Abstract
Austin was first isolated as a novel polyisoprenoid mycotoxin from Aspergillus ustus in 1976. Subsequently, some new austin-type meroterpenoids (ATMTs) have been continually found. This review attempts to give a comprehensive summary of progress on the isolation, chemical structural features, biological activities, and fungal biodiversity of 104 novel ATMTs from 5 genera of terrestrial- and marine-derived fungi reported from October 1976 to January 2023. The genera of Penicillium and Aspergillus are the two dominant producers, producing 63.5% and 30.8% of ATMTs, respectively. Moreover, about 26.9% of ATMTs display various pronounced bioactivities, including insecticidal, anti-inflammatory, cytotoxicity, antibacterial, and PTP1B inhibitory activities. The chemical diversity and potential activities of these novel fungal ATMTs are reviewed for a better understanding, and a relevant summary focusing on the source fungi and their taxonomy is provided to shed light on the future development and research of austin-type meroterpenoids.
1. Introduction
Microbial secondary metabolites differ from primary nutrients in that they are not essential for growth, but they play a vital role in the survival and adaptation of microbes in nature []. Fungi attract much attention from chemists and biologists due to the production of secondary metabolites with diverse structural skeletons and interesting bioactivities. Austin-type meroterpenoids (ATMTs) are a family of hybrid natural products with high diversity of intriguing scaffolds, but only 104 ATMTs have been characterized, which are a relatively rare branch of the terpenoid family and have often been isolated from fungi, especially from the genera Penicillium and Aspergillus [,,,,,,]. The naturally occurring meroterpenoids derived from 3,5-dimethylorsellinic acid (DMOA) incorporated with farnesyl pyrophosphate (FPP) are the most common subclass [,,,,], of which ATMTs are a class of special and important constituents. Based on the number of rings of the initial meroterpenoid in their biosynthesis pathway, ATMTs are classified into four categories, including tetracyclic, pentacyclic, hexacyclic, and heptacyclic systems. Interestingly, different types of ATMTs display broad and impressive biological activity [,,], including insecticidal, antiphlogistic, antimicrobial, and antineoplastic effects, etc.
To date, no individual and comprehensive identification of the chemical structures of ATMTs has been reported. Therefore, this review was prepared to provide an overall coverage of the chemical constituents of the ATMTs reported in the last five decades (from October 1976 to January 2023) originating from fungi according to a classification of their chemical skeletons (two databases were used for the search: SciFinder and Web of Science). This review will provide information on the isolation, chemical structural features, biological activities, and fungal biodiversity of ATMTs, which will facilitate further research and exploitation of these structures. In this review, the biosynthesis pathways of these ATMTs are not discussed as they have been extensively reviewed by Wang, Abe, and Brakhage [,,,].
2. Austin-Type Meroterpenoids Compounds
2.1. Tetracyclic Systems Austin-Type Meroterpenoids
2.1.1. Tetracyclic Systems-Rings A and B Are Spirocyclic
This subgroup of ATMTs is characterized by a tetracyclic spirocyclic systems with six members (1–6) (Figure 1), including austinoneol (1) that was obtained from a Penicillium sp. [] that was isolated from the root bark of Melia azedarach after surface sterilization and cultivated for three weeks on sterilized rice. The filamentous fungus Aspergillus nidulans has previously been found to produce two meroterpenoids, austinol and dehydroaustinol. The use of targeted deletions revealed that two separate gene clusters are required for meroterpenoid biosynthesis. One is a cluster of four genes including the polyketide synthase gene, ausA. The second is a cluster of 10 additional genes, including the prenyltransferase gene, ausN, located on a separate chromosome. Chemical analysis of mutant extracts enabled isolation of preaustinoid A5 (2), preaustinoid A4 (3), and preaustinoid A3 (4), which are either intermediates or shunt products from the biosynthetic pathway [] (Figure 1). The filamentous fungus Aspergillus oryzae NSAR1 is used as part of a heterologous fungal expression system; Aspergillus oryzae NSAR1 is a quadruple auxotrophic mutant strain (niaD−, sC−, ΔargB, adeA−) that is used to produce austinoid C (5) []. Furthermore, one unusual austin-type meroterpenoid is penicianstinoid C (6), which was the first ATMT with a unique 6/6/6/5 rearranged tetracyclic skeleton possessing two unusual spirocyclic moieties (2-oxaspiro[5.5]undeca-4,7-dien-3-one and 6-methylene-2-oxaspiro[4.5]decane-1,4-dione); it was obtained from the mangrove-derived fungus Penicillium sp. TGM112. In addition, this compound shows inhibitory activity against newly hatched larvae of Helicoverpa armigera Hubner (IC50 = 100 μg/mL) [].
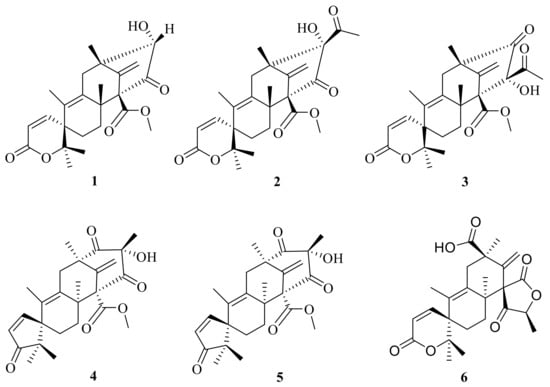
Figure 1.
Chemical structures of compounds 1–6.
2.1.2. Tetracyclic Systems-Rings A and B Are Bicyclic Fused
An endophytic fungus Penicillium sp. is cultured from solid rice medium and was isolated from the root bark of Melia azedarach. It produced two new tetracyclic system ATMTs, preaustiniod A (7) and B (11), and they exhibited moderate bacteriostatic effects on Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Bacillus sp. [] (Figure 2). Genome mining of the fungus Aspergillus oryzae NSAR1 led to the isolation of three tetracyclic system ATMTs, preaustinoid C (8), 5-hydroxyberkeleyone A (16), and berkeleyone A (17) []. By employing a large-scale culture approach, asperanstinoid E (9) was obtained from the fungus Aspergillus calidoustus, which was isolated from wetland soil collected at Dianchi Lake []. Penicianstinoid D (10), an unusual austin-type meroterpenoid with a 6/6/6/6 tetracyclic skeleton containing an octahydro-2H-chromen-2-one unit, was obtained from Penicillium sp. TGM112 []. The endophytic fungus Penicillium sp. derived from the root of Panax notoginseng yielded three new ATMTs, including preaustinoid B1 (12), preaustiniod A1 (18), and A2 (19) [] (Figure 2). Two additional meroterpenes were isolated and identified from rice cultures of Penicillium sp., a fungus obtained from the root bark of Melia azedarach. These new compounds were named preaustinoid B2 (13), and preaustinoid A3 (4) []. The undescribed meroterpenoid preaustinoid C (14) was isolated from Penicillium sp. RO-11, which was collected from the sediments of a hydrothermal spring located in the southwestern area of Saudi Arabia. Preaustinoid C showed significant activity against lipopolysaccharide (LPS)-induced NO production and a selective effect on IL-2 and IFN-γ gene regulation in activated Jurkat cells []. The mutant filamentous fungus Aspergillus nidulans has been found to produce protoaustinoid A (15) [].
