Abstract
Boletaceae, the largest and most diverse family of Boletales (Agaricomycetes and Basidiomycota), is both ecologically and economically important. Although many taxa have been described in China, the diversity of the family still remains incompletely understood. In the present study, Pseudophylloporus baishanzuensis gen. nov., sp. nov. and Rubroleccinum latisporus gen. nov., sp. nov. are proposed based on morphological and molecular phylogenetic analyses. These findings contribute to a deeper understanding of the diversity within the Boletaceae family.
1. Introduction
Boletaceae Chevall., the largest and most diverse family in Boletales (Agaricomycetes and Basidiomycota), has been in the spotlight in mycology [1,2]. With the rapid development of contemporary morphology and molecular phylogenetics, the comprehension of Boletaceae has significantly improved, leading to the discovery of numerous new taxa, especially in Asian and American regions [3,4,5,6,7,8,9,10,11,12,13,14]. Recently, Boletaceae has been divided into eight subfamilies, viz., Austroboletoideae G. Wu & Zhu L. Yang, Boletoideae Singer, Chalciporoideae G. Wu & Zhu L. Yang, Leccinoideae G. Wu & Zhu L. Yang, Phylloboletelloideae Dentinger, Tremble, Halling, T.W. Henkel & Moncalvo, Suillelloideae Dentinger, Tremble, Halling, T.W. Henkel & Moncalvo, Xerocomoideae Singer, and Zangioideae G. Wu, Y. C. Li & Zhu L. Yang [3,15,16,17]. Among these subfamilies, approximately 100 genera and 1200 species have been reported [3,4,14,15,16].
In China, people pay much attention to the family Boletaceae, for a significant number of species possess edibility and medicinal values, leading to considerable economic benefits [3,4,14,15]. Moreover, most species establish ectomycorrhizal symbiotic associations with host plants, including Fagaceae, Pinaceae, Dipterocarpaceae, and Myrtaceae, playing an important role in upholding the diversity and homeostasis of forest ecosystems [1,14,18,19,20,21,22,23]. In the subtropical regions of China, there are considerable forests dominated by Fagaceae trees, which provide good habitats for the growth and reproduction of boletes [3,14,24,25]. Although numerous boletes have been revealed in subtropical China [3,8,15,20], there are still many taxa awaiting to be uncovered. Recently, several collections of Boletaceae were made from the region (Zhejiang and Fujian Provinces); further morphology and molecular phylogenetic analyses confirm that these collections represent two novel genera. They are described in an effort to further demonstrate the diversity of Boletaceae in China.
2. Materials and Methods
2.1. Morphological Studies
The studied specimens were collected from Zhejiang and Fujian Provinces in China, then dried at 50–60 °C for 12 h and deposited in the Fungal Herbarium of Hainan Medical University (FHMU), Haikou City, Hainan Province of China. Field records and digital photographs of fresh specimens were made. Color documentation of fresh materials followed Kornerup and Wanscher [26]. Micromorphological features were observed and measured by 5% KOH solution or stained with 1% Congo Red. Sections of the pileipellis taken from the pileus between the center and margin, and sections of the stipitipellis were taken from the middle part along the longitudinal axis of the stipe [27,28]. All line drawings of microstructures were drawn by freehand. Basidiospores of dried specimens were examined with a JSM-7100F field emission scanning electron microscope (Tokyo, Japan). The number of measured basidiospores is given as n/m/p, where “n” represents the total number of basidiospores measured from “m” basidiomata of “p” collections. Dimensions of basidiospores were presented in the form (a–) b–e–c (–d), where the range b–c contains at least of 90% of the measured values (5th to 95th percentile), “a” and “d” were the extreme values, and “e” refers to the average length/width of basidiospores. Q refers to the length/width ratio of basidiospores; Qm refers to the average Q of basidiospores and is given with a standard deviation [29,30]. The size of the basidiospore was analyzed using SPSS Statistics Version 17.0 [31]. The terms referring to the size of the basidioma were based on Bas [32].
2.2. Molecular Procedures
Total genomic DNA was obtained with the Plant Genomic DNA Kit (KANGWEI Company, Taizhou, China) from materials dried with silica gel according to the manufacturer’s instructions. Fragments of three nuclear loci, including LR0R/LR5 [33,34] for the nuclear ribosomal large subunit RNA (28S), EF1-2F/EF1-2R [20] for the translation elongation factor 1-α gene (TEF1), and bRPB2-6F/bRPB2-7.1R [35] for the RNA polymerase II second largest subunit gene (RPB2), were used. The polymerase chain reaction (PCR) procedures were executed, referring to Xie et al. [31]. PCR products were checked in 1% (w/v) agarose gels, and positive reactions with a bright single band were purified and directly sequenced using an ABI 3730xl DNA Analyzer (Guangzhou Branch of BGI, Guangzhou, China) with the same primers used for PCR amplifications. The new generated DNA sequences were compiled using BioEdit v7.0.9 [36] and then uploaded to GenBank.
2.3. Dataset Assembly
There were eighteen new generated DNA sequences (six of 28S, six of TEF1, and six of RPB2) from six collections. For the concatenated dataset, the sequences of 28S, TEF1, and RPB2 from the new specimens were aligned with sequences of taxa from previous studies and GenBank (Table 1). Phlebopus portentosus (Berk. & Broome) Boedijn and Boletinellus merulioides (Schwein). Murrill were selected as the outgroup. To test for phylogenetic conflict among the different genes in the combined dataset, the phylogenetic trees based on 28S, TEF1, and RPB2 datasets were analyzed and conducted using the ML method to detect the topologies of the genes used. The results of the analyses showed that the different gene fragments were not in conflict. Then, three datasets (28S, TEF1, and RPB2) were aligned with MUSCLE v3.6 [37] and concatenated using Phyutility v2.2 for further analyses [38].

Table 1.
Taxa, vouchers, locations, and GenBank accession numbers of DNA sequences used in this study.
