Conversion of Soluble Compounds in Distillery Wastewater into Fungal Biomass and Metabolites Using Australian Ganoderma Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ganoderma Cultivation and Inoculum Preparation
2.3. Collection and Characterization of Distillery Wastewater
2.4. Culture Media Optimization
2.4.1. Wastewater Concentration and pH
2.4.2. Nitrogen Supplementation
- 0.5 and 2 g·L−1 of yeast extract;
- 0.11 g·L−1 and 0.43 g·L−1 of urea; and
- 0.24 g·L−1 and 0.94 g·L−1 of ammonium sulphate.
2.5. Metabolite Production in Stillage Versus Nutrient Medium
2.5.1. Glucan Quantification
2.5.2. Aqueous and Hexane Extraction
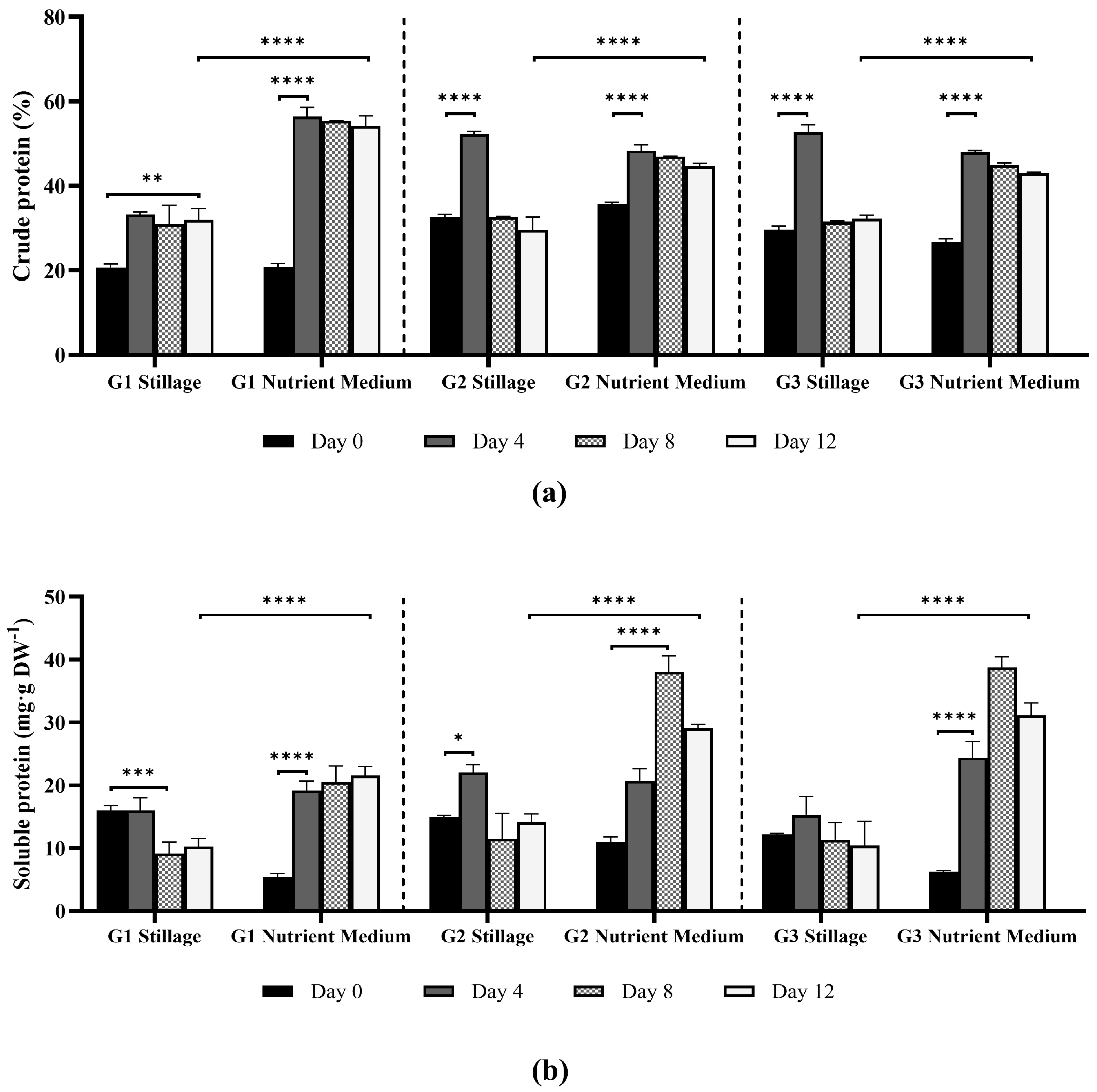
2.5.3. Crude and Soluble Proteins
2.5.4. Antioxidant Activity of Ganoderma Extracts
ABTS Radical-Scavenging Activity
Ferric Reducing Antioxidant Ability (FRAP)
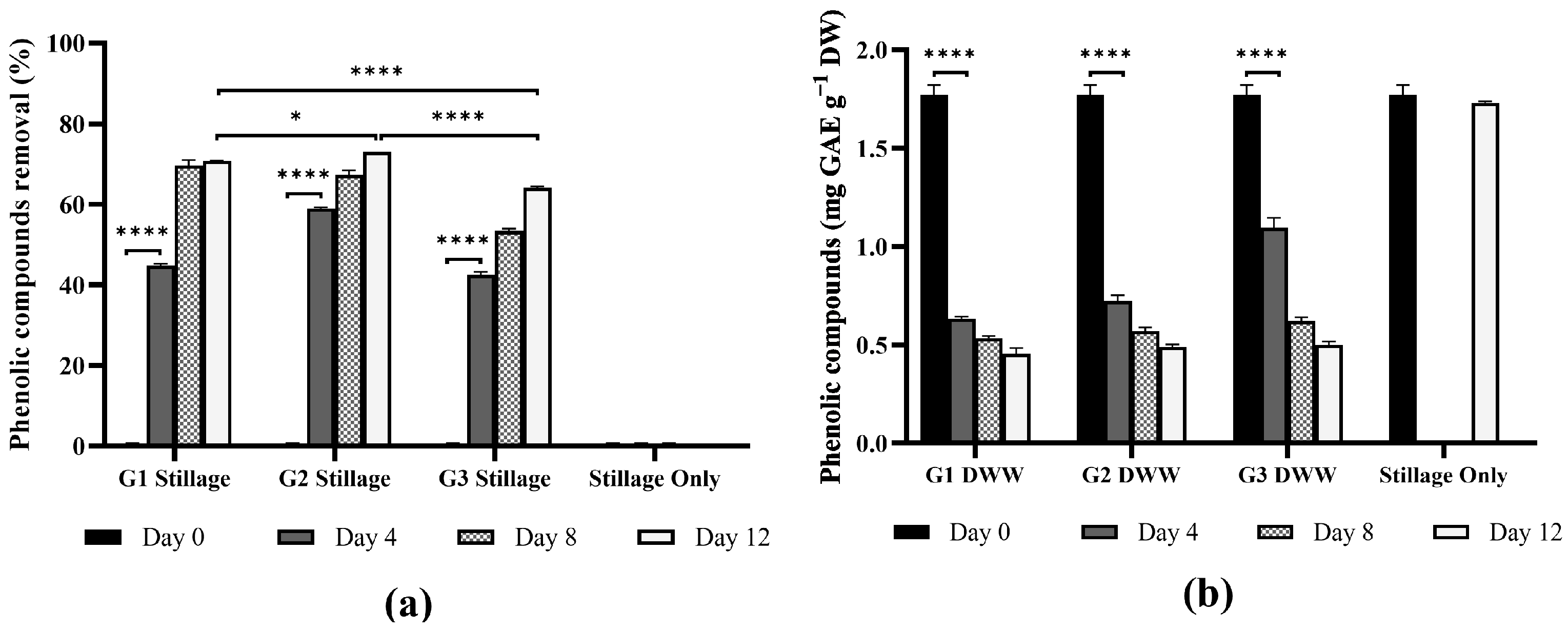
Total Phenolic Compounds
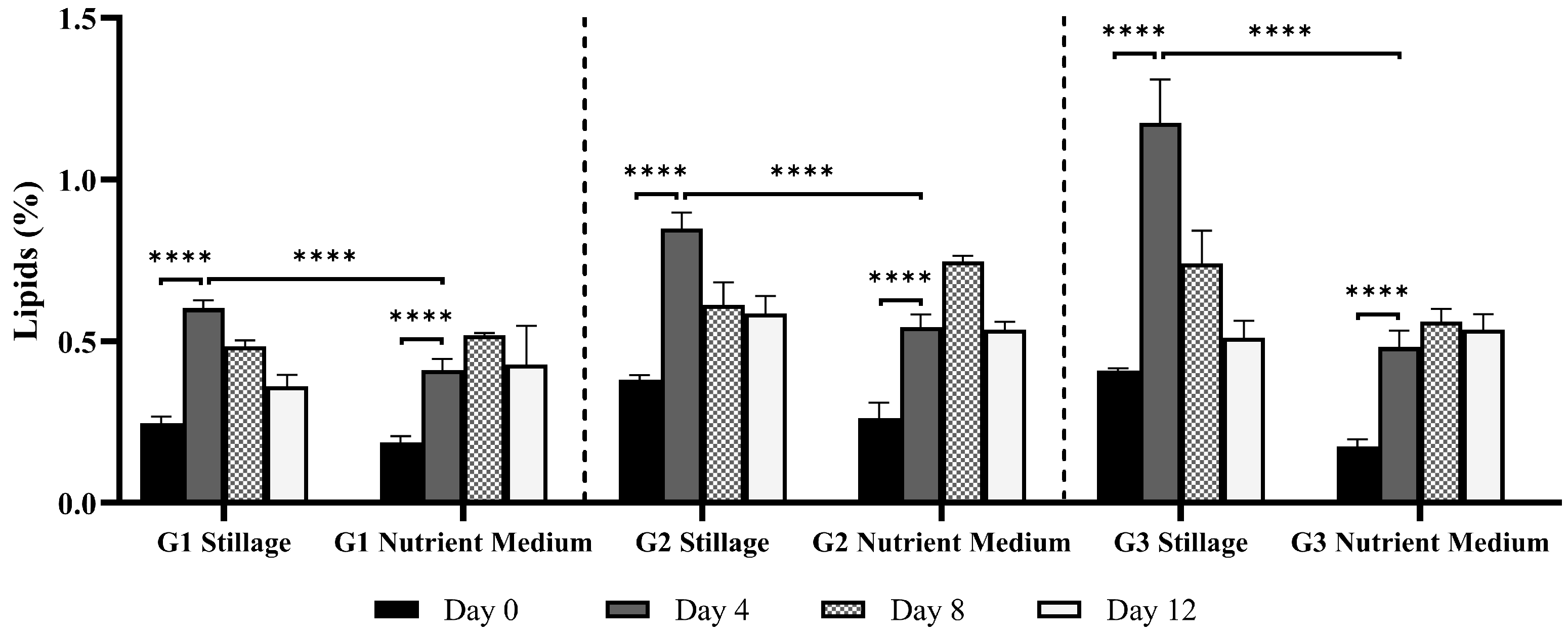
2.5.5. Total Lipids
2.5.6. Soluble Organic Carbon and Soluble Nitrogen
2.5.7. Statistical Analysis
3. Results and Discussion
3.1. Screening and Optimization of Growth Conditions of Australian Ganoderma Isolates
3.1.1. Stillage Concentration
3.1.2. Initial pH
3.1.3. Nitrogen Supplement
3.2. Ganoderma Biomass Synthesis in Stillage Versus a Nutrient Medium
3.2.1. Mycelial Biomass Synthesis
3.2.2. Extractable and Crude Protein Content in Ganoderma Biomass
3.2.3. Antioxidant Activity and Phenolic Content of Ganoderma Extracts
ABTS Assay
FRAP Assay
Phenolic Compounds in Ganoderma Biomass
3.2.4. Lipids in Ganoderma Biomass
3.2.5. β-Glucan Content in Ganoderma Mycelia
3.3. Bioremediation Potential of Ganoderma in Stillage
3.3.1. Removal of Phenolic Compounds
3.3.2. Removal of Soluble Organic Carbon (SOC) and Soluble Nitrogen (SN)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratna, S.; Rastogi, S.; Kumar, R. Current trends for distillery wastewater management and its emerging applications for sustainable environment. J. Environ. Manag. 2021, 290, 112544. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.L.; Paulillo, S.C.d.L.; Godoy, A.; Cherubin, R.A.; Lorenzi, M.S.; Giometti, F.H.C.; Bernardino, C.D.; Amorim Neto, H.B.d.; Amorim, H.V.d. Ethanol production in Brazil: A bridge between science and industry. Braz. J. Microbiol. 2016, 47, 64–76. [Google Scholar] [CrossRef]
- Sankaran, S.; Khanal, S.K.; Jasti, N.; Jin, B.; Pometto, A.L.; Van Leeuwen, J.H. Use of Filamentous Fungi for Wastewater Treatment and Production of High Value Fungal Byproducts: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 400–449. [Google Scholar] [CrossRef]
- Kaushik, G. Bioremediation of Industrial Effluents: Distillery Effluent. In Applied Environmental Biotechnology: Present Scenario and Future Trends; Springer: New Delhi, India, 2015; pp. 19–32. [Google Scholar]