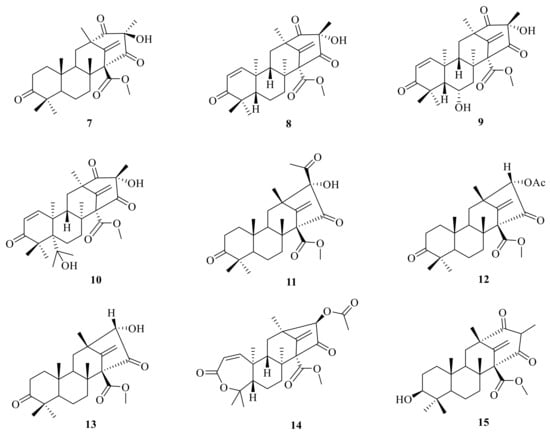
Figure 2.
Chemical structures of compounds 7–15.
In addition, peniscmeroterpenoid N (20) was isolated from the marine-derived fungus Penicillium sclerotiorum GZU-XW03-2 [], which was obtained from the intestinal tract of an Onchidium sp. collected from Xuwen in Guangdong province, China. The fungus Penicillium purpurogenum obtained from rotting fruit of the tree Averrhoa bilimbi growing in Sri Lanka yielded four new meroterpenoids, named dhilirolides F–I (21–24) []. In addition, 3,16-epoxy-preaustinoid D (25) is a rare austin meroterpenoid analogue with an open A ring that also features an undescribed oxygen bridge between C-3 and C-16 to construct an unexpected tetrahydrofuran ring; this was isolated and characterized from the fungus Aspergillus calidoustus [].
Furthermore, two new meroterpenoids, namely, 1-methoxy-hydropreaustinoid A1 (26) and hydroberkeleyone B (27), have been isolated through the aid of Liquid Chromatograph Mass Spectrometer (LC-MS) from the sponge-derived fungus Eupenicillium sp. 6A-9, and both of them have immune-suppressive activities with IC50 values of 42.3 and 28.5 μM, respectively []. Peniscmeroterpenoids F (28) and G (29) were also isolated from the marine-derived fungus Penicillium sclerotiorum GZU-XW03-2 []. SSW03M2 GY was isolated (4S, 5S, 7R, 9S, 11R, 12S)-1-methoxyberkeleyone C (30) from a Penicillium sp. that was derived from sediment at Seosan bay, South Korea; compound 30 showed anti-virulence activity by significantly inhibiting α-toxin (Hla) secreted by methicillin-resistant Staphylococcus aureus without growth inhibition at 10 μg/mL []. Peniscmeroterpenoids K–M (31–33) were also isolated from the marine-derived fungus Penicillium sclerotiorum GZU-XW03-2. Specifically, peniscmeroterpenoid K (31) was the first isolate where the C-24 was oxidized, and peniscmeroterpenoid M (33) (Figure 3) inhibited the production of nitric oxide (NO) in RAW264.7 cells with an IC50 value of 48.04 ± 2.51 μM [].
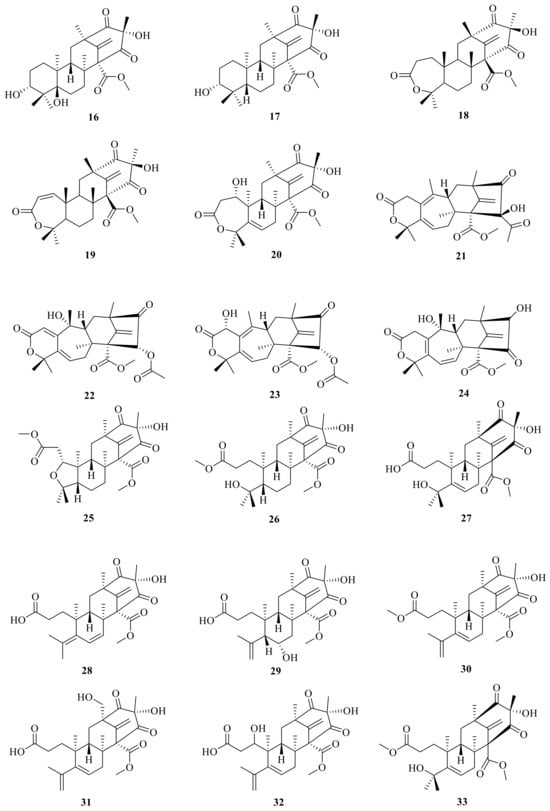
Figure 3.
Chemical structures of compounds 16–33.
Preaustinoid D (34) was isolated from the endophytic fungus Penicillium sp. T2-8 associated with Gastrodia elata and showed moderate activity against Candida albicans with an MIC of 128 μg/mL []. Bioinformatics analysis of the gene clusters in association with the qRT-PCR detection revealed the amplification of two key genes (clusters A and B) for the sponge-associated fungus Penicillium brasilianum WZXY-m122-9. Chromatographic separation of the EtOAc extract from the large-scale fermentation of this fungal strain resulted in the isolation of two new ATMTs, namely, brasilianoids D and E (35–36) []. The marine-derived fungal isolate Penicillium sp. SF-5497 resulted in the isolation of two new meroterpenoids, named preaustinoid A6 (37) and preaustinoid A7 (38); furthermore, preaustinoid A6 (37) (Figure 4) inhibited protein tyrosine phosphatase-1B (PTP1B) in a noncompetitive manner, with a Ki value of 17.0 µM [].