2.4. Phylogenetic Analyses
For the multi-gene (28S + TEF1 + RPB2) phylogenetic analyses, both maximum likelihood (ML) and Bayesian Inference (BI) were conducted. Maximum likelihood tree generation and bootstrap analyses were performed with the program RAxML 7.2.6 [77]. All parameters in the ML analysis were maintained at their default values, except the model set to GTRGAMMA [3]. Nonparametric bootstrapping with 1000 replicates was used to gain statistical support. MrBayes 3.1 was employed to implement the Markov Chain Monte Carlo (MCMC) technique for Bayesian analysis [78]. Two runs were established, each consisting of four chains, with sampling from the posterior distribution occurring every 100 generations. The default values for all other parameters were maintained and complemented in MrModeltest 2.3 [79]. Bayesian analysis of the combined nuclear dataset (28S + TEF1 + RPB2) was run for 40 million generations, and the average deviation of split frequencies was 0.003331. The first 25% generations of trees sampled were discarded as burn-in, and Bayesian posterior probabilities (PP) were then calculated for a majority consensus tree of the retained Bayesian trees. The best fit likelihood model for 28S, TEF1, and RPB2 were GTR + I + G, GTR + I + G, and SYM + I + G, respectively.
3. Results
3.1. Molecular Data
The combined dataset (28S + TEF1 + RPB2) consisted of 145 sequences with 2329 nucleotide sites, and the alignment was submitted to TreeBASE (S31712). The phylogram with branch lengths generated from RAxML and support values (BS and PP) are shown in Figure 1. The topologies of the phylogenetic trees generated from ML and BI analyses were identical, though statistical support for some branches showed slight differences. The existing molecular data demonstrated that our new collections formed two generic clades within Boletaceae (Figure 1).
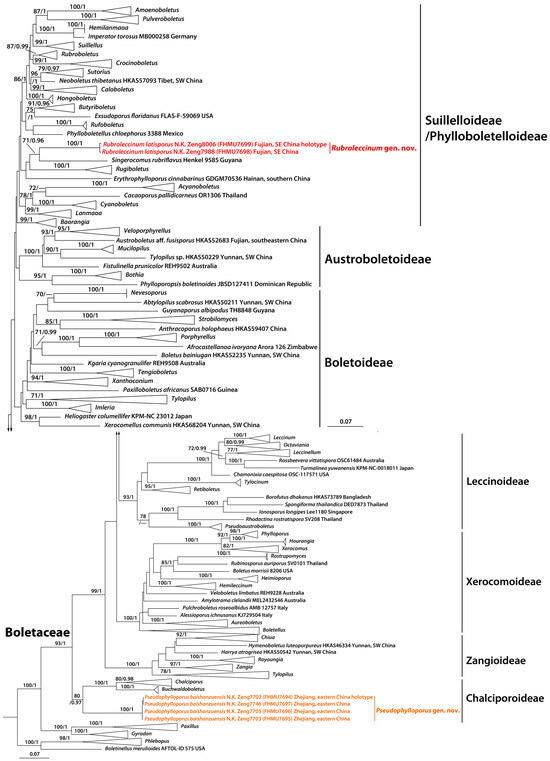
Figure 1.
A phylogram of Boletales inferred from a three-locus (28S, TEF1, and RPB2) dataset using RAxML. BS (≥70%) and PP (≥0.95) are indicated above the branches. Newly generated sequences are in color; SW: southwestern, NE: northeastern, and SE: southeastern.
3.2. Taxonomy
Pseudophylloporus N.K. Zeng, H.Z. Qin, W.F. Lin & L.G. Hu, gen. nov.
MycoBank: MB 855763.
Etymology—Named because of its phenotypic similarity to the genus Phylloporus.
Diagnosis—Differs from genera phylogenetically and morphologically close to the new genus by a lamellate hymenophore, lamellae usually forked, a blue-red-black color change of hymenophore and context when injured, smooth basidiospores, and a presence of clamp connections (Table 2).

Table 2.
Comparison of the genera morphologically and phylogenetically related to Pseudophylloporus.
Basidiomata pileate-stipitate with lamellate hymenophore. Pileus convex to plano-convex; surface dry to slightly viscous, nearly smooth, yellowish-brown to earthy yellow; context white to yellow, turning blue, then changing red, and finally black when injured when injured. Hymenophore lamellate, lamellate usually forked, yellow to yellowish-brown, turning blue, then changing red, and finally black when injured. Stipe central, solid, subcylindrical, base enlarged to subglobose; surface dry, tawny to pale brown, densely covered with pale brown scales; context pale yellow, turning blue, then changing red, and finally black when injured; basal mycelium yellowish. Basidiospores fusoid to elongate, smooth; pleuro- and cheilocystidia present; pileipellis a cutis. Clamp connections present in all tissues.
Type species—Pseudophylloporus baishanzuensis N.K. Zeng, H.Z. Qin, W.F. Lin & L.G. Hu
Pseudophylloporus baishanzuensis N.K. Zeng, H.Z. Qin, W.F. Lin & L.G. Hu, sp. nov.
MycoBank: MB 855764.

Figure 2.
Basidiomata of Pseudophylloporus and Rubroleccinum species. (a–f) Pseudophylloporus baishanzuensis ((a,b) from FHMU7694, holotype; (c–e) from FHMU7695; (f) from FHMU7697); (g–j) Rubroleccinum latisporus ((g,h) from FHMU7698; (i,j) from FHMU7699, holotype). Scale bars = 1 cm. Photographs by N.K. Zeng.
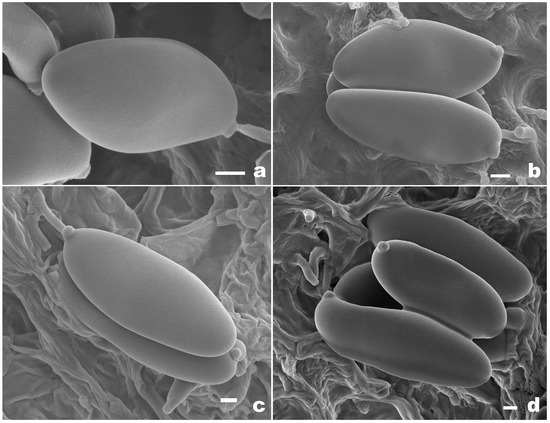
Figure 3.