- Rosa, F.M.; Mota, T.F.M.; Busso, C.; Arruda, P.V.d.; Brito, P.E.M.; Miranda, J.P.M.; Trentin, A.B.; Dekker, R.F.; Cunha, M.A.A.d. Filamentous Fungi as Bioremediation Agents of Industrial Effluents: A Systematic Review. Fermentation 2024, 10, 143. [Google Scholar] [CrossRef]
- Fernandes, J.M.C.; Sousa, R.; Fraga, I.; Sampaio, A.; Amaral, C.; Bezerra, R.M.F.; Dias, A.A. Fungal biodegradation and multi-level toxicity assessment of vinasse from distillation of winemaking by-products. Chemosphere 2020, 238, 124572. [Google Scholar] [CrossRef] [PubMed]
- Timm, T.G.; Schipmann, D.B.D.; Costa, T.M.; Tavares, L.B.B. Remediation of brewery wastewater and reuse for β-glucans production by basidiomycete fungi. Waste Biomass Valorization 2024, 15, 4629–4645. [Google Scholar] [CrossRef]
- Asad, S.; Gu, P.; Peng, C.; Huang, H.; Jiang, F.; Patabedige, N.; Karunarathna, S.C.; Hapuarachchi, K.K. Biotechnological potential of Ganoderma species: Current progress and future prospects. N. Z. J. Bot. 2024, 1–60. [Google Scholar] [CrossRef]
- Pilafidis, S.; Tsouko, E.; Sougleri, G.; Diamantopoulou, P.; Gkatzionis, K.; Ioannou, Z.; Sarris, D. Submerged cultivation of selected macro-fungi to produce mycelia rich in β-glucans and other bioactive compounds, valorizing side streams of the food industry. Carbon. Resour. Convers. 2024, 7, 100198. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Gardeli, C.; Papanikolaou, S. Impact of olive mill wastewaters on the physiological behavior of a wild-type new Ganoderma resinaceum isolate. Environ. Sci. Pollut. Res. Int. 2021, 28, 20570–20585. [Google Scholar] [CrossRef]
- Hsieh, C.; Hsu, T.-H.; Yang, F.-C. Production of polysaccharides of Ganoderma lucidum (CCRC36021) by reusing thin stillage. Process Biochem. 2005, 40, 909–916. [Google Scholar] [CrossRef]
- Lin, W.; Chen, L.; Tan, Z.; Deng, Z.; Liu, H. Application of filamentous fungi in microalgae-based wastewater remediation for biomass harvesting and utilization: From mechanisms to practical application. Algal Res. 2022, 62, 102614. [Google Scholar] [CrossRef]
- De Oliveira Campos, A.; Harrison, M.D.; Marshall, D.L.; Strong, P.J. Distributions of Lanostene-Derived Triterpenoids and Glucan Content in the Fruiting Bodies of the Australian Ganoderma Species. J. Fungi 2024, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J. A new method for measuring antioxidant activity. Biochem. Soc. Trans. 1993, 21, 95S. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1985, 1, 302–304. [Google Scholar]
- Melamane, X.; Strong, P.; Burgess, J. Treatment of wine distillery wastewater: A review with emphasis on anaerobic membrane reactors. S. Afr. J. Enol. Vitic. 2007, 28, 25–36. [Google Scholar] [CrossRef]