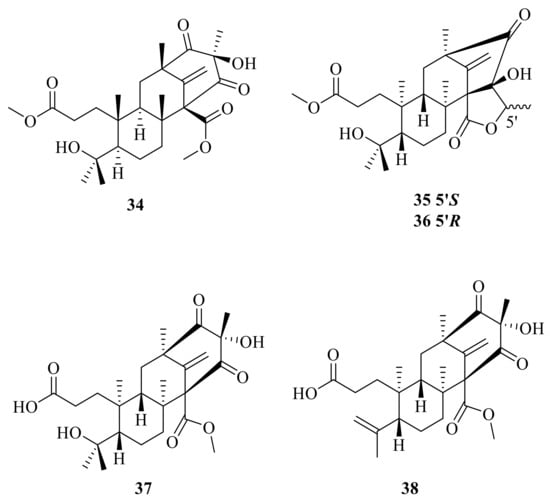
Figure 4.
Chemical structures of compounds 34–38.
2.2. Pentacyclic Systems Austin-Type Meroterpenoids
2.2.1. Pentacyclic Systems-Rings A and B Are Spirocyclic—Typical Austin-Type Meroterpenoids
Austin (39) (Figure 5) is a novel ATMT isolated from a strain of Aspergillus ustus that was found on stored black-eyed peas (Vigna sinensis). Austin (39) showed the gross signs of toxicity in cockerels, such as a general listlessness followed either by eventual improvement (250 mg/kg, oral) or ataxia and death (375 mg/kg, oral) [] (Figure 5). Austinol (40) and isoaustin (42) were isolated from an A. ustus strain []. Austinolide (41) and isoaustinone (44) are additional ATMTs produced by the rice cultures of Penicillium sp. []. Moreover, chemical examination of Aspergillus nidulans resulted the isolation of 11 β-hydroxyisoaustinone (43), (5′R)-isoaustinone (45), and neosuatinone (47) [].
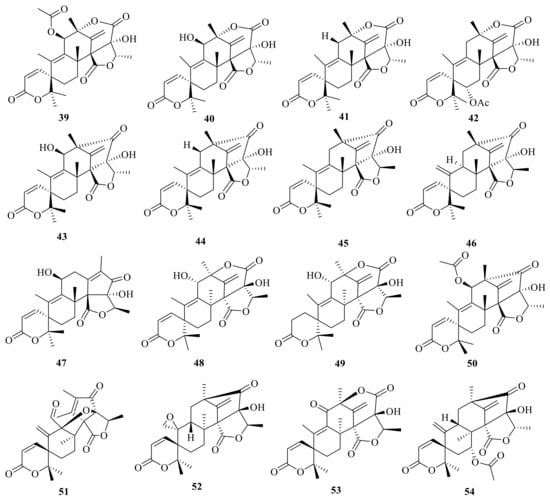
Figure 5.
Chemical structures of compounds 39–54.
Neoaustin (46) was obtained from a soil isolate of Penicillium sp. MG-11 []. Moreover, ED-2 (48) was isolated from a strain of Emericella nidulans var. dentata, and ED-2 (48) was hydrogenated with H2/PtO2 and afforded the crystalline dihydro-derivative ED-2H (49) []. In addition, 11β-acetoxyisoaustinone (50) was isolated from the seagrass-derived fungus Pestalotiopsis sp. PSU-ES194 []. In the same year, 11β-acetoxyisoaustinone (50) was also isolated from Penicillium sp., an endophytic fungus obtained from Dysosma versipellis []. Bioinformatics analysis of the synthetic gene clusters in association with the qRT-PCR detection of the marine-derived Penicillium brasilianum WZXY-m122-9 isolate yielded four new ATMTs, namely brasilianoids G–I (51–53) and brasilianoid L (54) (Figure 5). Compound 54 showed cytotoxicity activity against RAW264.7, IEC-6, and A549 with IC50 values of 84.67, 2.52 and 180.5 μg/mL, respectively [].
Moreover, asperaustins A–C (55–57) were isolated from a marine-derived Aspergillus sp. Compound 55 possesses an unusual spiro[4.5]deca-3,6-dien-2-one moiety with a unique 5/6/6/6/5 pentacyclic skeleton [] (Figure 6). Under the guidance of MS/MS-based molecular networking, 6-hydroxyisoaustinone (58) and 6-ketoisoaustinone (59) were isolated from the Penicillium sp. GDGJ-285 []. Penicianstinoid E (60), an unusual austin-type meroterpenoid that possesses a 6/5/6/6/6/5 fused hexacyclic skeleton with an uncommon five-membered ether ring system, was obtained from Penicillium sp. TGM112. Compound 60 showed inhibitory activity against newly hatched larvae of Helicoverpa armigera Hubner with an IC50 value of 200 μg/mL []. Asperanstinoid A was obtained from the fungus A. calidoustus and has the same structure as penicianstinoid E (60) [].
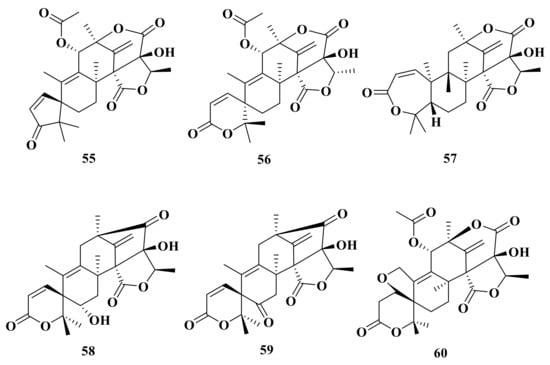
Figure 6.
Chemical structures of compounds 55–60.
2.2.2. Pentacyclic Systems-Rings A and B Are Bicyclic Fused
Dhilirolide D (61), a member of the family of secondary metabolites with a putative meroterpenoid biogenetic origin and unprecedented dhilirane and isodhilirane carbon skeletons, was isolated from a laboratory culture of P. purpurogenum collected in Sri Lanka [] (Figure 7). In addition, continued chemical investigation of laboratory cultures of P. purpurogenum yielded three new ATMTs, named dhilirolide E (62), dhilirolide K (63), and dhilirolide M (64) [].
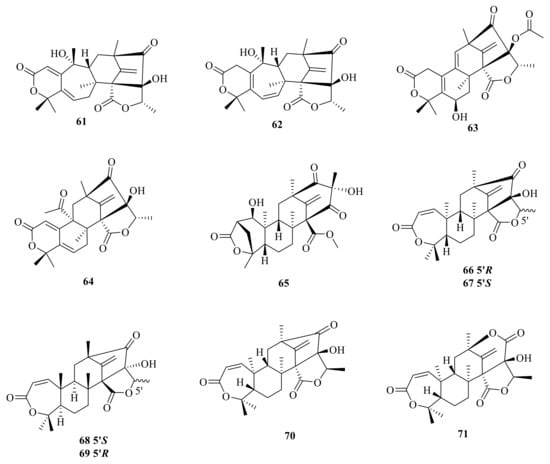
Figure 7.
