Variation in Growth, Morphology, and Fungicide Sensitivity Among Monilinia Species from South Tyrol’s Alpine Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi Isolates and Experimental Conditions
2.2. Phylogeny
2.3. Growth Rate and Colony Morphology on PDA
2.4. Mycelial Growth Rate and Morphology on Apple
2.5. Conidia Production and Characterization
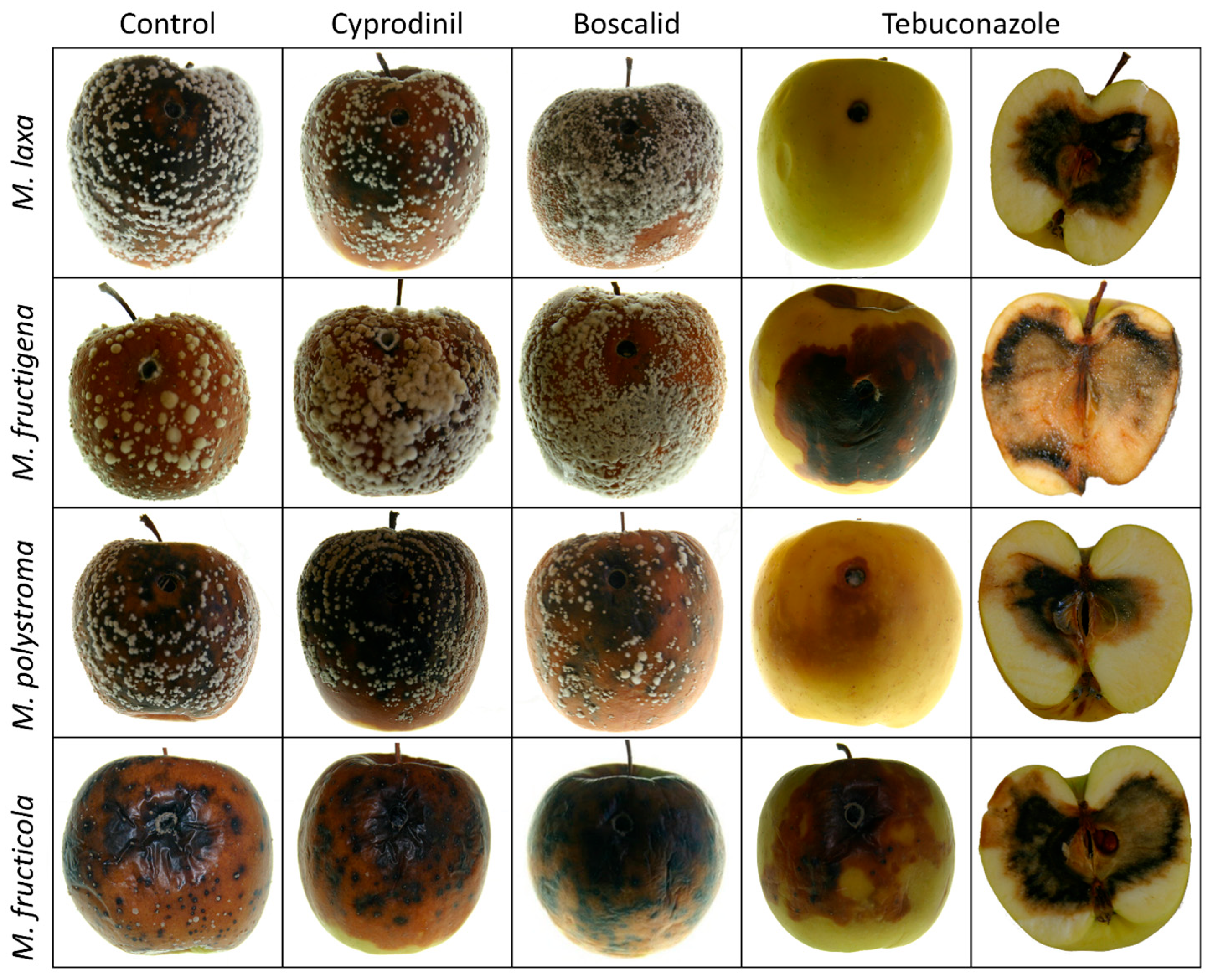
2.6. Fungicide Screening on Apples
2.7. Statistical Analyses
3. Results
3.1. Phylogeny
3.2. Growth Rate and Colony Morphology on PDA
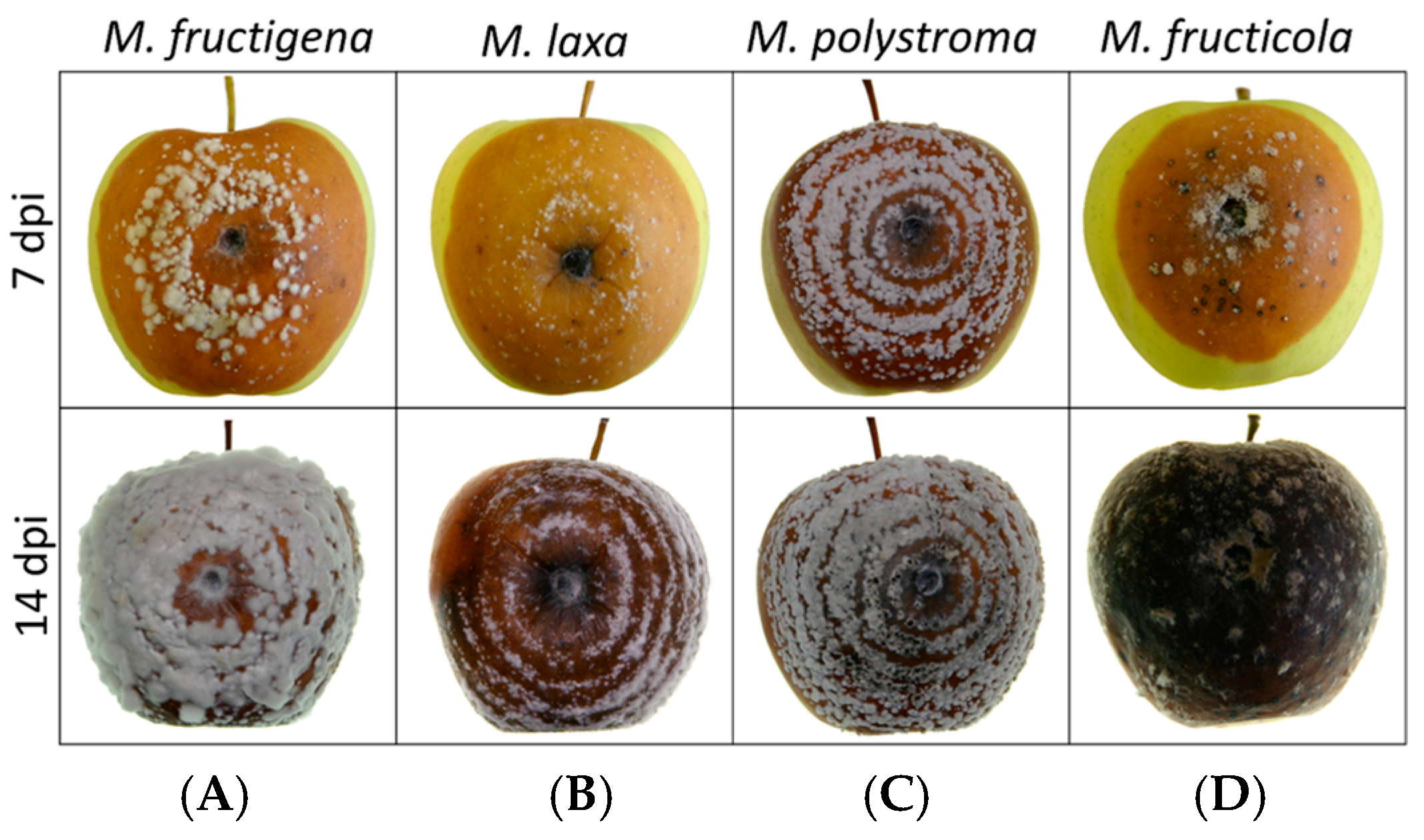
3.3. Mycelial Growth Rate and Colony Morphology on Apples
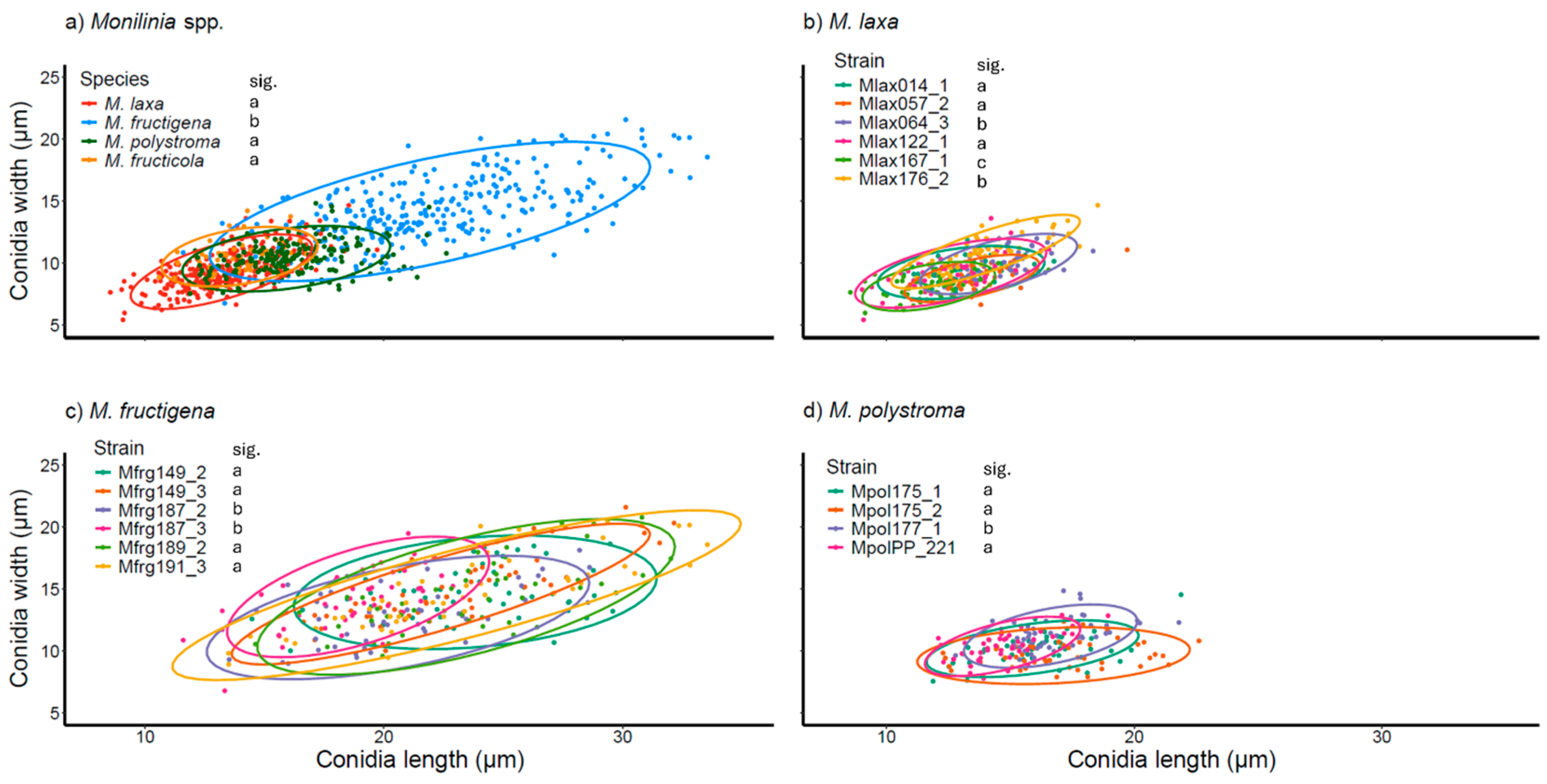
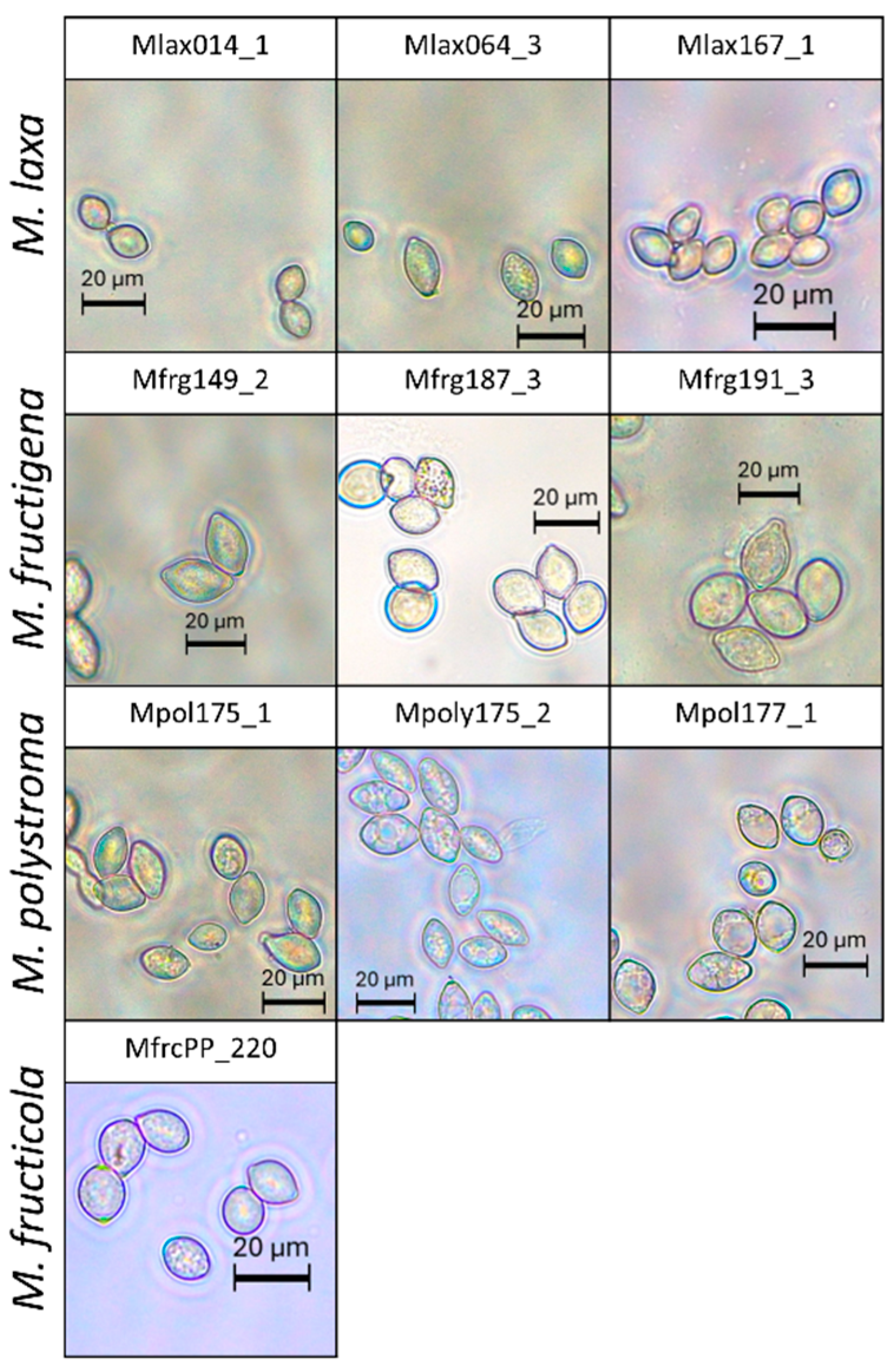
3.4. Conidia Production and Characterization from PDA, Apple, and TSA
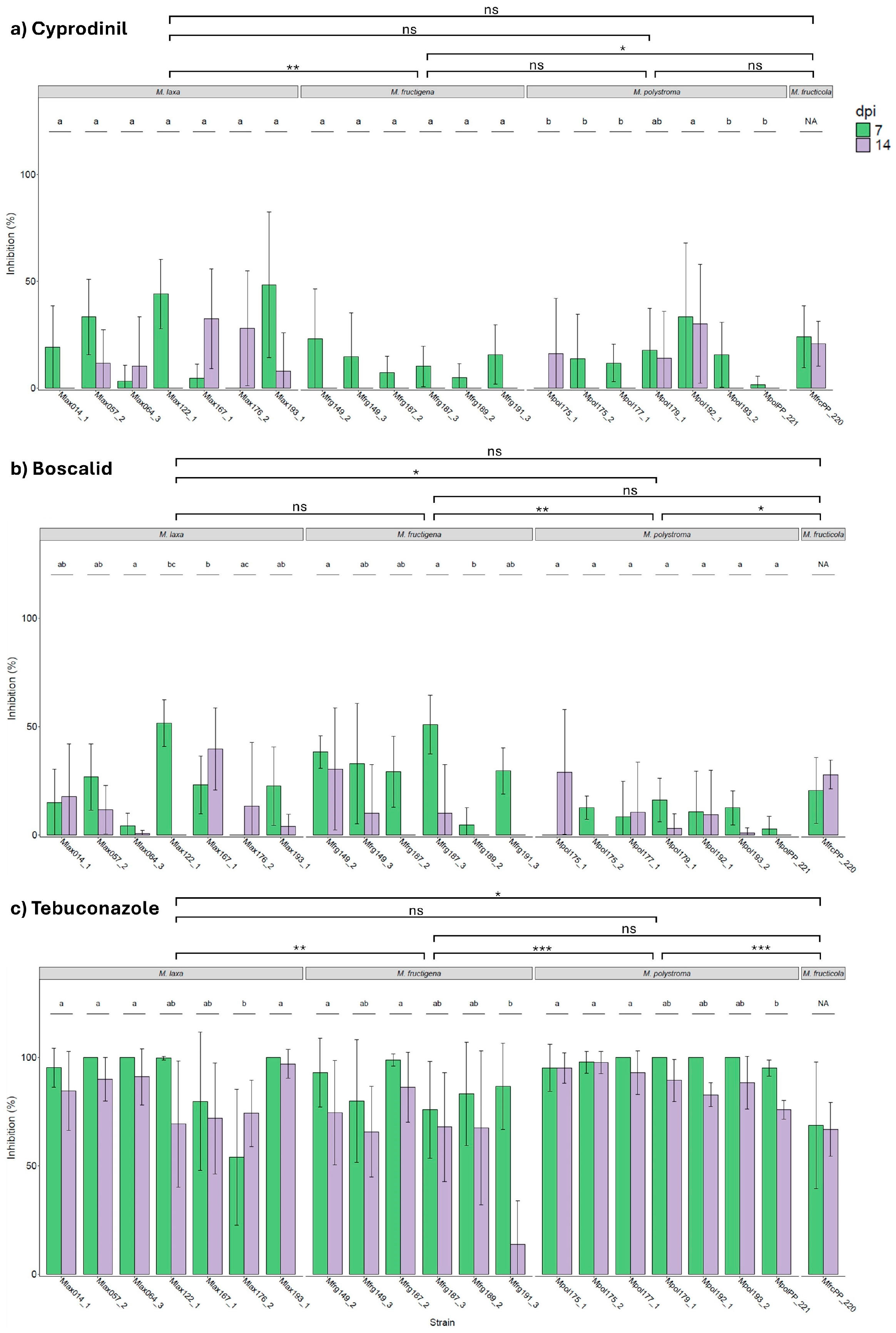
3.5. Fungicide Screening on Apples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abate, D.; Pastore, C.; Gerin, D.; de Miccolis Angelini, R.M.; Rotolo, C.; Pollastro, S.; Faretra, F. Characterization of Monilinia spp. populations on stone fruit in South Italy. Plant Dis. 2018, 102, 1708–1717. [Google Scholar] [CrossRef]
- Holb, I.J. Brown rot blossom blight of pome and stone fruits: Symptom, disease cycle, host resistance, and biological control. Int. J. Hortic. Sci. 2008, 14, 15–21. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Peach brown rot: Still in search of an ideal management option. Agriculture 2018, 8, 125. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Hu, M.-J.; Cox, K.D.; Schnabel, G.; Luo, C.-X. Monilinia species causing brown rot of peach in China. PLoS ONE 2011, 6, e24990. [Google Scholar] [CrossRef]
