Abstract
The haloacid dehalogenase (HAD) superfamily represents a large group of enzymes across diverse taxa. However, the characteristics and functional roles of HAD members in the destructive apple canker pathogen, Valsa mali strain Vm1 (Vm1), remain poorly understood, particularly regarding their expression during infection. In this study, the full-length cDNA sequence of the VmHAD gene from Vm1 was cloned using rapid amplification of cDNA ends (RACE) technology, and its bioinformatic properties, subcellular localization, and expression patterns during infection were characterized. The VmHAD cDNA was 1044 bp in length, containing a complete open reading frame (ORF) of 798 bp that encodes a 265 amino acid protein with a conserved HAD-like domain. Phylogenetic analysis revealed that VmHAD shares the highest similarity with the (S)-2-haloacid dehalogenase (accession no. KUI70710.1) from Cytospora mali 03-8, belonging to the L-2-haloacid dehalogenase family within the HAD hydrolase superfamily. Subcellular localization analysis using a transient expression system in Nicotiana benthamiana indicated that VmHAD is distributed in both the nucleus and cytoplasm. Expression profiling demonstrated that VmHAD was significantly upregulated during the infection of detached apple branches by Vm1, with relative expression levels increasing 3.13-, 4.25-, and 3.98-fold at 3, 5, and 7 days post-inoculation, respectively, compared with day 1, whereas no expression was detected in the uninoculated control. These findings identify VmHAD as a novel HAD family member in Vm1 and suggest that it plays a potential role in the infection process and pathogenicity. This work provides new insights into the molecular mechanisms underlying V. mali pathogenicity and contributes to the development of effective strategies for disease management.
1. Introduction
Apples (Malus domestica Borkh.) are one of the most widely cultivated and economically important fruit crops worldwide [,,]. Apple Valsa canker, caused by the fungal pathogen Valsa mali, is a destructive disease that severely reduces apple yield and quality, leading to significant economic losses in apple-producing regions [,,]. Previous studies have reported disease incidences ranging from 50% to 100%, with complete yield losses in older orchards in East Asia [,,,,,,]. Therefore, developing effective and sustainable management strategies against apple Valsa canker is of great importance.
Over the past decade, extensive research has focused on identifying the pathogenic factors and elucidating the infection mechanisms of V. mali. The virulence of V. mali has been attributed mainly to multiple pathogenic determinants, including cell wall-degrading enzymes (CWDEs), toxins, microRNA-like RNAs, effector proteins, secondary metabolites, and various pathogenic signaling regulators [,,,]. These factors collectively disrupt host cellular structure and metabolism, alter the chemical composition of apple tissues, and ultimately cause cell disintegration and branch decay. In particular, the secretion of CWDEs (such as xylanase, pectinase, and β-glucosidase) and the production of toxins (such as protocatechuic acid and p-hydroxybenzoic acid) are key virulence mechanisms that facilitate host cell wall degradation and tissue necrosis [,,,,]. Several genes associated with these processes, including VmXyl1, VmGlu2, VmXyl2, VmHbh1, and VmHbh4, have been identified and characterized [,,,]. In our previous transcriptomic analysis of the V. mali strain Vm1 (Vm1), a gene encoding a haloacid dehalogenase (HAD) superfamily enzyme, designated VmHAD, was identified and annotated to several metabolic pathways. These findings suggested that VmHAD may play a role in virulence metabolism or stress response signaling during host infection. However, its biological characteristics and potential function in Vm1 pathogenesis remain unknown.
The HAD superfamily represents one of the largest and most diverse groups of hydrolases, comprising enzymes such as dehalogenases, ATPases, phosphatases, phosphomutases, and phosphonatases [,,,]. Members of this family are widely distributed among prokaryotic and eukaryotic organisms and participate in numerous metabolic and regulatory processes. Various HAD enzymes have been structurally and biochemically characterized from different organisms, including phosphoserine phosphatase (SerB), phosphoglycolate phosphatase, phosphonacetaldehyde hydrolase, phosphoglucomutase, and inorganic pyrophosphatase from Methanococcus jannaschii [], Thermoplasma acidophilum [], Bacillus cereus [], Lactococcus lactis [], and Bacteroides thetaiotaomicron BT2127 [], as well as phosphatases YbiV and NagD from Escherichia coli K-12 [,]. Additionally, haloacid dehalogenases have been reported in Pseudomonas spp. [,], Xanthobacter autotrophicus [], and Staphylococcus lugdunensis (SLHAD1) []. While these studies have mainly focused on structural and biochemical features and their applications in environmental bioremediation, little is known about HAD enzymes in phytopathogenic fungi. Only a few HAD-related genes have been reported in plant pathogens, including cutA from Fusarium fujikuroi [], FoHAD-type II from F. oxysporum f. sp. momordicae [], FOXG-07877 from F. oxysporum f. sp. fragariae [], and Nem1 from Botryosphaeria dothidea [].
However, the biological characteristics and specific functions of the VmHAD gene in Vm1, particularly its expression dynamics during infection, have not been elucidated. Therefore, the objectives of this study were to (i) clone the full-length cDNA sequence of VmHAD gene from Vm1 and analyze its bioinformatic features; (ii) construct a recombinant expression vector to assess subcellular localization; (iii) analyze VmHAD gene expression during Vm1 infection of apple branches. The results of this study will enhance the understanding of the biological characteristics and functional roles of HAD enzymes in pathogenic fungi and may contribute to the development of effective strategies for apple Valsa canker management.
2. Materials and Methods
2.1. Fungal Preparation
The pathogen of Vm1 that causes apple Valsa canker was provided by the Laboratory of Plant Virology and Molecular Biology, College of Plant Protection, Gansu Agricultural University. The strain of Vm1 was cultured on potato dextrose agar (PDA) at 25 °C for 5 days for further experiments.