Chemical structures of compounds 61–71.
Chromatographic separation of the EtOAc extract of the large-scale fermentation of the sponge-associated P. brasilianum WZXY-m122-9 resulted in the isolation of three new ATMTs named brasilianoids A–C (65–67). Compound 65 showed significantly stimulated the expression of filaggrin and caspase-14 in HaCaT cells in dose-dependent manner; moreover, compounds 66 and 67 showed moderate inhibition against NO production in LPS-induced RAW 264.7 macrophages with IC50 values of 37.69 ± 5.25 μM and 33.76 ± 3.13 μM, respectively [] (Figure 7). Preaustinoids E–F (68–69) were isolated from the culture broth of a Penicillium sp. fungus collected from Chuja-do, Korea []. Asperaustin C (70) was isolated from a marine-derived Aspergillus sp. []. In addition, a new ATMT brasilianoid K (71) was isolated from the marine-derived fungus P. brasilianum WZXY-m122-9 [].
2.3. Hexacyclic Systems Austin-Type Meroterpenoids
2.3.1. Hexacyclic Systems-Rings A and B Are Spirocyclic
A new ATMT isoaustinone (72) was isolated from a strain of Aspergillus variecolor, and it inhibited the growth of newly third-instar larvae of Aedes aegypti with an LC50 value of 2.9 ppm [] (Figure 8). Dehydroaustin (73) and dehydroaustinol (74) were isolated from a soil fungus of Penicillium sp. MG-11. Compound 74 showed antimicrobial activity against Escherichia coli with an MIC value of 250 μg/mL and inhibited the growth of newly third-instar larvae of Aedes aegypti with an LC50 value of 7.3 ppm []. ED-1 (75) was isolated from a strain of Aspergillus ustus, and ED-1 (75) was hydrogenated with H2/PtO2 and afforded a crystalline dihydro derivative ED-1H (76) []. Acetoxydehydroaustin B′ (77) and 1,2-dihydro-acetoxydehydroaustin B′ (78) were isolated as a mixed crystal from the mangrove endophytic fungus Aspergillus sp. 085241B []. Furthermore, PF1364 (79) was isolated from Aspergillus sp. PF1364 and showed the control of pests and insects, such as the greenhouse whitefly [].
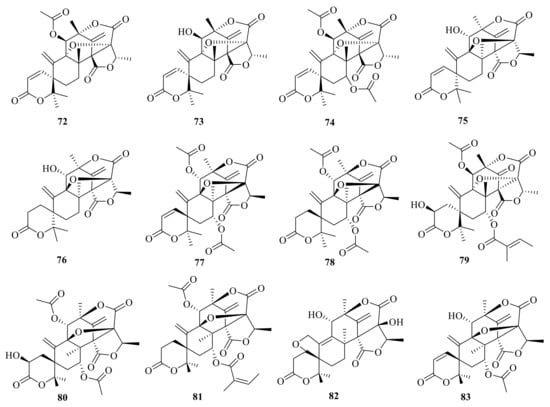
Figure 8.
Chemical structures of compounds 72–83.
2-Hydroacetoxydehydroaustin (80) was isolated from the mangrove endophytic fungus Aspergillus sp. 16-5c [] (Figure 8). In addition, 1,2-dehydro-terredehydroaustin (81) was isolated from the mangrove endophytic fungus Aspergillus terreus and inhibited nitric oxide (NO) production with an IC50 value of 42.3 μM []. Furanoaustinol (82) and 7-acetoxydehydroaustinol (83) (Figure 8) were isolated from the ethyl acetate extract of the marine-derived fungal strain Penicillium sp. SF-5497. Compound 82 weakly inhibited the activity of protein tyrosine phosphatase 1B in a dose-dependent manner with an IC50 value of 77.2 μM. In addition, compound 83 weakly suppressed the overproduction of nitric oxide in lipopolysaccharide-challenged BV2 microglial cells with an IC50 value of 61.0 μM [].
Asperaustins A–B (84–85) (Figure 9) were isolated from a marine-derived Aspergillus sp. []. Brasilianoid J (86) was isolated from the marine-derived fungus P. brasilianum WZXY-m122-9 [] (Figure 9). Penicianstinoids A–B (87-88) were obtained from the mangrove-derived fungus Penicillium sp. TGM112, and compounds 87 and 88 showed growth inhibition activity against newly hatched larvae of Helicoverpa armigera Hubner with IC50 values of 200 μg/mL. Compounds 87 and 88 displayed activity against Caenorhabditis elegans with EC50 values ranging from 9.4 ± 1.0 μg/mL to 38.2 ± 0.6 μg/mL []. In addition, 7-hydroxydehydroaustin (89) was isolated from the seagrass-derived fungus Pestalotiopsis sp. PSU-ES194 []. Austinone (90) was isolated from Penicillium sp. Y-5-2 [].
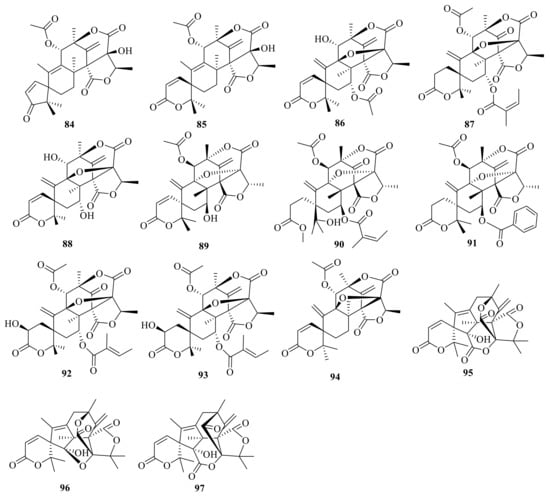
Figure 9.
Chemical structures of compounds 84–97.
