Computational Approaches for Discovering Virulence Factors in Coccidioides
Abstract
1. Introduction
2. Computational Framework for Fungal Virulence Discovery
3. Therapeutic Target Prioritization Framework
4. Coccidioides: A Model Pathogen for Computational Virulence Studies
5. Adhesins Mediate Host Attachment and Colonization
6. Membrane Transporters in Iron Acquisition and Drug Resistance
7. Secreted Signal Peptides Modulate Most Immunity and Tissue Invasion
8. Cell Wall Remodeling Enzymes During Immune Evasion
9. Secondary Metabolites as High Potential Therapeutic Targets
10. Structure-Based Drug Design: From Prediction to Therapeutics
11. Reverse Vaccinology: Computational Approaches to Vaccine Design
12. Validation Strategies Bridging Computation and Biology
13. Computational Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thambugala, K.M.; Daranagama, D.A.; Tennakoon, D.S.; Jayatunga, D.P.W.; Hongsanan, S.; Xie, N. Humans vs. Fungi: An Overview of Fungal Pathogens against Humans. Pathogens 2024, 13, 426. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: The World Health Organization (WHO) Fungal Priority Pathogens List in Response to Emerging Fungal Pathogens During the COVID-19 Pandemic. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e939088-1–e939088-3. [Google Scholar] [CrossRef] [PubMed]
- Rappleye, C.A.; Goldman, W.E. Defining Virulence Genes in the Dimorphic Fungi. Annu. Rev. Microbiol. 2006, 60, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R., III; Lewis, J.S., II; Nix, D.E.; Patterson, T.F. Current Concepts and Future Directions in the Pharmacology and Treatment of Coccidioidomycosis. Med. Mycol. 2019, 57, S76–S84. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.H.; Sayes, C.M.; Giesy, J.P.; Li, Y. Valley Fever under a Changing Climate in the United States. Environ. Int. 2024, 193, 109066. [Google Scholar] [CrossRef]
- The UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [CrossRef]
- Basenko, E.Y.; Shanmugasundram, A.; Böhme, U.; Starns, D.; Wilkinson, P.A.; Davison, H.R.; Crouch, K.; Maslen, G.; Harb, O.S.; Amos, B.; et al. What Is New in FungiDB: A Web-Based Bioinformatics Platform for Omics-Scale Data Analysis for Fungal and Oomycete Species. Genetics 2024, 227, iyae035. [Google Scholar] [CrossRef]
- Ahrendt, S.R.; Mondo, S.J.; Haridas, S.; Grigoriev, I.V. MycoCosm, the JGI’s Fungal Genome Portal for Comparative Genomic and Multiomics Data Analyses. Methods Mol. Biol. Clifton 2023, 2605, 271–291. [Google Scholar] [CrossRef]
- Johannesson, H.; Townsend, J.P.; Hung, C.-Y.; Cole, G.T.; Taylor, J.W. Concerted Evolution in the Repeats of an Immunomodulating Cell Surface Protein, SOWgp, of the Human Pathogenic Fungi Coccidioides Immitis and C. Posadasii. Genetics 2005, 171, 109–117. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Yu, J.-J.; Seshan, K.R.; Reichard, U.; Cole, G.T. A Parasitic Phase-Specific Adhesin of Coccidioides Immitis Contributes to the Virulence of This Respiratory Fungal Pathogen. Infect. Immun. 2002, 70, 3443–3456. [Google Scholar] [CrossRef]
- Diep, A.L.; Hoyer, K.K. Host Response to Coccidioides Infection: Fungal Immunity. Front. Cell. Infect. Microbiol. 2020, 10, 581101. [Google Scholar] [CrossRef] [PubMed]
- Perlin, M.H.; Andrews, J.; Toh, S.S. Essential Letters in the Fungal Alphabet: ABC and MFS Transporters and Their Roles in Survival and Pathogenicity. Adv. Genet. 2014, 85, 201–253. [Google Scholar] [CrossRef]
- Homer, C.M.; Voorhies, M.; Walcott, K.; Ochoa, E.; Sil, A. Transcriptomic Atlas throughout Coccidioides Development Reveals Key Phase-Enriched Transcripts of This Important Fungal Pathogen. PLoS Biol. 2025, 23, e3003066. [Google Scholar] [CrossRef] [PubMed]
- Zhgun, A.A. Fungal BGCs for Production of Secondary Metabolites: Main Types, Central Roles in Strain Improvement, and Regulation According to the Piano Principle. Int. J. Mol. Sci. 2023, 24, 11184. [Google Scholar] [CrossRef]
- Mitchell, N.M.; Grys, T.E.; Lake, D.F. Carbo-Loading in Coccidioides Spp.: A Quantitative Analysis of CAZyme Abundance and Resulting Glycan Populations. Glycobiology 2020, 30, 186–197. [Google Scholar] [CrossRef]
- Mead, H.L.; Roe, C.C.; Higgins Keppler, E.A.; Van Dyke, M.C.C.; Laux, K.L.; Funke, A.L.; Miller, K.J.; Bean, H.D.; Sahl, J.W.; Barker, B.M. Defining Critical Genes During Spherule Remodeling and Endospore Development in the Fungal Pathogen, Coccidioides posadasii. Front. Genet. 2020, 11, 483. [Google Scholar] [CrossRef]
