Species Identification and Fungicide Sensitivity of Fusarium spp. Causing Peanut Root Rot in Henan, China
Abstract
1. Introduction
2. Materials and Methods
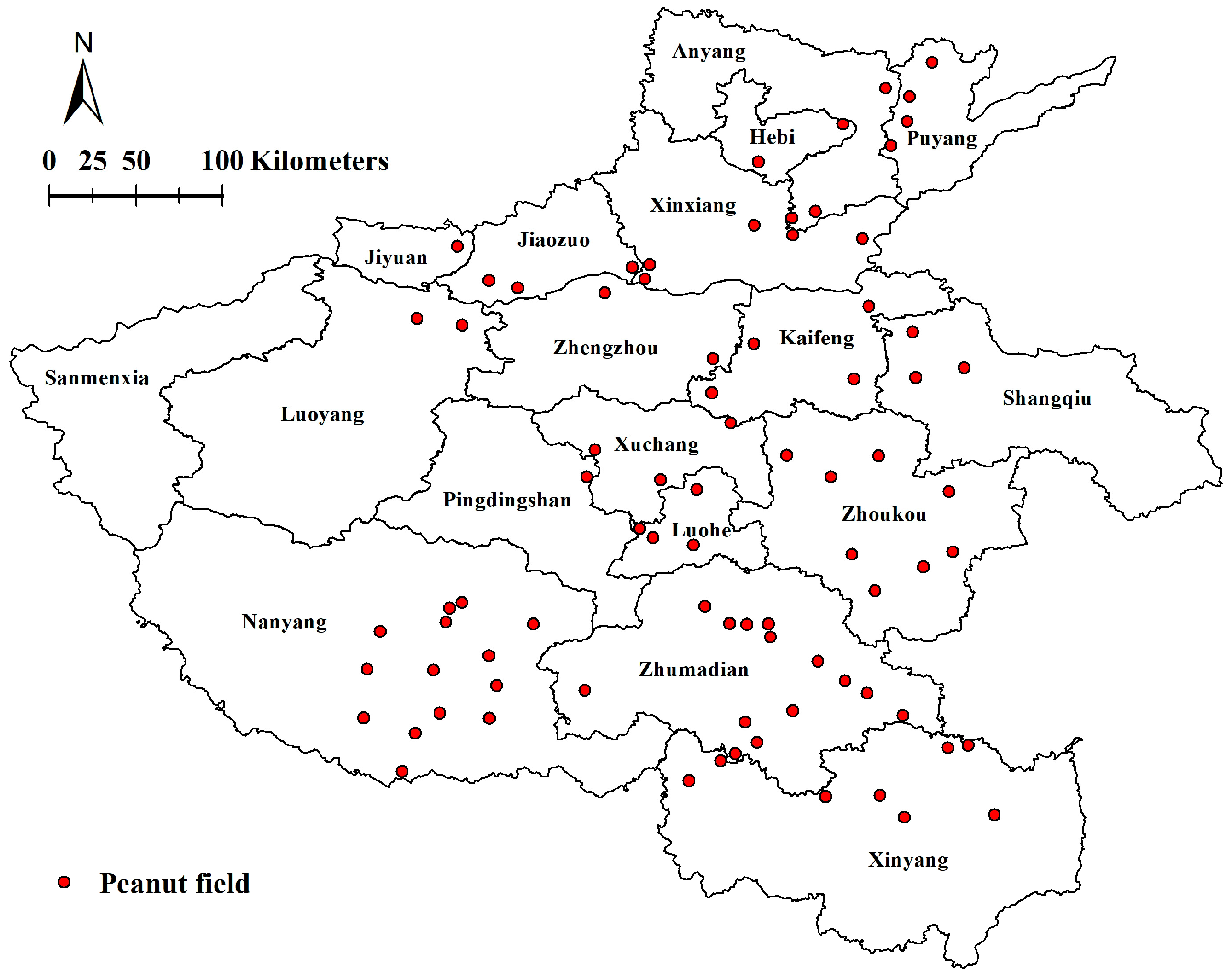
2.1. Sample Collection
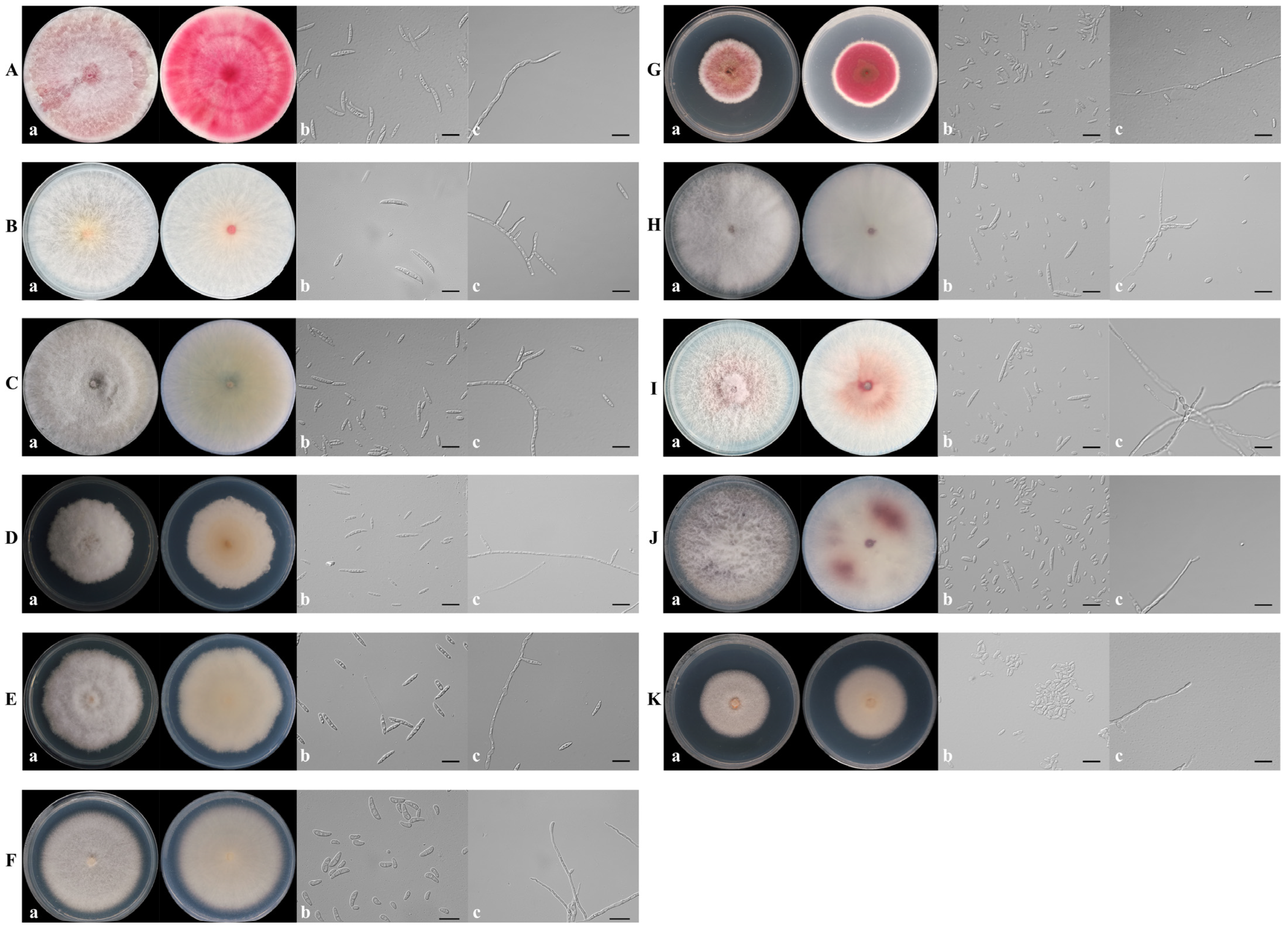
2.2. Isolation of Fungi and Morphological Observation