- Łebkowska, M.; Załęska-Radziwiłł, M. Application of white-rot fungi for biodegradation of refractory organic compounds—A review. Desalination Water Treat. 2014, 52, 3708–3713. [Google Scholar] [CrossRef]
- Agustin, F.; Juliyarsi, I. The effect of urea supplementation and incubation time in fermentation process of bagasse by using Ganoderma lucidum on the growth of G. lucidum and the nutritive value of bagasse. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Padang, Indonesia, 10–11 October 2019; p. 012016. [Google Scholar]
- Strong, P. Fungal remediation of Amarula distillery wastewater. World J. Microbiol. Biotechnol. 2010, 26, 133–144. [Google Scholar] [CrossRef]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Antoniou, T.; Merhautova, V.; Zervakis, G.I. Biodegradation and detoxification of olive mill wastewater by selected strains of the mushroom genera Ganoderma and Pleurotus. Chemosphere 2012, 88, 620–626. [Google Scholar] [CrossRef]
- Berovic, M.; Zhong, J.-J. Advances in production of medicinal mushrooms biomass in solid state and submerged bioreactors. Biochem. Eng. Biotechnol. Med. Mushrooms 2023, 184, 125–161. [Google Scholar] [CrossRef]
- Du, Z.; Dong, C.H.; Wang, K.; Yao, Y.J. Classification, Biological Characteristics and Cultivations of Ganoderma. Adv. Exp. Med. Biol. 2019, 1181, 15–58. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridi, L.M.; Katapodis, P.; Gonou-Zagou, Z.; Kapsanaki-Gotsi, E.; Christakopoulos, P. Growth and biomass production with enhanced β-glucan and dietary fibre contents of Ganoderma australe ATHUM 4345 in a batch-stirred tank bioreactor. Eng. Life Sci. 2011, 11, 65–74. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Kapoti, M.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom polysaccharides and lipids synthesized in liquid agitated and static cultures. Part I: Screening various mushroom species. Appl. Biochem. Biotechnol. 2012, 167, 536–551. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, Y.; Song, C. Proteins. In Essentials of Food Chemistry; Kan, J., Chen, K., Eds.; Springer: Singapore, 2021; pp. 49–121. [Google Scholar]
- Gmoser, R.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Post-treatment of fungal biomass to enhance pigment production. Appl. Biochem. Biotechnol. 2019, 189, 160–174. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Lenardon, M.D. Architecture of the dynamic fungal cell wall. Nat. Rev. Microbiol. 2023, 21, 248–259. [Google Scholar] [CrossRef]
- Ferrero, D.; Moscato, E.; Spina, F.; Cavalluzzi, M.M.; Rotondo, N.; Oddon, S.B.; Gargano, M.L.; Venturella, G.; Lentini, G.; Bertea, C.M. The fungal alternative: Insights on medicinal mushrooms-based protein-rich biomasses by submerged fermentation of agro-industrial by-products. Innov. Food Sci. Emerg. Technol. 2024, 95, 103721. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Kurbanoglu, E.; Algur, O.; Zulkadir, A. Submerged production of edible mushroom Agaricus bisporus mycelium in ram horn hydrolysate. Ind. Crops Prod. 2004, 19, 225–230. [Google Scholar] [CrossRef]