Ustusaustin A (91) was isolated and identified from a culture extract of Aspergillus ustus TK-5, which obtained from the inner tissue of the ascidian Pyuramomus []. Three 3,5-dimethylorsellinic acid (DMOA)-based meroterpenoids, namely asperanstinoids B–D (92–94) (Figure 9), were obtained from the fungus Aspergillus calidoustus, which was isolated from wetland soil collected at Dianchi Lake []. Under the guidance of MS/MS-based molecular networking, three novel skeleton meroterpenoids, peniclactones A–C (95–97) (Figure 9), were isolated from Penicillium sp. GDGJ-285 []. Peniclactones A–C (95–97) represent the first meroterpenoids featuring a 6/5/6/6/5/6 (95), 6/5/6/6/5/5 (96), and 6/5/6/5/5/6 (97) hexacyclic ring systems, respectively. Bioassays showed that 97 inhibited nitric oxide production in lipopolysaccharide-induced RAW 264.7 macrophage cells with an IC50 value of 39.03 μM.
2.3.2. Hexacyclic Systems-Rings A and B Are Bicyclic Fused
Dhilirolide L (98) and dhilirolide N (99) were isolated from P. purpurogenum, which was obtained from rotting fruit of a Averrhoa bilimbi tree growing in Sri Lanka. Compound 98 showed significant feeding inhibition and sublethal developmental disruption in the cabbage looper Trichoplusiani with a DC50 value of 5.9 μg/cm2 [] (Figure 10). Brasilianoid F (100) was obtained from the large-scale fermentation of P. brasilianum WZXY-m122-9 [].
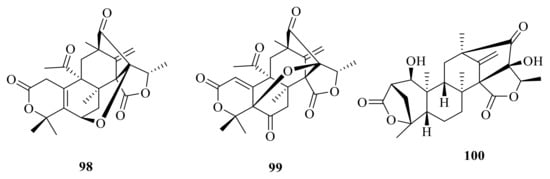
Figure 10.
Chemical structures of compounds 98–100.
2.4. Heptacyclic Systems Austin-Type Meroterpenoids
Heptacyclic Systems-Rings A and B Are Bicyclic Fused
Dhilirolides A–C (101–103) were isolated from laboratory cultures of the fruit-infecting fungus P. purpurogenum collected in Sri Lanka [] (Figure 11). The fungus P. purpurogenum obtained from rotting fruit of a A. bilimbi tree growing in Sri Lanka yielded one new austin-type meroterpenoid, named dhilirolide J (104) [].
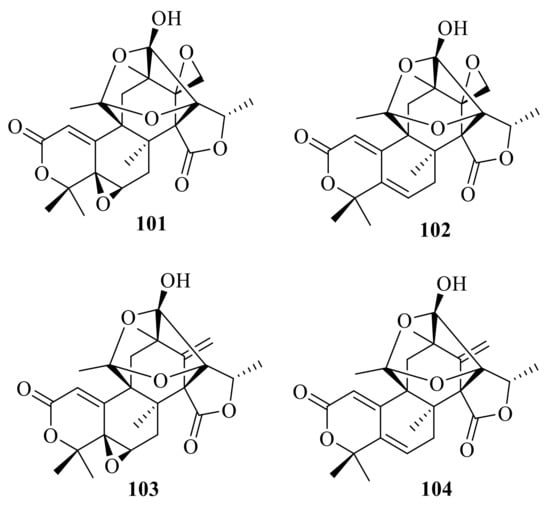
Figure 11.
Chemical structures of compounds 101–104.
3. Comprehensive Overview and Conclusions
To the best of our knowledge, investigations on the chemical constituents of ATMTs in the last five decades have reported fungal biodiversity, and total of 104 novel ATMTs from 5 genera of terrestrial- and marine-derived fungi have been reported in 40 research papers published from October 1976 to January 2023 (Table 1, Figure 12, Figure 13, Figure 14 and Figure 15). In total, 37% of the compounds are categorized as the tetracyclic type (up to 38 compounds) followed by pentacyclic (32%, 33), hexacyclic (27%, 29), and heptacyclic (4%, 4) types (Figure 12). This review summarizes the source, chemistry, and biological activities of the novel ATMTs. The majority of the ATMTs have been isolated since 2010, accounting for about 80% of all reported ATMTs (83/104) and 75% of the published articles (Figure 13). The increase was largely due to improvements in isolation procedures. All of the published ATMTs that have been isolated and identified in various filamentous fungi, most of them were produced by Penicillium (62%) and Aspergillus (30%), representing more than 90% of the secondary metabolites reported (Figure 14). The remaining (about 10%) were produced by Pestalotiopsis (2%), Eupenicillium (2%), and Emericella (4%).

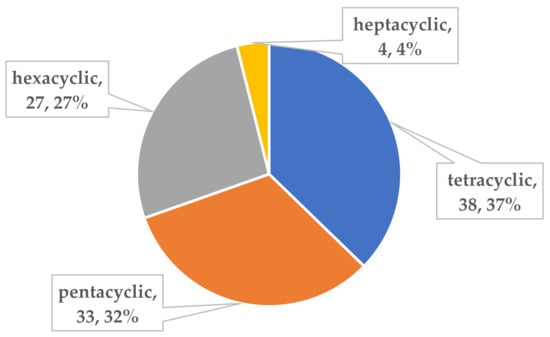
Figure 12.
Classification of all ATMTs compounds.
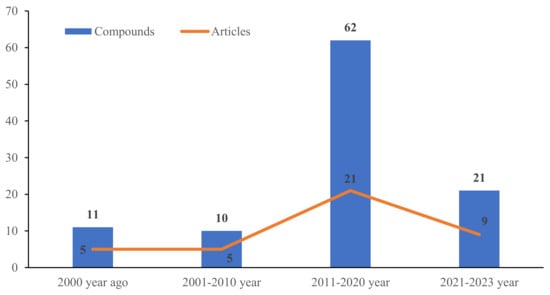
Figure 13.
The production of austin-type meroterpenoids and published articles per decade.
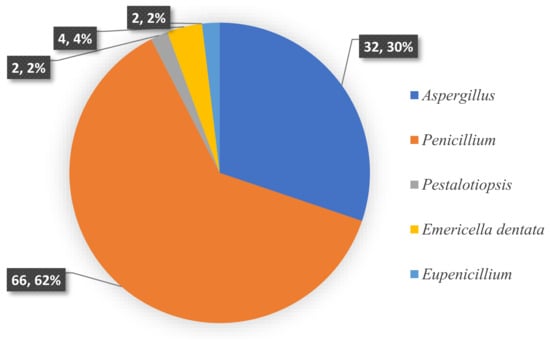
Figure 14.
Fungal species distribution of isolated austin-type meroterpenoids.