2.3. DNA Extraction, PCR Amplification and Sequencing
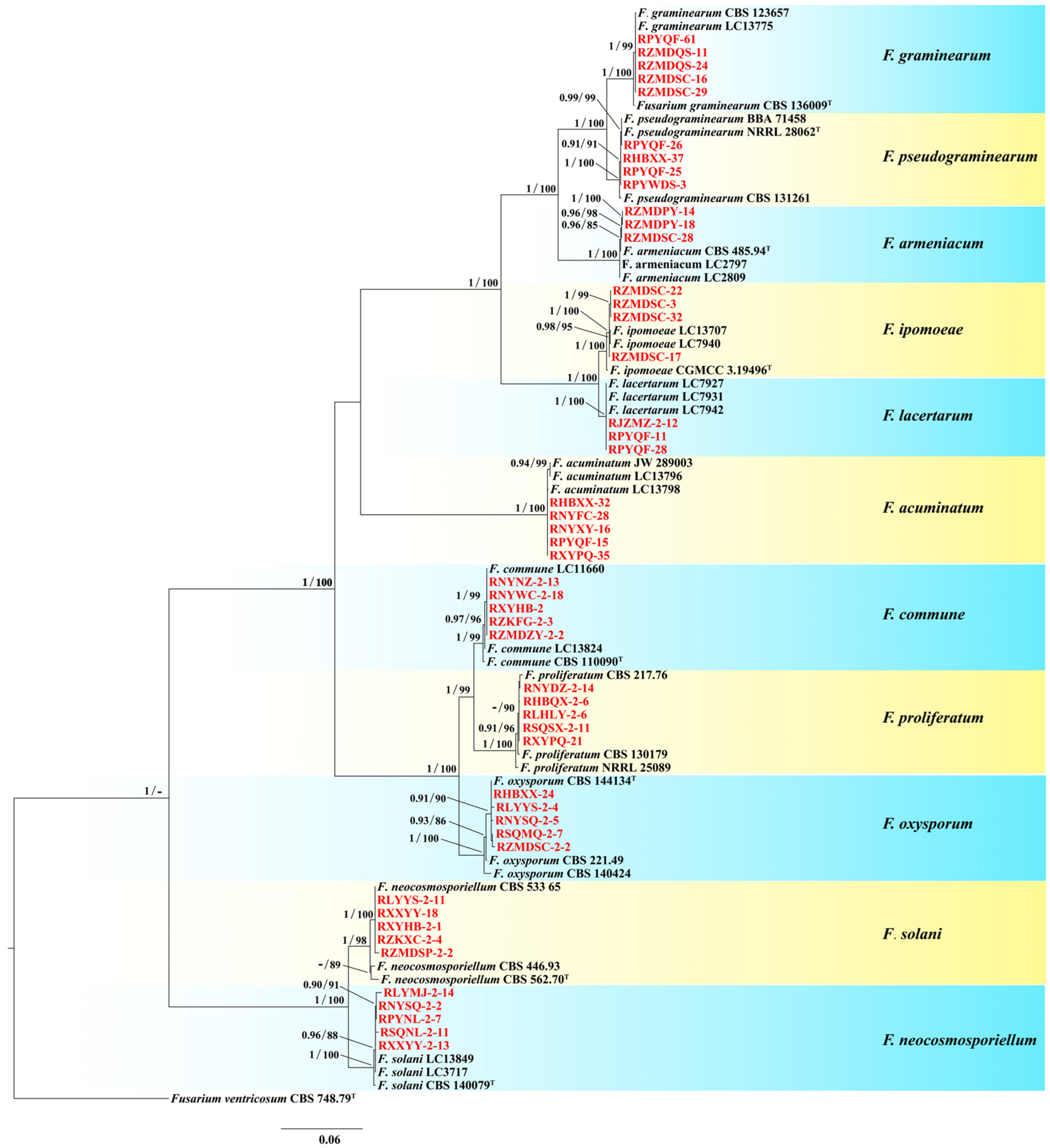
2.4. Phylogenetic Analysis
2.5. Prevalence
2.6. Pathogenicity Tests
2.7. Fungicide Sensitivity Assays
2.8. Statistical and Analysis
3. Results
3.1. Collection of Fungal Isolates
3.2. Species Identification and Phylogenetic Analysis
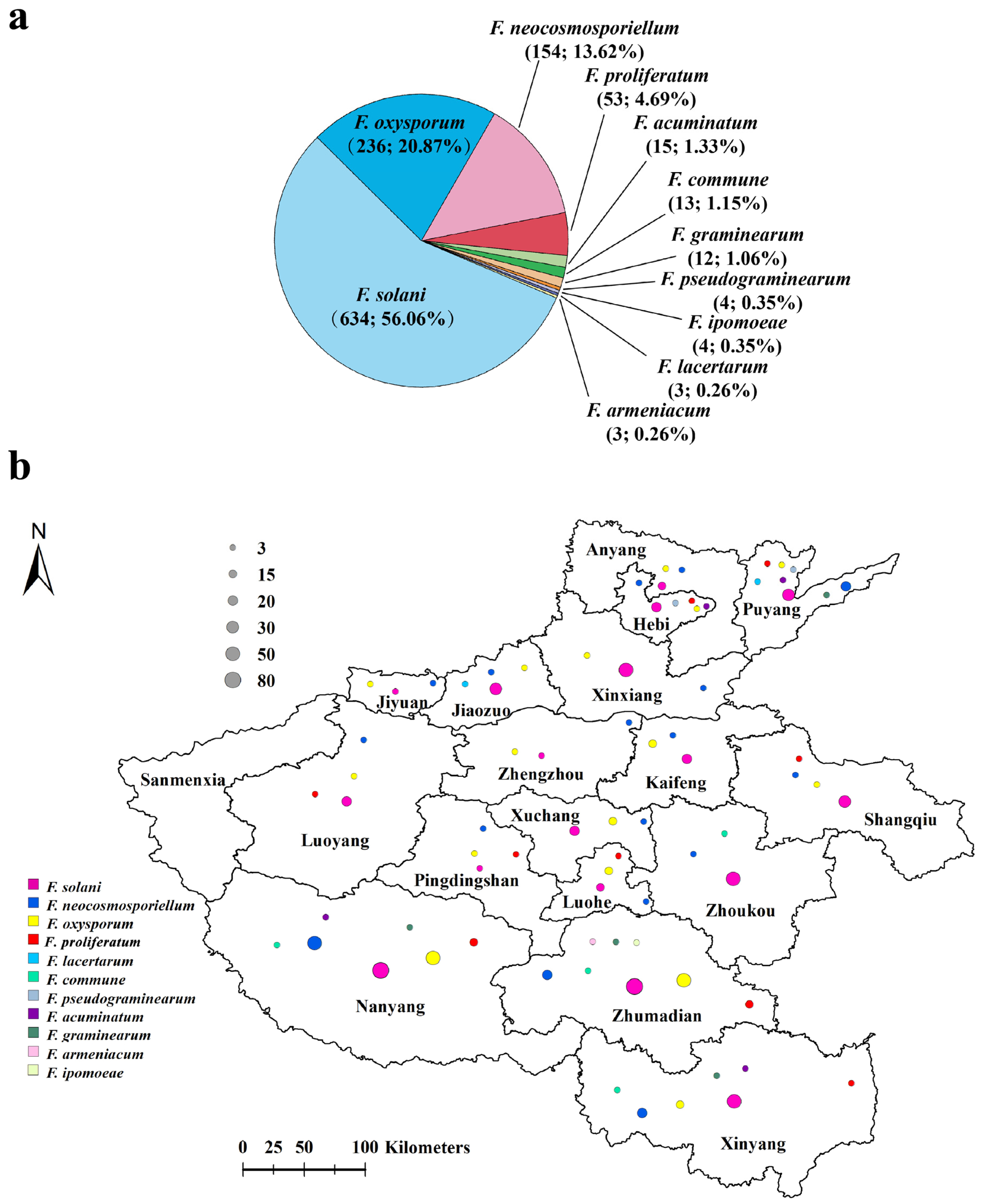