- Majumder, R.; Miatur, S.; Saha, A.; Hossain, S. Mycoprotein: Production and nutritional aspects: A review. Sustain. Food Technol. 2024, 2, 81–91. [Google Scholar] [CrossRef]
- Future Market Insights, Inc. Mycoprotein Market Outlook (2025 to 2035); Future Market Insights, Inc.: Newark, DE, USA, 2025. [Google Scholar]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-S.; Yu, Z.-R.; Wang, B.-J.; Wang, C.-C.; Weng, Y.-M.; Koo, M. Bioactive constituent characterization and antioxidant activity of Ganoderma lucidum extract fractionated by supercritical carbon dioxide. Sains Malays. 2015, 44, 1685–1691. [Google Scholar]
- Veljovic, S.; Veljovic, M.; Nikicevic, N.; Despotovic, S.; Radulovic, S.; Niksic, M.; Filipovic, L. Chemical composition, antiproliferative and antioxidant activity of differently processed Ganoderma lucidum ethanol extracts. J. Food Sci. Technol. 2017, 54, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Arslan, N.P.; Dawar, P.; Albayrak, S.; Doymus, M.; Azad, F.; Esim, N.; Taskin, M. Fungi-derived natural antioxidants. Crit. Rev. Food Sci. Nutr. 2023, 65, 1–24. [Google Scholar] [CrossRef]
- Sulkowska-Ziaja, K.; Zengin, G.; Gunia-Krzyzak, A.; Popiol, J.; Szewczyk, A.; Jaszek, M.; Rogalski, J.; Muszynska, B. Bioactivity and Mycochemical Profile of Extracts from Mycelial Cultures of Ganoderma spp. Molecules 2022, 27, 275. [Google Scholar] [CrossRef]
- Raseta, M.; Popovic, M.; Beara, I.; Sibul, F.; Zengin, G.; Krstic, S.; Karaman, M. Anti-Inflammatory, Antioxidant and Enzyme Inhibition Activities in Correlation with Mycochemical Profile of Selected Indigenous Ganoderma spp. from Balkan Region (Serbia). Chem. Biodivers. 2021, 18, e2000828. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.-S.; Tomescu, C.L.; Ionescu, A.-M. Natural bio-compounds from Ganoderma lucidum and their beneficial biological actions for anticancer application: A review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Cai, G.; Dong, H.; Liu, S.; Wu, W.; Yang, H. Comparative Evaluation of the Physiochemical Properties, and Antioxidant and Hypoglycemic Activities of Dendrobium officinale Leaves Processed Using Different Drying Techniques. Antioxidants 2023, 12, 1911. [Google Scholar] [CrossRef] [PubMed]
- Tajik, A.; Samadlouie, H.R.; Salek Farrokhi, A.; Ghasemi, A. Optimization of chemical conditions for metabolites production by Ganoderma lucidum using response surface methodology and investigation of antimicrobial as well as anticancer activities. Front. Microbiol. 2024, 14, 1280405. [Google Scholar] [CrossRef]