2.2. Fungal Total RNA Extraction and First-Strand cDNA Synthesis
For the total RNA extraction, the fresh mycelia of Vm1 were collected 5 days after inoculation on PDA media, and frozen with liquid nitrogen and stored at −80 °C for further experiments. The specific method for the total RNA extraction was used according to the manufacturer’s instructions for the Fungal RNA Extraction kit (OMEGA Bio-Tek, Norcross, GA, USA). The total RNA purity was assessed by OD260/280 and OD260/230 ratios. Integrity was checked by agarose gel electrophoresis. Thereafter, high-quality RNA samples were used for cDNA synthesis in accordance with the manufacturer’s instructions for the First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Haloacid Dehalogenase Gene (VmHAD) of Valsa mali Vm1 Cloning and Characterization
For cloning the full-length cDNA sequence of the VmHAD gene from Vm1, a fragment of the VmHAD gene was obtained from the transcriptome data in our previous work and used to design the primers (VmHAD-CF-F and VmHAD-CF-R). The core fragment of the VmHAD gene was cloned by using the cDNA of Vm1 as the template, and the VmHAD-CF-F and VmHAD-CF-R as primers to amplify. Thereafter, the synthesis of 5′ RACE and 3′ RACE cDNA fragments was carried out using the SMARTer® RACE 5′/3′ Kit (Takara Bio. Inc., Dalian, China), and the primers of VmHAD-5-1 and VmHAD-5-2, and VmHAD-3-1 and VmHAD-3-2 were designed to clone the 5′ RACE and 3′ RACE cDNA fragments, respectively. The full-length cDNA sequence of the VmHAD gene was verified using the primers of VmHAD-FL-F and VmHAD-FL-R. Primers were designed using Primer Premier 5.0 software, and synthesized and sequenced by Tsingke Biotechnology Co., Ltd., Beijing, China (Table S1). The open reading frame (ORF) of the VmHAD gene was analyzed by ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) (accessed on 15 May 2025) and then the corresponding amino acid sequences were deduced by DNAMAN 8.0 (Lynnon Biosoft, San Ramon, CA, USA). For the further analysis and characterization of the VmHAD protein, the multiple sequences of HAD amino acids from 7 species of plant pathogens were aligned and performed using DNAMAN 8.0. A maximum likelihood (ML) phylogenetic tree was constructed using the Mega 7.0 with a bootstrap value of 1000 replicates, according to the VmHAD and other reference HAD amino acid sequences.
2.4. VmHAD Bioinformatic Analysis
The related genes sequence was searched using BLAST in the NCBI databank (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi) (accessed on 15 May 2025); the physicochemical properties of the VmHAD protein were analyzed using EXPASY (https://web.expasy.org/protparam/) (accessed on 20 August 2025); the conservative structure domain was predicted using the NCBI database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (accessed on 15 May 2025); the membrane structure domain, signal peptide, phosphorylation sites, and protein secondary structure were analyzed using the TMHMM Server v. 2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/) (accessed on 15 May 2025), SignalP-5.0 Server (https://services.healthtech.dtu.dk/services/SignalP-5.0/) (accessed on 15 May 2025), NetPhos 3.1 Server (https://services.healthtech.dtu.dk/services/NetPhos/) (accessed on 15 May 2025), and SOPMA (https://npsa.lyon.inserm.fr/cgibin/npsa_automat.pl?page=/NPSA/npsa_sopma.html) (accessed on 20 August 2025), respectively; and the tertiary structure was constructed using the SWISS-MODEL (https://swissmodel.expasy.org/) (accessed on 15 May 2025).
2.5. VmHAD Gene Recombinant Vector Construction and Transformation
To monitor the subcellular localization of the VmHAD protein, the primers (VmHAD-C-F and VmHAD-C-R) were designed according to the full-length cDNA sequence of the VmHAD gene and used to clone the coding region (CDS) of the VmHAD gene (VmHAD-CDS). The primers (EF-VmHAD-F and EF-VmHAD-R) were designed to amplify the expression fragment of EF-VmHAD. Thereafter, EF-VmHAD was ligated into a pBWA(V)HS-GLosgfp plasmid using BsaI/Eco31I sites, and finally the recombinant plasmid (pBWA(V)HS-EF-VmHAD-GLosgfp) was transformed into E. coli DH5α. Finally, the positive single clones were selected for PCR verification using the primers of V-VmHAD-HS and V-VmHAD-D1. The verified positive single clones were used to extract the plasmids and then transformed into Agrobacterium tumefaciens GV3101. Thereafter, the single clones were observed and picked for PCR detection. Therefore, the cultured liquid of the verified clones was prepared for A. tumefaciens GV3101 transformation. Primers were designed using Primer Premier 5.0 software, and synthesized and sequenced by Tsingke Biotechnology Co., Ltd., Beijing, China (Table S1).
2.6. VmHAD Subcellular Location Analysis by Transient Expression
The transient expression of the VmHAD protein was performed in Nicotiana benthamiana. The A. tumefaciens GV3101 solutions carrying pBWA(V)HS-EF-VmHAD-GLosgfp and pBWA(V)HS-GLosgfp were injected into the leaves of 4-week-old N. benthamiana seedlings with three replications. Thereafter, the transformed N. benthamiana seedlings were incubated at 25 °C for 3 days after injection, and then fluorescence was observed with a confocal microscope (Nikon C2-ER, Tokyo, Japan).
2.7. VmHAD Gene Expression Characteristics Analysis During the Valsa mali Vm1 Infection Process by RT-qPCR
Healthy and uniform detached apple branches (cultivars, ‘Fuji’) with a diameter of approximately 6–7 mm were prepared for inoculation with Vm1 and VmHAD gene expression analysis during the infection process by RT-qPCR. The apple branches were disinfected with 1% sodium hypochlorite solution (NaOCl), rinsed with sterile water three times, and finally air-dried for further experiments. The cut edges of apple branches were sealed with paraffin wax and wounds were made on the surface. Thereafter, the disks of Vm1 were inoculated on the wounds of the detached apple branches, whereas the PDA disks without inoculation were used as controls. The inoculated branches were kept at 25 °C for 7 days. The tissue around the lesions of the branches in the treatment and control groups were collected after 1, 3, 5, and 7 days with a 2 day interval after inoculation, and stored at −80 °C for subsequent experiments. The total RNA extraction and First-Strand cDNA synthesis were performed according to the manufacturer’s instructions for the E.Z.N.A.® Plant RNA Kit (Tiangen Biotechnology, Beijing, China) and the First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA), respectively. The cDNA from the treatment and control groups was used as the template for determining the VmHAD gene expression characteristics after inoculation during the Vm1 infection process using the TB Green® Premix Ex Taq™ II kit (Takara, Dalian, China). Glucose–hexaphosphate dehydrogenase (G6PDH) (KC248180) and cytochrome enzyme (CYP) (KC248178) were selected as the internal reference genes. Primers for RT-qPCR determination were designed using Primer Premier 5.0 and named as q-VmHAD-F and q-VmHAD-R, G6PDH-F and G6PDH-R, CYP-F and CYP-R, and synthesized by Tsingke Biotechnology Co., Ltd., Beijing, China (Table S1). The stability of G6PDH and CYP was validated, and their melt curve analysis was evaluated before using them for VmHAD gene expression level determination. The samples in each treatment and the control were collected from three independently inoculated (treatment) or un-inoculated (control) branches, and each sample had three replications.