3.3. Prevalence of Fungal Species
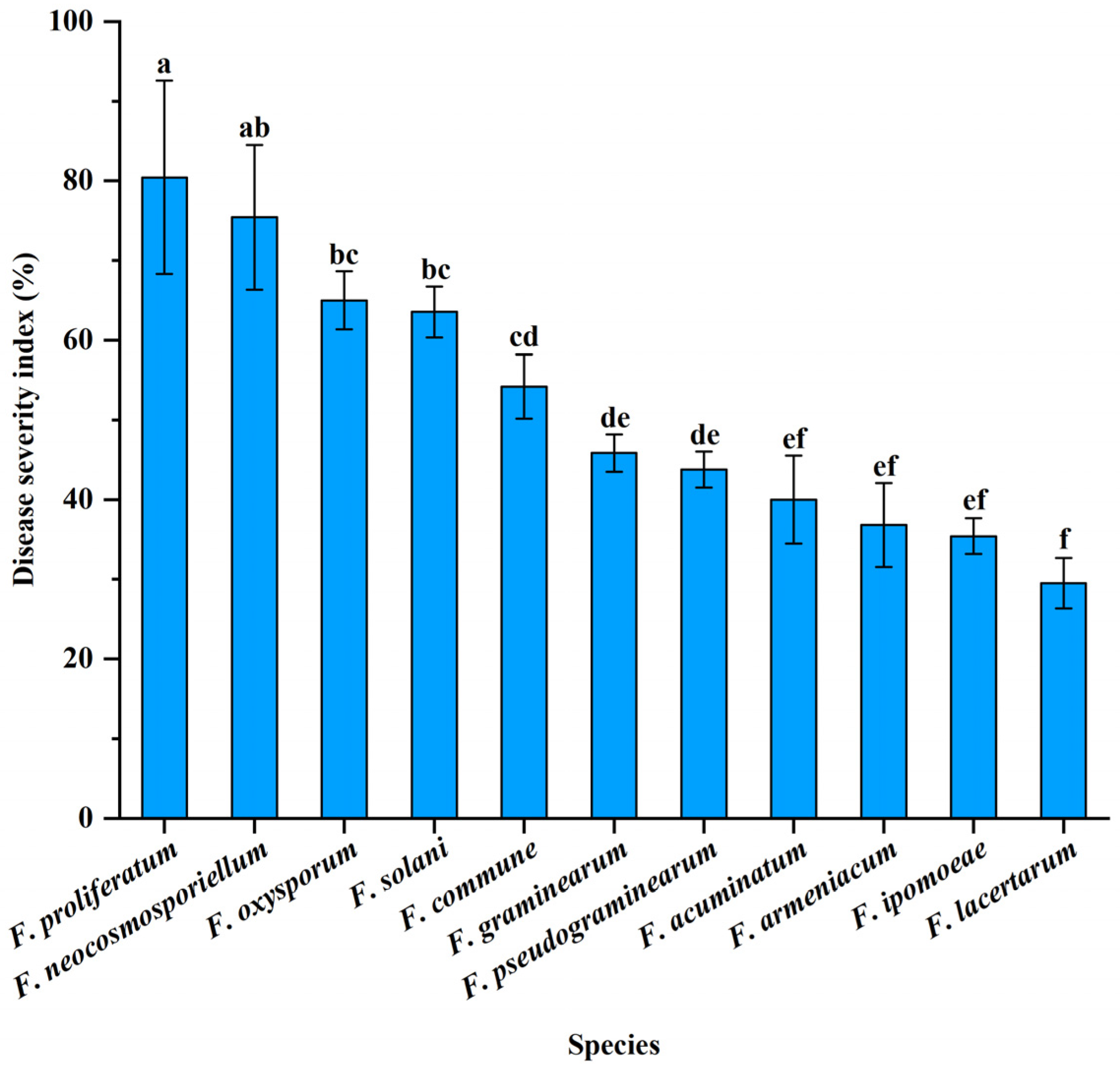
3.4. Pathogenicity Tests
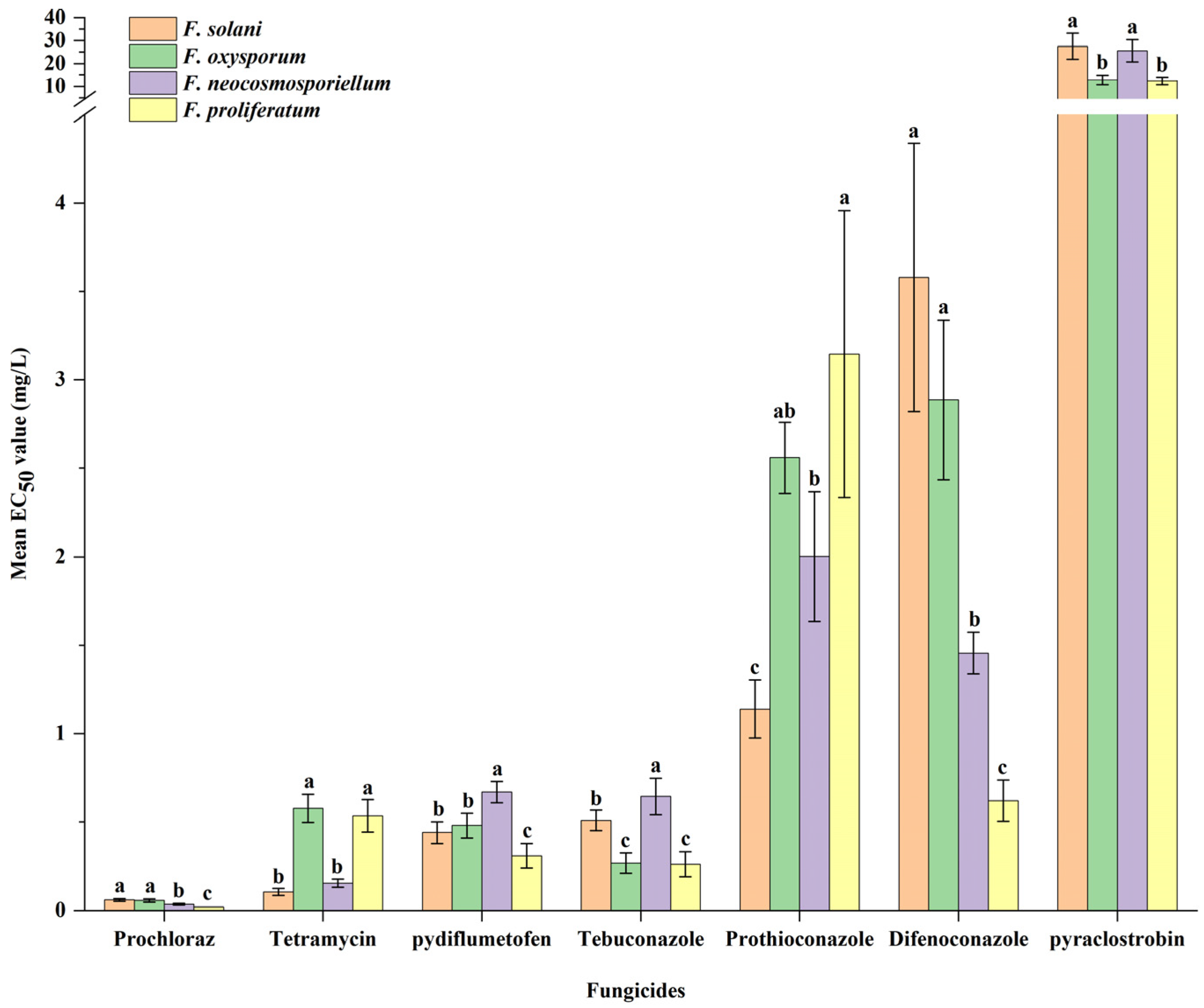
3.5. Fungicide Sensitivity Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRR | Peanut Root Rot |
| PDA | Potato Dextrose Agar |
| TEF1-α | The translation elongation factor 1-α |
| ML | Maximum Likelihood |
| BI | Bayesian Inference |
| MCMC | Markov Chain Monte Carlo |
| PP | Posterior Probability |
| BS | Bootstrap Values |
| FI | Isolation Frequency |
| DI | Disease Severity Index |
| DMSO | Dimethyl Sulfoxide |
| SHAM | Salicylhydroxamic Acid |
| ANOVA | Analysis of Variance |
| SD | Standard Deviation |
References
- Akram, N.A.; Shafiq, F.; Ashraf, M. Peanut (Arachis hypogaea L.): A prospective legume crop to offer multiple health benefits under changing climate. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization Statistical Database. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 February 2025).