- Mitchell, N.M.; Sherrard, A.L.; Dasari, S.; Magee, D.M.; Grys, T.E.; Lake, D.F. Proteogenomic Re-Annotation of Coccidioides Posadasii Strain Silveira. Proteomics 2018, 18, 1700173. [Google Scholar] [CrossRef]
- Whiston, E.; Taylor, J.W. Comparative Phylogenomics of Pathogenic and Nonpathogenic Species. G3 GenesGenomesGenetics 2015, 6, 235–244. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, H.; Guo, D.H.; Wang, Y.; Gu, J. Genomic Data Mining Reveals Abundant Uncharacterized Transporters in Coccidioides Immitis and Coccidioides posadasii. J. Fungi 2022, 8, 1064. [Google Scholar] [CrossRef]
- Lipke, P.N. What We Do Not Know about Fungal Cell Adhesion Molecules. J. Fungi 2018, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.W.J.; Bader, O.; de Boer, A.D.; Weig, M.; Chauhan, N. Adhesins in Human Fungal Pathogens: Glue with Plenty of Stick. Eukaryot. Cell 2013, 12, 470–481. [Google Scholar] [CrossRef]
- Briard, B.; Fontaine, T.; Samir, P.; Place, D.E.; Muszkieta, L.; Malireddi, R.K.S.; Karki, R.; Christgen, S.; Bomme, P.; Vogel, P.; et al. Galactosaminogalactan Activates the Inflammasome to Provide Host Protection. Nature 2020, 588, 688–692. [Google Scholar] [CrossRef]
- Lee, M.J.; Liu, H.; Barker, B.M.; Snarr, B.D.; Gravelat, F.N.; Al Abdallah, Q.; Gavino, C.; Baistrocchi, S.R.; Ostapska, H.; Xiao, T.; et al. The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLoS Pathog. 2015, 11, e1005187. [Google Scholar] [CrossRef]
- Alapan, D.; Bisweswar, O.; Prasenjit, S.; Prasanjit, D.; Arkapal, B. Recent Advances in the Clinical Development of Antifungal Vaccines: A Narrative Review. Front. Trop. Dis. 2024, 5, 1446477. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Ramachandran, S. Prediction of Virulence Factors Using Bioinformatics Approaches. Immunoinformatics 2014, 1184, 389–400. [Google Scholar] [CrossRef]
- Ramana, J.; Gupta, D. FaaPred: A SVM-Based Prediction Method for Fungal Adhesins and Adhesin-like Proteins. PLoS ONE 2010, 5, e9695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaudhuri, R.; Ansari, F.A.; Raghunandanan, M.V.; Ramachandran, S. FungalRV: Adhesin Prediction and Immunoinformatics Portal for Human Fungal Pathogens. BMC Genom. 2011, 12, 192. [Google Scholar] [CrossRef]
- Sachdeva, G.; Kumar, K.; Jain, P.; Ramachandran, S. SPAAN: A Software Program for Prediction of Adhesins and Adhesin-like Proteins Using Neural Networks. Bioinformatics 2005, 21, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Nath, A. Prediction and Molecular Insights into Fungal Adhesins and Adhesin like Proteins. Comput. Biol. Chem. 2019, 80, 333–340. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. dbCAN2: A Meta Server for Automated Carbohydrate-Active Enzyme Annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef]
- Barrett, K.; Hunt, C.J.; Lange, L.; Grigoriev, I.V.; Meyer, A.S. Conserved Unique Peptide Patterns (CUPP) Online Platform 2.0: Implementation of +1000 JGI Fungal Genomes. Nucleic Acids Res. 2023, 51, W108–W114. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H.; Reddy, V.S.; Moreno-Hagelsieb, G.; Hendargo, K.J.; Zhang, Y.; Iddamsetty, V.; Lam, K.J.K.; Tian, N.; Russum, S.; Wang, J.; et al. The Transporter Classification Database (TCDB): 2021 Update. Nucleic Acids Res. 2021, 49, D461–D467. [Google Scholar] [CrossRef] [PubMed]
- Alballa, M.; Butler, G. TooT-T: Discrimination of Transport Proteins from Non-Transport Proteins. BMC Bioinform. 2020, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Cunha, E.; Lagoa, D.; Faria, J.P.; Liu, F.; Henry, C.S.; Dias, O. TranSyT, an Innovative Framework for Identifying Transport Systems. Bioinformatics 2023, 39, btad466. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Sperschneider, J.; Dodds, P.N.; Gardiner, D.M.; Singh, K.B.; Taylor, J.M. Improved Prediction of Fungal Effector Proteins from Secretomes with EffectorP 2.0. Mol. Plant Pathol. 2018, 19, 2094–2110. [Google Scholar] [CrossRef]