- Byrde, R.; Willetts, H.J. The brown rot fungi of fruit: Their biology and control. Mycologia 1977, 69, 1240. [Google Scholar] [CrossRef]
- Rungjindamai, N.; Jeffries, P.; Xu, X.-M. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Petróczy, M.; Palkovics, L. First report of Monilia polystroma on apple in Hungary. Eur. J. Plant Pathol. 2009, 125, 343–347. [Google Scholar] [CrossRef]
- Balardin, R.; Madalosso, M.; Debortoli, M.; Lenz, G. Factors Affecting Fungicide Efficacy in the Tropics; Federal University of Santa Maria: Santa Maria, Brazil, 2010. [Google Scholar]
- Casals, C.; Teixidó, N.; Viñas, I.; Llauradó, S.; Usall, J. Control of Monilinia spp. on stone fruit by curing treatments. Postharvest Biol. Technol. 2010, 56, 19–25. [Google Scholar] [CrossRef]
- Garcia-Benitez, C.; Casals, C.; Usall, J.; Sánchez-Ramos, I.; Melgarejo, P.; de Cal, A. Impact of postharvest handling on preharvest latent infections caused by Monilinia spp. in nectarines. J. Fungi 2020, 6, 266. [Google Scholar] [CrossRef]
- Akhoon, B.A.; Gupta, S.K.; Dhar, M.K. Dissecting the genome, secretome, and effectome repertoires of Monilinia spp.: The causal agent of brown rot disease: A comparative analysis. Postharvest Biol. Technol. 2023, 195, 112120. [Google Scholar] [CrossRef]
- Adaskaveg, J.; Gubler, D.; Michailides, T.; Holtz, B. Efficacy and Timing of Fungicides, Bacteriocides, and Biologicals for Deciduous Tree Fruit, Nut, Strawberry and Vine Crops; Department of Plant Pathology, UC Davis: Davis, CA, USA, 2011. [Google Scholar]