2.8. Statistical Analysis
The data for the VmHAD gene expression characteristics in the present study were calculated using the 2−ΔΔCt method and analyzed using one-way ANOVA using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov method and p-value were used to check the normality of the sample distribution. The significant differences among the treatments were determined using the Duncan’s multiple range test at p < 0.05.
3. Results
3.1. Full-Length cDNA Sequence of VmHAD Gene Cloning and Characterization
The core fragment of the VmHAD gene from Vm1 was amplified by RT-PCR, with a length of 729 bp after it was sequenced (Figure 1A). Meanwhile, a 186 bp 5′ fragment (Figure 1B) and a 558 bp 3′ fragment (Figure 1C) were successfully cloned using the SMARTer® RACE 5′/3′ Kit and verified by sequencing. The full-length cDNA sequence of the VmHAD gene was ultimately obtained by splicing the 5′ and 3′ end sequences of the VmHAD gene with the fragment, producing a length of 1044 bp, which contained a complete ORF of 798 bp that encoded 265 amino acids (Figure 2). Thereafter, a 996 bp fragment was amplified to verify that the full-length cDNA sequence of the VmHAD gene was obtained correctly (Figure 1D). In addition, the VmHAD protein exhibited the highest similarity to Cytospora mali 03-8 (Figure 3) based on complete amino acid sequence analysis. The phylogenetic tree analysis revealed that it was closest to the (S)-2-haloacid dehalogenase (accession number KUI70710.1) of C. mali 03-8, with clustering in the same clade with a bootstrap support rate of 100% (Figure 4).
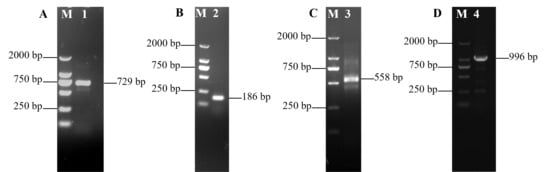
Figure 1.
Full-length cDNA sequence of VmHAD gene of V. mali Vm1 cloning and characterization: M: D2000 marker; (A): lane 1, core fragment; (B): lane 2, 5′ RACE fragment; (C): lane 3, 3′ RACE fragment; (D): lane 4, full-length cDNA fragment verified.
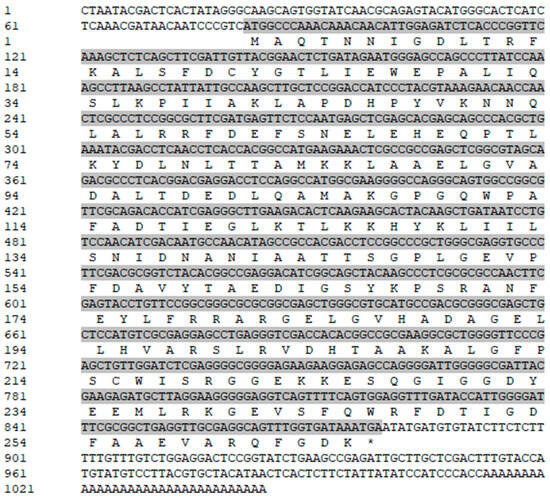
Figure 2.
Full-length cDNA and amino acid sequences of VmHAD gene of V. mali Vm1. Gray shaded sequences represent the ORF region; “*” represents the stop codon.
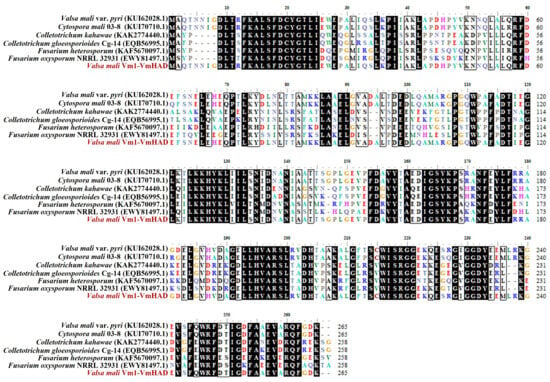
Figure 3.
Amino acid sequence alignment of VmHAD protein. Multiple sequences were aligned using DNAMAN 8.0.
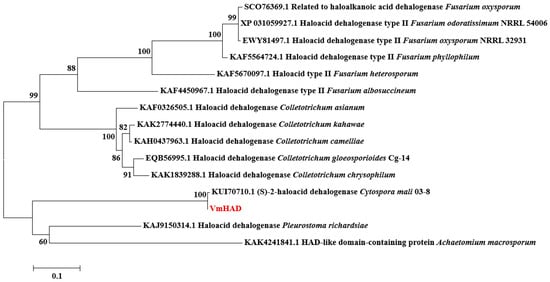
Figure 4.
Phylogenetic tree construction of VmHAD protein. A maximum likelihood (ML) phylogenetic tree was constructed with a bootstrap value of 1000 replicates.
3.2. VmHAD Bioinformation Analysis
The results revealed that the VmHAD protein comprises 265 amino acids, with a molecular formula of C1317H2049N357O395S6 and an approximate molecular weight of 29.4 kDa. The theoretical isoelectric point (pI), aliphatic index (AI), grand average of hydrophilicity (GRAVY), and instability index were 5.47, 83.36, −0.358, and 38.20, respectively. The predominant amino acids included alanine (11.7%), leucine (10.6%), and glycine (7.9%), and there were also 38 negatively charged residues (Asp + Glu) and 31 positively charged residues (Arg + Lys) (Table 1). The conserved domain structure analysis revealed that the VmHAD protein had a particularly matched HAD-like junction domain at positions 14-234 of the amino acid sequences and belongs to the L-2-haloacid dehalogenase family within the HAD-like superfamily (Figure 5A). However, the VmHAD protein did not contain a signal peptide and transmembrane regions. Furthermore, the phosphorylation site prediction revealed that the VmHAD protein contains 34 potential phosphorylation sites, including 12 serine (Ser, S) phosphorylation sites at positions S17, S34, S63, S134, S146, S165, S169, S199, S214, S218, S226, and S243; 14 threonine (Thr, T) phosphorylation sites at positions T4, T11, T23, T72, T80, T81, T97, T117, T123, T144, T145, T159, T205, and T250; and 8 tyrosine (Tyr, Y) phosphorylation sites at positions Y21, Y48, Y75, Y128, Y158, Y166, Y175, and Y233 (Figure 5B). The hydrophilicity analysis also confirmed that it is a hydrophilic protein (Figure 5C). The predictive secondary structure of the VmHAD protein indicated that there are four spatial conformations. Among them, the α-helix has the highest proportion, accounting for 49.06% of the amino acid residues, followed by random coils and extended chains accounting for 34.72% and 10.57%, respectively, whereas the β-sheets had the lowest proportion at 5.66% (Figure 6A). The tertiary structure of the VmHAD protein indicated that it encompasses amino acids from the 1st to the 265th position. The similarity between the sequence and the template sequence was 96.98%, with a coverage rate of 0.93 (Figure 6B).

