1. Introduction
Autophagy in eukaryotes is a highly conserved process to maintain homeostasis by degradation and recycling of damaged organelles or superfluous cellular components [
1]. The process of autophagy is modulated by a series of autophagy-related (ATG) proteins [
2]. Multiple studies have demonstrated that autophagy plays important roles in pathogenicity of various fungal pathogens [
1]. At present, over 40 ATGs have been identified in yeast and their homologs are well conserved in the sequenced plant pathogenic fungi [
3]. These ATGs are widely involved in the regulation of pathogenicity in plant pathogenic fungi [
4,
5,
6].
ATG16, a subunit of the ATG12-ATG5-ATG16 complex which functions in a manner analogous to an E3-like enzyme, is required for ATG8 lipidation, a process of ATG8 conjugation to phosphatidylethanolamine in the autophagosomal membrane, which is an essential step for the phagophore membrane expansion and the autophagosome formation [
7,
8]. Studies in yeast have shown that ATG16 is required for autophagy and functions structurally as a homodimer mediated by a coiled-coil domain (CCD) which is also responsible for the combination of ATG16 and ATG12 [
9,
10]. In yeast, ATG16 forms homodimers via its coiled-coil domain (CCD) and interacts with the ATG12-ATG5 conjugate through its N-terminal ATG5-binding motif to assemble the functional complex [
11]. In human pathogenic
Acanthamoeba castellanii, AcATG16 was involved in autophagosome formation of mitochondrial autophagy and played an essential role in the encystation [
12]. In the insect-pathogenic fungus
Beauveria bassiana, deletion of
BbATG16 resulted in defects in asexual development, tolerance to oxidative stress, virulence and autophagosome formation [
13].
Current research on the role of ATG16 in plant pathogenic fungi is limited, with only one reported study in
Magnaporthe oryzae.
M. oryzae ATG16 (MoATG16) physically interacted with Clp1, a PHD domain-containing protein, and colocalized at preautophagosomal structures (PASs) and autophagosomes, and the loss of Clp1 displayed defects in fungal morphology and development, glycogen metabolism, plant infection and virulence [
14]. However, the precise molecular function of MoATG16 in autophagy regulation and the mechanistic basis of MoATG16-Clp1 remain unclear.
Natural rubber, as an irreplaceable important strategic resource and industrial raw material, plays an important role in the transportation and military industries. As the main source of natural rubber, rubber tree (
Hevea brasiliensis) is an economically vital tropical tree species in numerous tropical countries, whose productivity directly underpins the sustainability of the global natural rubber industry [
15].
Colletotrichum gloeosporioides can cause rubber tree anthracnose and result in serious loss of natural rubber production [
16]. In this study,
C. gloeosporioides ATG16 (
CgATG16) was amplified and its functional roles in the development and virulence by generating deletion mutant (Δ
CgATG16) was studied. This study enriched our understanding of the roles of ATG16 in plant pathogenic fungi.
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
The wild-type (WT) strain of
Colletotrichum gloeosporioides used in this manuscript was isolated in 2015 from anthracnose-infected leaves of
Hevea brasiliensis at the Xiqing plantation in Danzhou City, Hainan Province. The fungal strains used in this study were cultured on potato dextrose agar (PDA: 200.0 g/L potato extract, 20.0 g/L dextrose, 20.0 g/L agar; pH 6.0) at 28 °C under dark conditions [
17].
2.2. Bioinformatics Analysis
The sequence of
CgATG16 was derived from the transcriptome database of
C. gloeosporioides (transcript ID: PV866801). The ORF sequence of ATG16 was obtained from a transcriptome database of
C. gloeosporioides. Using this sequence as the template, specific amplification primers for
CgATG16 were designed by using Primer Premier 5.0 software. The sequence of
CgATG16 was subsequently verified by RT-PCR amplification and sequencing. All primers synthesized by Beijing Tsingke Biotechnology Co., Ltd. (Beijing, China) are listed in
Supplementary Table S1 (Table S1). The amino acid sequence of CgATG16 was deduced by DNAMAN (version 7,0,2,176) software. Signal peptides and transmembrane domains were predicted using the online tools NovoPro SignalP 5.0 (
https://novopro.cn/tools/signalp.html (accessed on 10 April 2024)) and DeepTMHMM 1.0.39 (
https://dtu.biolib.com/DeepTMHMM (accessed on 10 April 2024)), respectively. Conserved domains were predicted using SMART 7 (
http://smart.embl-heidelberg.de/ (accessed on 13 September 2024)). Protein secondary structures were predicted using JPRED 4 (
https://www.compbio.dundee.ac.uk/jpred/ (accessed on 15 December 2024)). Sequence alignment of the N-terminal (ATG5-interacting motif) regions of different ATG16 proteins was carried out using the Multiple Sequence Alignment program at the NCBI COBALT tool (
https://www.ncbi.nlm.nih.gov/tools/cobalt/ (accessed on 12 April 2024)). WebLogo (version 2.8.2) was used for making logos of N-terminal ATG5-interacting motif [
18].
2.3. Quantitative RT-PCR Analysis
To explore the expression pattern of
CgATG16, samples were prepared from
C. gloeosporioides mycelia, conidia, germinated conidia, appressoria, and rubber tree leaves inoculated with the fungus, following previously described protocols. For mycelial RNA extraction, conidial suspensions were inoculated into complete medium (CM) to an initial concentration of 1 × 10
3 conidia·mL
−1, and incubated at 28 °C with shaking at 120 rpm for 2 days. Mycelia were then harvested for subsequent RNA isolation. For germinated conidium and appressorium samples, conidial suspensions (1 × 10
5 conidia·mL
−1) were inoculated onto plastic plates and incubated at 28 °C for 4 h (for germinated conidia) and 24 h (for appressoria), respectively. The germinated conidia and appressoria were collected using a cell scraper. For RNA extraction during the fungal infection of rubber tree leaves, conidial suspensions were sprayed onto rubber tree leaves. Inoculated leaves were harvested at 0, 1, 2, and 3 days post-inoculation (dpi), respectively, and immediately frozen at −80 °C for total RNA extraction. Fungal total RNA was extracted using Trizol Reagent (Invitrogen, Waltham, MA, USA), following the method described previously. For plant total RNA extraction, the Polysaccharide- and Polyphenol-Rich Plant Total RNA Extraction Kit (TIANGEN Biotech, DP441) was used according to the manufacturer’s instructions. Reverse transcription was performed using SPARK script II All-in-one RT SuperMix for qPCR (With gDNA Eraser) (sparkjade, AG0305-B, Shandong, China). Quantitative real-time PCR (qRT-PCR) analysis was conducted on a Quant Studio™ 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The designed primers were validated for specificity using NCBI Primer Blast (
https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome (accessed on 15 July 2024)). The β-tubulin-1 gene (Cgβ-tub1) was used as the internal reference gene for normalizing gene expression levels in
C. gloeosporioides. The relative expression levels of the target genes were calculated using the 2
−ΔΔCt method, with four technical replicates for each sample [
17].