- Min, H.; Li, H.H.; Zhou, L.; Mao, Q.; Hao, R. Recent Occurrence of Peanut Diseases in Henan Province and Integrated Application of Disease Management Strategies. China Plant Prot. 2024, 44, 74–77. [Google Scholar]
- Fuhlbohm, M.F.; Tatnell, J.R.; Ryley, M.J. Neocosmospora vasinfecta is pathogenic on peanut in Queensland. Australas. Plant Dis. Notes 2007, 2, 3–4. [Google Scholar] [CrossRef]
- Dau, V.T.; Pham, L.T.; Luong, T.M.; Huynh, L.M.T.; Tran, N.T.; Ho, T.D.; Hoang, H.M.T.; Phan, H.T.; Burgess, L.W. First report of Neocosmospora vasinfecta associated with the root rot complex of peanuts in Vietnam. Australas. Plant Dis. Notes 2010, 5, 79–81. [Google Scholar] [CrossRef]
- Zaman, N.; Ahmed, S. Survey of root rot of groundnut in rainfed areas of Punjab, Pakistan. Afr. J. Biotechnol. 2012, 11, 4791–4794. [Google Scholar] [CrossRef]
- Casasnovas, F.; Fantini, E.N.; Palazzini, J.M.; Giaj-Merlera, G.; Chulze, S.N.; Reynoso, M.M.; Torres, A.M. Development of amplified fragment length polymorphism (AFLP)-derived specific primer for the detection of Fusarium solani aetiological agent of peanut brown root rot. J. Appl. Microbiol. 2013, 114, 1782–1792. [Google Scholar] [CrossRef]
- Abd-Elmagid, W.M.; Aly, M.M.E.S.; El-Sharkawy, R.M. Control of peanut root and pod rots diseases using certain bioagents. J. Phytopathol. Pest. Manag. 2020, 7, 79–90. [Google Scholar]
- Sridhar, P.; Venkateshbabu, G.; Hemalakshmi, D.; Kirthika, V.M.; Palani, P. New disease and first report of marasmioid fungus, Marasmius palmivorus (Sharples), causing white root rot in Arachis hypogaea L. Lett. Appl. Microbiol. 2022, 75, 368–377. [Google Scholar] [CrossRef]
- Debele, S.; Fininsa, C.; Dejene, M.; Tana, T. Distribution of groundnut (Arachis hypogaea L.) root rot complex and associated pathogens in eastern Ethiopia. Afr. J. Plant Sci. 2023, 17, 18–29. [Google Scholar] [CrossRef]
- Xu, M.L.; Yang, J.G.; Wang, F.L.; Wu, J.X.; Chi, Y.C. First report of Rhizopus arrhizus (syn. R. oryzae) causing root rot of peanut in China. Plant Dis. 2015, 99, 1448. [Google Scholar] [CrossRef]
- Xu, M.L.; Yang, J.G.; Wu, J.X.; Chi, Y.C.; Xie, L.H. First Report of Aspergillus niger Causing Root Rot of Peanut in China. Plant Dis. 2015, 99, 284. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lei, T.; Yuan, H.; Chen, S. Occurrence of root rot caused by Fusarium fujikuroi and Fusarium proliferatum on peanut in China. Plant Dis. 2023, 107, 940. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.; Guo, Z.; Song, X.; Xu, M.; He, K.; Zhang, X.; Chi, Y. First report of peanut root rot caused by Fusarium acuminatum in Shandong Province, China. Plant Dis. 2023, 107, 2882. [Google Scholar] [CrossRef]
- Erazo, J.G.; Palacios, S.A.; Pastor, N.; Giordano, F.D.; Rovera, M.; Reynoso, M.M.; Venisse, J.S.; Torres, A.M. Biocontrol mechanisms of Trichoderma harzianum ITEM 3636 against peanut brown root rot caused by Fusarium solani RC 386. Biol. Control 2021, 164, 104774. [Google Scholar] [CrossRef]
- Rojo, F.G.; Reynoso, M.M.; Ferez, M.; Chulze, S.N.; Torres, A.M. Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Prot. 2007, 26, 549–555. [Google Scholar] [CrossRef]
- Shanmugam, V.; Senthil, N.; Raguchander, T.; Ramanathan, A.; Samiyappan, R. Interaction of Pseudomonas fluorescens with Rhizobium for their effect on the management of peanut root rot. Phytoparasitica 2002, 30, 169–176. [Google Scholar] [CrossRef]