- Rustin, P.; Munnich, A.; Rötig, A. Succinate dehydrogenase and human diseases: New insights into a well-known enzyme. Eur. J. Hum. Genet. 2002, 10, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Lee, H.B. Molecular mechanisms of succinate dehydrogenase inhibitor resistance in phytopathogenic fungi. Res. Plant Dis. 2020, 26, 1–7. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Förster, H.; Thompson, D.F. Identification and etiology of visible quiescent infections of Monilinia fructicola and Botrytis cinerea in sweet cherry fruit. Plant Dis. 2000, 84, 328–333. [Google Scholar] [CrossRef]
- Zeng, F.; Arnao, E.; Zhang, G.; Olaya, G.; Wullschleger, J.; Sierotzki, H.; Ming, R.; Bluhm, B.H.; Bond, J.P.; Fakhoury, A.M.; et al. Characterization of quinone outside inhibitor fungicide resistance in Cercospora sojina and development of diagnostic tools for its identification. Plant Dis. 2015, 99, 544–550. [Google Scholar] [CrossRef]
- Spitaler, U.; Oettl, S.; Deltedesco, E. First report of brown rot caused by Monilinia polystroma on quince in Italy. Plant Dis. 2023, 107, 229. [Google Scholar] [CrossRef]
- Spitaler, U.; Pfeifer, A.; Deltedesco, E.; Hauptkorn, S.; Oettl, S. Detection of Monilinia spp. by a multiplex real-time PCR assay and first report of Monilinia fructicola in South Tyrol (northern Italy). J. Plant Dis. Prot. 2022, 129, 1013–1020. [Google Scholar] [CrossRef]
- Deltedesco, E.; Oettl, S.; Spitaler, U. First report of brown rot caused by Monilinia polystroma on sweet cherry and almond in Italy. Plant Dis. 2023, 107, 2252. [Google Scholar] [CrossRef]