Basidiospores of Pseudophylloporus and Rubroleccinum species from herbarium materials under SEM. (a,b) Pseudophylloporus baishanzuensis (FHMU7694, holotype); (c,d) Rubroleccinum latisporus (FHMU7699, holotype). Scale bars: 1 μm. Photographs by H.Z. Qin.
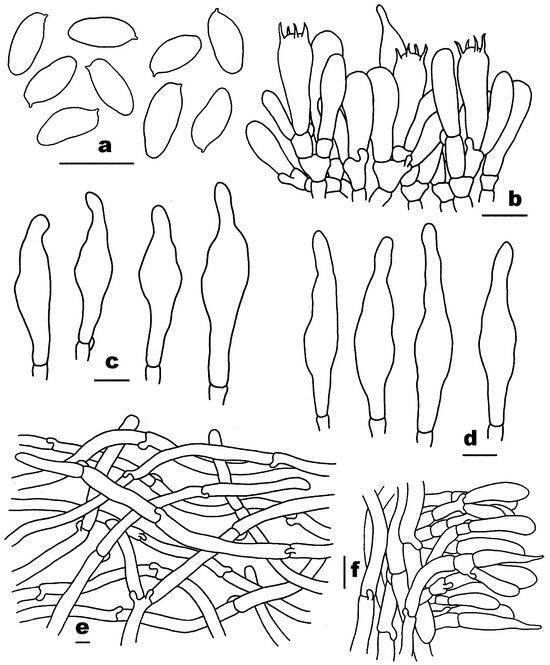
Figure 4.
Microscopic features of Pseudophylloporus baishanzuensis (FHMU7694, holotype). (a) Basidiospores. (b) Basidia and pleurocystidium. (c) Pleurocystidia. (d) Cheilocystidia. (e) Pileipellis. (f) Stipitipellis. Scale bars = 10 μm. Drawings by H.Z. Qin.
Etymology—Latin “baishanzuensis”, referring to the name of the type locality.
Holotype—CHINA. Zhejiang Province: Lishui City, Qingyuan County, Baishanzu National Forest Park, elev. 1300 m, 12 August 2023, N.K. Zeng7702 (FHMU7694). GenBank accession number: 28S = PQ330210, TEF1 = PQ330110, RPB2 = PQ330114.
Diagnosis—The new species is characterized by a blue-red-black color change of hymenophore and context when injured, forked lamellae, yellowish basal mycelia, a cutis pileipellis, and a presence of clamp connections.
Description—Basidiomata is very small to small-sized. Pileus 1.2–3.6 cm diam, convex to plano-convex, becoming applanate with age; surface dry to slightly viscous, smooth, yellowish-brown (5A5), earthy yellow (5A6–5B6) to pale brown (5B5–8); margin incurved, slightly straight when mature; context 0.1–0.6 cm thick in the center of the pileus, white (2A1), pale yellow (2A2) to yellow (2A4), turning blue (24B6), then changing red (10A6), and finally black when injured. Hymenophore lamellate, decurrent; lamellae 0.1–0.3 cm in height, subdistant, usually forked, yellow (1A5–2A5) to yellowish-brown (2B5, 3B5–6), turning blue (24C7) quickly, then changing red (10A6), and finally black (10F7) when injured. Stipe 1.7–3 × 0.3–0.5 cm, central, solid, subcylindrical, base enlarged to subglobose; surface dry, tawny (3A5–3B5) to pale brown (4B4–5), densely covered with pale brown (3B5) sometimes reddish-brown (6B8) scales; context pale yellow (2A2) to yellowish-brown (4A5), turning blue (24B6), then changing red (10A6), and finally black when injured; basal mycelium yellowish (2A4). Odor indistinct.
Basidiospores [160/8/4] 7–9.26–10.5 (–11) × 3–3.99–4 (–5) μm, Q = 2–2.57 (–3), Qm = 2.32 ± 0.16, fusoid to elongate, slightly thick-walled (0.8–1 μm), smooth, pale yellow to yellow in KOH. Basidia 18–33 × 5–9 μm, clavatet o subcylindric, slightly thick-walled (up to 0.8 μm), 4-spored, colorless to yellowish in KOH; sterigmata 2–5 μm in length. Hymenophoral trama composed of slightly thin-walled (up to 0.5 μm) hyphae, 5–15 μm wide, colorless to yellowish in KOH. Pleurocystidia 42–62 × 7–14 μm, subfusiform, slightly thick-walled (up to 1 μm), pale yellow to yellow in KOH, no encrustations. Cheilocystidia 38–74 × 10–15 μm, subfusiform, slightly thick-walled (up to 1 μm), yellow in KOH, no encrustations. Pileipellis a cutis 140–380 μm thick, composed of slightly thick-walled (up to 1 μm) hyphae, subparallel to slightly interwoven, 3–11 μm wide, colorless in KOH; terminal cells 41–119 × 6–14 μm, clavate or subcylindrical. Pileal trama composed of slightly thick-walled (up to 1 μm) hyphae, 8–23 μm wide, colorless in KOH. Stipitipellis a trichoderm-like structure 25–75 μm thick, composed of slightly thick-walled (up to 1 μm) hyphae, 3–9 μm wide, yellowish in KOH; terminal cells 28–39 × 5–7 μm, clavate to subcylindrical. Stipe trama composed of parallel hyphae, slightly thick-walled (up to 1 μm), 5–31 μm wide, subcylindrical, yellowish in KOH. Clamp connections are present in all tissues.
Habitat—Solitary or gregarious on the ground in forests dominated by fagaceous trees.
Known distribution—Eastern China (Zhejiang Province).