- Tan, W.C.; Kuppusamy, U.R.; Phan, C.W.; Tan, Y.S.; Raman, J.; Anuar, A.M.; Sabaratnam, V. Ganoderma neo-japonicum Imazeki revisited: Domestication study and antioxidant properties of its basidiocarps and mycelia. Sci. Rep. 2015, 5, 12515. [Google Scholar] [CrossRef]
- Dong, Q.; He, D.; Ni, X.; Zhou, H.; Yang, H. Comparative study on phenolic compounds, triterpenoids, and antioxidant activity of Ganoderma lucidum affected by different drying methods. J. Food Meas. Charact. 2019, 13, 3198–3205. [Google Scholar] [CrossRef]
- Sudheer, S.; Taha, Z.; Manickam, S.; Ali, A.; Cheng, P.G. Development of antler-type fruiting bodies of Ganoderma lucidum and determination of its biochemical properties. Fungal Biol. 2018, 122, 293–301. [Google Scholar] [CrossRef]
- Mohammadifar, S.; Fallahi Gharaghoz, S.; Asef Shayan, M.R.; Vaziri, A. Comparison between antioxidant activity and bioactive compounds of Ganoderma applanatum (Pers.) Pat. and Ganoderma lucidum (Curt.) P. Karst from Iran. Iran. J. Plant Physiol. 2020, 11, 3417–3424. [Google Scholar]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Merhautova, V.; Zervakis, G.I. Olive mill wastewater biodegradation potential of white-rot fungi--Mode of action of fungal culture extracts and effects of ligninolytic enzymes. Bioresour. Technol. 2015, 189, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Vu, D. Effects of extraction solvents on phytochemicals and bioactivities of Ganoderma lucidum. Egypt. J. Chem. 2023, 66, 581–588. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Cabello, K.P.; Lugo-Flores, M.A.; Rivera-Palafox, P.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Esqueda, M.; Gaitan-Hernandez, R.; Ayala-Zavala, J.F. Antioxidant Properties and Industrial Uses of Edible Polyporales. J. Fungi 2021, 7, 196. [Google Scholar] [CrossRef]
- Lim, T.C.; Ilham, Z.; Wan-Mohtar, W. Production of Ganodiesel from the biomass of Ganoderma lucidum in air-L-shaped bioreactor (ALSB). Heliyon 2024, 10, e35170. [Google Scholar] [CrossRef]
- Diamantis, I.; Melanouri, E.-M.; Dedousi, M.; Panagopoulou, I.; Papanikolaou, S.; Stoforos, N.G.; Diamantopoulou, P. Sustainable and eco-friendly conversions of olive mill wastewater-based media by Pleurotus pulmonarius cultures. Fermentation 2022, 8, 129. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Liu, Y. Supercritical carbon dioxide extraction of Ganoderma lucidum spore lipids. LWT 2016, 70, 16–23. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Microbial lipids for foods. Trends Food Sci. Technol. 2022, 119, 593–607. [Google Scholar] [CrossRef]
- Timm, T.G.; Costa, T.M.; Alberton, M.D.; Helm, C.V.; Tavares, L.B.B. Mushroom β-glucans: Application and innovation for food industry and immunotherapy. Appl. Microbiol. Biotechnol. 2023, 107, 5035–5049. [Google Scholar] [CrossRef]