- Angeli, S.S.; de Mio, L.L.M.; Amorim, L. Comparative analysis of Monilinia fructicola and M. laxa isolates from Brazil: Monocyclic components of peach brown rot. Cienc. Rural 2017, 47, e20160300. [Google Scholar] [CrossRef]
- van Leeuwen, G.C.; Baa Yen, R.P.; Holb, I.J.; Jeger, M.J. Distinction of the Asiatic brown rot fungus Monilia polystroma sp. nov. from M. fructigena. Mycol. Res. 2002, 106, 444–451. [Google Scholar] [CrossRef]
- Petróczy, M.; Szigethy, A.; Palkovics, L. Monilinia species in Hungary: Morphology, culture characteristics, and molecular analysis. Trees 2012, 26, 153–164. [Google Scholar] [CrossRef]
- Vasić, M.; Vico, I.; Jurick, W.M.; Duduk, N. Distribution and characterization of Monilinia spp. causing apple fruit decay in Serbia. Plant Dis. 2018, 102, 359–369. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. ISBN 9780123721808. [Google Scholar]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular evolutionary genetic analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, X.; Chen, S.; Schnabel, E.; Schnabel, G. Characterization of Monilinia fructicola strains resistant to both propiconazole and boscalid. Plant Dis. 2013, 97, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2015, 67, 48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Wickham, H. Getting Started with ggplot2. In ggplot2; Wickham, H., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 11–31. ISBN 978-3-319-24275-0. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Soft. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Guan, G.-X.; Nokes, L.; Braun, U.; Liu, S.-Y.; Pfister, D.H. Secondary DNA barcodes (CAM, GAPDH, GS, and RpB2) to characterize species complexes and strengthen the powdery mildew phylogeny. Front. Ecol. Evol. 2022, 10, 918908. [Google Scholar] [CrossRef]