2.4. Construction of CgATG16 Knockout and Complementary Strains
The
CgAtg16 gene knockout strains were constructed using the split-marker strategy based on the principle of homologous recombination [
19]. Briefly, the coding region of the CgATG16 on the genome was determined based on the ORF sequence of
CgATG16. Then, based on the genomic sequence of
C. gloeosporioides, the upstream of the coding region of the
CgATG16 (5′-flanking regions, 624 bp) and the downstream of the coding region of the
CgATG16 (3′-flanking regions, 761 bp) were, respectively, amplified from the genomic DNA of
C. gloeosporioides. Meanwhile, the acetolactate synthase gene (SUR) cassette, which confers resistance to chlorimuron ethyl (a sulfonylurea herbicide) to the strain, was cloned from the vector pCB1532. Subsequently, primer pairs CgAtg16-5F/SURspl-R and SURspl-F/CgAtg16-3R were used, respectively, to amplify the fusion fragments of “flanking region-truncated SUR resistance gene. These fragments served as materials for subsequent protoplast transformation [
20].
Protoplast preparation and transformation were performed as described in our laboratory-established protocol [
21]. Conidia were inoculated into 200 mL of potato broth to an initial concentration of 10
5 conidia·mL
−1, and the culture was incubated at 28 °C with shaking at 150 rpm for 24 h. Mycelia were then harvested by filtration through a nylon membrane, washed twice with 1 M sorbitol, and transferred to 1 M sorbitol supplemented with 10 mg·mL
−1 lysing enzyme (Sigma-Aldrich (Oakville, ON, Canada)). The mycelial suspension was incubated at 28 °C with shaking at 100 rpm for 3 h to induce cell wall degradation. Protoplasts were collected via filtration through a nylon membrane, followed by centrifugation at 2000 rpm and 4 °C. After two additional washes, the protoplasts were resuspended in STC buffer (1 M sorbitol, 50 mM Tris-HCl, 10 mM CaCl
2, pH 7.4) to a final concentration of 10
8 CFU·mL
−1. For the transformation process, 100 mg of the fusion fragment was mixed with 200 μL of protoplasts, and the mixture was incubated on ice for 20 min. Next, 1 mL of 40% polyethylene glycol (PEG, dissolved in STC buffer) was added, and the mixture was further incubated at 28 °C for 20 min. An amount of 5 mL of liquid regeneration medium (composed of 1 g·L
−1 yeast extract, 1 g·L
−1 casein, and 6 M sucrose) was added to the mixture, which was then incubated at 28 °C with shaking at 100 rpm for 4 h. The regenerated protoplasts were mixed with regeneration medium (preheated to 50 °C) containing 1% agar, gently homogenized, and spread onto Petri dishes. Once the agar solidified, an equal volume of regeneration medium supplemented with 1% agar and 100 μg·mL
−1 chlorimuron ethyl was overlaid to select transformants.
Two independent PCR reactions using primers CgATG16-d5F/R and CgATG16-d3F/R were conducted to verify the correct integration of the resistant transformants. Positive transformants were further verified by 1) single-conidium purification (to eliminate heterokaryons); and 2) sequencing of homologous recombination junctions (performed by Tsingke Biotechnology (Beijing, China)). To generate the complementary strain, the sequences of CgATG16 with its promotor were ligated into the complementary vector pGPDA with HPT (hygromycin phosphotransferase gene) cassette. Protoplast preparation and transformation of CgATG16 knockout mutant were performed as above. The positive transformants of complementary strains were further confirmed through the PCR diagnosis of CgATG16 ORF.
2.5. Pathogenicity Assay
Conidia of C. gloeosporioides were suspended in a solution of 0.5% Malt Extract Broth (Difco, Franklin Lakes, NJ, USA) to a final concentration of 2 × 105 conidia/mL. Using 0.5% Malt Extract Broth as negative control, 5 µL of conidial suspensions were inoculated onto the wounded detached “light green” leaves of rubber tree variety 73-3-97. The inoculated leaves were kept in a moist chamber at 28 °C under natural illumination and the disease symptoms were scored at 4 days post-inoculation.
A conidial suspension with a concentration of 2 × 105 conidia/mL was used, and 5 μL droplets of this suspension were inoculated onto the pre-wounded sites of detached “light green” leaves of the rubber tree cultivar 7-33-97, which were maintained in a greenhouse. The disease symptoms were scored at 4 days post inoculation. Each experiment contained three replicates of at least 10 leaves.
2.6. Fungal Growth, Conidiation and Germination Assay
For fungal growth assay, 5 mm diameter hyphae agar disks were taken and inoculated into PDA medium for 5 days, and the colony morphology were observed and the colony diameters were recorded.
For conidiation assay, conidia of indicated strains were harvested from the strains growing on PDA medium for 7 days and inoculated into 50 mL liquid complete medium (CM) (1% Glucose, 0.1% Trace element solution, 0.1% Vitamin solution, 5% Nitrate solution, 0.2% Peptone, 0.1%Yeast extract, and 0.1% Acid—hydrolyzed casein per liter) to the final conidia concentration of 104/mL. The conidia numbers were counted under a microscope after incubation at 28 °C with shaking (120 rpm) for 3 days.
For conidia germination assay, spores were resuspended in 1 mL of 2% YCS (2% sucrose, 0.1% yeast extract, 0.1% ac-id-hydrolyzed casein), centrifuged at 5000 r/min for 30 s, and this centrifugation–washing step was repeated twice. The spore pellet was resuspended in 1 mL of 2% YCS, and the spore concentration was adjusted to 5 × 105 spores/mL. A 20 μL aliquot of the spore suspension was inoculated onto clean Petri dishes, which were then moisturized and incubated in a 28 °C incubator. Samples were prepared at 2 h and 4 h for microscopic observation; images were captured, and statistical analysis of germination rates was performed.
The experiments were repeated three times, with four replicates for each sample and ten microscope fields surveyed for every replicate.
2.7. Assays for Fungal Biomass and Melanin Content
A piece of cellophane was cut to fit the size of a Petri dish, weighed, and then sterilized. The sterilized cellophane was moistened with sterile water and carefully placed onto fresh PDA medium using sterile tweezers. Freshly cultured test strains (wild-type, mutant, and corresponding complemented strain) with consistent growth status were punched into agar plugs of uniform size using a sterile cork borer. Each agar plug was inoculated at the center of the cellophane, and the Petri dishes were labeled accordingly. All cultures were sealed in plastic bags and incubated at 28 °C for 5 days. After measuring the colony diameter using the cross method, the cellophane with the mycelium was collected, dried, and weighed. All data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test to determine significant differences between groups. Statistical significance was defined as a p-value < 0.05. The experiment was independently repeated three times.
For melanin content determination, the procedure was performed following the instructions of the GENMED Fungal/Yeast Melanin Colorimetric Quantification Kit (GMS50365.3). Briefly, the concentration of fresh spore suspensions of the strains was adjusted to 1 × 104 spores/mL, and conidiospores were inoculated into CM medium. The cultures were incubated at 28 °C with shaking at 160 rpm for 3 days, after which the mycelia were filtered, collected, and dried. The dried mycelia were rapidly ground into a fine powder in liquid nitrogen. For each mycelial sample, three aliquots of 0.05 g each were weighed out for subsequent melanin extraction. Melanin was extracted according to the kit instructions, and its content was quantified using a microplate reader at a wavelength of 360 nm. The absorbance values were recorded, and the melanin content of each sample was calculated using a standard curve (the standard curve was generated based on the measurements of the standard supplied with the kit) (unit: μg/mg DW). Three biological replicates were performed for this assay.