- Raja Mohan, K.; Balabaskar, P. Survey on the incidence of groundnut root rot disease in cuddalore district of Tamil Nadu and assessing the cultural characters and pathogenicity of Macrophomina phaseolina (Tassi.) Goid. Asian J. Sci. Technol. 2012, 3, 90–94. [Google Scholar]
- Ahmed, M.S.; Sallam, N.M.; Mohamed, A.A.; Hassan, M.H. Effect of mycorrhiza and biofertilisers on reducing the incidence of Fusarium root and pod rot diseases of peanut. Arch. Phytopathol. Plant Prot. 2013, 46, 868–881. [Google Scholar] [CrossRef]
- Sakhuja, P.K.; Sethi, C.L. Frequency of occurrence of various plants parasitic nematodes and root-rot fungi on groundnut in Punjab. Indian J. Nematol. 1985, 15, 191–194. [Google Scholar]
- Li, X.G.; De Boer, W.D.; Zhang, Y.N.; Ding, C.F.; Zhang, T.L.; Wang, X.X. Suppression of soil-borne Fusarium pathogens of peanut by intercropping with the medicinal herb Atractylodes lancea. Soil Biol. Biochem. 2018, 116, 120–130. [Google Scholar] [CrossRef]
- Pan, X.; Yan, S.W.; Hu, X.Y.; Du, P.Q.; Zhang, X.T.; Zhou, L.; Gao, F. Isolation and Identification of Fusarium from Roots, Stems and Pods of Peanut in Henan Province. J. Peanut Sci. 2023, 52, 25–32. [Google Scholar] [CrossRef]
- Guan, L.; Guo, B.B.; Wang, X.K.; Zhang, D.X.; Wang, K.; Liu, F. Control Efficacies of Two Preparations of Difenoconazole and Fluazinam by Seed-Coating Against Peanut Soil-Borne Fungal Diseases. Sci. Agric. Sin. 2015, 48, 2176–2186. [Google Scholar] [CrossRef]
- Guan, L.; Guo, B.B.; Wang, X.K.; Li, B.X.; Zhang, D.X.; Liu, F. Seed-coating treatment of four fungicides against peanut crown rot and root rot diseases. Acta Phytophylacica Sin. 2016, 43, 842–849. [Google Scholar] [CrossRef]
- Oddino, C.M.; Marinelli, A.D.; Zuza, M.; March, G.J. Influence of crop rotation and tillage on incidence of brown root rot of peanut caused by Fusarium solani in Argentina. Can. J. Plant Pathol. 2008, 30, 575–580. [Google Scholar] [CrossRef]
- Tunali, B.; Nicol, J.M.; Hodson, D.; Uçkun, Z.; Büyük, O.; Erdurmuş, D.; Hekimhan, H.; Aktaş, H.; Akbudak, M.A.; Bağci, S.A. Root and Crown Rot Fungi Associated with Spring, Facultative, and Winter Wheat in Turkey. Plant Dis. 2008, 92, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. (Eds.) The Fusarium Laboratory Manual; Blackwell: Ames, IO, USA, 2006. [Google Scholar]
- Chang, X.; Dai, H.; Wang, D.; Zhou, H.; He, W.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.; Shang, J.; et al. Identification of Fusarium species associated with soybean root rot in Sichuan Province, China. Eur. J. Plant Pathol. 2018, 151, 563–577. [Google Scholar] [CrossRef]
- Özer, G.; Paulitz, T.C.; Imren, M.; Alkan, M.; Muminjanov, H.; Dababat, A.A. Identity and Pathogenicity of Fungi Associated with Crown and Root Rot of Dryland Winter Wheat in Azerbaijan. Plant Dis. 2020, 104, 2149–2157. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: SanDiego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Reeb, V.; Lutzoni, F.; Roux, C. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenet. Evol. 2004, 32, 1036–1060. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Rivedal, H.M.; Tabima, J.F.; Stone, A.G.; Johnson, K.B. Identity and Pathogenicity of Fungi Associated with Root, Crown, and Vascular Symptoms Related to Winter Squash Yield Decline. Plant Dis. 2022, 106, 1660–1668. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest V2. Program Distributed by the Author Evolutionary Biology Centre; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Huelsenbeck, J.; Rannala, B. Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Syst. Biol. 2004, 53, 904–913. [Google Scholar] [CrossRef]