- Sperschneider, J.; Dodds, P.N. EffectorP 3.0: Prediction of Apoplastic and Cytoplasmic Effectors in Fungi and Oomycetes. Mol. Plant-Microbe Interact. 2022, 35, 146–156. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The Database of Proteolytic Enzymes, Their Substrates and Inhibitors. Nucleic Acids Res. 2014, 42, D503–D509. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Khaldi, N.; Seifuddin, F.T.; Turner, G.; Haft, D.; Nierman, W.C.; Wolfe, K.H.; Fedorova, N.D. SMURF: Genomic Mapping of Fungal Secondary Metabolite Clusters. Fungal Genet. Biol. 2010, 47, 736–741. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Prihoda, D.; Palicka, A.; Soukup, J.; Klempir, O.; Rampula, L.; Durcak, J.; Wurst, M.; Kotowski, J.; Chang, D.; et al. A Deep Learning Genome-Mining Strategy for Biosynthetic Gene Cluster Prediction. Nucleic Acids Res. 2019, 47, e110. [Google Scholar] [CrossRef]
- Almeida, H.; Palys, S.; Tsang, A.; Diallo, A.B. TOUCAN: A Framework for Fungal Biosynthetic Gene Cluster Discovery. NAR Genom. Bioinform. 2020, 2, lqaa098. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Le Guilloux, V.; Schmidtke, P.; Tuffery, P. Fpocket: An Open Source Platform for Ligand Pocket Detection. BMC Bioinform. 2009, 10, 168. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Ene, I.V.; Farrer, R.A.; Hirakawa, M.P.; Agwamba, K.; Cuomo, C.A.; Bennett, R.J. Global Analysis of Mutations Driving Microevolution of a Heterozygous Diploid Fungal Pathogen. Proc. Natl. Acad. Sci. USA 2018, 115, E8688–E8697. [Google Scholar] [CrossRef] [PubMed]
- Dijck, P.V.; Sjollema, J.; Cammue, B.P.A.; Lagrou, K.; Berman, J.; d’Enfert, C.; Andes, D.R.; Arendrup, M.C.; Brakhage, A.A.; Calderone, R.; et al. Methodologies for in Vitro and in Vivo Evaluation of Efficacy of Antifungal and Antibiofilm Agents and Surface Coatings against Fungal Biofilms. Microb. Cell 2018, 5, 300–326. [Google Scholar] [CrossRef] [PubMed]
- Rohl, C.A.; Strauss, C.E.M.; Misura, K.M.S.; Baker, D. Protein Structure Prediction Using Rosetta. Methods Enzymol. 2004, 383, 66–93. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Z.; Zhou, C.; Tang, X.; Hu, X.; Tian, G.; Yang, J.; Yao, Y. Protein Structure Prediction via Deep Learning: An in-Depth Review. Front. Pharmacol. 2025, 16, 1498662. [Google Scholar] [CrossRef]
- Golan, N.; Schwartz-Perov, S.; Landau, M.; Lipke, P.N. Structure and Conservation of Amyloid Spines From the Candida Albicans Als5 Adhesin. Front. Mol. Biosci. 2022, 9, 926959. [Google Scholar] [CrossRef]
- Burnham-Marusich, A.R.; Zayac, K.R.; Galgiani, J.N.; Lewis, L.; Kozel, T.R. Antigenic Relatedness between Mannans from Coccidioides Immitis and Coccidioides Posadasii Spherules and Mycelia. J. Fungi 2024, 10, 89. [Google Scholar] [CrossRef]
- Víglaš, J.; Olejníková, P. An Update on ABC Transporters of Filamentous Fungi—From Physiological Substrates to Xenobiotics. Microbiol. Res. 2021, 246, 126684. [Google Scholar] [CrossRef]
- Li, L.X.; Rautengarten, C.; Heazlewood, J.L.; Doering, T.L. UDP-Glucuronic Acid Transport Is Required for Virulence of Cryptococcus neoformans. mBio 2018, 9, e02319-17. [Google Scholar] [CrossRef]
- Gerstein, A.C.; Jackson, K.M.; McDonald, T.R.; Wang, Y.; Lueck, B.D.; Bohjanen, S.; Smith, K.D.; Akampurira, A.; Meya, D.B.; Xue, C.; et al. Identification of Pathogen Genomic Differences That Impact Human Immune Response and Disease during Cryptococcus Neoformans Infection. mBio 2019, 10, e01440-19. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Banerjee, A.; Khandelwal, N.K.; Dhamgaye, S. The ABCs of Candida Albicans Multidrug Transporter Cdr1. Eukaryot. Cell 2015, 14, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Basenko, E.Y.; Pulman, J.A.; Shanmugasundram, A.; Harb, O.S.; Crouch, K.; Starns, D.; Warrenfeltz, S.; Aurrecoechea, C.; Stoeckert, C.J.; Kissinger, J.C.; et al. FungiDB: An Integrated Bioinformatic Resource for Fungi and Oomycetes. J. Fungi 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Carlin, A.F.; Beyhan, S.; Peña, J.F.; Stajich, J.E.; Viriyakosol, S.; Fierer, J.; Kirkland, T.N. Transcriptional Analysis of Coccidioides Immitis Mycelia and Spherules by RNA Sequencing. J. Fungi 2021, 7, 366. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Beyhan, S.; Stajich, J.E. Evaluation of Different Gene Prediction Tools in Coccidioides Immitis. J. Fungi 2023, 9, 1094. [Google Scholar] [CrossRef]
- Neafsey, D.E.; Barker, B.M.; Sharpton, T.J.; Stajich, J.E.; Park, D.J.; Whiston, E.; Hung, C.-Y.; McMahan, C.; White, J.; Sykes, S.; et al. Population Genomic Sequencing of Coccidioides Fungi Reveals Recent Hybridization and Transposon Control. Genome Res. 2010, 20, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida Albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, A.; Zeng, L.; Hou, S.; Wang, M.; Li, L.; Cai, Z.; Zhong, G. The Putative Polysaccharide Synthase AfCps1 Regulates Aspergillus Fumigatus Morphogenesis and Conidia Immune Response in Mouse Bone Marrow-Derived Macrophages. J. Microbiol. 2021, 59, 64–75. [Google Scholar] [CrossRef]