2.8. Appressorium Development and Penetration Ability Assay
For appressorium development assay, 20 μL drops of conidial suspensions were placed on a hydrophobic plastic Petri dish and incubated at 28 °C. The conidial morphology was observed at 0, 2, 4, and 8 h post-incubation, and the percentages of appressorium formation were determined under a microscope at 8 h post-incubation. Moreover, in order to identify the potential signaling pathway by which CgATG16 functions, the following treatments at the respective final concentrations were added to the conidial suspensions and analyzed at 12 h:200 nM rapamycin (Rap; Beyotime Biotechnology, Shanghai, China) and 10 mM 8-bromoadenosine 3′,5′-cyclic monophosphate sodium salt (8-Br-cAMP; Sparkjade, Jinan, China).
The invasive growth assay was performed on onion epidermis which was sprayed with 2.5 × 105/mL of conidia and placed on agar medium plate at 28 °C for 12 h, and the invasion was observed under a microscope. The experiments were repeated three times, and at least 100 conidia were detected per replicate. The conidia were randomly selected from different microscopic fields of view.
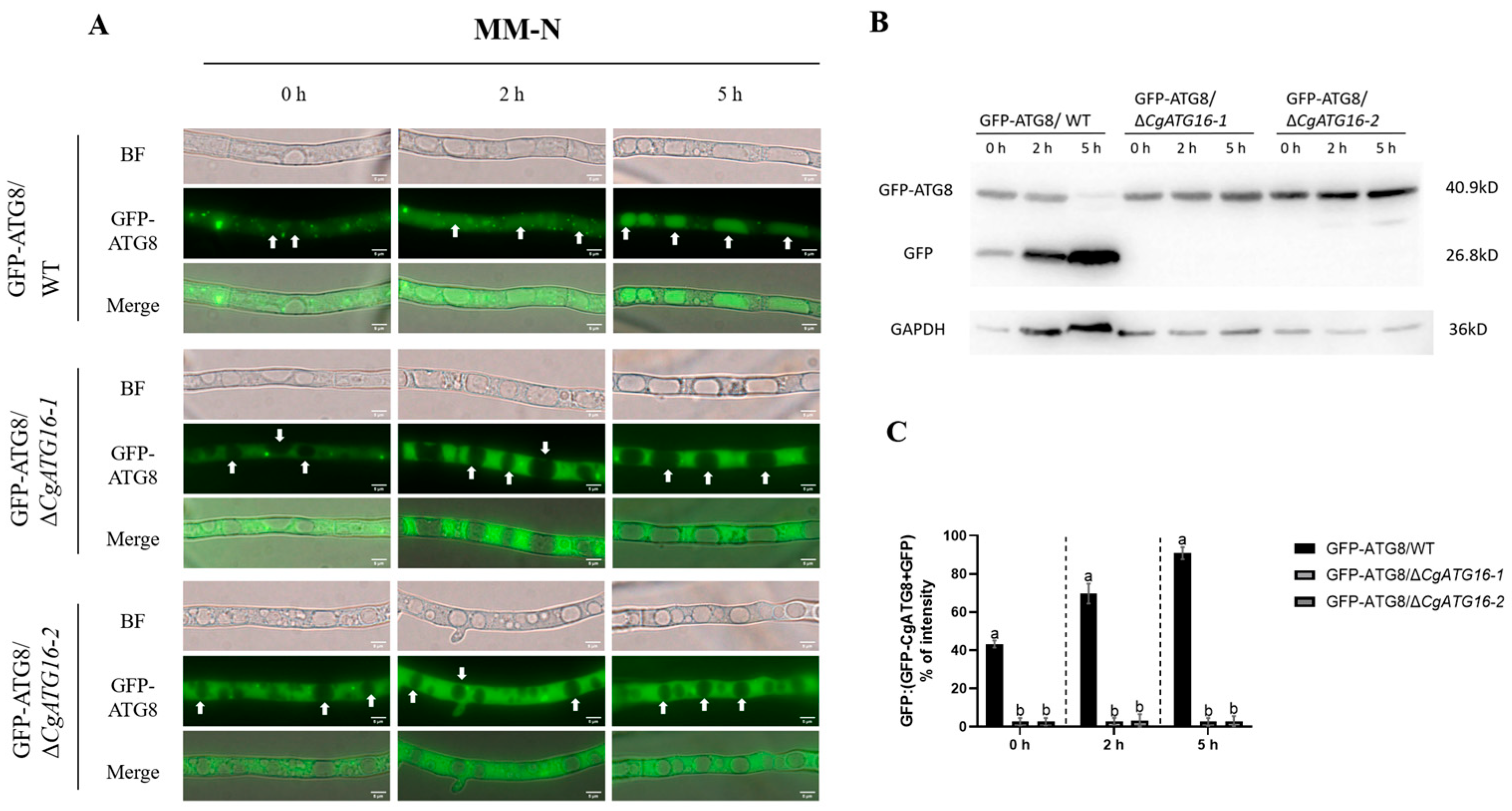
2.9. Analysis of Nitrogen Starvation Induced Autophagy
Fresh spore suspensions were prepared using liquid CM medium, and 20 mL of spore suspension with a concentration of 5 × 105 spores/mL was obtained. The spore suspension was cultured at 28 °C with shaking at 120 rpm for 16–24 h to harvest mycelia.
The cultured mycelia were aspirated and evenly distributed into several 2 mL centrifuge tubes. For each tube, 1.5 mL of sterile ddH
2O was added, followed by gentle shaking to mix thoroughly. The mixture was centrifuged at 4000 rpm for 1 min; the supernatant was carefully discarded, and residual liquid at the bottom was removed. This centrifugation–washing step was repeated twice to eliminate nutrients from the CM medium. Subsequently, the mycelia were treated with MM-N (MM-N: 0.05% Potassium chloride, 0.1% Potassium dihydrogen phosphate, 0.05% Magnesium sulfate heptahydrate, 0.001% Ferrous sulfate heptahydrate, 3% sucrose, and 0.02% Trace element solution per liter) medium supplemented with PMSF solution (final concentration: 4 mmol/L) to induce autophagy under nitrogen starvation conditions, and cultured at 28 °C with shaking at 120 rpm for 2 h and 5 h, respectively. Among them, PMSF can inhibit vacuolar degradation, which facilitates the observation of the presence of autophagosomes within vacuoles [
22]. Observations were performed under a fluorescence microscope, followed by statistical analysis. The experiment was independently repeated three times, with no fewer than ten fields of view observed per repetition.