- Bozoğlu, T.; Derviş, S.; Imren, M.; Amer, M.; Özdemir, F.; Paulitz, T.C.; Morgounov, A.; Dababat, A.A.; Özer, G. Fungal Pathogens Associated with Crown and Root Rot of Wheat in Central, Eastern, and Southeastern Kazakhstan. J. Fungi 2022, 8, 417. [Google Scholar] [CrossRef]
- Bilgi, V.N.; Bradley, C.A.; Khot, S.D.; Grafton, K.F.; Rasmussen, J.B. Response of Dry Bean Genotypes to Fusarium Root Rot, Caused by Fusarium solani f. sp. phaseoli, Under Field and Controlled Conditions. Plant Dis. 2008, 92, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Yan, L.; Naeem, M.; Khaskheli, M.I.; Zhang, H.; Gong, G.; Zhang, M.; Song, C.; Yang, W.; Liu, T.; et al. Maize/Soybean Relay Strip Intercropping Reduces the Occurrence of Fusarium Root Rot and Changes the Diversity of the Pathogenic Fusarium Species. Pathogens 2020, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, C.; Gilardi, G.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Genetic diversity and virulence of italian strains of Fusarium oxysporum isolated from Eustoma grandiflorum. Eur. J. Plant Pathol. 2015, 141, 83–97. [Google Scholar] [CrossRef]
- Cruz Jimenez, D.R.; Ellis, M.L.; Munkvold, G.P.; Leandro, L.F.S. Isolate–cultivar interactions, in vitro growth, and fungicide sensitivity of Fusarium oxysporum isolates causing seedling disease on soybean. Plant Dis. 2018, 102, 1928–1937. [Google Scholar] [CrossRef]
- Wenham, K. Investigation into the Emerging Soil Borne Disease of Peanut–Neocosmospora Root Rot. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2018. [Google Scholar]
- Hou, Y.P.; Mao, X.W.; Wang, J.X.; Zhan, S.W.; Zhou, M.G. Sensitivity of Fusarium asiaticum to a novel succinate dehydrogenase inhibitor fungicide pydiflumetofen. Crop Prot. 2017, 96, 237–244. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]


| Locus | Primer | Direction | Sequence (5′ to 3′) |
|---|---|---|---|
| ITS | ITS1 | Forward | TCCGTAGGTGAACCTGCGG |
| ITS4 | Reverse | TCCTCCGCTTATTGATATGC | |
| TEF-1a | EF1 | Forward | ATGGGTAAGGARGACAAGAC |
| EF2 | Reverse | GGARGTACCAGTSATCATG | |
| RPB2 | 5f2 | Forward | GGGGWGAYCAGAAGAAGGC |
| 7cr | Reverse | CCCATRGCTTGYTTRCCCAT | |
| TUB2 | T1 | Forward | AACATGCGTGAGATTGTAAGT |
| T2 | Reverse | TAGTGACCCTTGGCCCAGTTG | |
| CAM | CL1 | Forward | GARTWCAAGGAGGCCTTCTC |
| CL2A | Reverse | TTTTTGCATCATGAGTTGGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Chen, L.; Wang, Q.; He, L.; Duan, Y.; Mao, X.; Zhou, L. Species Identification and Fungicide Sensitivity of Fusarium spp. Causing Peanut Root Rot in Henan, China. J. Fungi 2025, 11, 433. https://doi.org/10.3390/jof11060433
Li M, Chen L, Wang Q, He L, Duan Y, Mao X, Zhou L. Species Identification and Fungicide Sensitivity of Fusarium spp. Causing Peanut Root Rot in Henan, China. Journal of Fungi. 2025; 11(6):433. https://doi.org/10.3390/jof11060433
Chicago/Turabian StyleLi, Min, Liting Chen, Qinqin Wang, Leiming He, Yun Duan, Xuewei Mao, and Lin Zhou. 2025. "Species Identification and Fungicide Sensitivity of Fusarium spp. Causing Peanut Root Rot in Henan, China" Journal of Fungi 11, no. 6: 433. https://doi.org/10.3390/jof11060433
APA StyleLi, M., Chen, L., Wang, Q., He, L., Duan, Y., Mao, X., & Zhou, L. (2025). Species Identification and Fungicide Sensitivity of Fusarium spp. Causing Peanut Root Rot in Henan, China. Journal of Fungi, 11(6), 433. https://doi.org/10.3390/jof11060433