Additional specimens examined—CHINA. Zhejiang Province: Lishui City, Qingyuan County, Baishanzu National Forest Park, elev. 1300 m, 12 August 2023, N.K. Zeng7703 (FHMU7695); same location and date, N.K. Zeng7705(FHMU7696); same location and date, N.K. Zeng7746 (FHMU7697).
Rubroleccinum N.K. Zeng, H.Z. Qin & H. Zeng, gen. nov.
MycoBank: MB 855749.
Etymology—Latin “Rubro-” means a stipe punctuated with red scabers, and “-leccinum” refers to the morphological similarities of the new genus with leccinoid mushrooms.
Diagnosis—Differs from genera phylogenetically and morphologically close to the new genus by a red-tinged basidioma, a stipe punctuated with red to reddish-brown scabers, yellow basal mycelia, a blue-red color change of hymenophore and context when injured, and a trichoderm pileipellis (Table 3).

Table 3.
Comparison of the genera morphologically and phylogenetically related to Rubroleccinum.
Basidiomata pileate-stipitate with tubular hymenophore. Pileus convex to plano-convex; surface dry, nearly smooth, reddish-orange to grayish-yellow; context yellow, changing blue, then turning red when injured. Hymenophore brilliant yellow to yellow, changing blue, then turning red when injured. Stipe central, solid, subcylindrical; surface dry, punctuated with red to reddish-brown scabers; context yellow, changing blue, then turning red when injured; basal mycelium yellow. Basidiospores cylindrical to fusoid, smooth; pleuro- and cheilocystidia present; pileipellis is a trichoderm. Clamp connections are absent in all tissues.
Type species—Rubroleccinum latisporus N.K. Zeng, H.Z. Qin & H. Zeng
Rubroleccinum latisporus N.K. Zeng, H.Z. Qin & H. Zeng, sp. nov.
MycoBank: MB 855750.
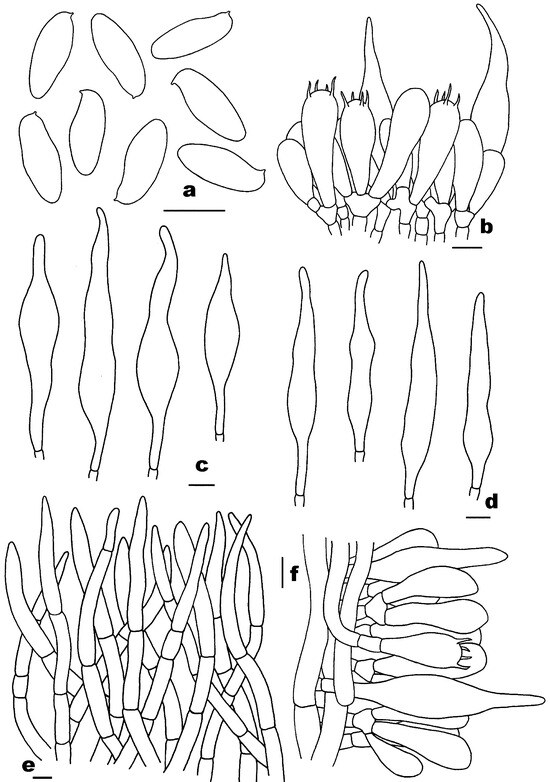
Figure 5.
Microscopic features of Rubroleccinum latisporus (FHMU7699, holotype). (a) Basidiospores. (b) Basidia and pleurocystidia. (c) Pleurocystidia. (d) Cheilocystidia. (e) Pileipellis. (f) Stipitipellis. Scale bars = 10 μm. Drawings by H.Z. Qin.
Etymology—Latin “latisporus” refers to the wide basidiospores.
Holotype—CHINA. Fujian Province: Wuyishan City, Wuyi Mountain National Forest Park, elev. 1100 m, 17 August 2023, N.K. Zeng8006 (FHMU7699). GenBank accession number: 28S = PQ325254, TEF1 = PQ330107, RPB2 = PQ330109.
Diagnosis—The new species is characterized by a red-tinged basidioma, a stipe punctuated with red to reddish-brown scabers, yellow basal mycelia, a blue-red color change of hymenophore and context when injured, wide basidiospores, and a trichodermal pileipellis with cuspidal apex of terminal cells.
Description—Basidiomata very small to medium-sized. Pileus 2–5.5 cm diam, subhemispherical when young, then convex to plano-convex; surface dry, nearly smooth, orange (5A7–8) to reddish-orange (6A8) when young, then grayish-yellow (4A3–4) to reddish-brown (6B8–7C7); margin incurved; context 0.5–1.25 cm thick in the center of the pileus, yellow (3A5–7), changing blue (24D7), then turning red when injured. Hymenophore poroid, depressed around apex of stipe, slightly decurrent; pores angular to subround, brilliant yellow (2A6–7) to yellow (4A6), changing blue (24D7), then turning red when injured; tubes 0.4–3 cm in length, yellow (4A6), changing blue (24D7), then turning red when injured. Stipe 2.9–5.1 × 0.5–1.5 cm, central, solid, subcylindrical; surface dry, yellow (4A6), punctuated with red (9A7–8) to reddish-brown (8C7–8) scabers; context yellow (3A6), changing blue (24D7), then turning red when injured; basal mycelium yellow (2A4). Odor indistinct.