2.10. Protein Extraction and Western Blot Analysis
Spore suspensions of the strains were cultured in liquid CM medium at 28 °C with shaking at 120 rpm for 24 h. The mycelia were washed with ddH2O and then transferred to MM-N medium, followed by incubation at 28 °C with shaking at 120 rpm for 2 h and 5 h, respectively. Mycelia were collected, ground into powder in liquid nitrogen, and resuspended in 1 mL of lysis buffer (Lysis Buffer: 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1% Triton-X-100, 1 mM PMSF, and 1× protease inhibitor cocktail per liter) containing a protease inhibitor cocktail. The mixture was vortexed to ensure thorough mixing, and the lysate was centrifuged at 12,000× g for 20 min at 4 °C. The supernatant containing proteins was collected. The protein concentration was determined and quantified using the Bradford Protein Assay Kit (detergent-compatible) (Beyotime, P0006C, Shanghai, China). Subsequently, proteins were analyzed by 12% SDS-PAGE and then subjected to Western blot using a 1:10,000 dilution of anti-GFP primary antibody (ABclonal, AE012, Wuhan, China), and the anti-GAPDH primary antibody (ProteinTech Group, 60004-1-Ig, Wuhan, China) was used as an internal reference control. A Goat anti-Mouse IgG (H + L) (Thermo, 31160, Waltham, MA, USA) was used as the secondary antibody with a dilution of 1:20,000. Finally, protein signals were detected using the BeyoECL Plus chemiluminescence kit (Beyotime, P0018S, Shanghai, China) and analyzed with ImageJ (version 1.53e) software.
The phosphorylation level of p70-S6 kinase 1 (S6K1), a functional orthologue of yeast Sch9 and a TOR substrate, was used to indirectly reflect the activity of TOR [
23]. To detect S6K/Sch9 phospho-status, WT, Δ
CgATG16 and Res-Δ
CgATG16 were grown in CM, as above, and the mycelia were transferred to fresh CM with and without 200 nM Rap for 8 h. Mycelia harvested from the second growth regime were washed with distilled water three times and finely ground in liquid nitrogen. Equal amounts of mycelia powder were used for total protein extraction in a freshly prepared cell lysis buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% (
w/
v) glycerol, 5% β-mercaptoethanol) supplemented with protease inhibitors (5 mM EDTA, 1 mM PMSF, 1× cocktail) and phosphatase inhibitors (5 mM Na
3VO
4), followed by denaturation at 95 °C for 3 min. The cell lysates were cleared by centrifugation at 16,000×