- Poniatowska, A.; Michalecka, M.; Puławska, J. Phylogenetic relationships and genetic diversity of Monilinia spp. isolated in Poland based on housekeeping- and pathogenicity-related gene sequence analysis. Plant Pathol. 2021, 70, 1640–1650. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Niu, C.-W.; Chen, X.-Y.; Guo, L.-Y. Monilinia Species Associated with Brown Rot of Cultivated Apple and Pear Fruit in China. Plant Dis. 2016, 100, 2240–2250. [Google Scholar] [CrossRef]
- Martini, C.; Mari, M. Monilinia fructicola, Monilinia laxa (Monilinia rot, brown rot). In Postharvest Decay; Academic Press: Cambridge, MA, USA, 2014; pp. 233–265. [Google Scholar] [CrossRef]
- Verde-Yáñez, L.; Usall, J.; Teixidó, N.; Vall-Llaura, N.; Torres, R. Deciphering the effect of light wavelengths in Monilinia spp. DHN-Melanin production and their interplay with ROS metabolism in M. fructicola. J. Fungi 2023, 9, 653. [Google Scholar] [CrossRef]
- Munda, A. First report of brown rot on peach caused by Monilia polystroma in Slovenia. Plant Dis. 2015, 99, 1281. [Google Scholar] [CrossRef]
- Avenot, H.F.; Quattrini, J.; Puckett, R.; Michailides, T.J. Different levels of resistance to cyprodinil and iprodione and lack of fludioxonil resistance in Botrytis cinerea isolates collected from pistachio, grape, and pomegranate fields in California. Crop Prot. 2018, 112, 274–281. [Google Scholar] [CrossRef]