- Homer, C.M.; Voorhies, M.; Walcott, K.; Ochoa, E.; Sil, A. Transcriptomic Atlas of the Morphologic Development of the Fungal Pathogen Coccidioides Reveals Key Phase-Enriched Transcripts. BioRxiv 2024. [Google Scholar] [CrossRef]
- Van Dyke, M.C.C.; Thompson, G.R.; Galgiani, J.N.; Barker, B.M. The Rise of Coccidioides: Forces Against the Dust Devil Unleashed. Front. Immunol. 2019, 10, 2188. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-Y.; Seshan, K.R.; Yu, J.-J.; Schaller, R.; Xue, J.; Basrur, V.; Gardner, M.J.; Cole, G.T. A Metalloproteinase of Coccidioides Posadasii Contributes to Evasion of Host Detection. Infect. Immun. 2005, 73, 6689–6703. [Google Scholar] [CrossRef] [PubMed]
- Hage, H.; Rosso, M.-N. Evolution of Fungal Carbohydrate-Active Enzyme Portfolios and Adaptation to Plant Cell-Wall Polymers. J. Fungi 2021, 7, 185. [Google Scholar] [CrossRef]
- Bains, R.K.; Nasseri, S.A.; Wardman, J.F.; Withers, S.G. Advances in the Understanding and Exploitation of Carbohydrate-Active Enzymes. Curr. Opin. Chem. Biol. 2024, 80, 102457. [Google Scholar] [CrossRef]
- Hopke, A.; Brown, A.J.P.; Hall, R.A.; Wheeler, R.T. Dynamic Fungal Cell Wall Architecture in Stress Adaptation and Immune Evasion. Trends Microbiol. 2018, 26, 284–295. [Google Scholar] [CrossRef]
- Mittal, J.; Ponce, M.G.; Gendlina, I.; Nosanchuk, J.D. Histoplasma Capsulatum: Mechanisms for Pathogenesis. Curr. Top. Microbiol. Immunol. 2019, 422, 157–191. [Google Scholar] [CrossRef]
- Garfoot, A.L.; Shen, Q.; Wüthrich, M.; Klein, B.S.; Rappleye, C.A. The Eng1 β-Glucanase Enhances Histoplasma Virulence by Reducing β-Glucan Exposure. mBio 2016, 7, e01388-15. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Kasper, L.; Gresnigt, M.S.; Brunke, S.; Hube, B. “Under Pressure”—How Fungi Evade, Exploit, and Modulate Cells of the Innate Immune System. Semin. Immunol. 2023, 66, 101738. [Google Scholar] [CrossRef]
- Hameed, S.; Hans, S.; Singh, S.; Dhiman, R.; Monasky, R.; Pandey, R.P.; Thangamani, S.; Fatima, Z. Revisiting the Vital Drivers and Mechanisms of β-Glucan Masking in Human Fungal Pathogen, Candida albicans. Pathogens 2021, 10, 942. [Google Scholar] [CrossRef]
- de Assis, L.J.; Bain, J.M.; Liddle, C.; Leaves, I.; Hacker, C.; Peres da Silva, R.; Yuecel, R.; Bebes, A.; Stead, D.; Childers, D.S.; et al. Nature of β-1,3-Glucan-Exposing Features on Candida Albicans Cell Wall and Their Modulation. mBio 2022, 13, e02605-22. [Google Scholar] [CrossRef]
- Childers, D.S.; Avelar, G.M.; Bain, J.M.; Pradhan, A.; Larcombe, D.E.; Netea, M.G.; Erwig, L.P.; Gow, N.A.R.; Brown, A.J.P. Epitope Shaving Promotes Fungal Immune Evasion. mBio 2020, 11, e00984-20. [Google Scholar] [CrossRef]
- Lu, T.; Yao, B.; Zhang, C. DFVF: Database of Fungal Virulence Factors. Database J. Biol. Databases Curation 2012, 2012, bas032. [Google Scholar] [CrossRef]
- Vivek-Ananth, R.P.; Mohanraj, K.; Vandanashree, M.; Jhingran, A.; Craig, J.P.; Samal, A. Comparative Systems Analysis of the Secretome of the Opportunistic Pathogen Aspergillus Fumigatus and Other Aspergillus Species. Sci. Rep. 2018, 8, 6617. [Google Scholar] [CrossRef]
- Jin, J.-H.; Lee, K.-T.; Hong, J.; Lee, D.; Jang, E.-H.; Kim, J.-Y.; Lee, Y.; Lee, S.-H.; So, Y.-S.; Jung, K.-W.; et al. Genome-Wide Functional Analysis of Phosphatases in the Pathogenic Fungus Cryptococcus neoformans. Nat. Commun. 2020, 11, 4212. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between Secondary Metabolism and Fungal Development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Riedling, O.; Walker, A.S.; Rokas, A. Predicting Fungal Secondary Metabolite Activity from Biosynthetic Gene Cluster Data Using Machine Learning. Microbiol. Spectr. 2024, 12, e03400-23. [Google Scholar] [CrossRef]
- Dubin, C.A.; Voorhies, M.; Sil, A.; Teixeira, M.M.; Barker, B.M.; Brem, R.B. Genome Organization and Copy-Number Variation Reveal Clues to Virulence Evolution in Coccidioides posadasii. J. Fungi 2022, 8, 1235. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.P d.C.; Brancini, G.T.P.; de Castro, P.A.; Valero, C.; Ferreira Filho, J.A.; Silva, L.P.; Rocha, M.C.; Malavazi, I.; Pontes, J.G.d.M.; Fill, T.; et al. Aspergillus Fumigatus G-Protein Coupled Receptors GprM and GprJ Are Important for the Regulation of the Cell Wall Integrity Pathway, Secondary Metabolite Production, and Virulence. mBio 2020, 11, e02458-20. [Google Scholar] [CrossRef]