g for 15 min at 4 °C, and equal volumes of total proteins in lysates were resolved by 10% SDS-PAGE and then transferred to a PVDF membrane. The phosphorylation status of S6K1/Sch9 was monitored using an anti-phospho-p70 S6 kinase antibody (Beyotime Biotechnology, Shanghai, China) diluted 1:5000 in Western Primary Antibody Dilution Buffer (Sparkjade, ED0013), and the signals were normalized to those of p70 S6 kinase and GAPDH, which served as internal references. Protein signals were detected using the BeyoECL Plus chemiluminescence kit (Beyotime, P0018S, Shanghai, China) and analyzed with ImageJ software.
2.11. cAMP Content Assay
After 24 h of massive induction of appressorium formation on hydrophobic surfaces, the appressoria were collected into 1.5 mL centrifuge tubes using a cell scraper, followed by weighing. Dilute HCl (0.1 mol/L) was then added at a ratio of 10% (w/v). After dissolution by vortexing, the mixture was centrifuged at 1000 rpm for 10 min at room temperature, and the supernatant was collected. The cyclic adenosine monophosphate (cAMP) content was determined according to the instructions of the Fungus cAMP ELISA KIT (GOY-L0141, Shanghai Guyan, Shanghai, China).
2.12. Statistical Analysis
Statistical significance analyses were performed using SPSS Statistics version 21.0. Data with a single variable were analyzed by one-way analysis of variance, and mean separations were performed by Duncan’s multiple range test. Differences at p < 0.05 were considered significant.
4. Discussion
Autophagosome formation is an essential step in the occurrence of autophagy [
7,
8]. ATG16, as one of the “core” ATG genes firstly identified in yeast in 1999, was present in all eukaryotic species and required for the formation of autophagosomes [
11,
28]. During canonical autophagy, ATG12-ATG5-ATG16 complex acts as an E3 ligase mediating Atg8 conjugation to phosphatidylethanolamine (PE) on membranes, thereby promoting the expansion of the autophagosome membrane [
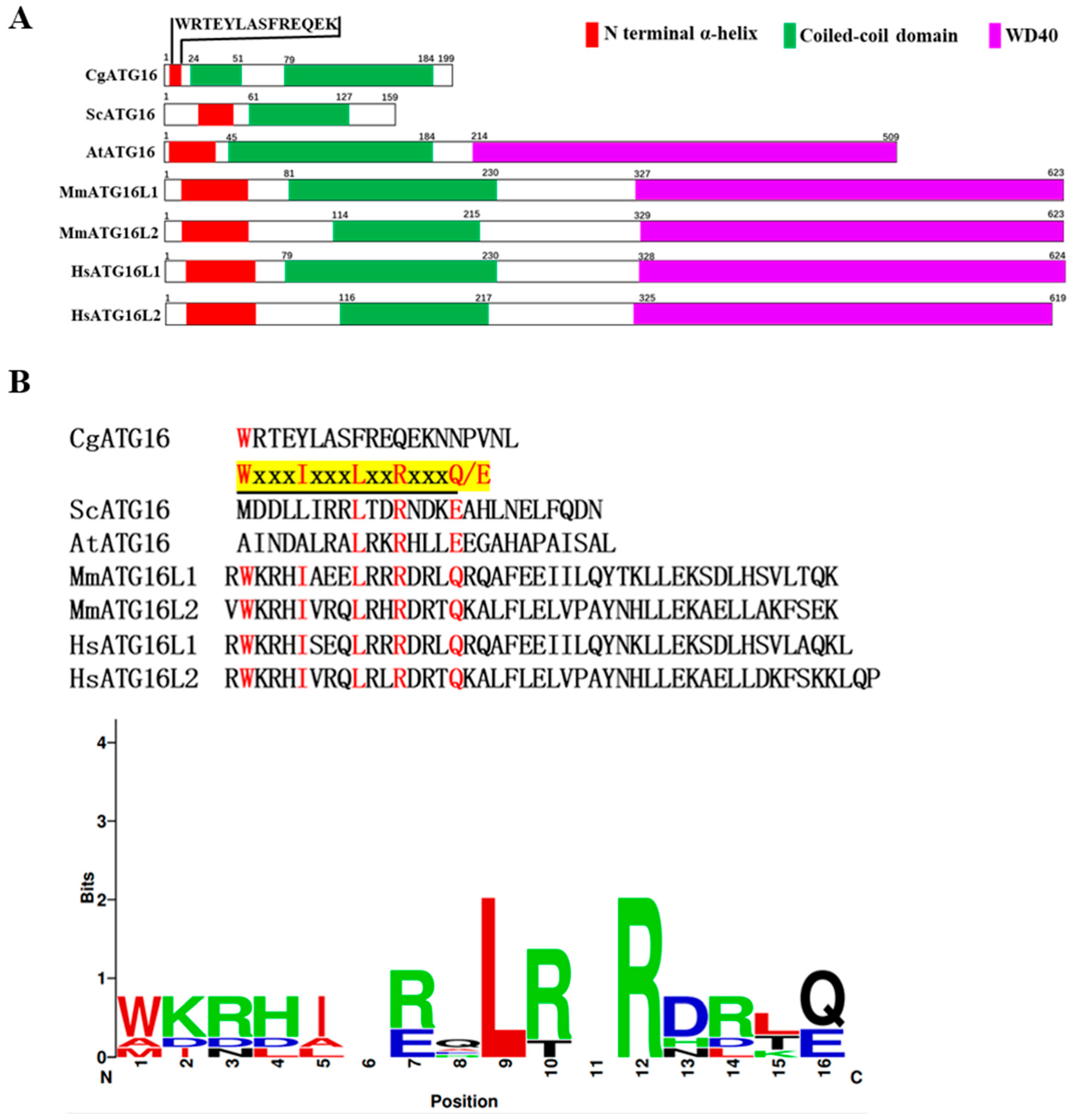
29]. The ATG16 proteins in eukaryotic species usually contained an N-terminal α-helix (ATG5-interacting motif) and had a CCD, as well as the WD40 domain that is unique to higher eukaryotic species, and was considered to be a scaffold protein that interacted with ATG12-ATG5 protein conjugates via its N-terminal ATG5-interacting motif and self-assembles through its coiled-coil domain (CCD) [
24,
30,
31]. Our 199-amino-acid CgATG16 harbored an N-terminal α-helix and two CCDs, but lacked WD40 domain (
Figure 1A), which was consistent with the structural characteristics of ATG16 proteins in lower eukaryotic species such as yeast. Studies in yeast had shown that yeast ATG16 contained a coiled-coil domain (CCD), which mediated both its own dimerization and interaction with ATG21 [
31,
32]. Recent studies had further confirmed that the dimerization and peripheral membrane-binding activity of yeast ATG16 depended on two distinct protein regions of CCD [