Table 1.
Physicochemical properties of VmHAD protein.
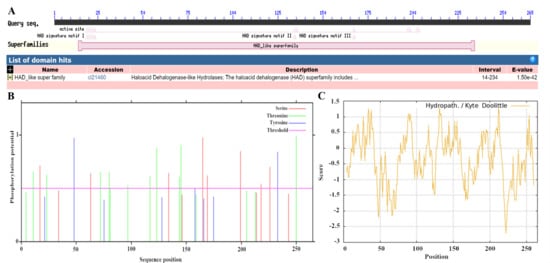
Figure 5.
Bioinformation of VmHAD protein. (A): Conserved domain analysis; (B): phosphorylation sites prediction; (C): hydrophilic and hydrophobic properties analysis.
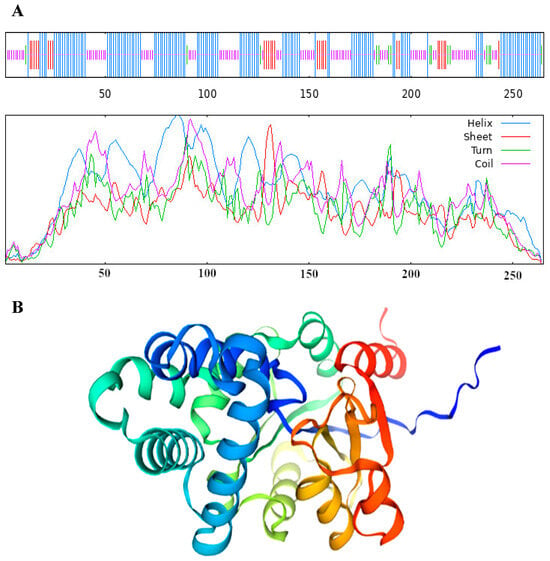
Figure 6.
Secondary structure (A) and tertiary structure (B) of VmHAD prediction.
3.3. Subcellular Localization Vector of VmHAD Gene Construction and Transformation
For monitoring the subcellular localization of the VmHAD gene, a 798 bp fragment of CDS was obtained and sequenced (Figure 7A). Thereafter, an 835 bp expression fragment (EF-VmHAD) was amplified (Figure 7B) and ligated into the double-digested pBWA(V)HS-GLosgfp plasmid (Figure 7C). Finally, a single band with a length of approximately 680 bp was obtained in each of transformed clones after successful transformation into E. coli DH5α (Figure 7D, lanes 2–11), whereas the clone that transformed the empty plasmid did not yield any bands (Figure 7D, lane 1), and also the blank control showed no bands (Figure 7D, lane 12). Finally, a 680 bp fragment was amplified from each of the eight well-growing single clones after transformation into A. tumefaciens GV3101 (Figure 7E, lanes 1–8).
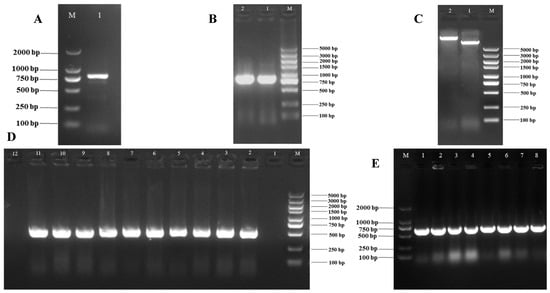
Figure 7.
Subcellular localization vector of VmHAD gene construction and transformation. M: D2000 and D5000 markers; (A): CDS fragment of VmHAD gene cloning, where line 1 represents CDS fragment; (B): expression fragment cloning, where lines 1–2 represent the expression fragment of EF-VmHAD; (C): recombinant plasmid construction, where line 1 represents the empty plasmid of pBWA(V)HS-GLosgfp and line 2 represents the recombinant plasmid of pBWA(V)HS-EF-VmHAD-GLosgfp; (D): recombinant plasmid transformation, where line 1 represents the transformed empty plasmid (pBWA(V)HS-GLosgfp) of E. coli DH5α clone detected by PCR verification, lines 2–11 represent the transformed recombinant plasmid (pBWA(V)HS-EF-VmHAD-GLosgfp) of E. coli DH5α clones, and line 12 represents the blank control; (E): A. tumefaciens GV3101 clone detection, where lines 1–8 represent the transformed recombinant plasmid (pBWA(V)HS-EF-VmHAD-GLosgfp) of A. tumefaciens GV3101 clones detected by PCR verification.
3.4. VmHAD Subcellular Location Analysis
The expression location of the VmHAD protein in N. benthamiana leaves was observed at the subcellular level using laser confocal microscopy. The results showed that the VmHAD protein was possibly located in both the nucleus and cytoplasm after being injected into the N. benthamiana leaves using the transient expression system (Figure 8).
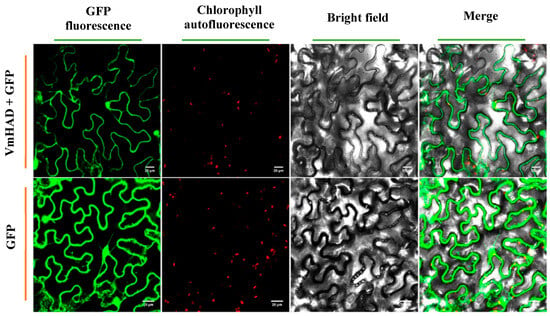
Figure 8.
Localization of VmHAD protein expressed transiently in N. benthamiana leaves at the subcellular level. The size of the scale bars represents 20 µm.