- Scott, J.; Sueiro-Olivares, M.; Thornton, B.P.; Owens, R.A.; Muhamadali, H.; Fortune-Grant, R.; Thomson, D.; Thomas, R.; Hollywood, K.; Doyle, S.; et al. Targeting methionine synthase in a fungal pathogen causes a metabolic imbalance that impacts cell energetics, growth, and virulence. mBio 2020, 11, e01985-20. [Google Scholar] [CrossRef]
- Dardani, G.; Guarnaccia, V.; Nari, L.; Testempasis, S.I.; Karaoglanidis, G.S.; Gullino, M.L. Identification of pathogens causing brown rot of stone fruit in Cuneo province (Italy) and assessment of sensitivity to azoxystrobin, cyprodinil, fenhexamid, fludioxonil, and tebuconazole. Phytopathol. Mediterr. 2023, 62, 455–465. [Google Scholar] [CrossRef]
- Egüen, B.; Melgarejo, P.; de Cal, A. The effect of fungicide resistance on the structure of Monilinia laxa populations in Spanish peach and nectarine orchards. Eur. J. Plant Pathol. 2016, 145, 815–827. [Google Scholar] [CrossRef]
- Hrustić, J.; Mihajlović, M.; Grahovac, M.; Delibašić, G.; Tanović, B. Fungicide sensitivity, growth rate, aggressiveness and frost hardiness of Monilinia fructicola and Monilinia laxa isolates. Eur. J. Plant Pathol. 2018, 151, 389–400. [Google Scholar] [CrossRef]
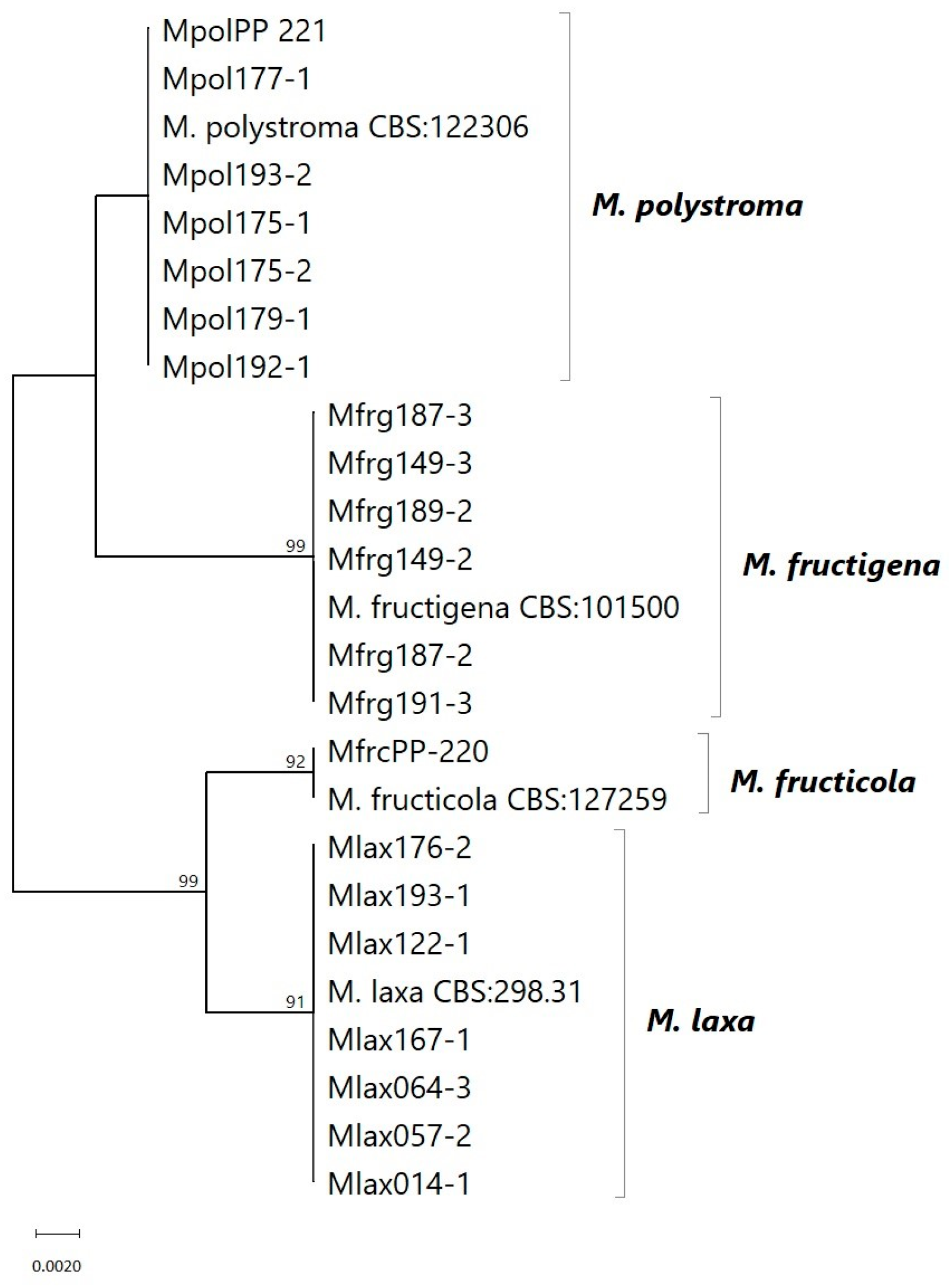
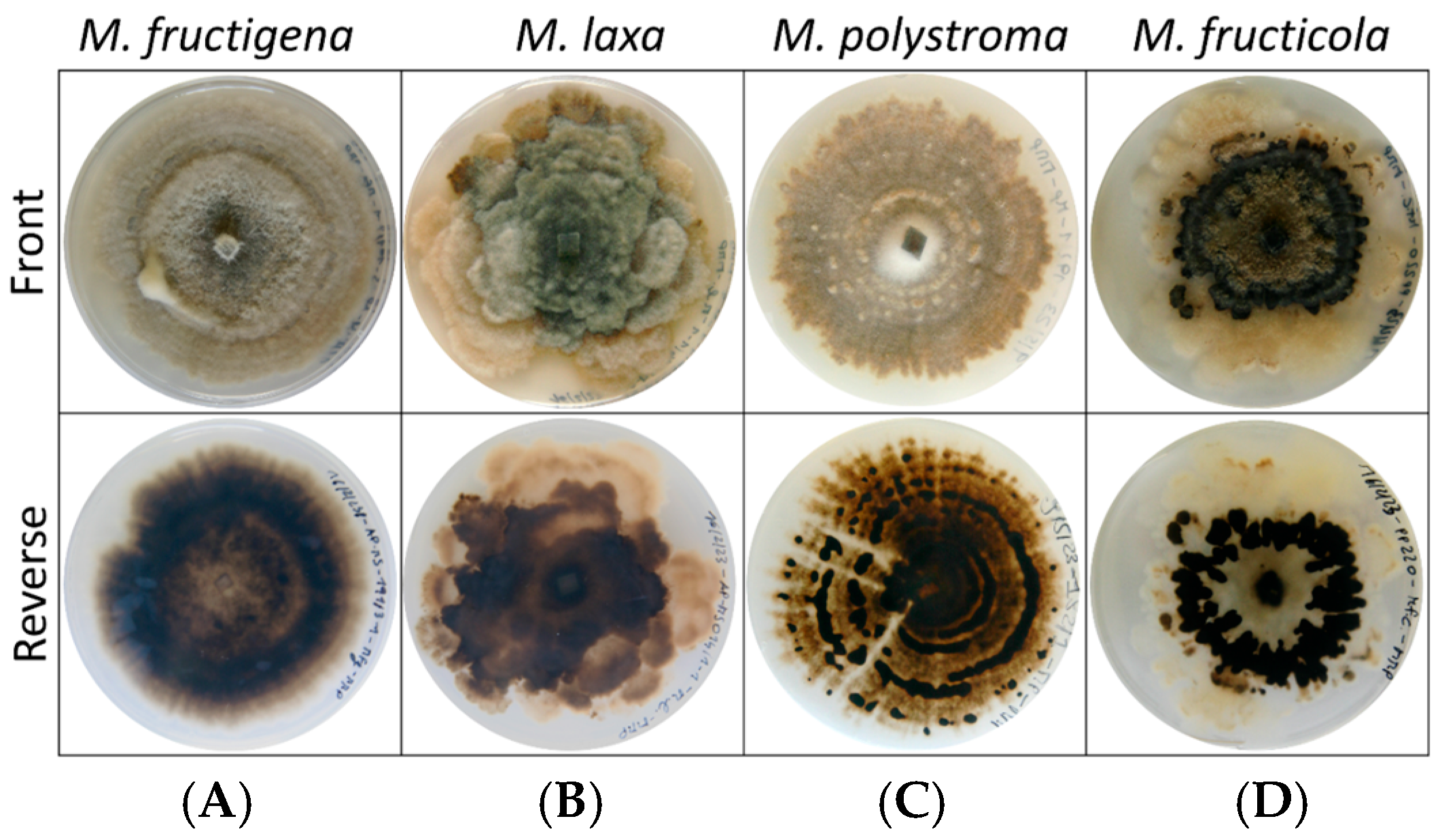
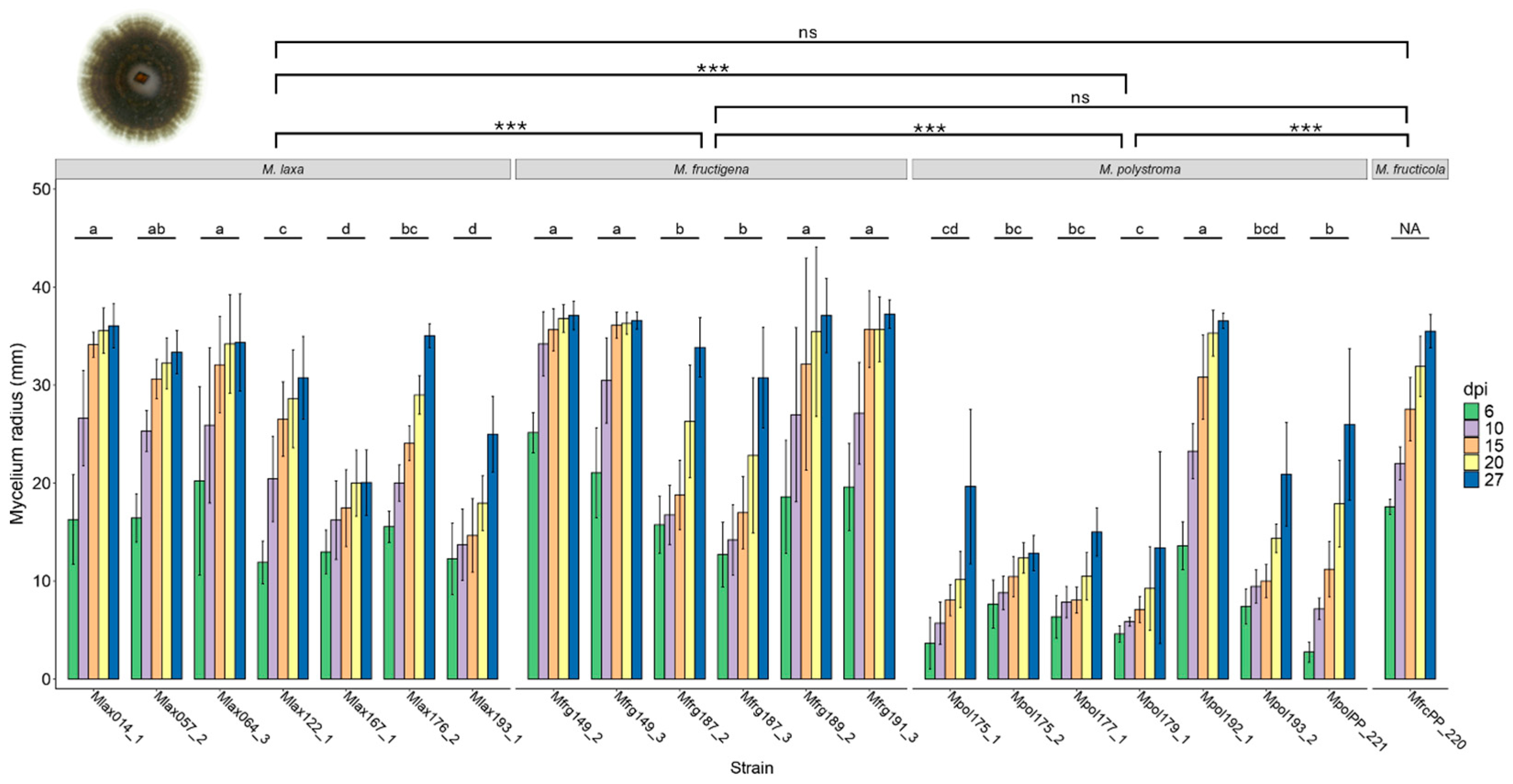
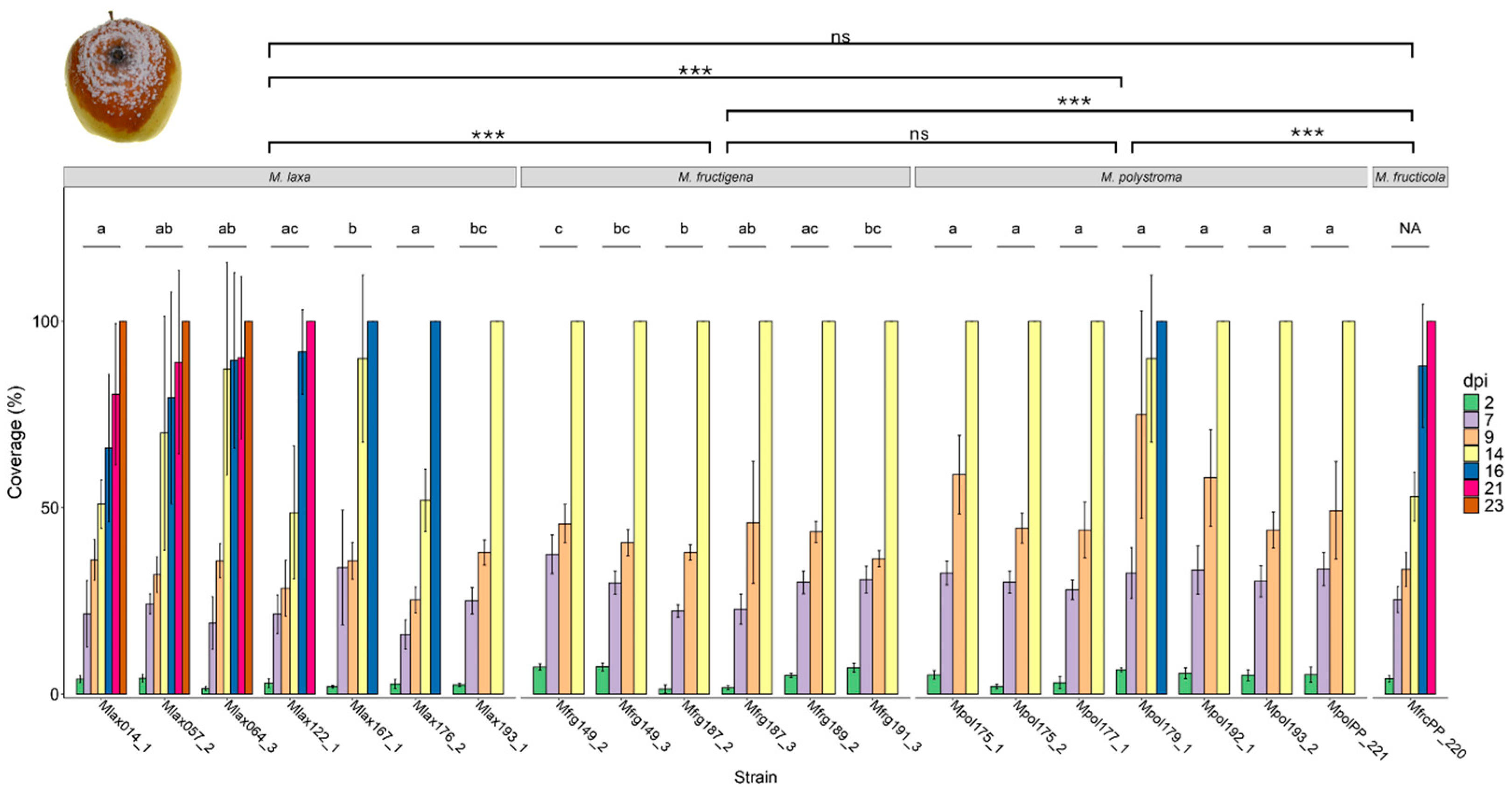
| Species | Strain ID | Host Fruit | Sampling Sites * |
|---|---|---|---|
| M. laxa | Mlax014_1 | Plum | Jenesien/San Genesio |
| Mlax057_2 | Peach | Partschins/Parcines | |
| Mlax064_3 | Cherry | Allitz/Lasa | |
| Mlax122_1 | Cherry | Ritten/Renon | |
| Mlax167_1 | Apricot | Kollmann/Colma | |
| Mlax176_2 | Peach | Piglon/Piccolungo | |