9]. We noticed that CgATG16 contained two CCDs (
Figure 1A); however, it was still unknown whether these two CCDs are related to dimerization and membrane-binding activity. By crystal structure assay, it was found that the 33 residues containing conserved sequence W-x-x-x-I-x-x-x-L-x-x-R-x-x-x-Q/E at N-terminal of human ATG16 are sufficient to bind to ATG5, which also was further confirmed by biochemical and cell biological analyses. In yeast, although only a partially conserved sequence “XXXXXXXX- L-x-x-R-x-x-x-Q/E” exists, ATG16 still interacts with ATG5 [
24,
25]. In this study, N-terminal helix of CgATG16 contained only 13 amino acid residues (WRTEYLASFREQEK), which has an extremely low homology with conserved sequence “W-x3-I-x3-L-x2-R-x3-Q/E” (
Figure 1B). At present, we have not yet clarified whether CgATG16 interacts with CgATG5. Furthermore, if CgATG16 and CgATG5 do indeed interact with each other, then what is the domain in CgATG16 that binds to CgATG5? Obviously, it is one of the core directions of our subsequent research to investigate the potential interaction mechanism between CgATG16 and CgATG5, which will help elucidate the molecular basis of CgATG16-mediated autophagy in
C. gloeosporioides.
Previous studies on the function of ATG16 were mainly conducted in yeast and animal cells, focusing on its role as a component of ATG12-ATG5-ATG16 complex in regulating the formation of autophagosomes [
24,
33,
34]. The functional characterization of ATG16 in pathogenic fungi was still limited, particularly in plant pathogenic fungi. AcATG16 of human pathogenic Acanthamoeba was confirmed to be involved in mitochondrial autophagy and encystation [
12]. BbATG16, the homolog ATG16 of insect-pathogenic fungus
Beauveria bassiana, contributed to asexual development, tolerance to oxidative stress, virulence and autophagosome formation [
13]. In plant pathogenic fungus
Magnaporthe oryzae, ATG16 (MoATG16) interacts with the PHD-domain protein Clp1 to regulate fungal development and virulence [
14].
Fusarium graminearum FgATG16 indirectly regulated aerial hyphae development, asexual sporulation, deoxynivalenol (DON) synthesis, and virulence during wheat infection by maintaining the normal operation of the autophagy pathway [
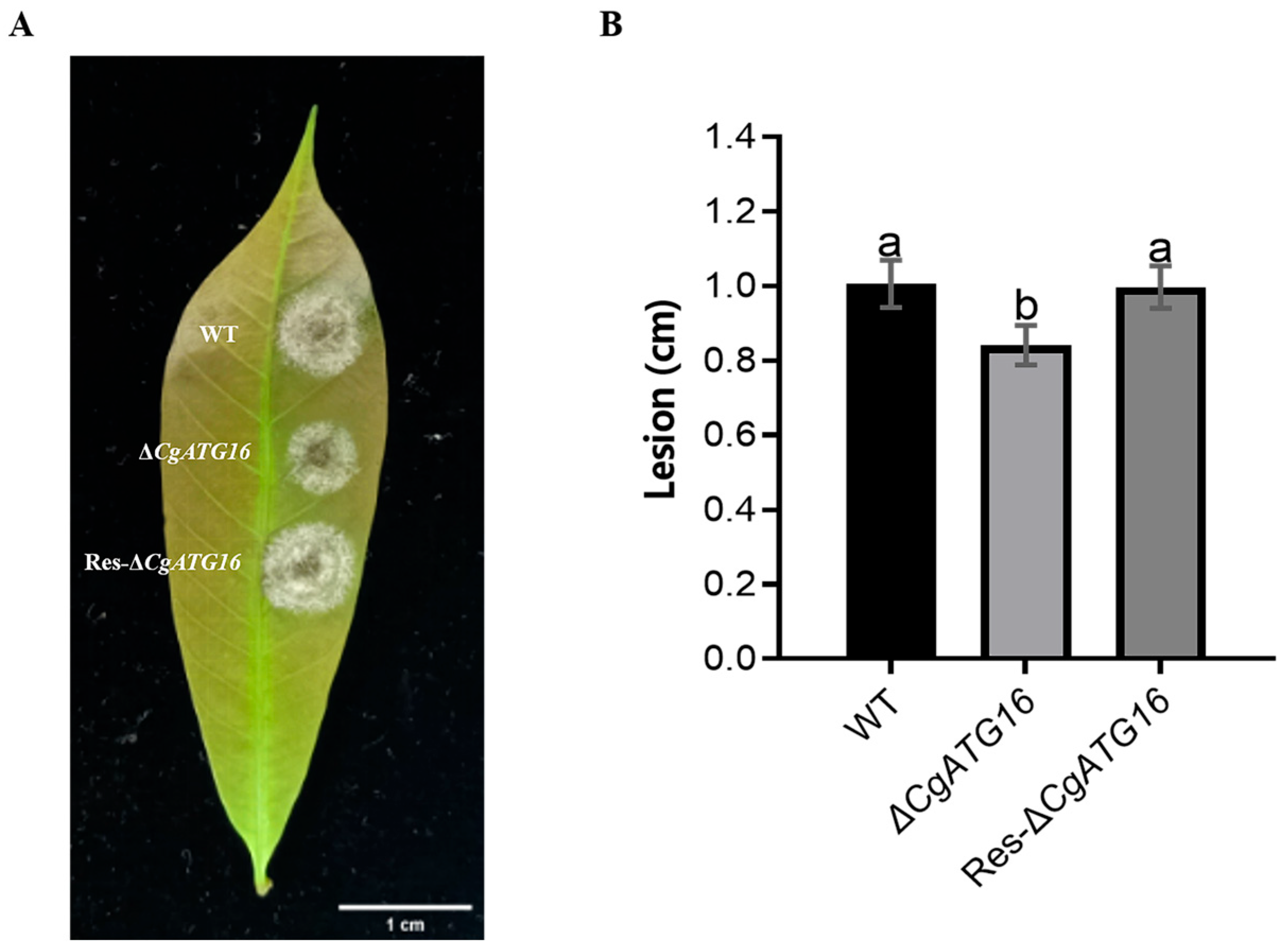
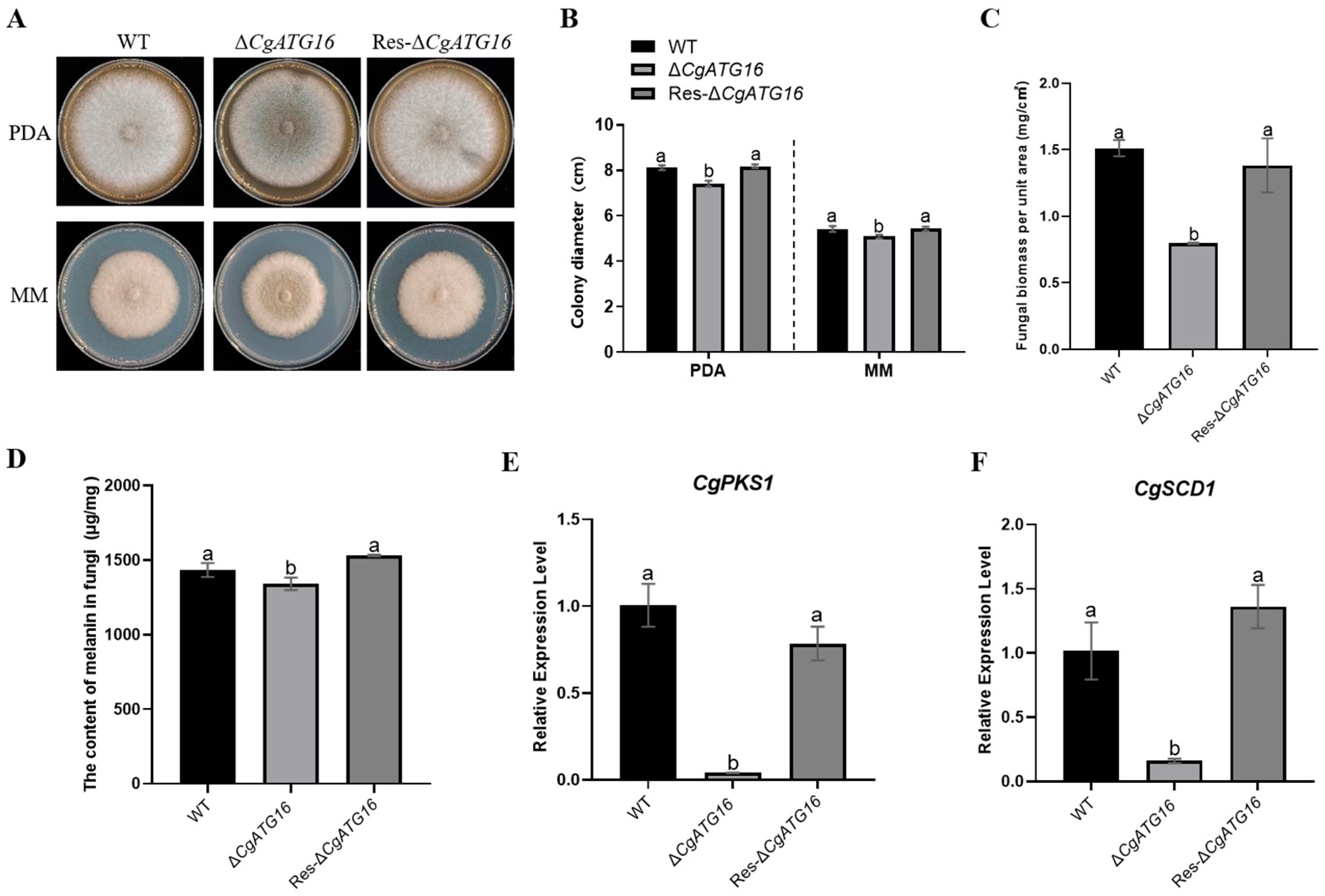
35]. In our study, we experimentally demonstrated the contribution of CgATG16 to mycelial growth rate and biomass (
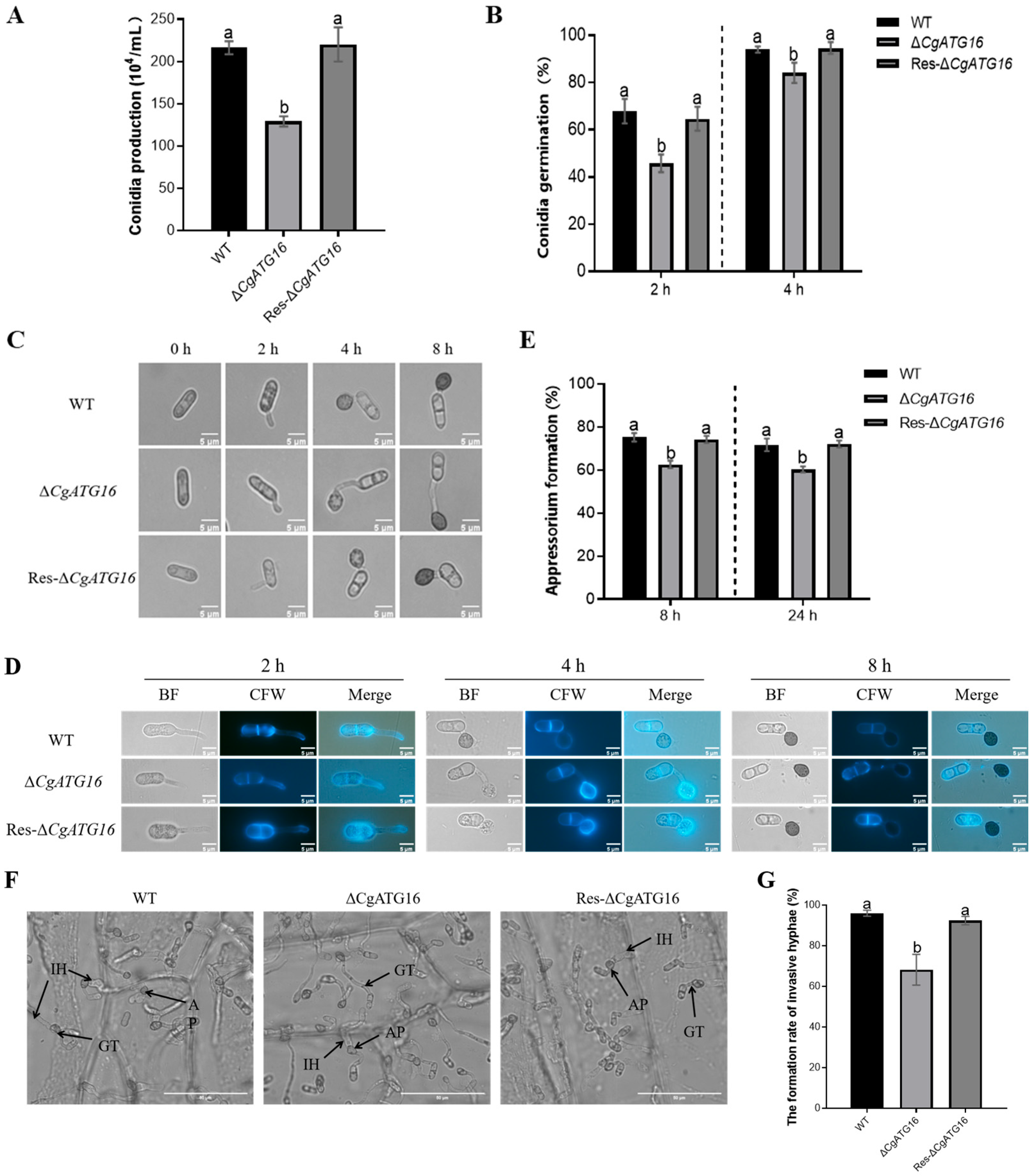
Figure 4), the development of the infection structure and primary invasion (
Figure 5), which might be the mechanism by which ATG16 regulated pathogenicity of
C. gloeosporioides to the rubber tree (
Figure 3).