3.5. VmHAD Gene Expression Characteristics Analysis
Compared with the control, the VmHAD gene was significantly expressed during the infection process of Vm1 on detached apple branches at different time points, whereas an expression level was not found in the control branches (no inoculation). The expression level of the VmHAD gene was significantly increased during Vm1 infection, with significant difference from day 1 to 5 after inoculation with Vm1 on the detached apple branches, and stability expressed at days 5 and 7. The relative expression level of the VmHAD gene was increased by 3.13-, 4.25-, and 3.98-fold at 3, 5, and 7 days after inoculation in comparison to day 1, with the highest expression level after 5 days (Figure 9).
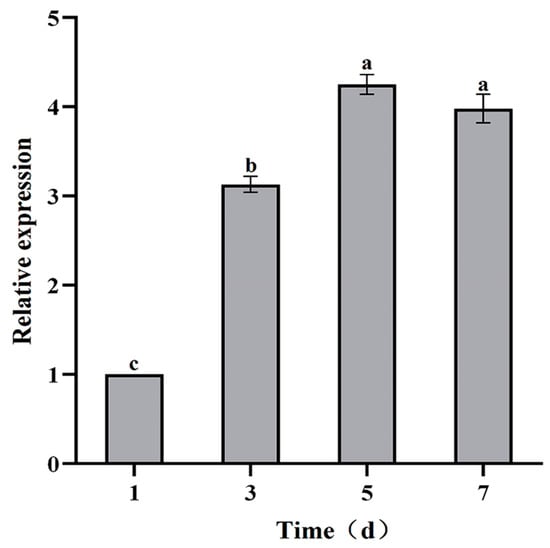
Figure 9.
The expression characteristics of VmHAD gene analysis at different time points during the V. mali Vm1 infection process. Line bars represent the standard errors (SEs) of the means of replications, and the different letters indicate the significant difference among the different time points during the infection process at p < 0.05 level according to Duncan’s new multiple range test.
4. Discussion
HAD-like hydrolases have been found in organisms with 479,051 sequences in databases (InterPro IPR023214) and 33 major families [,]. The number of HAD genes that has been reported in different bacteria has ranged from 10 to 20, as well as 100 in humans, 115 in Arabidopsis thaliana (InterPro database), and more than 45 in Saccharomyces cerevisiae in their individual genomes []. However, the majority of the HADs’s biological and structural characteristics and even their functions remain uncharacterized []. In our present study, a novel VmHAD gene from Vm1 with full-length cDNA sequences of 1044 bp were cloned using RACE technology, and its genetic relationship was closest to the (S)-2-halogenated dehalogenase (accession number KUI70710.1) of C. mali 03-8, with a bootstrap support rate of 100%, which belongs to the L-2-haloacid dehalogenase family of the HAD hydrolase superfamily. Previous studies revealed that 2-haloacid dehalogenase in microorganisms is widespread in nature, and has been classified into four types including the D-2-haloacid dehalogenase, L-2-haloacid dehalogenase, configuration-inverting DL-2-haloacid dehalogenase, and configuration-retaining DL-2-haloacid dehalogenase, according to the substrate specificities and product configurations []. To date, the biochemical characterization of 2-haloacid dehalogenases has been studied, including the L-2-haloacid dehalogenase from the thermophilic archaeon Sulfolobus tokodaii [], the DL-2-haloacid dehalogenase gene from Burkholderia cepacia [], and even a novel L-2-haloacid dehalogenase (L-2-DhlB) (25 kDa) has been isolated from the strain of Ancylobacter aquaticus UV5, and it was found that it belongs to the family of L-2-haloacid dehalogenase []. However, specific functions, such as their role in pathogenicity, have not been fully explored recently.
In addition, our study found that the ORF of the VmHAD gene was 798 bp, corresponding to 265 amino acids, and with a particularly matched junction domain that was HAD-like. Kumar et al. (2016) [] investigated the L-2-DhlB of A. aquaticus UV5 with a putative conserved domain of a hypothetical HAD-like superfamily and subfamily IA, and a 693 bp ORF sequence corresponding to 230 amino acids; Ren et al. (2023) found that the HAD-like superfamily Nem1 gene from the pathogen of B. dothidea contains a transmembrane domain and a characteristic HAD-like domain []. Additionally, the cDNA fragment of MoNEM1 from Magnaporthe oryzae that contains an ORF encoding 537 amino acids and a conserved HAD-like superfamily domain was also investigated []. However, the differences in ORF length and domain structure in our present study and previous studies may indicate the differences in their potential function in different organisms, especially the pathogenic functions.
Phosphorylation is an important post-translational modification process that plays a significant role in regulating the activity, stability, and subcellular localization of proteins. In our present study, we predicted that the phosphorylation site of the VmHAD protein contains 34 potential phosphorylation sites, and found that it is possibly located in both the nucleus and cytoplasm of N. benthamiana at the subcellular level. Consistently, Lee et al. (2022) reported that the haloacid dehalogenase-like phosphatase AtHAD1 was involved in repressing the ABA response and expressed in the Arabidopsis nucleus and cytoplasm []. Thus, we hypothesis that this dual localization indicates that the VmHAD protein could participate in various metabolic processes, such as the possible functions of the VmHAD gene involved in modulating nuclear signaling pathways or in cytoplasmic metabolic processes. However, work related to the quantitative assessment and the localization of nuclear and cytoplasmic markers will be performed in the future.
Furthermore, we found that the VmHAD gene was significantly expressed during the Vm1 infection process on the detached apple branches. The expression level was significantly increased with infection process time increased from day 1 to 5, and with significant differences from 3 to 7 days in comparison to day 1. Thus, our result indicates that the function of the VmHAD gene may play a potential role in the pathogenicity of Vm1. Ren et al. (2023) found that the Nem1 homolog in B. dothidea was significantly upregulated during the infection process, which indicates that the Nem1 gene is important for the pathogenicity of B. dothidea []. Other previous studies found that Nem1 was involved in regulating mycelial growth and conidiation but not in conidial morphology or germination, including the filamentous fungi of Aspergillus fumigatus, M. oryzae, and F. graminearum [,,,]. Zhang et al. (2023) found that the expression level of FoHAD-type II plays an important role in the pathogenic process of F. oxysporum f. sp. momordicae []. Fang et al. (2014) found that the FOXG-07877 gene that codes for the HAD superfamily of hydrolases showed a downregulated expression in the strawberry wilt pathogen (with a lower-toxin strain of F. oxysporum f. sp. fragariae) by analyzing the different expression genes in different pathogenic strains at the protein level in comparison to the higher toxin strain []. In addition, Garcia-Martinez et al. (2014) reported that the cutA gene from F. fujikuroi that encodes the protein of the haloacid dehalogenase family and its functions were involved in osmotic stress and glycerol metabolism []. However, the specific pathogenic mechanisms and pathways for HAD genes in plant pathogens are little researched; in particular, the molecular pathogenic mechanisms and regulatory pathways involving VmHAD virulence in Vm1 are unknown and will be further studied in future.