Basidiospores [80/4/2] 12.5–14.44–16 (–17.5) × 5–5.67–6 μm, Q = (2.17–) 2.27–2.83 (–3.1), Qm = 2.56 ± 0.18, cylindrical to fusoid, slightly thick-walled (up to 1 μm), smooth, pale yellow to yellowish brown in KOH. Basidia 28–43 × 10–14 μm, subclavate or subcylindric, thin- to slightly thick-walled (0.5–0.8 μm), 4-spored, yellowish in KOH; sterigmata 2–7 μm in length. Hymenophoral trama composed of slightly thick-walled (up to 1 μm) hyphae, 4–13 μm wide, colorless to yellowish in KOH. Pleurocystidia 58–106 × 11–17 μm, abundant, subfusiform, thin- to slightly thick-walled (0.5–0.8 μm), pale yellow to yellow in KOH, no encrustations. Cheilocystidia 39–100 × 7–14 μm, abundant, subfusiform, thin- to slightly thick-walled (0.5–1 μm), yellowish to yellow, or colorless in KOH, no encrustations. Pileipellis a trichoderm 100–200 μm thick, composed of thin- to slightly thick-walled (0.5–1 μm) hyphae, 4–10 μm wide, pale yellow to yellow in KOH, usually with granular contents; terminal cells 35–77 × 5–11 μm, clavate to subcylindrical, with cuspidal apex. Pileal trama composed of slightly thick-walled (up to 1 μm) hyphae, 4–12 μm wide, yellowish in KOH. Stipitipellis a trichoderm-like structure 170–400 μm thick, composed of slightly thick-walled (up to 1 μm) hyphae, 3–9 μm wide, yellowish in KOH; terminal cells 40–102 × 4–8 μm, subcylindrical to cylindrical. Stipe trama composed of parallel hyphae, slightly thick-walled (0.8–1 μm), 4–13 μm wide, subcylindrical, yellowish in KOH. Clamp connections absent in all tissues.
Habitat—Solitary or gregarious on the ground in forests dominated by fagaceous trees.
Known distribution—Southeastern China (Fujian Province).
Additional specimen examined—CHINA. Fujian Province: Wuyishan City, Wuyi Mountain National Forest Park, elev. 1100 m, 17 August 2023, N.K. Zeng7988 (FHMU7698).
4. Discussion
The phylogenetic analyses showed that the new genus Pseudophylloporus is a member of subfamily Chalciporoideae within Boletaceae (Figure 1). The lamellate hymenophore of Pseudophylloporus is reminiscent of several other genera, viz., Phylloporus Quél., Phyllobolites Singer, Phylloboletellus Singer, Phylloporopsis Angelini, A. Farid, Gelardi, M.E. Smith, Costanzo, & Vizzini, Erythrophylloporus Ming Zhang & T.H. Li, and Paxilloboletus Furneaux, De Kesel & F.K. Khan. Phylloporus, a genus of subfamily Xerocomoideae, morphologically differs from Pseudophylloporus by usually not forked lamellae, basidiospores with bacillate ornamentation, and clamp connections usually absent [4,5,20]. Phyllobolites, a genus with undefined subfamily ranking, is different by a membranous ring deriving from a partial veil, a pileal context unchanging or changing blue when injured, a hymenophore turning sienna or rust-color to chestnut when injured, verrucose basidiospores with slight rugulose, and an absence of clamp connections [12,80,81]. Phylloboletellus, a member of subfamily Phylloboletelloideae, can be distinguished from Pseudophylloporus by a blue (without red) color change of hymenophore and context when injured; basidiospores with longitudinal, continuous, or bifurcate ribs; and sometimes scarce clamp connections [12,81,82]. Phylloporopsis, a genus of subfamily Austroboletoideae, is characterized by a hymenophore sometimes sub-boletinoid, lamellae usually not forked, a blue (without red) color change of the hymenophore and context when injured, and an absence of clamp connections [12]. Erythrophylloporus, a member of subfamily Suillelloideae, differs from Pseudophylloporus by an orange, reddish-orange to yellowish-red basidioma, a red hymenophore, lamellae usually not forked, a vivid yellow to orange yellow context changing dark violet to blackish blue when injured, and an absence of clamp connections [51]. Paxilloboletus, a member of subfamily Boletoideae, is distinguished from Pseudophylloporus by all tissues unchanging in color when injured and an absence of clamp connections [13].
Phylogenetically, Pseudophylloporus is closely related to Buchwaldoboletus Pilát and Chalciporus Bataille (Figure 1). However, Buchwaldoboletus has a poroid hymenophore, a blue (without red) color change of hymenophore and context when injured, an absence of clamp connections, and usually a saprophytic habit [39]. Chalciporus differs from Pseudophylloporus by a poroid hymenophore, hymenophore and context unchanging or turning bluish when injured, and an absence of clamp connections [39]. The molecular data indicated that the new genus Rubroleccinum is assigned to the subfamily Suillelloideae, which has been recognized as an independent subfamily based on the whole genome sequences [16]. Despite the inability to differentiate the subfamilies Suillelloideae and Phylloboletelloideae based on our multi-locus (28S + TEF1 + RPB2) phylogenetic analysis, it is sufficient to demonstrate that Rubroleccinum exhibits distinct phylogenetic variations (Figure 1).
The obvious scabers on stipe of Rubroleccinum are reminiscent of several other genera, viz., Hemileccinum Šutara, Leccinellum Bresinsky & Manfr. Binder, Leccinum Gray, and Sutorius Halling, Nuhn & N.A. Fechner. Hemileccinum, a member of subfamily Xerocomoideae, can be distinguished from Rubroleccinum by a tissue unchanging in color when injured, irregularly warty basidiospores, and a hyphoepithelium pileipellis [4,68,83,84]. Leccinellum, a genus of subfamily Leccinoideae, is characterized by a whitish or yellow hymenophore unchanging or staining brownish to ferruginous, or at first reddish then blackish when injured, scabrous squamules over the surface of stipe brown to blackish, and an epithelium pileipellis [85,86]. Leccinum, also a member of subfamily Leccinoideae, has a whitish or yellow hymenophore, a white to cream context unchanging or staining blue or red when injured, and scabrous to dotted squamules on the stipe brown to blackish [15,87,88,89,90]. Sutorius, also a genus of subfamily Suillelloideae, differs from Rubroleccinum by a hymenophore dark purple, purplish red or purplish brown, and a context without color change or staining blue to dark blue when injured [39,44,91,92].