Fungal melanin is a complex secondary metabolite and has a variety of functional properties and biological activities [
36]. In melanin-producing pathogenic fungi, melanin played a critical role in conidiation, appressorium development, cell wall integrity and in response to biotic and abiotic stresses [
37,
38,
39], and also contributed to invasion through mediating the buildup of high pressure in the appressorium providing the essential driving force for mechanical penetration [
40]. In our study, the melanin content of Δ
CgATG16 was significantly lower than that of WT (
Figure 4D). Fungal melanin synthesis was catalyzed by a series of enzymes by melanin synthesis [
41]. In
C. gloeosporioides, polyketide synthase CgPKS1 and scytalone dehydratase CgSCD1 were demonstrated to be involved in melanin synthesis and pathogenicity [
26,
27]. Therefore, we investigated the transcriptional expression of
CgPKS1 and
CgSCD1 in WT, Δ
CgATG16 and Res-Δ
CgATG16, and the results showed that the expression levels of
CgPKS1 and
CgSCD1 in Δ
CgATG16 were significantly lower than that in WT (
Figure 4E), suggesting that
CgATG16 modulated melanin synthesis through CgPKS1 and CgSCD1. Considering that CgATG16 was required for efficient infection of
C. gloeosporioides on rubber tree leaves and onion epidermal cells (
Figure 3 and
Figure 5F–G), we speculated that the regulation of CgATG16 on virulence may be related to melanin-mediated infection structure development and infection process.
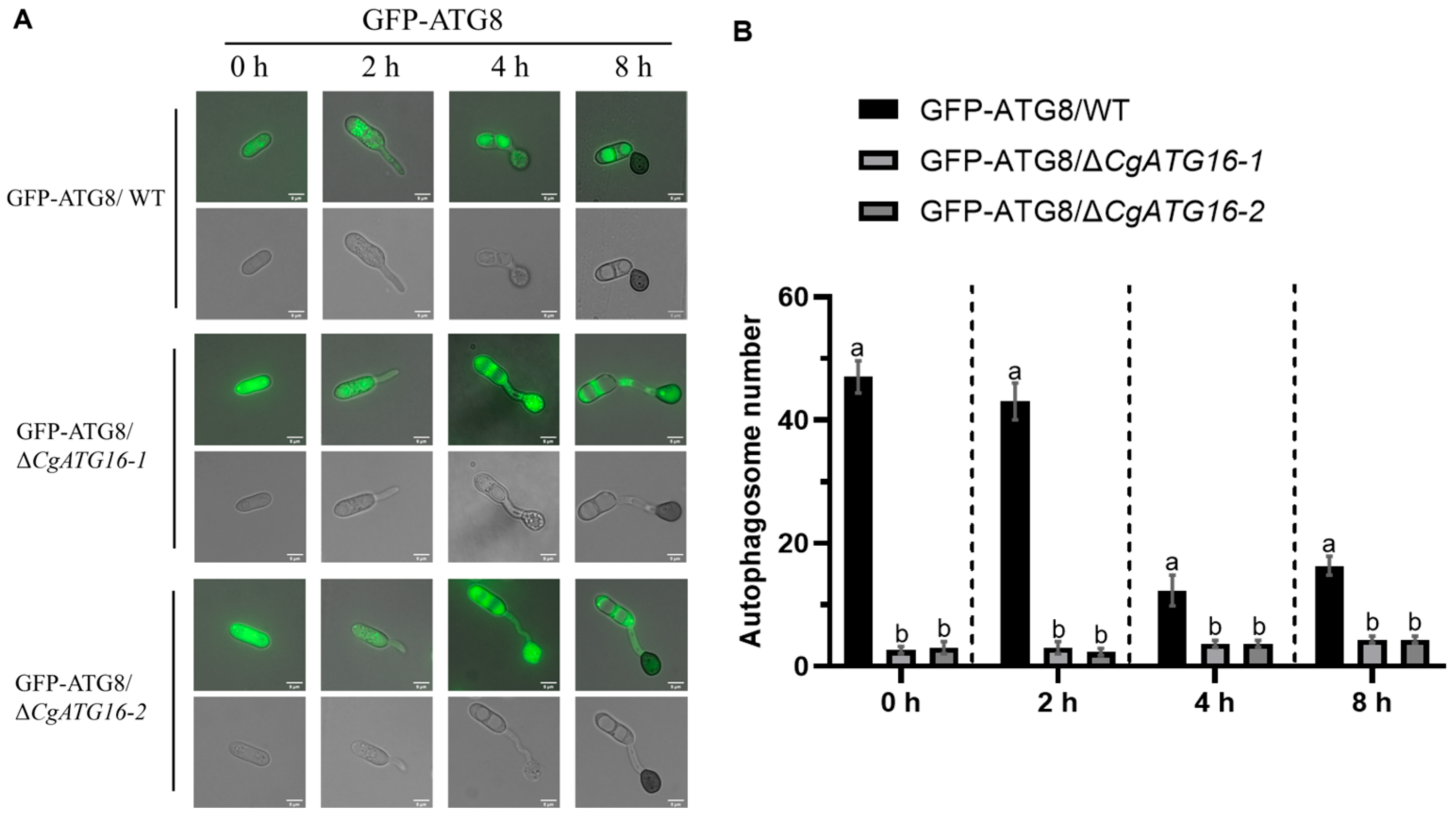
As is well known, ATG16 plays a crucial role during autophagy. The formation of fungal functional appressorium requires the transfer of conidial cell contents through an autophagic recycling into the developing appressorium [
42]. The appressorium formation process in
C. gloeosporioides is accompanied by the occurrence of autophagy [
17]. Studies in
M. oryzae revealed that autophagy pathway is linked to appressorium morphogenesis, maturation, penetration and pathogenicity [
3,
43,
44]. In the autophagy process, PEylation process of ATG8, the key step for autophagosome formation, was catalyzed by ATG16 complex [
29]. The functional studies of these related ATG proteins in plant pathogenic fungi contribute to the understanding of the roles of autophagy in pathogenesis. In
M. oryzae, deletion of MoATG5 and MoATG8 markedly disrupted autophagosome formation, conidiation, appressorium-mediated penetration, and pathogenicity [
40]. Similarly,
C. orbiculare CoATG8 and
C. scovillei CsATG8 were required for autophagy occurrence, appressorium development and full virulence on host plants [
41,
42]. In our study, the absence of CgATG16 not only resulted in defective autophagy and appressorium development, but also resulted in impaired growth and sporulation (
Figure 6 and
Figure 7), suggesting the complex functions of ATG16 in the autophagy-mediated pathogenicity of plant pathogenic fungi.