- Gauthier, G.M. Fungal Dimorphism and Virulence: Molecular Mechanisms for Temperature Adaptation, Immune Evasion, and In Vivo Survival. Mediat. Inflamm. 2017, 2017, 8491383. [Google Scholar] [CrossRef]
- Gluck-Thaler, E.; Haridas, S.; Binder, M.; Grigoriev, I.V.; Crous, P.W.; Spatafora, J.W.; Bushley, K.; Slot, J.C. The Architecture of Metabolism Maximizes Biosynthetic Diversity in the Largest Class of Fungi. Mol. Biol. Evol. 2020, 37, 2838–2856. [Google Scholar] [CrossRef]
- Condon, D.E.; Schroeder, B.K.; Rowley, P.A.; Ytreberg, F.M. Discovery of Novel Targets for Important Human and Plant Fungal Pathogens via Automated Computational Pipeline HitList. PLoS ONE 2025, 20, e0323991. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Li, J.; Liu, J.; Dai, X.; Zhu, A.; Xiao, Q.; Qian, W.; Li, H.; Guo, L.; et al. Cryo-EM Structure of the β-1,3-Glucan Synthase FKS1-Rho1 Complex. Nat. Commun. 2025, 16, 2054. [Google Scholar] [CrossRef]
- Walker, L.A.; Munro, C.A. Caspofungin Induced Cell Wall Changes of Candida Species Influences Macrophage Interactions. Front. Cell. Infect. Microbiol. 2020, 10, 164. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Lv, Q.; Yan, L.; Wang, Y.; Jiang, Y. The Fungal CYP51s: Their Functions, Structures, Related Drug Resistance, and Inhibitors. Front. Microbiol. 2019, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Mohid, S.A.; Biswas, K.; Won, T.; Mallela, L.S.; Gucchait, A.; Butzke, L.; Sarkar, R.; Barkham, T.; Reif, B.; Leipold, E.; et al. Structural Insights into the Interaction of Antifungal Peptides and Ergosterol Containing Fungal Membrane. Biochim. Biophys. Acta BBA—Biomembr. 2022, 1864, 183996. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Qi, A.; Hoekstra, W.J.; Schotzinger, R.J.; York, J.D.; Guengerich, F.P.; Lepesheva, G.I. Structural Analyses of Candida Albicans Sterol 14α-Demethylase Complexed with Azole Drugs Address the Molecular Basis of Azole-Mediated Inhibition of Fungal Sterol Biosynthesis. J. Biol. Chem. 2017, 292, 6728–6743. [Google Scholar] [CrossRef] [PubMed]
- Hast, M.A.; Nichols, C.B.; Armstrong, S.M.; Kelly, S.M.; Hellinga, H.W.; Alspaugh, J.A.; Beese, L.S. Structures of Cryptococcus Neoformans Protein Farnesyltransferase Reveal Strategies for Developing Inhibitors that Target Fungal Pathogens*. J. Biol. Chem. 2011, 286, 35149–35162. [Google Scholar] [CrossRef]
- Chua, S.M.H.; Wizrah, M.S.I.; Luo, Z.; Lim, B.Y.J.; Kappler, U.; Kobe, B.; Fraser, J.A. Structural Features of Cryptococcus Neoformans Bifunctional GAR/AIR Synthetase May Present Novel Antifungal Drug Targets. J. Biol. Chem. 2021, 297, 101091. [Google Scholar] [CrossRef]
- Karelina, M.; Noh, J.J.; Dror, R.O. How Accurately Can One Predict Drug Binding Modes Using AlphaFold Models? eLife 2023, 12, RP89386. [Google Scholar] [CrossRef] [PubMed]
- Malisi, C.; Schumann, M.; Toussaint, N.C.; Kageyama, J.; Kohlbacher, O.; Höcker, B. Binding Pocket Optimization by Computational Protein Design. PLoS ONE 2012, 7, e52505. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xia, C.; Pan, X.; Shen, H. BindWeb: A Web Server for Ligand Binding Residue and Pocket Prediction from Protein Structures. Protein Sci. Publ. Protein Soc. 2022, 31, e4462. [Google Scholar] [CrossRef]
- Mareuil, F.; Torchet, R.; Ruano, L.C.; Mallet, V.; Nilges, M.; Bouvier, G.; Sperandio, O. InDeepNet: A Web Platform for Predicting Functional Binding Sites in Proteins Using InDeep. Nucleic Acids Res. 2025, 53, W324–W329. [Google Scholar] [CrossRef]
- Noske, J.; Kynast, J.P.; Lemm, D.; Schmidt, S.; Höcker, B. PocketOptimizer 2.0: A Modular Framework for Computer-aided Ligand-binding Design. Protein Sci. Publ. Protein Soc. 2023, 32, e4516. [Google Scholar] [CrossRef] [PubMed]
- Zemla, A.T.; Allen, J.E.; Kirshner, D.; Lightstone, F.C. PDBspheres: A Method for Finding 3D Similarities in Local Regions in Proteins. NAR Genom. Bioinforma. 2022, 4, lqac078. [Google Scholar] [CrossRef]
- Ahlawat, V.; Sura, K.; Singh, B.; Dangi, M.; Chhillar, A.K. Bioinformatics Approaches in the Development of Antifungal Therapeutics and Vaccines. Curr. Genom. 2024, 25, 323–333. [Google Scholar] [CrossRef]