- Caseiro, C.; Dias, J.N.R.; de Andrade Fontes, C.M.G.; Bule, P. From cancer therapy to winemaking: The molecular structure and applications of β-glucans and β-1, 3-glucanases. Int. J. Mol. Sci. 2022, 23, 3156. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- PW Consulting Chemical; Energy Research Center. Ganoderma Lucidum Extract Market; PW Consulting Agency: Kowloon, Hong Kong, 2025. [Google Scholar]
- Suwanno, S.; Phutphat, C. 1,3-β-glucan Content of Local Medicinal Mushrooms from the Southern Region of Thailand. Walailak J. Sci. Technol. 2018, 15, 189–200. [Google Scholar] [CrossRef]
- Cortina-Escribano, M.; Pihlava, J.M.; Miina, J.; Veteli, P.; Linnakoski, R.; Vanhanen, H. Effect of Strain, Wood Substrate and Cold Treatment on the Yield and beta-Glucan Content of Ganoderma lucidum Fruiting Bodies. Molecules 2020, 25, 4732. [Google Scholar] [CrossRef]
- Guo, J.; Tang, C.; Liu, Y.; Shi, J.; Vunduk, J.; Tang, C.; Feng, J.; Zhang, J. Innovative submerged directed fermentation: Producing high molecular weight polysaccharides from Ganoderma lucidum. Food Chem. 2025, 471, 142759. [Google Scholar] [CrossRef]
- Mejía, S.M.V.; de Francisco, A.; Bohrer, B. A comprehensive review on cereal β-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020, 60, 3693–3704. [Google Scholar] [CrossRef]
- [iUNIK] Beta Glucan Daily Moisture Cream. Available online: www.iunikcos.com/CREAM/?idx=204 (accessed on 22 May 2025).
- Mikucka, W.; Zielinska, M. Individual phenolic acids in distillery stillage inhibit its biomethanization. Energies 2022, 15, 5377. [Google Scholar] [CrossRef]
- Ariste, A.F.; Batista-García, R.A.; Vaidyanathan, V.K.; Raman, N.; Vaithyanathan, V.K.; Folch-Mallol, J.L.; Jackson, S.A.; Dobson, A.D.; Cabana, H. Mycoremediation of phenols and polycyclic aromatic hydrocarbons from a biorefinery wastewater and concomitant production of lignin modifying enzymes. J. Clean. Prod. 2020, 253, 119810. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Zervakis, G.I. Comparative examination of the olive mill wastewater biodegradation process by various wood-rot macrofungi. BioMed Res. Int. 2014, 2014, 482937. [Google Scholar] [CrossRef]
- Matos, A.; Bezerra, R.; Dias, A. Screening of fungal isolates and properties of Ganoderma applanatum intended for olive mill wastewater decolourization and dephenolization. Lett. Appl. Microbiol. 2007, 45, 270–275. [Google Scholar] [CrossRef]
- Dasgupta, D.; Barman, S.; Sarkar, J.; Mridha, D.; Labrousse, P.; Roychowdhury, T.; Acharya, K.; Sarkar, J.; Chakraborty, N. Mycoremediation of different wastewater toxicants and its prospects in developing value-added products: A review. J. Water Process Eng. 2024, 58, 104747. [Google Scholar] [CrossRef]
- Ahmed, P.M.; de Figueroa, L.I.C.; Pajot, H.F. Dual Purpose of ligninolytic- basidiomycetes: Mycoremediation of bioethanol distillation vinasse coupled to sustainable bio-based compounds production. Fungal Biol. Rev. 2020, 34, 25–40. [Google Scholar] [CrossRef]