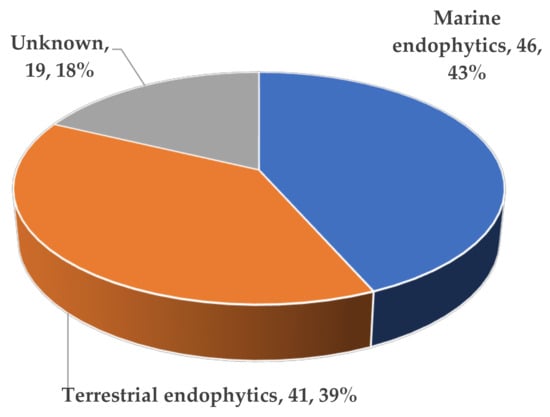
Figure 15.
Fungal producers of austin-type meroterpenoids.
In terms of the source distribution of compounds, one surprising discovery was that all of these novel ATMTs were isolated from fungi, including 39% terrestrial- and 43% marine-derived endophytic fungi, much more than those produced by unknown sources (18%) (Figure 15). The results showed that these kinds of ATMTs are a very special class of fungi metabolites, and more chemical ecological research needs to be carried out. Overall, this review provides a comprehensive overview of the diverse chemical structures and bioactive properties of 104 new ATMTs that have been isolated from fungi in the last five decades. Nearly 26% of the ATMTs showed bioactivities. About 7% exhibited inhibitory effects on NO production. Interestingly, about 9% ATMTs have been showed to possess selective insecticidal activity, about 2% exhibit antimicrobial activity, and 8% demonstrate other activities, including cytotoxicity and antityrosinase activity (Figure 16), revealing the untapped potential of activity ATMTs in pesticides and medicinal applications. However, for most of the isolated ATMTs, the lack of activity analysis and pharmacodynamic evaluation limit their application.
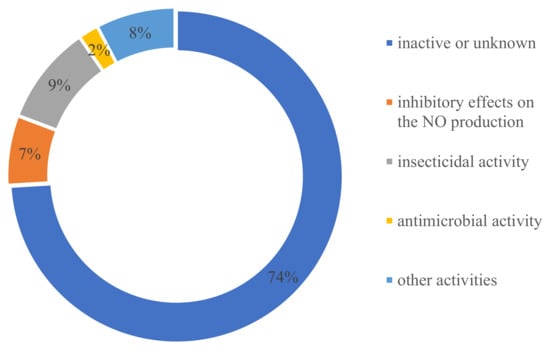
Figure 16.
Bioactivity distribution of austin-type meroterpenoids.
In summary, a total 104 of ATMTs have been isolated and characterized to date since the initial discovery of austin-type meroterpenoids in 1976. Although ATMTs have a distinct a unique chemical skeleton and potential biological activities, the unavailability of large amounts of natural austin-type meroterpenoids and purification challenges due to their structural complexity hindering efficient chemical synthesis have hindered further research. Most of the ATMTs with novel skeletons and biological activities have been discovered in recent years. The further development and application of these compounds is important, and the identification of promising lead compounds for the development of drugs is a critical future direction of study.
Author Contributions
Conceptualization, M.B. and W.-F.Z.; methodology, J.-L.H. and C.-J.C.; validation, Y.-H.L., C.-H.G. and R.-P.W.; data curation, J.-L.H. and C.-J.C.; writing—original draft preparation, J.-L.H. and M.B.; writing—review and editing, M.B.; supervision, W.-F.Z.; funding acquisition, M.B. and W.-F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Guangxi (No. 2023GXNSFAA026313 and 2021GXNSFBA220072), Hainan Province Science and Technology Special Fund (No. ZDKJ2021035), the Innovation Platform for Academicians of Hainan Province (YSPTZX202130), National Natural Science Foundation of China (Nos. 32260041 and 31960012), Hainan Provincial Natural Science Foundation of China (No. 321RC545), and Hainan Normal University graduate research innovation research project (No. hsyx2022-59).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, J.P.; Wu, Y.F.; Yuan, B.C.; Liu, D.; Zhu, K.; Huang, J.; Proksch, P.; Lin, W.H. DMOA-based meroterpenoids with diverse scaffolds from the sponge-associated fungus Penicillium brasilianum. Tetrahedron 2019, 75, 2193–2205. [Google Scholar] [CrossRef]
- Wen, H.L.; Yang, X.L.; Liu, Q.; Li, S.J.; Li, Q.; Zang, Y.; Chen, C.M.; Wang, J.P.; Zhu, H.C.; Zhang, Y.H. Structurally diverse meroterpenoids from a marine-derived Aspergillus sp. Fungus. J. Nat. Prod. 2020, 83, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Chexal, K.K.; Springer, J.P.; Clardy, J.; Cole, R.J.; Kirksey, J.W.; Dorner, J.W.; Cutler, B.J.; Strawter, B.J. Austin, a novel polyisoprenoid mycotoxin from Aspergillus ustus. J. Am. Chem. Soc. 1976, 98, 6748–6750. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, B.T.M.; Sallum, W.S.T.; Takahashi, J.A. Austin, dehydroaustin and other metabolites from Penicillium brasilianum. Quim. Nova 2010, 33, 1044–1046. [Google Scholar] [CrossRef]
- Zhang, G.J.; Sun, S.W.; Zhu, T.J.; Lin, Z.J.; Gu, J.Y.; Li, D.H.; Gu, Q.Q. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry 2011, 72, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Katsube, Y.; Ishido, H.; Yamazaki, M.; Maebayashi, Y. The absolute configuration of desacetylaustin isolated from Emericella nidulans var. dentate. Chem. Pharm. Bull. 1980, 28, 2270–2271. [Google Scholar] [CrossRef]
- Simpson, T.J.; Stenzel, D.J.; Bartlett, A.J.; Brien, E.O.; Holker, J.S.E. Studies on fungal metabolites. Part 3. 13C NMR spectral and structural studies on austin and new related meroterpenoids from Aspergillus ustus, Aspergillus variecolor, and Penicillium diversum. J. Chem. Soc. Perkin Trans. 1982, 1, 2687–2692. [Google Scholar] [CrossRef]
- Park, J.S.; Quang, T.H.; Yoon, C.S.; Kim, H.J.; Sohn, J.H.; Oh, H. Furanoaustinol and 7-acetoxydehydroaustinol: New meroterpenoids from a marine-derived fungal strain penicillium sp. sf-5497. J. Antibiot. 2018, 71, 557–563. [Google Scholar] [CrossRef]