- Sunita; Sajid, A.; Singh, Y.; Shukla, P. Computational Tools for Modern Vaccine Development. Hum. Vaccines Immunother. 2020, 16, 723–735. [Google Scholar] [CrossRef]
- Bhargav, A.; Fatima, F.; Chaurasia, P.; Seth, S.; Ramachandran, S. Computer-Aided Tools and Resources for Fungal Pathogens: An Application of Reverse Vaccinology for Mucormycosis. Monoclon. Antibodies Immunodiagn. Immunother. 2022, 41, 243–254. [Google Scholar] [CrossRef]
- Basu, A. In Silico Epitope-Based Vaccine Prediction against Fungal Infection Aspergillosis. Challenges 2022, 13, 29. [Google Scholar] [CrossRef]
- Ochoa, R.; Cardim-Pires, T.R.; Sant’Anna, R.; Cossio, P.; Foguel, D. Connection between MHC Class II Binding and Aggregation Propensity: The Antigenic Peptide 10 of Paracoccidioides Brasiliensis as a Benchmark Study. Comput. Struct. Biotechnol. J. 2023, 21, 1746–1758. [Google Scholar] [CrossRef]
- Scorzoni, L.; Alves de Paula E Silva, A.C.; de Oliveira, H.C.; Tavares Dos Santos, C.; de Lacorte Singulani, J.; Akemi Assato, P.; Maria Marcos, C.; Teodoro Oliveira, L.; Ferreira Fregonezi, N.; Rossi, D.C.P.; et al. In Vitro and In Vivo Effect of Peptides Derived from 14-3-3 Paracoccidioides Spp. Protein. J. Fungi 2021, 7, 52. [Google Scholar] [CrossRef]
- Castro-Lopez, N.; Hung, C.-Y. Immune Response to Coccidioidomycosis and the Development of a Vaccine. Microorganisms 2017, 5, 13. [Google Scholar] [CrossRef]
- Campuzano, A.; Pentakota, K.D.; Liao, Y.-R.; Zhang, H.; Wiederhold, N.P.; Ostroff, G.R.; Hung, C.-Y. A Recombinant Multivalent Vaccine (rCpa1) Induces Protection for C57BL/6 and HLA Transgenic Mice against Pulmonary Infection with Both Species of Coccidioides. Vaccines 2024, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Hurtgen, B.J.; Hung, C.-Y.; Ostroff, G.R.; Levitz, S.M.; Cole, G.T. Construction and Evaluation of a Novel Recombinant T Cell Epitope-Based Vaccine against Coccidioidomycosis. Infect. Immun. 2012, 80, 3960–3974. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Robb, E.J.; Powell, D.A.; Bowen, R.A.; Bosco-Lauth, A.; Hartwig, A.; Porter, S.M.; Trinh, H.; Moale, H.; Bielefeldt-Ohmann, H.; et al. ΔCps1 Vaccine Protects Dogs against Experimentally Induced Coccidioidomycosis. Vaccine 2021, 39, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Shubitz, L.F.; Orbach, M.J.; Mandel, M.A.; Powell, D.A.; Klein, B.S.; Robb, E.J.; Ohkura, M.; Seka, D.J.; Tomasiak, T.M.; et al. Vaccines to Prevent Coccidioidomycosis: A Gene-Deletion Mutant of Coccidioides Posadasii as a Viable Candidate for Human Trials. J. Fungi 2022, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved Predictions of MHC Antigen Presentation by Concurrent Motif Deconvolution and Integration of MS MHC Eluted Ligand Data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef]
- Paul, S.; Sidney, J.; Sette, A.; Peters, B. TepiTool: A Pipeline for Computational Prediction of T Cell Epitope Candidates. Curr. Protoc. Immunol. 2016, 114, 18.19.1–18.19.24. [Google Scholar] [CrossRef]
- Singh, H.; Raghava, G.P.S. ProPred: Prediction of HLA-DR Binding Sites. Bioinformatics 2001, 17, 1236–1237. [Google Scholar] [CrossRef]
- Clifford, J.N.; Høie, M.H.; Deleuran, S.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-3.0: Improved B-Cell Epitope Prediction Using Protein Language Models. Protein Sci. Publ. Protein Soc. 2022, 31, e4497. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Naneva, L.; Doytchinova, I.; Bangov, I. AllergenFP: Allergenicity Prediction by Descriptor Fingerprints. Bioinforma. Oxf. Engl. 2014, 30, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Patiyal, S.; Dhall, A.; Pande, A.; Arora, C.; Raghava, G.P.S. AlgPred 2.0: An Improved Method for Predicting Allergenic Proteins and Mapping of IgE Epitopes. Brief Bioinform. 2021, 22, bbaa294. [Google Scholar] [CrossRef] [PubMed]
- Rapin, N.; Lund, O.; Bernaschi, M.; Castiglione, F. Computational Immunology Meets Bioinformatics: The Use of Prediction Tools for Molecular Binding in the Simulation of the Immune System. PLoS ONE 2010, 5, e9862. [Google Scholar] [CrossRef]
