0.31 a | 7.57 ± 0.27 a | 7.68 ± 0.31 a |
| NO3−-N (mg/g) | 4.26 ± 2.93 a | 2.53 ± 1.86 ab | 2.32 ± 1.65 b |
| NH4+-N (g/kg) | 4.42 ± 2.24 a | 3.35 ± 2.14 ab | 3.07 ± 0.93 b |
| TN (mg/kg) | 2.48 ± 1.74 a | 2.75 ± 1.99 a | 1.07 ± 0.41 b |
| TP (mg/g) | 1.26 ± 0.38 b | 1.66 ± 0.42 a | 0.95 ± 0.12 c |
| AP (mg/g) | 5.61 ± 1.14 a | 5.42 ± 1.73 a | 3.91 ± 1.62 b |
| AK (mg/g) | 3.05 ± 1.78 a | 3.72 ± 1.73 a | 2.92 ± 1.23 a |
| SOC (g/kg) | 6.52 ± 1.93 a | 6.29 ± 1.24 ab | 5.02 ± 1.28 b |
| MBC (g/kg) | 1.21 ± 0.62 a | 1.14 ± 0.70 a | 0.58 ± 0.74 b |
| MBN (g/kg) | 1.14 ± 0.53 a | 1.11 ± 0.49 a | 0.68 ± 0.13 b |
| S-SC (nmol/g/h) | 2.66 ± 0.59 a | 2.28 ± 0.52 b | 2.32 ± 0.06 b |
| S-ALP (nmol/g/h) | 2.63 ± 0.20 a | 1.54 ± 0.41 b | 1.10 ± 0.11 b |
| S-UE (nmol/g/h) | 3.58 ± 2.37 a | 2.67 ± 1.39 ab | 1.97 ± 0.81 b |
| GRSP (mg/g) | 4.36 ± 0.60 a | 4.38 ± 0.61 a | 4.35 ± 0.63 a |
| RB (g) | 2.07 ± 1.83 a | 1.82 ± 1.00 a | 1.20 ± 0.97 b |
| RC (%) | 90.43 ± 5.07 a | 57.54 ± 2.77 b | 35.40 ± 1.71 c |
| HLD (m/g) | 4.01 ± 0.45 a | 2.23 ± 0.25 b | 1.90 ± 0.08 c |
| H (cm) | 77.67 ± 15.44 a | 74.19 ± 15.96 b | 70.04 ± 17.07 c |
| BDH (mm) | 7.50 ± 1.89 a | 7.23 ± 2.00 b | 7.11 ± 2.09 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Wang, S.; Song, Y.; Xie, L.; Xiao, B.; Guo, X. Arbuscular Mycorrhizal Fungi Promote Soil Respiration Primarily Through Mediating Microbial and Root Biomass in Rocky Desertification Habitat. J. Fungi 2025, 11, 616. https://doi.org/10.3390/jof11090616
Zhao S, Wang S, Song Y, Xie L, Xiao B, Guo X. Arbuscular Mycorrhizal Fungi Promote Soil Respiration Primarily Through Mediating Microbial and Root Biomass in Rocky Desertification Habitat. Journal of Fungi. 2025; 11(9):616. https://doi.org/10.3390/jof11090616
Chicago/Turabian StyleZhao, Shuang, Shaojun Wang, Yali Song, Lingling Xie, Bo Xiao, and Xiaofei Guo. 2025. "Arbuscular Mycorrhizal Fungi Promote Soil Respiration Primarily Through Mediating Microbial and Root Biomass in Rocky Desertification Habitat" Journal of Fungi 11, no. 9: 616. https://doi.org/10.3390/jof11090616
APA StyleZhao, S., Wang, S., Song, Y., Xie, L., Xiao, B., & Guo, X. (2025). Arbuscular Mycorrhizal Fungi Promote Soil Respiration Primarily Through Mediating Microbial and Root Biomass in Rocky Desertification Habitat. Journal of Fungi, 11(9), 616. https://doi.org/10.3390/jof11090616