| Mlax193_1 | Quince | Kaltern/Caldaro | |
| M. fructigena | Mfrg149_2 | Plum | Kollmann/Colma |
| Mfrg149_3 | Plum | Kollmann/Colma | |
| Mfrg187_2 | Apple | Terlan/Terlano | |
| Mfrg187_3 | Apple | Terlan/Terlano | |
| Mfrg189_2 | Almond | Piglon/Piccolungo | |
| Mfrg191_3 | Almond | Piglon/Piccolungo | |
| M. polystroma | Mpol175_1 | Cherry | Laimburg |
| Mpol175_2 | Cherry | Laimburg | |
| Mpol177_1 | Almond | Piglon/Piccolungo | |
| Mpol179_1 | Almond | Piglon/Piccolungo | |
| Mpol192_1 | Quince | Kaltern/Caldaro | |
| Mpol193_2 | Quince | Kaltern/Caldaro | |
| MpolPP_221 | Apple | Japan (CBS 102686) | |
| M. fructicola | MfrcPP_220 | Cherry | Bari, Italy (CBS 144849) |
| Active Ingredient | Trade Name | Manufacturer/Distributor | Composition (AI) | Applied Dose (AI) |
|---|---|---|---|---|
| Boscalid | Cantus® | BASF Italia S.p.A. (Cesano Maderno, Italy) | 500 g kg−1 | 200 mg L−1 |
| Cyprodinil | Chorus® | Syngenta Italia S.p.A. (Milan, Italy) | 500 g kg−1 | 250 mg L−1 |
| Tebuconazole | Folicur® WG | Bayer CropScience S.r.l. (Milan, Italy) | 250 g kg−1 | 125 mg L−1 |
| Species | Isolate | Sporulation | |||||
|---|---|---|---|---|---|---|---|
| PDA | dpi | Apple | dpi | TSA | dpi | ||
| M. laxa | Mlax014_1 | - | - | - | - | X | 6 |
| Mlax057_2 | - | - | - | - | X | 12 | |
| Mlax064_3 | - | - | - | - | X | 6 | |
| Mlax122_1 | - | - | - | - | X | 6 | |
| Mlax167_1 | X | 12 | - | - | X | 12 | |
| Mlax176_2 | - | - | - | - | X | 6 | |
| Mlax193_1 | - | - | - | - | X | 12 | |
| M. fructigena | Mfrg149_2 | - | - | X | 9 | X | 6 |
| Mfrg149_3 | - | - | X | 9 | X | 7 | |