- Mead, H.L.; Kollath, D.R.; Itogawa, A.N.; Blackmon, A.V.; Morales, M.M.; Bryant, M.L.; Teixeira, M.d.M.; Barker, B.M. Using Double Cut in Vitro Assembled CRISPR/Cas9 to Modify the Genome of Coccidioides posadasii. BioRxiv 2023. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Stevens, D.A.; Hung, C.-Y.; Beyhan, S.; Taylor, J.W.; Shubitz, L.F.; Duttke, S.H.; Heidari, A.; Johnson, R.H.; Deresinski, S.C.; et al. Coccidioides Species: A Review of Basic Research: 2022. J. Fungi 2022, 8, 859. [Google Scholar] [CrossRef]
- Mendoza Barker, M.; Saeger, S.; Campuzano, A.; Yu, J.-J.; Hung, C.-Y. Galleria Mellonella Model of Coccidioidomycosis for Drug Susceptibility Tests and Virulence Factor Identification. J. Fungi 2024, 10, 131. [Google Scholar] [CrossRef]
- Kirsch, E.J.; Greene, R.T.; Prahl, A.; Rubin, S.I.; Sykes, J.E.; Durkin, M.M.; Wheat, L.J. Evaluation of Coccidioides Antigen Detection in Dogs with Coccidioidomycosis. Clin. Vaccine Immunol. 2012, 19, 343–345. [Google Scholar] [CrossRef]
- Koistinen, K.; Mullaney, L.; Bell, T.; Zaki, S.; Nalca, A.; Frick, O.; Livingston, V.; Robinson, C.G.; Estep, J.S.; Batey, K.L.; et al. Coccidioidomycosis in Nonhuman Primates: Pathologic and Clinical Findings. Vet. Pathol. 2018, 55, 905–915. [Google Scholar] [CrossRef]
- de Oliveira, A.R.; Oliveira, L.N.; Chaves, E.G.A.; Weber, S.S.; Bailão, A.M.; Parente-Rocha, J.A.; Baeza, L.C.; de Almeida Soares, C.M.; Borges, C.L. Characterization of Extracellular Proteins in Members of the Paracoccidioides Complex. Fungal Biol. 2018, 122, 738–751. [Google Scholar] [CrossRef]
- Begum, N.; Lee, S.; Portlock, T.J.; Pellon, A.; Nasab, S.D.S.; Nielsen, J.; Uhlen, M.; Moyes, D.L.; Shoaie, S. Integrative Functional Analysis Uncovers Metabolic Differences between Candida Species. Commun. Biol. 2022, 5, 1013. [Google Scholar] [CrossRef] [PubMed]
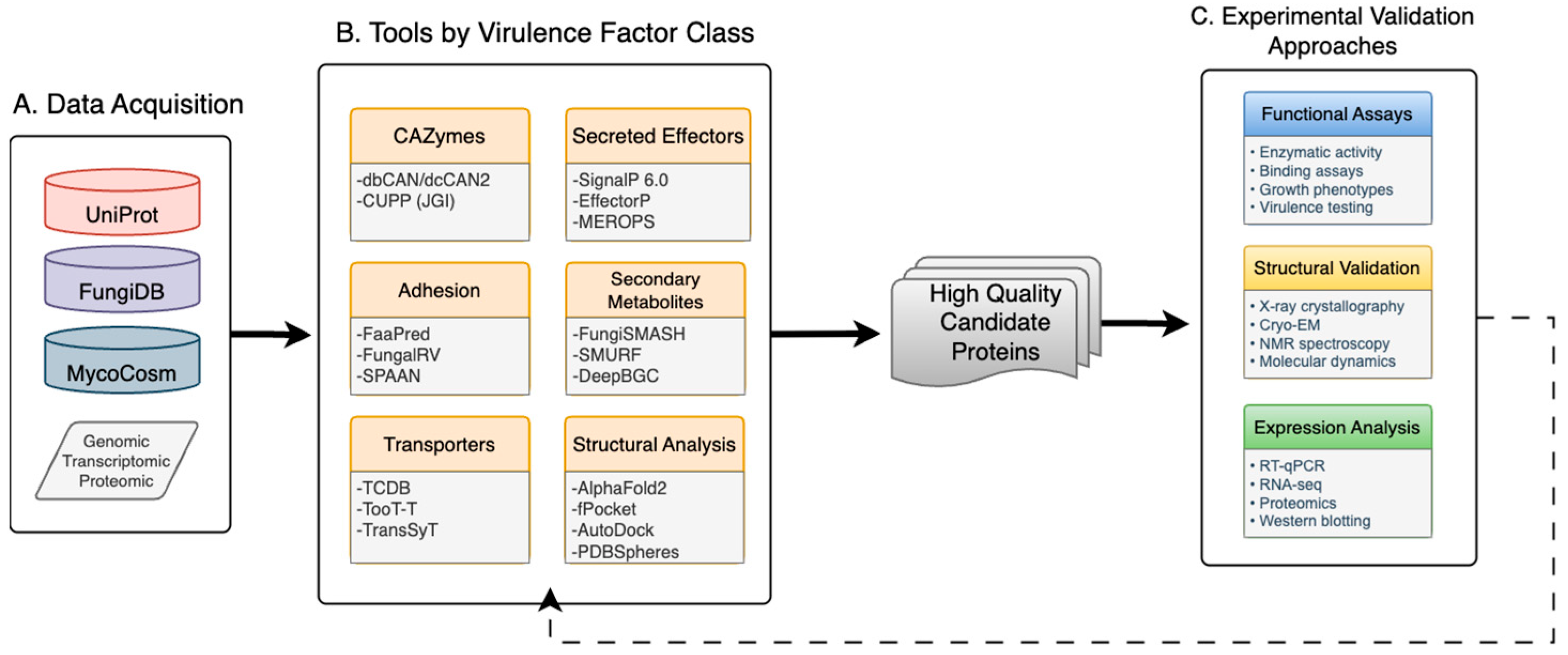
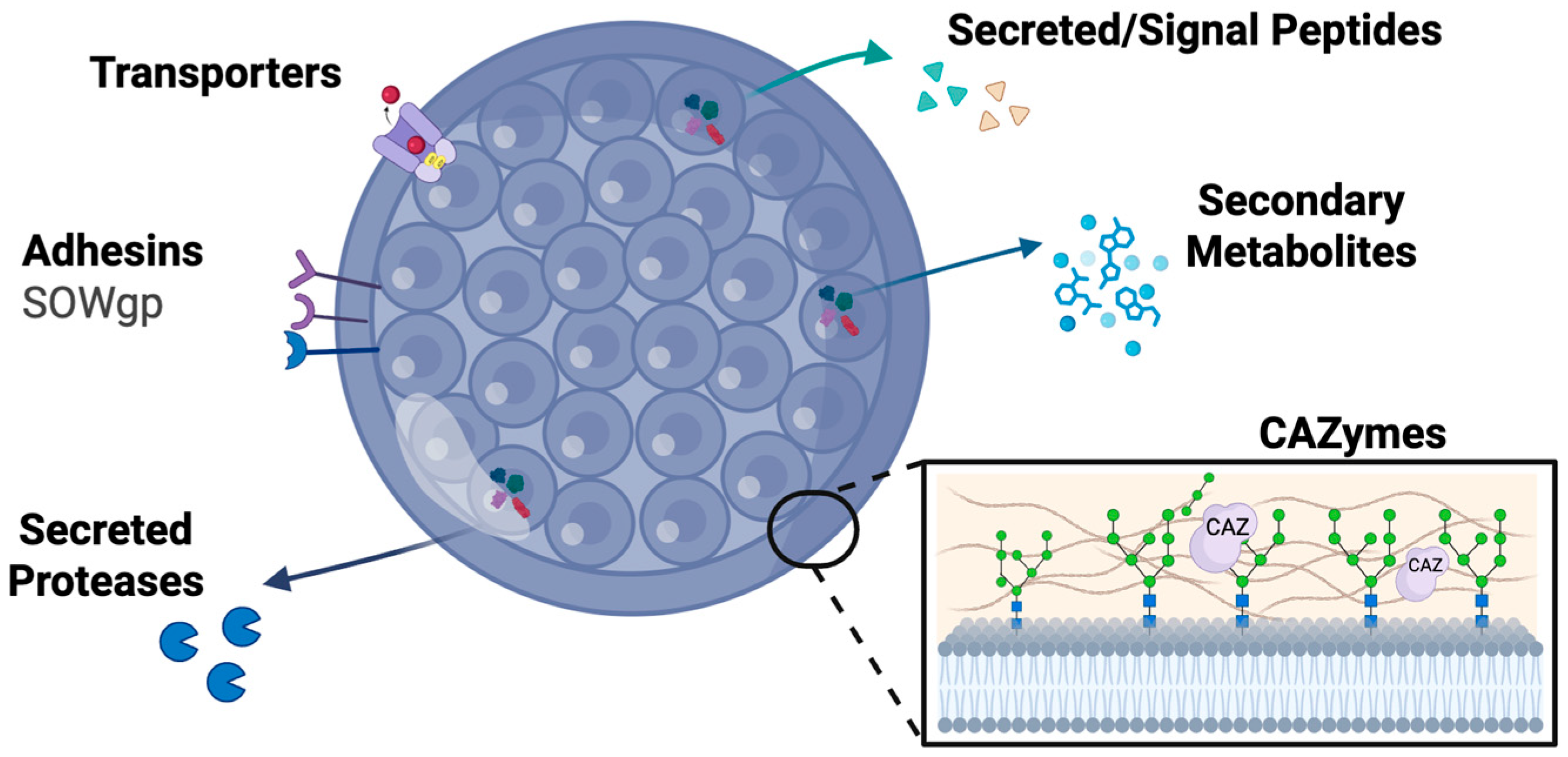
| Virulence Factor Class | Function in Pathogenesis | Computational Signatures | Example Proteins in Coccidioides | Conserved Across Species | Therapeutic Target Potential |
|---|---|---|---|---|---|
| CAZymes | Cell wall remodeling, immune evasion, morphological transitions | Glycoside hydrolases, Glycosyltransferases, Carbohydrate Transferases, Polysaccharide Lyases families; signal peptides | β-1,3-glucanases, β-1,6-glucanases | High (Candida, Aspergillus, Histoplasma) | Moderate—metabolic redundancy |
| Adhesins | Host cell attachment, colonization, biofilm formation | Lack conserved domains; rich in Ser/Thr; repetitive sequences | SOWgp (spherule outer wall glycoprotein) | Low—host-specific adaptations | High—species-specific targets |
| Transporters | Nutrient acquisition, drug efflux, stress resistance | Transmembrane domains; ABC, MFS families | Sit1-like iron transporters, ABC efflux pumps | High—essential metabolic functions | High—druggable targets |
| Iron Acquisition | Host iron sequestration, immune suppression | Siderophore biosynthesis clusters; iron-binding domains | Siderophore transporters, iron reductases | High—conserved iron metabolism | High—iron-limiting strategies |
| Secreted Proteases | Tissue invasion, immune evasion, host protein degradation | Signal peptides; protease domains (M, S, C families) | Mep1 metalloproteinase, serine carboxypeptidases | High—convergent evolution | Moderate—protease inhibition |
| Secreted Effectors | Host immune modulation, virulence enhancement | Signal peptides; small size; cysteine-rich | Cysteine-rich proteins, secreted hydrolases | Variable—pathogen-specific | High—immunotherapy targets |
| Secondary Metabolites | Immune suppression, tissue damage, antibiosis | BGC organization; NRPS, Polyketide synthase domains | Putative mycotoxin clusters | Moderate—chemical diversity | High—small molecule inhibition |
| Membrane Proteins | Stress response, cell wall integrity, morphogenesis | Transmembrane domains; GPI anchors | Cell surface glycoproteins, stress sensors | High—essential cellular functions | High—membrane-accessible targets |
| Morphogenesis Factors | Yeast-hyphal transitions, spherule development | Stage-specific expression; cytoskeletal interactions | Spherule-specific transcription factors | Moderate—dimorphic fungi | High—morphology disruption |
| Virulence Factor Class | Tool Name | Method/Algorithm | Input Required | Key Features | Performance Metrics | Limitations | Reference |
|---|---|---|---|---|---|---|---|
| CAZymes | dbCAN | HMM-based annotation | Protein sequences (FASTA) | Comprehensive CAZy family classification, batch processing | 98% accuracy; 2 min per 1000 proteins | Requires manual curation for novel families | [30] |