- Beltran-Flores, E.; Pla-Ferriol, M.; Martinez-Alonso, M.; Gaju, N.; Blanquez, P.; Sarra, M. Fungal bioremediation of agricultural wastewater in a long-term treatment: Biomass stabilization by immobilization strategy. J. Hazard. Mater. 2022, 439, 129614. [Google Scholar] [CrossRef]
- Prost-Boucle, S.; Pelus, L.; Becheau, E.; Cervoise, L.; Troesch, S.; Molle, P. Combination of sequencing batch reactor and vertical flow treatment wetlands: A full-scale experience for rum distillery wastewater treatment in a tropical climate. Nat.-Based Solut. 2023, 3, 100056. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Chaudhari, P.K. Physicochemical treatment of distillery wastewater—A review. Chem. Eng. Commun. 2015, 202, 1098–1117. [Google Scholar] [CrossRef]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef]
- Mohd Hanafiah, Z.; Wan Mohtar, W.H.M.; Abu Hasan, H.; Jensen, H.S.; Klaus, A.; Wan-Mohtar, W. Performance of wild-Serbian Ganoderma lucidum mycelium in treating synthetic sewage loading using batch bioreactor. Sci. Rep. 2019, 9, 16109. [Google Scholar] [CrossRef] [PubMed]
- Mooralitharan, S.; Mohd Hanafiah, Z.; Abd Manan, T.S.B.; Muhammad-Sukki, F.; Wan-Mohtar, W.A.A.Q.I.; Wan Mohtar, W.H.M. Vital conditions to remove pollutants from synthetic wastewater using Malaysian Ganoderma lucidum. Sustainability 2023, 15, 3819. [Google Scholar] [CrossRef]
- Kalra, A.; Gupta, A. Microbiological treatment of distillery wastewater focusing on colorant decolorization and resource recovery: A review. Rev. Environ. Sci. Bio/Technol. 2023, 22, 175–204. [Google Scholar] [CrossRef]
- Davis, M.A.; Wong, K.H. Nitrogen metabolism in filamentous fungi. In Cellular and Molecular Biology of Filamentous Fungi; American Society for Microbiology Press: Washngton, CO, USA, 2010; pp. 325–338. [Google Scholar]
- Basu, A. Characteristics of distillery wastewater. J. (Water Pollut. Control Fed.) 1975, 47, 2184–2190. [Google Scholar]
- Kadhim, N.F.; Mohammed, W.J.; Al Hussaini, I.M.; Al-Saily, H.; Ali, R.N. The efficiency of some fungi species in wastewater treatment. J. Water Land. Dev. 2021, 50, 248–254. [Google Scholar] [CrossRef]
- Wu, W.; Li, S.; Xie, P.; Li, X.; Chang, H.; Ho, S.-H. Algal-fungal interactions and biomass production in wastewater treatment: Current status and future perspectives. Algal Res. 2023, 70, 103021. [Google Scholar] [CrossRef]
- Hanafiah, Z.M.; Mohtar, W.H.M.W.; El Messaoudi, N.; Miyah, Y.; Maged, A.; Knani, S.; Graba, B.; Gafforov, Y.; Wan, W.A.A.Q.I. Wastewater treatment using Ganoderma species via ganoremediation bioreactor: A comprehensive review. Bioresour. Technol. Rep. 2025, 30, 102105. [Google Scholar] [CrossRef]
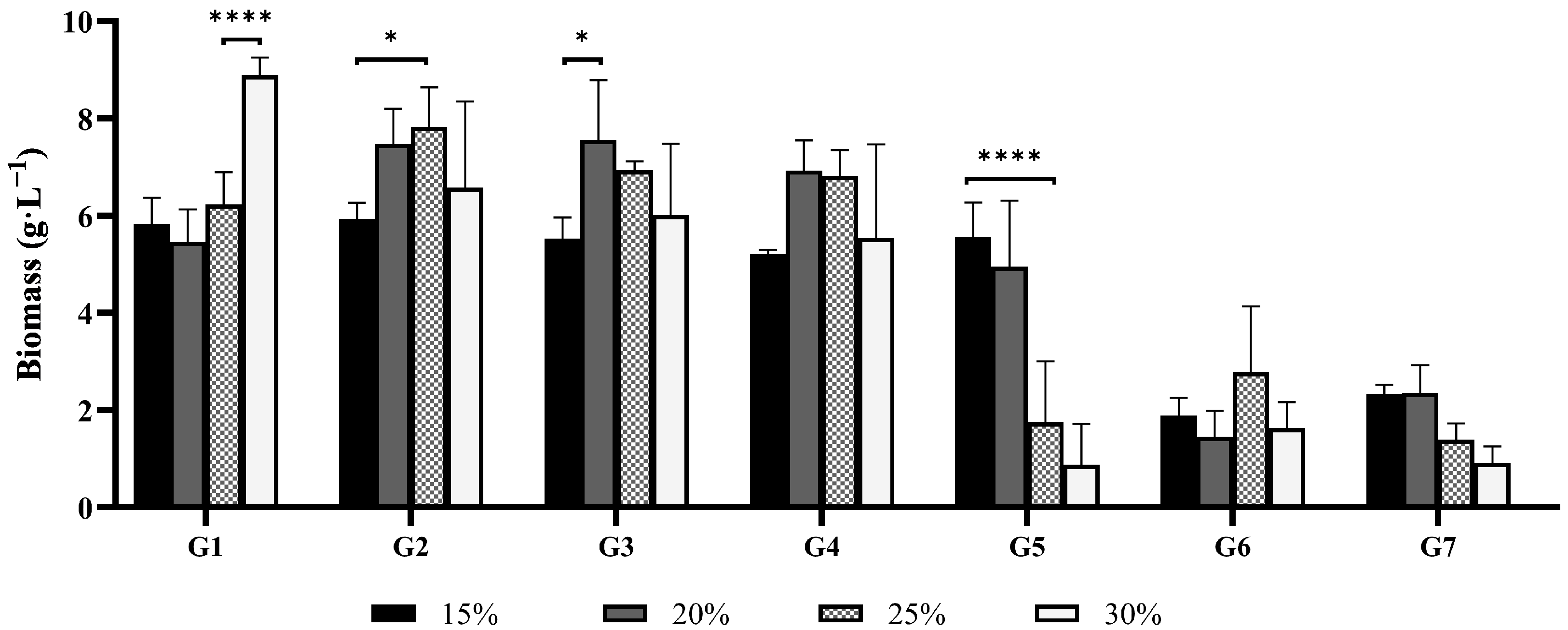
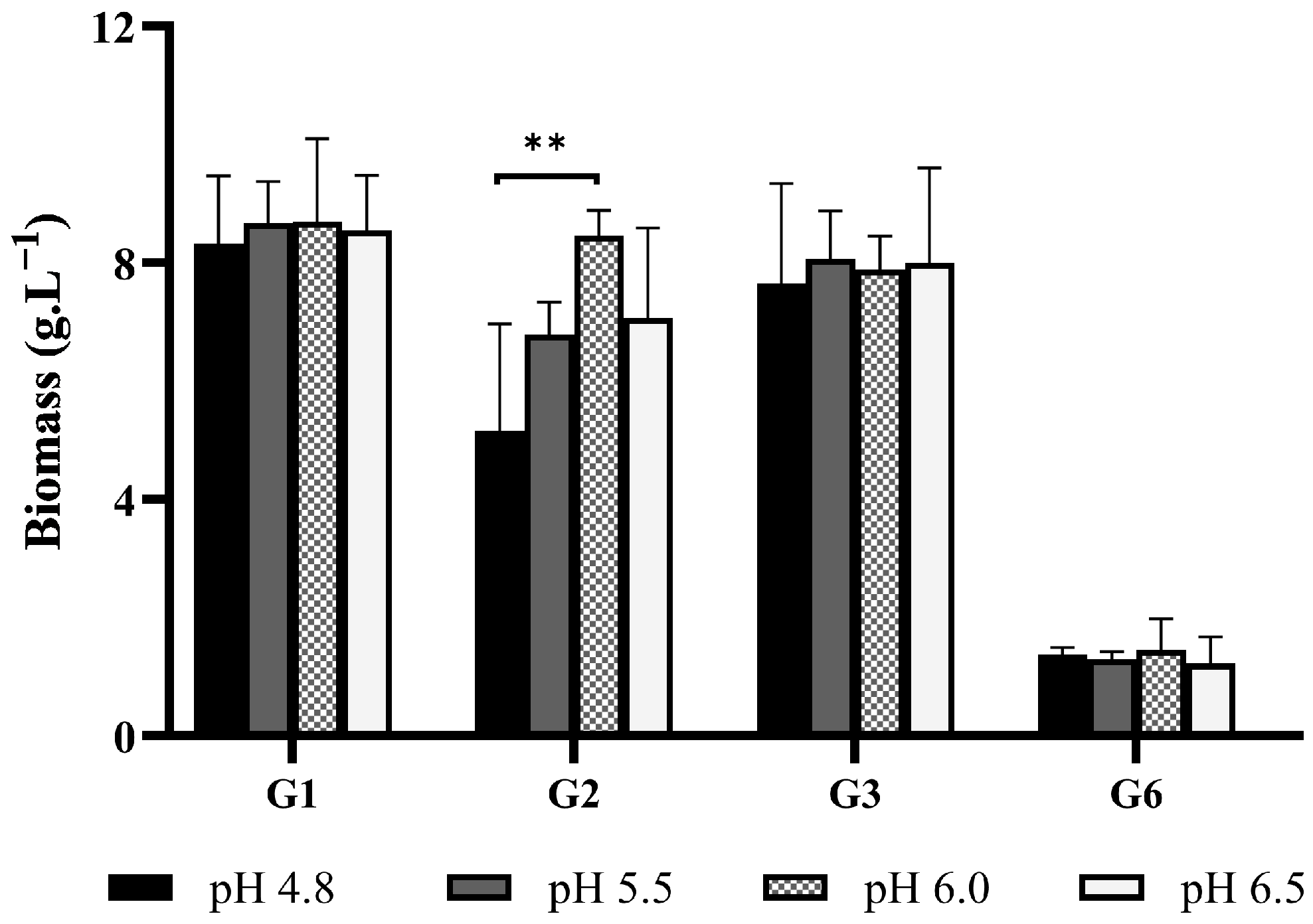