Phylogenetically, Rubroleccinum is closely related to Singerocomus T.W. Henkel & M.E. Sm. (Figure 1). However, Singerocomus can be distinguished from Rubroleccinum by all tissues unchanging in color when injured [7].
In the Boletaceae, abundant taxa exhibit poroid hymenophore [1,2,3,4,7,8,9]. However, more and more boletes with lamellate hymenophore have been discovered recently, and these genera are distributed across different subfamilies within Boletaceae (Figure 1), which indicated that the trait of lamellate hymenophore is a multiple occurrence event evolutionarily [4,5,12,13,20,51,80,81,82].
In the subtropical regions of China, there is a rich diversity of Boletaceae species [1,2,3,4]. Among them, many boletes such as Butyriboletus spp. and Neoboletus spp. are commercially traded for edibility, which are contributing to economic benefits [6,8,15,39,93,94]. It is noteworthy that the consumption records for the newly identified genera Rubroleccinum and Pseudophylloporus have not been documented at a collected location. Further studies including toxicity assessments of the two genera should be conducted. Although the edibility of Rubroleccinum and Pseudophylloporus remains unclear, they are symbiotic with trees of Fagaceae, influencing the growth and nutrient uptake of trees and other ecological processes [1,95,96,97]. Revealing the ecological roles of Rubroleccinum and Pseudophylloporus is also an interesting study, which enhances our understanding of the complexity and stability of ecological networks, facilitating more effective conservation efforts for subtropical forests of China.
5. Conclusions
Although abundant taxa of Boletaceae have been revealed, the diversity of this family has not been completely resolved. In this work, Pseudophylloporus baishanzuensis gen. nov., sp. nov. and Rubroleccinum latisporus gen. nov., sp. nov. are described based on morphological and molecular phylogenetic analyses. These findings contribute to a deeper understanding of the diversity within the Boletaceae family.
Author Contributions
Conceptualization, Z.-Q.L. and N.-K.Z.; Methodology, Performing the experiment, and Formal analysis, H.-Z.Q. and Y.W.; Resources, N.-K.Z., W.-F.L., H.Z., L.-G.H., B.-R.K. and Z.-H.Z.; Writing—original draft preparation, H.-Z.Q.; Writing—review and editing, Z.-Q.L. and N.-K.Z.; Supervision, N.-K.Z.; Project administration, N.-K.Z.; Funding acquisition, N.-K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 32460003 and 32160001); Hainan Institute of National Park, HINP, KY-24ZK02; Hainan Province Science and Technology Special Fund (ZDYF2023RDYL01); Fujian Provincial Natural Science Foundation (2023J01379); and the Project of FAAS (XTCXGC2021007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this study have been deposited in NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and Mycobank (https://www.mycobank.org/page/Home/MycoBank).
Acknowledgments
The first author is very grateful to Yun-Xiao Han, Run Tian, and Chang Xu, Hainan Medical University, and Xu Zhang, Hainan Normal University, for their help with the molecular data analyses. Thanks are due to Qingyuan Conservation Center, Qianjiangyuan-Baishanzu National Park, and Wuyi Mountain National Park Administration, for their kind help during the field investigations.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Wang, Y.; Wang, L.Y.; Dai, D.; Qi, Z.X.; Zhang, Z.H.; Liu, Y.J.; Hu, J.J.; Zhang, P.; Li, Y.; Zhang, B. Boletaceae in China: Taxonomy and phylogeny reveal a new genus, two new species, and a new record. Front. Microbiol. 2023, 13, 1052948. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Angelini, C.; Biketova, A.Y.; Ercole, E.; Svetasheva, T.Y.; Miller, K.O.; de la Fuente, J.I.; García-Jiménez, J.; Vizzini, A. Coccoloba-associated xerocomoid boletes (Boletaceae) from the Caribbean and Mexico: Tropicoboletus ruborculus gen. et comb. nov., revision of Xerocomus coccolobae, phylogenetic assessment of Singerocomus guadelupae comb. nov., and type studies of Xerocomus caeruleonigrescens, X. cuneipes, and X. pseudoboletinus var. pini-caribaeae. Mycol. Prog. 2023, 22, 29. [Google Scholar] [CrossRef]
- Wu, G.; Li, H.J.; Horak, E.; Wu, K.; Li, G.M.; Yang, Z.L. New taxa of Boletaceae from China. Mycosphere 2023, 14, 754–776. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, X.; Xu, C.; Xie, H.J.; Wu, L.L.; Wang, Y.; Tang, L.P.; Hao, Y.J.; Zhao, K.; Jiang, S.; et al. The subfamily Xerocomoideae (Boletaceae, Boletales) in China. Stud. Mycol. 2023, 106, 95–197. [Google Scholar] [CrossRef]
- Neves, M.A.; Binder, M.; Halling, R.; Hibbett, D.; Soytong, K. The phylogeny of selected Phylloporus species, inferred from NUC-LSU and ITS sequences, and descriptions of new species from the old world. Fungal Divers. 2012, 55, 109–123. [Google Scholar] [CrossRef]
- Arora, D.; Frank, J.L. Clarifying the butter Boletes: A new genus, Butyriboletus, is established to accommodate Boletus sect. Appendiculati, and six new species are described. Mycologia 2014, 106, 464–480. [Google Scholar] [CrossRef]