5. Conclusions
In conclusion, a detailed characterization of a novel haloacid dehalogenase superfamily gene (VmHAD) from Vm1 was completed and monitored at the molecular and subcellular levels. Our results found that the VmHAD protein was closest to the (S)-2-halogenated dehalogenase of C. mali 03-8, which belongs to the L-2-haloacid dehalogenase of the HAD hydrolase superfamily. In addition, it was possibly located in both the nucleus and cytoplasm after being transformed into the N. benthamiana at the subcellular level. We also found that VmHAD expression was induced during Vm1 infection. Our results indicate that the function of the VmHAD protein was related to the Vm1 infection process and may play a potential role in the pathogenicity of Vm1. However, the specific pathogenic functions and mechanisms for the VmHAD protein will be further identified using gene knocking out technology and the quantitative assessment of its localization will be performed in future work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11120827/s1, Table S1. Primers for VmHAD gene cloning, subcellular localization, and expression characteristics determination.
Author Contributions
Conceptualization, B.X.; data curation, X.C. and C.D.; formal analysis, X.C. and S.Z.; funding acquisition, S.Z.; methodology, X.C., J.L., and S.Z.; project administration, S.Z. and B.X.; software, X.C., S.Z., and F.T.; supervision, B.X.; writing—original draft, S.Z.; writing—review and editing, B.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Major Science and Technology Projects of Gansu Province (project 22ZD6NA045); Key R & D Project in Gansu Province (project 23YFNA0020); Key National Research and Development Program Project (2023YFD1401400); Fuxi Outstanding Talent Cultivation Program, Gansu Agricultural University (Gaufx-03J03); and the Lanzhou Science and Technology Project (2023-QN-171).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to the College of Plant Protection, Gansu Agricultural University, and the plasmid provided by the Wuhan Biorun Bio-Tech CO., LTD., and Alejandro CalderónUrrea’s comments and suggestions for the manuscript designing and writing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cornille, A.; Giraud, T.; Smulders, M.J.M.; Roldan-Ruiz, I.; Gladieux, P. The domestication and evolutionary ecology of apples. Trends Genet. 2014, 30, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.B.; Bai, Y.; Sun, H.H.; Wang, N.; Ma, Y.M.; Li, M.J.; Wang, X.; Jiao, C.; Legall, N.; Mao, L.Y.; et al. Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat. Commun. 2017, 8, 249. [Google Scholar] [CrossRef]
- Daccache, M.A.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Impact of the physicochemical composition and microbial diversity in apple juice fermentation process: A review. Molecules 2020, 25, 3698. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.X.; Wei, X.Y.; Xiao, Y.X.; Sun, Y.; Biggs, A.R.; Gleason, M.L.; Shang, S.P.; Zhu, M.Q.; Guo, Y.Z.; Sun, G.Y. Management of Valsa canker on apple with adjustments to potassium nutrition. Plant Dis. 2016, 100, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Hu, T.L.; Wang, Y.A.; Luo, Y.; Michailides, T.J.; Cao, K.Q. New understanding on infection processes of Valsa canker of apple in China. Eur. J. Plant Pathol. 2016, 146, 531–540. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Wang, X.H.; Chen, K.M.; Han, C.Y.; Guan, H.P.; Wang, Y.; Zhao, Y.R. Feasibility and potential of terahertz spectral and imaging technology for apple Valsa canker detection: A preliminary investigation. Spectrochim. Acta 2025, 327, 125308. [Google Scholar] [CrossRef]
- Cao, K.Q.; Guo, L.Y.; Li, B.H.; Sun, G.Y.; Chen, H.J. Investigations on the occurrence and control of apple canker in China. Plant Protect. 2009, 35, 114–117. [Google Scholar]
- Vasilyeva, L.; Kim, W.G. Valsa mali Miyabe et Yamada, the causal fungus of apple tree canker in east Asia. Mycobiology 2000, 28, 153–157. [Google Scholar] [CrossRef]
- Wang, X.L.; Shi, C.M.; Gleason, M.L.; Huang, L.L. Fungal species associated with apple Valsa canker in east Asia. Phytopathol. Res. 2020, 2, 14. [Google Scholar] [CrossRef]
- Abe, K.; Kotoda, N.; Kato, H.; Soejima, J. Resistance sources to Valsa canker (Valsa ceratosperma) in a germplasm collection of diverse Malus species. Plant Breed. 2007, 126, 449–453. [Google Scholar] [CrossRef]
- Wang, X.L.; Zang, R.; Yin, Z.Y.; Kang, Z.S.; Huang, L.L. Delimiting cryptic pathogen species causing apple Valsa canker with multilocus data. Ecol. Evol. 2014, 4, 1369–1380. [Google Scholar] [CrossRef]
- Li, Z.P.; Yin, Z.Y.; Fan, Y.Y.; Xu, M.; Kang, Z.S.; Huang, L.L. Candidate effector proteins of the necrotrophic apple canker pathogen Valsa mali can suppress BAX-induced PCD. Front. Plant Sci. 2015, 6, 579. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Li, X.S.; Bozorov, T.A.; Ma, R.; Ma, J.B.; Zhang, Y.H.; Yang, H.L.; Li, L.; Zhang, D.Y. Characterization and pathogenicity of six Cytospora strains causing stem canker of wild apple in the Tianshan Forest, China. Forest Pathol. 2020, 50, e12587. [Google Scholar] [CrossRef]