- Geris, R.; Rodrigues-Fo, E.; Silva, H.H.G.; Silva, I.G. Larvicidal effects of fungal meroterpenoids in the control of Aedes aegypti L., the main vector of dengue and yellow fever. Chem. Biodiver. 2010, 5, 341–345. [Google Scholar] [CrossRef]
- Stierle, D.B.; Stierle, A.A.; Patacini, B.; Mcintyre, K.; Girtsman, T.; Bolstad, E. Berkeleyones and related meroterpenes from a deep water acidmine waste fungus that inhibit the production of interleukin 1-β from induced inflammasomes. J. Nat. Prod. 2011, 74, 2273–2277. [Google Scholar] [CrossRef]
- Qi, B.; Liu, T.; Mo, T.; Zhu, Z.; Li, J.; Wang, J.; Shi, X.; Zeng, K.; Wang, X.; Tu, P.; et al. 3,5-dimethylorsellinic acid derived meroterpenoids from Penicillium chrysogenum MT-12, an endophytic fungus isolated from Huperzia serrate. J. Nat. Prod. 2017, 80, 2699–2707. [Google Scholar] [CrossRef]
- Centko, R.M.; Williams, D.E.; Patrick, B.O.; Akhtar, Y.; Chavez, M.A.G.; Wang, Y.A.; Isman, M.; Silva, E.D.; Andersen, R.J. Dhilirolides E–N, meroterpenoids produced in culture by the fungus Penicillium purpurogenum collected in Sri Lanka: Structure elucidation, stable isotope feeding studies, and insecticidal activity. J. Org. Chem. 2014, 79, 3327–3335. [Google Scholar] [CrossRef]
- Silva, E.D.; Williams, D.E.; Jayanetti, D.R.; Centko, R.M.; Patrick, B.O.; Wijesunder, R.L.C.; Andersen, R.J. Dhilirolides A-D, meroterpenoids produced in culture by the fruit-infecting fungus Penicillium purpurogenum collected in Sri Lanka. Org. Lett. 2011, 13, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Furutani, S.; Ihara, M.; Ling, Y.; Yang, X.; Kai, K.; Hayashi, H.; Matsuda, K. Meroterpenoid chrodrimanins are selective and potent blockers of insect GABA-gated chloride channels. PLoS ONE 2015, 10, e0122629. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zheng, C.J.; Huang, G.L.; Mei, R.Q.; Wang, B.; Luo, Y.P.; Zheng, C.; Niu, Z.G.; Chen, G.Y. Bioactive meroterpenoids and isocoumarins from the mangrove-derived fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Valiante, V.; Mattern, D.J.; Schueffler, A.; Horn, F.; Walther, G.; Scherlach, K.; Petzke, L.; Dickhaut, J.; Guthke, R.; Hertweck, C.; et al. Discovery of an extended austinoid biosynthetic pathway in Aspergillus calidoustus. ACS Chem. Biol. 2017, 12, 1227–1234. [Google Scholar] [CrossRef]
- Lo, H.C.; Entwistle, R.; Guo, C.J.; Ahuja, M.; Szewczyk, E.; Hung, J.H.; Chiang, Y.M.; Oakley, B.R.; Wang, C.C. Two separate gene clusters encode the biosynthetic pathway for the meroterpenoids austinol and dehydroaustinol in Aspergillus nidulans. J. Am. Chem. Soc. 2012, 134, 4709–4720. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Awakawa, T.; Wakimoto, T.; Abe, I. Spiro-ring formation is catalyzed by a multifunctional dioxygenase in austinol biosynthesis. J. Am. Chem. Soc. 2013, 135, 10962–10965. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Iwabuchi, T.; Fujimoto, T.; Awakawa, T.; Nakashima, Y.; Mori, T.; Zhang, H.P.; Hayashi, F.; Abe, I. Discovery of key dioxygenases that diverged the paraherquonin and acetoxydehydroaustin pathways in Penicillium brasilianum. J. Am. Chem. Soc. 2016, 138, 12677–12761. [Google Scholar] [CrossRef]
- Mattern, D.J.; Valiante, V.; Horn, F.; Petzke, L.; Brakhage, A.A. Rewiring of the austinoid biosynthetic pathway in filamentous fungi. ACS Chem. Biol. 2017, 12, 2927–2933. [Google Scholar] [CrossRef]
- Dos Santos, R.M.G.; Rodrigues-Filho, E. Structures of meroterpenes produced by Penicillium sp., an endophytic fungus found associated with Melia azedarach. J. Braz. Chem. Soc. 2003, 14, 722–727. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Chen, G.Y. Austins-type meroterpenoids from a mangrove-derived Penicillium sp. J. Nat. Prod. 2021, 84, 2104–2110. [Google Scholar] [CrossRef]
- Dos Santos, R.M.G.; Rodrigues-Fo, E. Meroterpenes from Penicillium sp. found in association with Melia azedarach. Phytochemistry 2002, 61, 907–912. [Google Scholar] [CrossRef]
- Mo, S.Y.; Yin, J.; Ye, Z.; Li, F.L.; Lin, S.; Zhang, S.T.; Yang, B.Y.; Yao, J.; Wang, J.P.; Hu, Z.X.; et al. Asperanstinoids A–E: Undescribed 3,5-dimethylorsellinic acid-based meroterpenoids from Aspergillus calidoustus. Phytochemistry 2021, 190, 112892. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.M.G.; Rodrigues-Fo, E.Z. Further meroterpenes produced by Penicillium sp. an endophyte obtained from Melia azedarach. Z. Naturforsch. 2003, 58, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Fill, T.P.; Pereira, G.K.; Dos Santos, R.M.G.; Rodrigues-Fo, E. Four additional meroterpenes produced by Penicillium sp. found in association with Melia azedarach. possible biosynthetic intermediates to austin. Z. Naturforsch. 2007, 62, 1035–1044. [Google Scholar] [CrossRef]