- Henkel, T.W.; Obase, K.; Husbands, D.; Uehling, J.K.; Bonito, G.; Aime, M.C.; Smith, M.E. New Boletaceae taxa from Guyana: Bnderoboletus segoi gen. and sp. nov., Guyanaporus albipodus gen. and sp. nov., Singerocomus rubriflavus gen. and sp. nov., and a new combination for Xerocomus inundabilis. Mycologia 2016, 108, 157–173. [Google Scholar] [CrossRef]
- Chai, H.; Liang, Z.Q.; Xue, R.; Jiang, S.; Luo, S.H.; Wang, Y.; Wu, L.L.; Tang, L.P.; Chen, Y.; Hong, D.; et al. New and noteworthy boletes from subtropical and tropical China. MycoKeys 2019, 46, 55. [Google Scholar] [CrossRef]
- Deng, H.; Wang, Y.; Lei, J.R.; Chen, Z.Z.; Liang, Z.Q.; Zeng, N.K. Four new species of Strobilomyces (Boletaceae, Boletales) from Hainan island, tropical China. J. Fungi 2023, 9, 1128. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Lumyong, S.; Raspé, O. Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys 2019, 54, 1–29. [Google Scholar] [CrossRef]
- Magnago, A.C.; Alves-Silva, G.; Henkel, T.W.; da Silveira, R.M.B. New genera, species, and combinations of Boletaceae from Brazil and Guyana. Mycologia 2022, 114, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Farid, A.; Gelardi, M.; Angelini, C.; Franck, A.R.; Costanzo, F.; Kaminsky, L.; Ercole, E.; Baroni, T.J.; White, A.L.; Garey, J.R.; et al. Phylloporus and Phylloboletellus are no longer alone: Phylloporopsis gen. nov. (Boletaceae), a new smooth-spored lamellate genus to accommodate the American species Phylloporus boletinoides. Fungal Syst. Evol. 2018, 2, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Badou, S.A.; Furneaux, B.; De Kesel, A.; Khan, F.K.; Houdanon, R.D.; Ryberg, M.; Yorou, N.S. Paxilloboletus gen. nov., a new lamellate bolete genus from tropical Africa. Mycol. Prog. 2022, 21, 243–256. [Google Scholar] [CrossRef]
- Ayala-Vásquez, O.; Pérez-Moreno, J.; Pinzón, J.P.; Garibay-Orijel, R.; García-Jiménez, J.; de la Fuente, J.I.; Venegas-Barrera, C.S.; Martínez-Reyes, M.; Montoya, L.; Bandala, V.; et al. Broadening the knowledge of Mexican boletes: Addition of a new genus, seven new species, and three new combinations. J. Fungi 2023, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feng, B.; Xu, J.; Zhu, X.T.; Li, Y.C.; Zeng, N.K.; Hosen, M.I.; Yang, Z.L. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers. 2014, 69, 93–115. [Google Scholar] [CrossRef]
- Tremble, K.; Henkel, T.; Bradshaw, A.; Domnauer, C.; Brown, L.M.; Thám, L.X.; Furci, G.; Aime, M.C.; Moncalvo, J.M.; Dentinger, B. A revised phylogeny of Boletaceae using whole genome sequences. Mycologia 2024, 116, 392–408. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, CT, USA, 2008. [Google Scholar]
- Wu, G.; Li, M.X.; Horak, E.; Yang, Z.L. Phylogenetic analysis reveals the new genus Amoenoboletus from Asia and New Zealand. Mycologia 2022, 114, 144–156. [Google Scholar] [CrossRef]
- Becerra, A.G.; Zak, M.R. The ectomycorrhizal symbiosis in South America: Morphology, colonization, and diversity. In Diversity and Biotechnology of Ectomycorrhizae; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–41. [Google Scholar] [CrossRef]
- Zeng, N.K.; Tang, L.P.; Li, Y.C.; Tolgor, B.; Zhu, X.T.; Zhao, Q.; Yang, Z.L. The genus Phylloporus (Boletaceae, Boletales) from China: Morphological and multilocus DNA sequence analyses. Fungal Divers. 2013, 58, 73–101. [Google Scholar] [CrossRef]
- Pérez-Moreno, J.; Guerin-Laguette, A.; Flores, A.R.; Qiang, F. Mushrooms, Humans and Nature in a Changing World; Springer Nature: Basel, Switzerland, 2020; p. 473. [Google Scholar] [CrossRef]
- Pérez-Moreno, J.; Guerin-Laguette, A.; Rinaldi, A.C.; Yu, F.; Verbeken, A.; Hernández-Santiago, F.; Martínez Reyes, M. Edible mycorrhizal fungi of the world: What is their role in forest sustainability, food security, biocultural conservation and climate change? Plants People Planet 2020, 3, 471–490. [Google Scholar] [CrossRef]
- Husbands, D.R.; Henkel, T.W.; Bonito, G.; Vilgalys, R.; Smith, M.E. New species of Xerocomus (Boletales) from the Guiana Shield, with notes on their mycorrhizal status and fruiting occurrence. Mycologia 2013, 105, 422–435. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Z.; Wang, Q.C.; Wang, Y.L.; Hu, H.W.; He, J.Z.; Zheng, Y.; Yang, Y. Compartment and plant identity shape tree mycobiome in a subtropical forest. Microbiol. Spectr. 2022, 10, e0134722. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Deng, T.; Boufford, D.E. Origins and evolution of plant diversity in the Hengduan Mountains, China. Plant Divers. 2017, 39, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kornerup, A.; Wanscher, J.H. Taschenlexikon der Farben, 3rd ed.; Muster-Schmidt Verlag: Göttingen, Germany, 1981; p. 242. [Google Scholar]
- Zeng, N.K.; Wu, G.; Li, Y.C.; Liang, Z.Q.; Yang, Z.L. Crocinoboletus, a new genus of Boletaceae (Boletales) with unusual boletocrocin polyene pigments. Phytotaxa 2014, 175, 133–140. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, R.; Tang, L.P.; Liang, Z.Q.; Zhang, W.H.; Jiang, S.; Wang, C.K.; Zeng, N.K. Morphological and phylogenetic evidence reveal three new species of Aureoboletus (Boletaceae, Boletales) from China. Phytotaxa 2022, 567, 127–148. [Google Scholar] [CrossRef]
- Zeng, N.K.; Liang, Z.Q.; Tang, L.P.; Li, Y.C.; Yang, Z.L. The genus Pulveroboletus (Boletaceae, Boletales) in China. Mycologia 2017, 109, 422–442. [Google Scholar] [CrossRef]