| Mfrg187_2 | - | - | X | 14 | X | 7 | |
| Mfrg187_3 | - | - | X | 9 | X | 6 | |
| Mfrg189_2 | - | - | X | 14 | X | 7 | |
| Mfrg191_3 | - | - | X | 21 | X | 6 | |
| M. polystroma | Mpol175_1 | - | - | - | - | X | 6 |
| Mpol175_2 | - | - | - | - | X | 12 | |
| Mpol177_1 | - | - | - | - | X | 12 | |
| Mpol179_1 | - | - | - | - | - | - | |
| Mpol192_1 | - | - | - | - | - | - | |
| Mpol193_2 | - | - | - | - | - | - | |
| MpolPP_221 | - | - | - | - | X | 6 | |
| M. fructicola | MfrcPP_220 | - | - | X | 16 | X | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, M.M.; Oettl, S.; Deltedesco, E.; Pii, Y.; Spitaler, U. Variation in Growth, Morphology, and Fungicide Sensitivity Among Monilinia Species from South Tyrol’s Alpine Orchards. J. Fungi 2025, 11, 690. https://doi.org/10.3390/jof11100690
Pagano MM, Oettl S, Deltedesco E, Pii Y, Spitaler U. Variation in Growth, Morphology, and Fungicide Sensitivity Among Monilinia Species from South Tyrol’s Alpine Orchards. Journal of Fungi. 2025; 11(10):690. https://doi.org/10.3390/jof11100690
Chicago/Turabian StylePagano, Melanie M., Sabine Oettl, Evi Deltedesco, Youry Pii, and Urban Spitaler. 2025. "Variation in Growth, Morphology, and Fungicide Sensitivity Among Monilinia Species from South Tyrol’s Alpine Orchards" Journal of Fungi 11, no. 10: 690. https://doi.org/10.3390/jof11100690
APA StylePagano, M. M., Oettl, S., Deltedesco, E., Pii, Y., & Spitaler, U. (2025). Variation in Growth, Morphology, and Fungicide Sensitivity Among Monilinia Species from South Tyrol’s Alpine Orchards. Journal of Fungi, 11(10), 690. https://doi.org/10.3390/jof11100690