- Orfali, R.; Perveen, S.; Peng, J.N.; Alqahtani, A.S.; Nasr, F.A.; Ahmed, M.Z.; Luciano, P.; Chianese, G.; Al-Taweel, A.M.; Taglialatela-Scafati, O. Penicillactonin and preaustinoid C, lactone-containing metabolites from a hot spring sediment Penicillium sp. Fitoterapia 2022, 163, 105330. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Chen, J.; Pan, W.C.; Tan, S.L.; Cui, H.; Zhao, Z.X. Seven new meroterpenoids from the fungus Penicillium sclerotiorum GZU-XW03-2. Fitoterapia 2023, 165, 105428. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Mo, S.Y.; Yin, J.; Zhang, S.T.; Gu, S.S.; Ye, Z.; Wang, J.P.; Hu, Z.X.; Zhang, Y.H. Structurally diverse metabolites from a soil-derived fungus Aspergillus calidoustus. Bioorg. Chem. 2022, 127, 105988. [Google Scholar] [CrossRef]
- Gu, B.B.; Wu, W.; Liu, L.Y.; Tang, J.; Zeng, Y.J.; Wang, S.P.; Sun, F.; Li, L.; Yang, F.; Lin, H.W. 3,5-dimethylorsellinic acid derived meroterpenoids from Eupenicillium sp. 6A-9, a fungus isolated from the marine sponge Plakortis simplex. Eur. J. Org. Chem. 2018, 1, 48–59. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X.C.; Pan, W.C.; Liu, X.; Tan, S.L.; Cui, H.; Zhao, Z.X. Meroterpenoids from the fungus Penicillium sclerotiorum GZU-XW03-2 and their anti-inflammatory activity. Phytochemistry 2022, 202, 113307. [Google Scholar] [CrossRef]
- Ku, H.; Lee, Y.; Lee, S.; Lee, J.W.; Kang, H.S.; Joo, H.S.; Shim, S.H. New meroterpenoids from a soil-derived fungus Penicillium sp. SSW03M2 GY and their anti-virulence activity. J. Antibiot. 2023, 76, 57–64. [Google Scholar] [CrossRef]
- Duan, R.T.; Zhou, H.; Yang, Y.B.; Li, H.T.; Dong, J.W.; Li, X.Z.; Chen, G.Y.; Zhao, L.X.; Ding, Z.T. Antimicrobial meroterpenoids from the endophytic fungus penicillium sp. T2-8 associated with Gastrodia elata. Phytochem. Lett. 2016, 18, 197–201. [Google Scholar] [CrossRef]
- Zhang, J.P.; Yuan, B.C.; Liu, D.; Gao, S.; Prokschm, P.; Lin, W.H. Brasilianoids A–F, new meroterpenoids from the sponge-associated fungus Penicillium brasilianum. Front. Chem. 2018, 6, 314. [Google Scholar] [CrossRef]
- Park, J.S.; Quang, T.H.; Nguyen, T.; Sohn, J.H.; Oh, H. New preaustinoids from a marine-derived fungal strain Penicillium sp. SF-5497 and their inhibitory effects against PTP1B activity. J. Antibiot. 2019, 76, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Mukaihara, M.; Murao, S.; Arai, M.; Lee, A.L.; Clardy, J. Acetoxydehydroaustin, a new bioactive compound, and related compound neoaustin from Penicillium sp. MG-11. Biosci. Biotechnol. Biochem. 1994, 58, 334–338. [Google Scholar] [CrossRef]
- Arunpanichlert, J.; Rukachaisirikul, V.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. Meroterpenoid, isocoumarin and phenol derivatives from the seagrass-derived fungus Pestalotiopsis sp. PSU-ES194. Tetrahedron 2015, 71, 882–888. [Google Scholar] [CrossRef]
- Li, J.W.; Duan, R.G.; Zou, J.H.; Chen, R.D.; Dai, J.G. Meroterpenoids and isoberkedienolactone from endophytic fungus Penicillium sp. associated with Dysosma versipellis. Acta Pharm. Sin. B 2014, 49, 913–920. [Google Scholar]
- Mo, T.X.; Huang, X.S.; Zhang, W.X.; Schäberle, T.F.; Qin, J.K.; Zhou, D.X.; Qin, X.Y.; Xu, Z.L.; Li, J.; Yang, R.Y. A series of meroterpenoids with rearranged skeletons from an endophytic fungus Penicillium sp. GDGJ-285. Org. Chem. Front. 2021, 8, 2232–2241. [Google Scholar] [CrossRef]
- Hwang, J.Y.; You, M.J.; Oh, O.C.; Oh, K.B.; Shin, J.H. New meroterpenoids from a Penicillium sp. Fungus. Nat. Prod. Sci. 2018, 24, 253–258. [Google Scholar] [CrossRef]
- Maebayashi, Y.K.; Okuyama, E.; Yamazaki, M.K.; Katsube, Y.K. Structure of ED-1 isolated from Emericella dentate. Chem. Pharm. Bull. 1982, 30, 1911–1912. [Google Scholar] [CrossRef]
- Song, Y.X.; Qiao, L.T.; Wang, J.J.; Zeng, H.M.; She, Z.G.; Miao, C.D.; Hong, K.; Gu, Y.C.; Liu, L.; Lin, Y.C. New meroterpenes from the mangrove endophytic fungus Aspergillus sp. 085241B. Helv. Chim. Acta 2011, 94, 1875–1880. [Google Scholar] [CrossRef]
- Akira, H.; Mariko, T.; Takeshi, T.; Kazuhiko, O.; Masaaki, M. PF1364 of Aspergilus for use as horticultural and agriculture pesticide (or insecticide). Jpn. Kokai Tokkyo Koho 2010, JA 2010018586 A 20100128. [Google Scholar]
- Long, Y.H.; Hui, C.; Liu, X.L.; Xiao, Z.E.; Wen, S.T.; She, Z.G.; Huang, X.S. Acetylcholinesterase inhibitory meroterpenoid from a mangrove endophytic fungus Aspergillus sp. 16-5c. Molecules 2017, 22, 727. [Google Scholar] [CrossRef]
- Liu, Z.M.; Liu, H.J.; Chen, Y.; She, Z.G. A new anti-inflammatory meroterpenoid from the fungus Aspergillus terreus H010. Nat. Prod. Res. 2018, 32, 2652–2656. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.P.; Shi, Y.T.; Auckloo, B.N.; Hassan, S.S.; Akhter, N.; Wang, K.W.; Ye, Y.; Chen, S.H.; Tao, X.Y.; Wu, B. Isolation and antibiotic screening of fungi from a hydrothermal vent site and characterization of secondary metabolites from a Penicillium isolate. Mar. Biotechnol. 2017, 19, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, L.; Li, X.M.; Li, H.L.; Konuklugil, B.; Wang, B.G. Ustusaustin A: A new neuraminidase inhibitory meroterpene from the ascidian-derived endophytic fungus Aspergillus ustus TK-5. Nat. Prod. Res. 2021, 35, 4939–4944. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).