- Ke, X.W.; Yin, Z.Y.; Song, N.; Dai, Q.Q.; Voegele, R.T.; Liu, Y.Y.; Wang, H.Y.; Gao, X.N.; Kang, Z.S.; Huang, L.L. Transcriptome profiling to identify genes involved in pathogenicity of Valsa mali on apple tree. Fungal Genet. Biol. 2014, 68, 31–38. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Liu, H.Q.; Li, Z.P.; Ke, X.W.; Dou, D.L.; Gao, X.N.; Song, N.; Dai, Q.Q.; Wu, Y.X.; Xu, J.R.; et al. Genome sequence of Valsa canker pathogens uncovers a potential adaptation of colonization of woody bark. New Phytol. 2015, 208, 1202–1216. [Google Scholar] [CrossRef]
- Sun, G.C.; Xie, S.C.; Tang, L.; Zhao, C.; Zhang, M.; Hang, L.L. Comparative genomics of five Valsa species gives insights on their pathogenicity evolution. G3 2023, 13, jkac312. [Google Scholar] [CrossRef]
- Feng, H.; Wang, C.L.; He, Y.T.; Tang, L.; Han, P.L.; Liang, J.H.; Huang, L.L. Apple Valsa canker: Insights into pathogenesis and disease control. Phytopathol. Res. 2023, 5, 45. [Google Scholar] [CrossRef]
- Wang, C.X.; Li, C.; Li, B.H.; Li, G.F.; Dong, X.L.; Wang, G.P.; Zhang, Q.M. Toxins produced by Valsa mali var. mali and their relationship with pathogenicity. Toxins 2014, 6, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Gao, X.N.; Chen, J.L.; Yin, Z.Y.; Feng, H.; Huang, L.L. The feruloyl esterase genes are required for full pathogenicity of the apple tree canker pathogen Valsa mali. Mol. Plant Pathol. 2018, 19, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- McCombe, C.L.; Greenwood, J.R.; Solomon, P.S.; Williams, S.J. Molecular plant immunity against biotrophic, hemibiotrophic, and necrotrophic fungi. Essays Biochem. 2022, 66, 581–593. [Google Scholar] [CrossRef]
- Wang, F.; Ye, L. Biotrophic fungal pathogens: A critical overview. Appl. Biochem. Biotechnol. 2023, 195, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Li, T.; Shi, X.P.; Saleem, M.; Li, B.H.; Liang, W.X.; Wang, C.X. Deletion of endo-β-1, 4-xylanase VmXyl1 impacts the virulence of Valsa mali in apple tree. Front. Plant Sci. 2018, 9, 663. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, C.L.; Sun, C.C.; Saleem, M.; Li, P.L.; Li, B.H.; Wang, C.X. β-glucosidase VmGlu2 contributes to the virulence of Valsa mali in apple tree. Front. Microbiol. 2021, 12, 695112. [Google Scholar] [CrossRef]
- Cui, X.Y.; Li, X.K.; Li, S.; Huang, Y.; Liu, N.; Lian, S.; Li, B.H.; Wang, C.X. Xylanase VmXyl2 is involved in the pathogenicity of Valsa mali by regulating xylanase activity and inducing cell necrosis. Front. Plant Sci. 2024, 15, 1342714. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.L.; Sun, C.L.; Gao, L.Y.; Saleem, M.; Li, B.H.; Wang, C.C. Hydroxybenzoate hydroxylase genes underlying protocatechuic acid production in Valsa mali are required for full pathogenicity in apple trees. Mol. Plant Pathol. 2021, 22, 1370–1382. [Google Scholar] [CrossRef]
- Aravind, L.; Galperin, M.Y.; Koonin, E.V. The catalytic domain of the P-type ATPase has the haloacid dehalogenase fold. Trends Biochem. Sci. 1998, 23, 127–129. [Google Scholar] [CrossRef]
- Collet, J.F.; Stroobant, V.; Pirard, M.; Delpierre, G.; Van Schaftingen, E. A new class of phosphotransferases phosphorylated on an aspartate residue in an amino-terminal DXDX (T/V) motif. J. Biol. Chem. 1998, 273, 14107–14112. [Google Scholar] [CrossRef]
- Pandey, B.K.; Mehra, P.; Verma, L.; Bhadouria, J.; Giri, J. OsHAD1, a haloacid dehalogenase-like ATPase, enhances phosphate accumulation. Plant Physiol. 2017, 174, 2316–2332. [Google Scholar] [CrossRef]
- May, A.; Berger, S.; Hertel, T.; Kock, M. The Arabidopsis thaliana phosphate starvation responsive gene AtPPsPase1 encodes a novel type of inorganic pyrophosphatase. Biochim. Biophys. Acta 2011, 1810, 178–185. [Google Scholar] [CrossRef]
- Wang, W.; Kim, R.; Jancarik, J.; Yokota, H.; Kim, S.H. Crystal structure of phosphoserine phosphatase from Methanococcus jannaschii, a hyperthermophile, at 1.8 A resolution. Structure 2001, 9, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yakunin, A.F.; Kuznetsova, E.; Xu, X.; Pennycooke, M.; Gu, J.; Cheung, F.; Proudfoot, M.; Arrowsmith, C.H.; Joachimiak, A.; et al. Structure and function-based characterization of a new phosphoglycolate phosphatase from Thermoplasma acidophilum. J. Biol. Chem. 2004, 279, 517–526. [Google Scholar] [CrossRef]
- Morais, M.C.; Zhang, W.H.; Baker, A.S.; Zhang, G.F.; Dunaway-Mariano, D.; Allen, K.N. The crystal structure of Bacillus cereus phosphonoacetaldehyde hydrolase: insight into catalysis of phosphorus bond cleavage and catalytic diversification within the HAD enzyme superfamily. Biochemistry 2000, 39, 10385–10396. [Google Scholar] [CrossRef]
- Lahiri, S.D.; Zhang, G.F.; Dunaway-Mariano, D.; Allen, K.N. Caught in the act: The structure of phosphorylated β-phosphoglucomutase from Lactococcus lactis. Biochemistry 2002, 41, 8351–8359. [Google Scholar] [CrossRef]
- Huang, H.; Patskovsky, Y.; Toro, R.; Farelli, J.D.; Pandya, C.; Almo, S.C.; Allen, K.N.; Dunaway-Mariano, D. Divergence of structure and function in the haloacid dehalogenase enzyme superfamily: Bacteroides thetaiotaomicron BT2127 is an inorganic pyrophosphatase. Biochemistry 2011, 50, 8937–8949. [Google Scholar] [CrossRef]
- Roberts, A.; Lee, S.Y.; McCullagh, E.; Silversmith, R.E.; Wemmer, D.E. YbiV from Escherichia coli K12 is a HAD phosphatase. Proteins 2005, 58, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, L.W.; Dunaway-Mariano, D.; Allen, K.N. Structure and activity analyses of Escherichia coli K-12 NagD provide insight into the evolution of biochemical function in the haloalkanoic acid dehalogenase superfamily. Biochemistry 2006, 45, 1183–1193. [Google Scholar] [CrossRef]