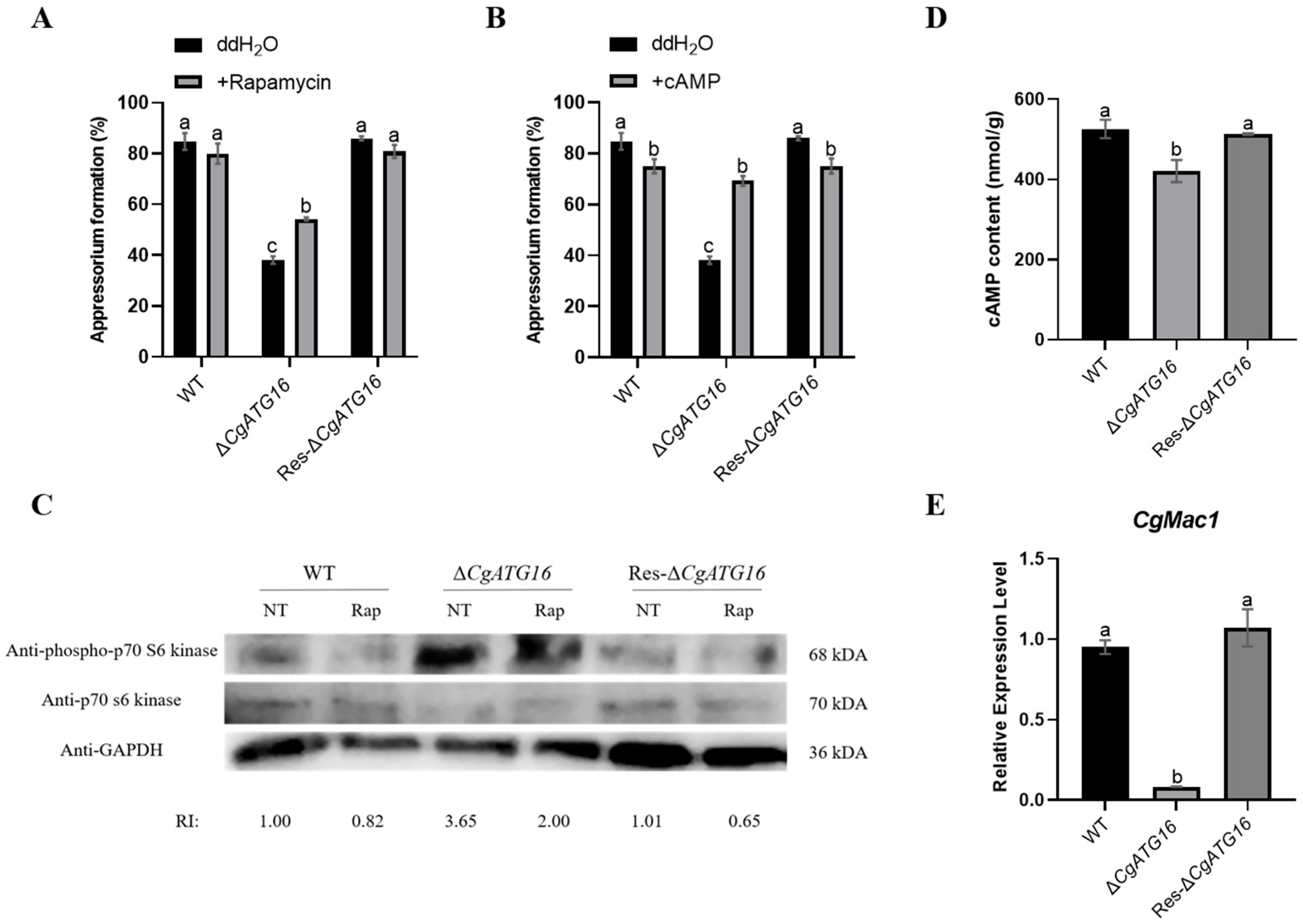
Multiple signaling pathways were involved in appressorial formation and development in plant pathogenic fungi. In
Magnaporthe oryzae, activated cAMP/PKA signaling and inactivated TOR signaling (TORoff) were required for appressorium formation and development. The cAMP/PKA signaling was required for appressorium formation initiation and activated the cAMP production by the adenylate cyclase Mac1 [
45]. TOR signaling was mediated by TOR kinase and inactivated TOR kinase (TORoff) induced autophagy and appressorium formation [
45,
46]. In our study, exogenous addition of rapamycin partially restored the appressorial formation rate of
CgATG16 deletion strain (
Figure 8A) and the phosphorylation level of S6K1 in
CgATG16 deletion strain was significantly increased (
Figure 8C), indicating that CgATG16 might function on appressorium formation by modulating TOR activity. Our data also demonstrated that exogenous addition of cAMP restored the appressorial formation rate of
CgATG16 deletion strain (
Figure 8B), indicating that cAMP signaling was also involved in CgATG16-mediated appressorial formation. Furthermore, we proved that the content of cAMP in
CgATG16 deletion strain was significantly lower than that in WT with decreased
CgMac1 gene expression (
Figure 8D,E), suggesting that the cAMP synthesis mediated by CgMac1 was essential for CgATG16 mediated appressorial formation.
Taken together, our data revealed that CgATG16 was required for full pathogenicity of C. gloeosporioides. Given that CgATG16 was involved in mycelium growth, conidiation, appressorium formation, melanin production, and also required for mycelium autophagy and the autophagy during appressorium formation and development, we speculated that CgATG16 contributed significantly to pathogenicity by regulating the mycelium growth, melanin synthesis, invasion structure formation, and this process might be related to autophagy mediated by TOR and cAMP signaling. However, the mechanism by which CgATG16 regulates the TOR and cAMP signaling pathway remains to be further investigated.