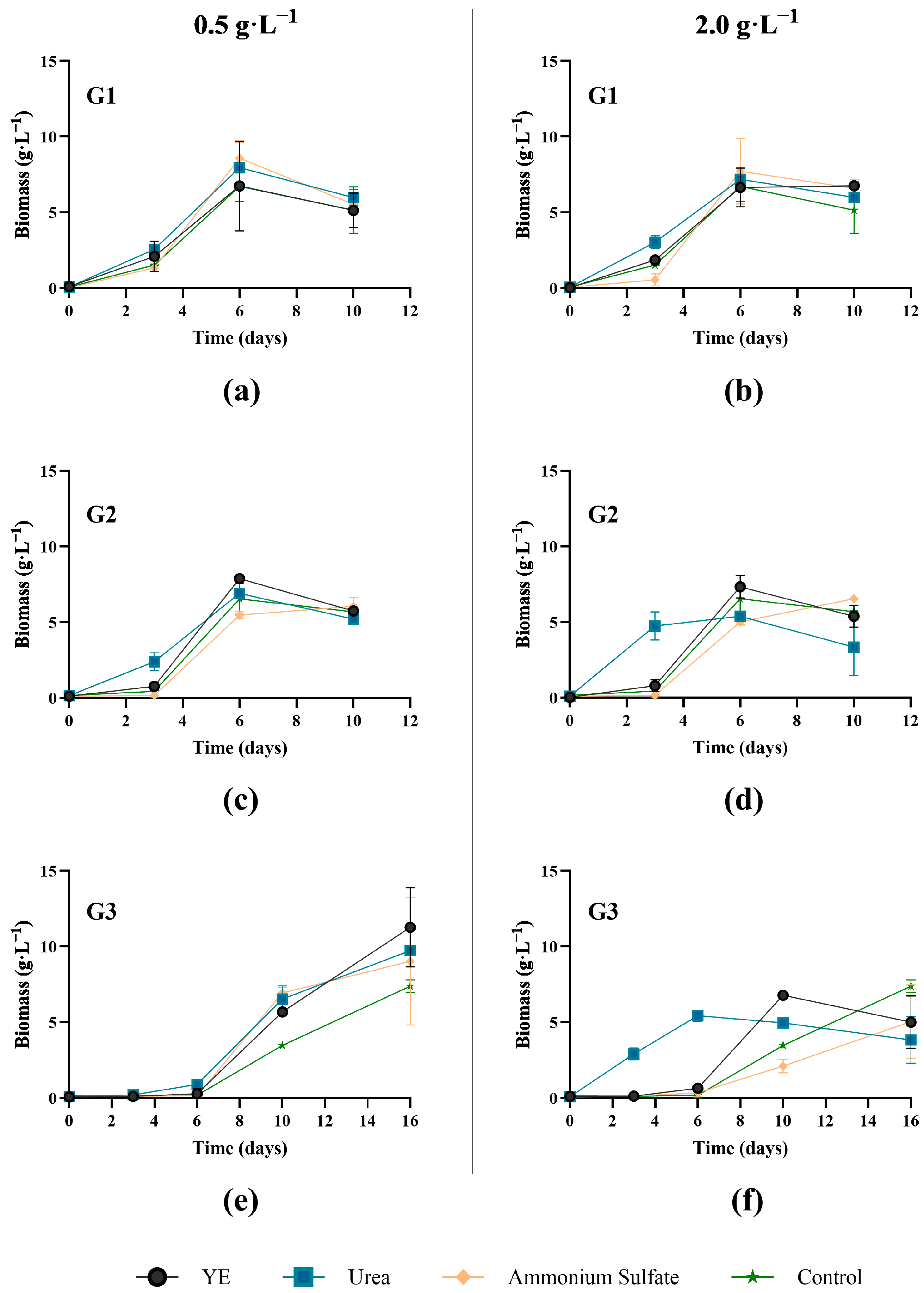
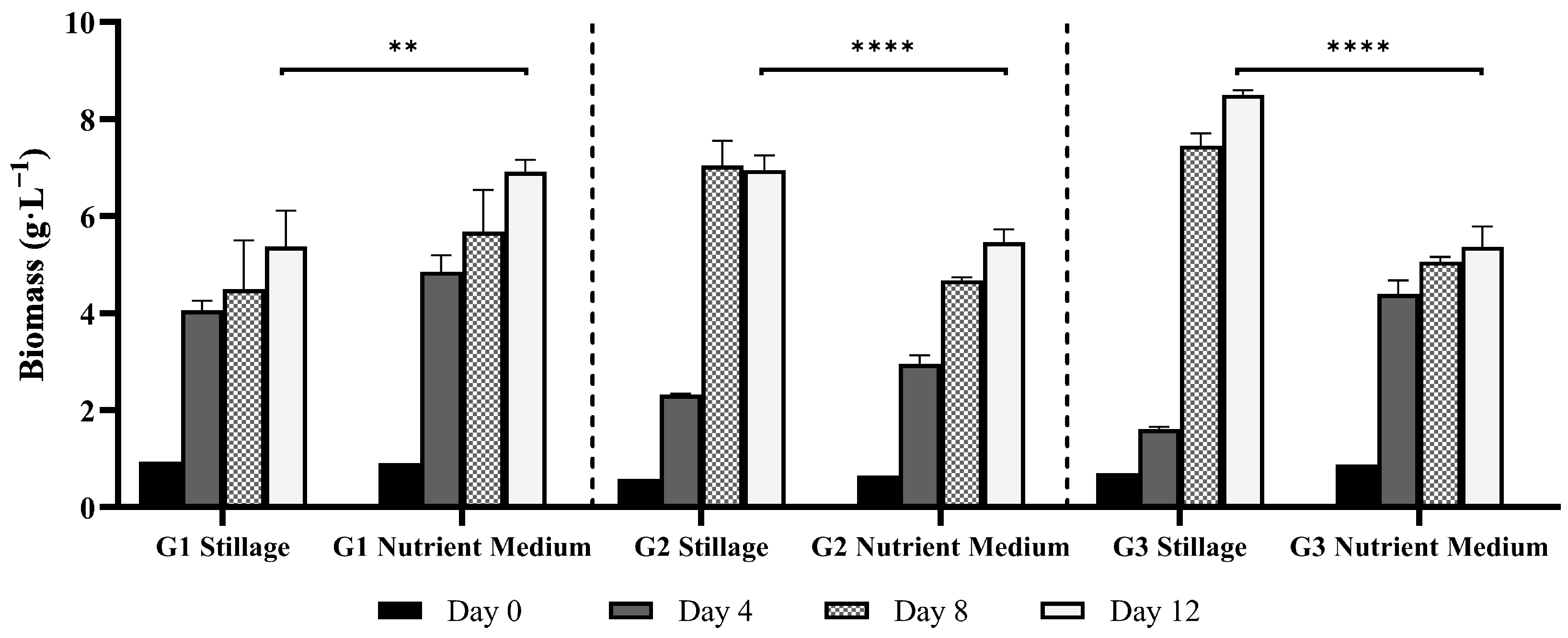



| Isolate | Description |
|---|---|
| G1 | Laccate, fan-shaped pileus. Dark brown becoming lighter at margins. Concentric zones on pileus surface. Margin and pore surface are cream white. Pore layer is thin, with thicker context tissue. |
| G2 | Laccate, kidney-shaped pileus. Dark-brown surface with dark-brown margins. Concentric zones on pileus surface. Pore surface darkened due to physical damage (common in Ganoderma species). Pore layer thicker than context tissue. |
| G3 | Laccate, kidney-shaped pileus. Dark brown becoming lighter at margins. Concentric zones on pileus surface. Both margin and pore surface are cream white. Context tissue is thicker than pore layer. |
| G4 | Laccate, kidney-shaped pileus. Medium-brown color becoming lighter at margins. Margin and pore layer are cream white. Context tissue thicker than pore layer. |
| G5 | Laccate, kidney-shaped pileus. Medium-brown pileus becoming lighter at margin. Margin and pore surface are cream white. Context tissue thicker than pore layer. |
| G6 | Laccate, fan-shaped pileus. Dark brown with concentric zones on surface. Margin dark. Cream white pore surface, thicker than context tissue. |
| G7 | Laccate, fan-shaped pileus. Medium brown with concentric zones. Margins and pore tissue are cream white. Pore layer thicker than context tissue. |
| Parameter | Value |
|---|---|
| pH | 4.85 |
| Soluble organic carbon | 14.7 g·L−1 |
| Total nitrogen | 0.7 g·L−1 |
| Total phenolic content | 1.8 g GAE·L−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, A.D.O.; Wahalathanthrige, H.J.; Russell, S.; Harrison, M.D.; Strong, P.J. Conversion of Soluble Compounds in Distillery Wastewater into Fungal Biomass and Metabolites Using Australian Ganoderma Isolates. J. Fungi 2025, 11, 432. https://doi.org/10.3390/jof11060432
Campos ADO, Wahalathanthrige HJ, Russell S, Harrison MD, Strong PJ. Conversion of Soluble Compounds in Distillery Wastewater into Fungal Biomass and Metabolites Using Australian Ganoderma Isolates. Journal of Fungi. 2025; 11(6):432. https://doi.org/10.3390/jof11060432
Chicago/Turabian StyleCampos, Aline D. O., Hashini J. Wahalathanthrige, Shane Russell, Mark D. Harrison, and Peter James Strong. 2025. "Conversion of Soluble Compounds in Distillery Wastewater into Fungal Biomass and Metabolites Using Australian Ganoderma Isolates" Journal of Fungi 11, no. 6: 432. https://doi.org/10.3390/jof11060432
APA StyleCampos, A. D. O., Wahalathanthrige, H. J., Russell, S., Harrison, M. D., & Strong, P. J. (2025). Conversion of Soluble Compounds in Distillery Wastewater into Fungal Biomass and Metabolites Using Australian Ganoderma Isolates. Journal of Fungi, 11(6), 432. https://doi.org/10.3390/jof11060432