| dbCAN2 | Multi-method integration | Protein/genomic sequences | Combines HMM, DIAMOND, Hotpep methods | Sensitivity 95.6%; specificity 97.8% | Computationally intensive | [30] | |
| CUPP (JGI) | Machine learning pipeline | Assembled genomes | Automated functional annotation | Applied to JGI MycoCosm database | Limited to JGI-hosted genomes | [31] | |
| Adhesins | FaaPred | Support Vector Machine | Protein sequences | High specificity for fungal adhesins | Sensitivity 82.6%; accuracy 86% (fungal dataset) | Limited training dataset | [26] |
| FungalRV | Hidden Markov Model | Protein sequences | User-friendly web interface | Sensitivity 82.4%; precision 92.3%; accuracy 99% (fungal dataset) | Moderate sensitivity | [27] | |
| SPAAN | Neural network | Protein sequences | Non-homology based | 89% accuracy (bacteria); 65–75% (fungi) | Originally designed for bacteria | [28] | |
| Transporters | TCDB | Homology-based search | Protein sequences | Comprehensive transporter classification | Contains 20,000+ characterized transporters | Manual annotation required | [32] |
| TooT-T | Machine learning | Protein sequences | High accuracy for transport prediction | 94% accuracy; 15–20% improvement over BLAST | Limited fungal-specific training | [33] | |
| TransSyT | Multi-feature analysis | Protein sequences | Outperforms traditional methods | F1-score 0.91; precision 89% | Requires computational expertise | [34] | |
| Secreted Effectors | SignalP 6.0 | Deep learning | Protein sequences | High accuracy signal peptide prediction | Precision 94%; recall 91%; <5 min per 10,000 sequences | May miss non-classical secretion | [35] |
| EffectorP | Machine learning | Protein sequences | Distinguishes effectors from other secreted proteins | Sensitivity 92%; specificity 88% | Limited training on fungal effectors | [36,37] | |
| MEROPS | Database search | Protein sequences | Protease classification and annotation | 5000+ characterized proteases; E-value < 1 × 10−5 threshold | Requires homology to know proteases | [38] | |
| Secondary Metabolites | FungiSMASH | Rule-based + ML | Genomic sequences | Fungi-specific BGC detection | 85–95% detection of known BGCs; 20% more sensitive than SMURF | Requires complete genome assemblies | [39] |
| SMURF | Rule-based | Genomic sequences | Web-based, user-friendly | Effective for canonical BGC architectures | Less sensitive than FungiSMASH | [40] | |
| DeepBGC | Deep learning | Genomic sequences | Predicts bioactivity with confidence scores; exploits Pfam domains | BGC detection: 79% precision, 74% recall; bioactivity: 51–68% accuracy | Requires large training datasets | [41] | |
| TOUCAN | Machine Learning | Genomic sequences | Outperforms FungiSMASH and DeepBGC | Precision 98%; Recall 91%; F1 score 0.98 on Aspergillus niger and A. nidulans | Possible overprediction of cluster bounds; requires post-process filtering | [42] | |
| Structural Analysis | AlphaFold2 | Deep learning | Protein sequences | Highly accurate structure prediction | Median pLDDT > 90 for ordered regions; CASP14 GDT_TS 92.4 | Limited to single chain proteins | [43] |
| fpocket | Geometric algorithm | Protein structures (PDB) | Druggable pocket identification | 94% success rate; 2–5 s per protein | Sensitive to structure quality | [44] | |
| AutoDock | Molecular docking | Protein structure and ligands | Virtual screening capabilities | RMSD < 2 Å (78% of test cases); 10,000× faster than prior version | Requires expert parameter tuning | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, A.D.; Senthil, V.; Hoyer, K.K. Computational Approaches for Discovering Virulence Factors in Coccidioides. J. Fungi 2025, 11, 754. https://doi.org/10.3390/jof11100754
Daniel AD, Senthil V, Hoyer KK. Computational Approaches for Discovering Virulence Factors in Coccidioides. Journal of Fungi. 2025; 11(10):754. https://doi.org/10.3390/jof11100754
Chicago/Turabian StyleDaniel, Arianna D., Vikram Senthil, and Katrina K. Hoyer. 2025. "Computational Approaches for Discovering Virulence Factors in Coccidioides" Journal of Fungi 11, no. 10: 754. https://doi.org/10.3390/jof11100754
APA StyleDaniel, A. D., Senthil, V., & Hoyer, K. K. (2025). Computational Approaches for Discovering Virulence Factors in Coccidioides. Journal of Fungi, 11(10), 754. https://doi.org/10.3390/jof11100754