- Hisano, T.; Hata, Y.; Fujii, T.; Liu, J.Q.; Kurihara, T.; Esaki, N.; Soda, K. Crystal structure of L-2-haloacid dehalogenase from Pseudomonas sp. YL. An alpha/beta hydrolase structure that is different from the alpha/beta hydrolase fold. J. Biol. Chem. 1996, 271, 20322–20330. [Google Scholar] [CrossRef] [PubMed]
- Schmidberger, J.W.; Wilce, J.A.; Weightman, A.J.; Wilce, M.C.J. Purification, crystallization and preliminary crystallographic analysis of DehI, a group I α-haloacid dehalogenase from Pseudomonas putida strain PP3. Acta Crystallogr. Sect. F 2008, 64, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Ridder, I.S.; Rozeboom, H.J.; Kalk, K.H.; Janssen, D.B.; Dijkstra, B.W. Three-dimensional structure of L-2-haloacid dehalogenase from Xanthobacter autotrophicus GJ10 complexed with the substrate-analogue formate. J. Biol. Chem. 1997, 272, 33015–33022. [Google Scholar] [CrossRef]
- Kaur, H.; Rode, S.; KP, S.; Mahto, J.K.; Alam, M.S.; Gupta, D.N.; Kar, B.; Singla, J.; Kumar, P.; Sharma, A.K. Characterization of haloacid dehalogenase superfamily acid phosphatase from Staphylococcus lugdunensis. Arch. Biochem. Biophys. 2024, 753, 109888. [Google Scholar] [CrossRef]
- García-Martínez, J.; Castrillo, M.; Avalos, J. The gene cutA of Fusarium fujikuroi, encoding a protein of the haloacid dehalogenase family, is involved in osmotic stress and glycerol metabolism. Microbiology 2014, 160, 26–36. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wen, C.Y.; Li, F.X.; Wei, C.X.; Du, H.Y.; Zhong, R.R.; Zhao, Y. Function analysis of haloacid dehalogenase type II gene FoHAD-type II in Fusarium oxysporum f. sp. momordicae. Acta Phytopathol. Sin. 2023, 53, 195–206. [Google Scholar]
- Fang, X.L.; Barbetti, M.J. Differential protein accumulations in isolates of the strawberry wilt pathogen Fusarium oxysporum f. sp. fragariae differing in virulence. J. Proteom. 2014, 108, 223–237. [Google Scholar] [CrossRef]
- Ren, W.C.; Zhang, Y.H.; Zhu, M.Q.; Liu, Z.Q.; Lian, S.; Wang, C.X.; Li, B.H.; Liu, N. The phosphatase cascade Nem1/Spo7-Pah1 regulates fungal development, lipid homeostasis, and virulence in Botryosphaeria dothidea. Microbiol. Spectr. 2023, 11, e0388122. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Tatusov, R.L. Computer analysis of bacterial haloacid dehalogenases defines a large superfamily of hydrolases with diverse specificity. Application of an iterative approach to database search. J. Mol. Biol. 1994, 244, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, A.M.; Allen, K.N.; Dunaway-Mariano, D.; Aravind, L. Evolutionary genomics of the HAD superfamily: Understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J. Mol. Biol. 2006, 361, 1003–1034. [Google Scholar] [CrossRef]
- Kuznetsova, E.; Nocek, B.; Brown, G.; Makarova, K.S.; Flick, R.; Wolf, Y.I.; Khusnutdinova, A.; Evdokimova, E.; Jin, K.; Tan, K.; et al. Functional diversity of haloacid dehalogenase superfamily phosphatases from Saccharomyces cerevisiae: Biochemical, structural, and evolutionary insights. J. Biol. Chem. 2015, 290, 18678–18698. [Google Scholar] [CrossRef] [PubMed]
- Pathira Kankanamge, L.S.; Ruffner, L.A.; Touch, M.M.; Pina, M.; Beuning, P.J.; Ondrechen, M.J. Functional annotation of haloacid dehalogenase superfamily structural genomics proteins. Biochem. J. 2023, 480, 1553–1569. [Google Scholar] [CrossRef]
- Zakary, S.; Oyewusi, H.A.; Huyop, F. Genomic analysis of Mesorhizobium loti strain tono reveals dehalogenases for bioremediation. J. Trop. Life Sci. 2021, 11, 67–77. [Google Scholar] [CrossRef]
- Rye, C.A.; Isupov, M.N.; Lebedev, A.A.; Littlechild, J.A. Biochemical and structural studies of a L-haloacid dehalogenase from the thermophilic archaeon Sulfolobus tokodaii. Extremophiles 2009, 13, 179–190. [Google Scholar] [CrossRef]
- Ohkouchi, Y.; Koshikawa, H.; Terashima, Y. Cloning and expression of DL-2-haloacid dehalogenase gene from Burkholderia cepacia. Water Sci. Technol. 2000, 42, 261–268. [Google Scholar] [CrossRef]
- Kumar, A.; Pillay, B.; Olaniran, A.O. L-2-haloacid dehalogenase from Ancylobacter aquaticus UV5: Sequence determination and structure prediction. Int. J. Biol. Macromol. 2016, 83, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiao, T.L.; Liu, X.H.; Lin, F.C.; Wu, W.R. Functional characterization of a NEM1-like gene in Magnaporthe oryzae. Agric. Sci. China 2011, 10, 1385–1390. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.; Kim, T.; Hwang, J.; Lee, J. AtHAD1, a haloacid dehalogenase-like phosphatase, is involved in repressing the ABA response. Biochem. Bioph. Res. Commun. 2022, 587, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Winkelströter, L.K.; Dolan, S.K.; Fernanda Dos Reis, T.; Bom, V.L.P.; Alves de Castro, P.; Hagiwara, D.; Alowni, R.; Jones, G.W.; Doyle, S.; Brown, N.A.; et al. Systematic global analysis of genes encoding protein phosphatases in Aspergillus fumigatus. G3 2015, 5, 1525–1539. [Google Scholar] [CrossRef]
- Liu, N.; Yun, Y.Z.; Yin, Y.N.; Hahn, M.; Ma, Z.H.; Chen, Y. Lipid droplet biogenesis regulated by the FgNem1/Spo7-FgPah1 phosphatase cascade plays critical roles in fungal development and virulence in Fusarium graminearum. New Phytol. 2019, 223, 412–429. [Google Scholar] [CrossRef]
- Yun, Y.Z.; Liu, Z.Y.; Yin, Y.N.; Jiang, J.H.; Chen, Y.; Xu, J.R.; Ma, Z.H. Functional analysis of the Fusarium graminearum phosphatome. New Phytol. 2015, 207, 119–134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).