- Xu, C.; Liang, Z.Q.; Su, M.S.; Jiang, S.; Chen, Y.; Fan, Y.G.; Zeng, N.K. Austroboletus brunneisquamus (Boletaceae, Boletales), a new ectomycorrhizal fungus from a tropical rainforest, China. Forests 2021, 12, 1438. [Google Scholar] [CrossRef]
- Xie, H.J.; Tang, L.P.; Mu, M.; Fan, Y.G.; Jiang, S.; Su, M.S.; Liang, Z.Q.; Zeng, N.K. A contribution to knowledge of Gyroporus (Gyroporaceae, Boletales) in China: Three new taxa, two previous species, and one ambiguous taxon. Mycol. Prog. 2022, 21, 71–92. [Google Scholar] [CrossRef]
- Bas, C. Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 1969, 5, 285–573. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- James, T.Y.; Kauff, F.; Schoch, C.; Matheny, P.B.; Hofstetter, V.; Cox, C.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef]
- Matheny, P.B. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogenetics Evol. 2005, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Dunn, C.W. Phyutility: A phyloinformatics tool for trees, alignments andmolecular data. Bioinformatics 2008, 24, 715–716. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.C.; Zhu, X.T.; Zhao, K.; Han, L.H.; Cui, Y.Y.; Li, F.; Xu, J.P.; Yang, Z.L. One hundred noteworthy boletes from China. Fungal Divers. 2016, 81, 25–188. [Google Scholar] [CrossRef]
- Orihara, T.; Smith, M.E. Unique phylogenetic position of the African truffle-like fungus, Octaviania ivoryana (Boletaceae, Boletales), and the proposal of a new genus, Afrocastellanoa. Mycologia 2017, 109, 323–332. [Google Scholar] [CrossRef]
- Gelardi, M.; Simonini, G.; Ercole, E.; Vizzini, A. Alessioporus and Pulchroboletus (Boletaceae, Boletineae), two novel genera for Xerocomus ichnusanus and X. roseoalbidus from the European Mediterranean basin: Molecular and morphological evidence. Mycologia 2014, 106, 1168–1187. [Google Scholar] [CrossRef]
- Lebel, T.; Davoodian, N.; Bloomfield, M.; Syme, K.; May, T.W.; Hosaka, K.; Castellano, M.A. A mixed bag of sequestrate fungi from five different families: Boletaceae, Russulaceae, Psathyrellaceae, Strophariaceae, and Hysterangiaceae. Swainsona 2022, 36, 33–65. [Google Scholar]
- Nuhn, M.E.; Binder, M.; Taylor, A.F.; Halling, R.E.; Hibbett, D.S. Phylogenetic overview of the Boletineae. Fungal Biol. 2013, 117, 479–511. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, K.; Li, Y.C.; Zeng, N.K.; Feng, B.; Halling, R.E.; Yang, Z.L. Four new genera of the fungal family Boletaceae. Fungal Divers. 2015, 81, 1–24. [Google Scholar] [CrossRef]
- Nuhn, M.E. Molecular ecology of Boletinellus merulioides and systematics of the Boletineae; Clark University ProQuest Dissertations Publishing: Worcester, MA, USA, 2016. [Google Scholar]
- Hosen, M.; Feng, B.; Wu, G.; Zhu, X.T.; Li, Y.C.; Yang, Z.L. Borofutus, a new genus of Boletaceae from tropical Asia: Phylogeny, morphology and taxonomy. Fungal Divers. 2013, 58, 215–226. [Google Scholar] [CrossRef]
- Halling, R.E.; Baroni, T.J.; Binder, M. A new genus of Boletaceae from eastern North America. Mycologia 2007, 99, 310–316. [Google Scholar] [CrossRef]
- Zeng, N.K.; Su, M.S.; Liang, Z.Q.; Yang, Z.L. A geographical extension of the North American genus Bothia (Boletaceae, Boletales) to East Asia with a new species B. fujianensis from China. Mycol. Prog. 2015, 14, 1015. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, G.; Feng, B.; Yang, Z.L. Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycol. Prog. 2014, 13, 1127–1136. [Google Scholar] [CrossRef]
- Kuo, M.; Ortiz-Santana, B. Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycologia 2020, 112, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, T.H. Erythrophylloporus (Boletaceae, Boletales), a new genus inferred from morphological and molecular data from subtropical and tropical China. Mycosystema 2018, 37, 1111–1126. [Google Scholar] [CrossRef]
- Biketova, A.Y.; Gelardi, M.; Smith, M.E.; Simonini, G.; Healy, R.A.; Taneyama, Y.; Vasquez, G.; Kovács, Á.; Nagy, L.G.; Wasser, S.P.; et al. Reappraisal of the genus Exsudoporus (Boletaceae) worldwide based on multi-gene phylogeny, morphology and biogeography, and insights on Amoenoboletus. J. Fungi 2022, 8, 101. [Google Scholar] [CrossRef]
- Halling, R.E.; Nuhn, M.; Osmundson, T.; Fechner, N.; Trappe, J.M.; Soytong, K.; Arora, D.; Hibbett, D.S.; Binder, M. Affinities of the Boletus chromapes group to Royoungia and the description of two new genera, Harrya and Australopilus. Aust. Syst. Bot. 2012, 25, 418–431. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Raspé, O.; Lumyong, S. Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R. rostratispora from Thailand. MycoKeys 2018, 29, 63. [Google Scholar] [CrossRef]
- Dentinger, B.T.; Ammirati, J.F.; Both, E.E.; Desjardin, D.E.; Halling, R.E.; Henkel, T.W.; Moreau, P.A.; Nagasawa, E.; Soytong, K.; Taylor, A.F.; et al. Molecular phylogenetics of porcini mushrooms (Boletus section Boletus). Mol. Phylogenet. Evol. 2010, 57, 1276–1292. [Google Scholar] [CrossRef]
- Binder, M.; Larsson, K.H.; Matheny, P.B.; Hibbett, D.S. Amylocorticiales ord. nov. and Jaapiales ord. nov.: Early diverging clades of agaricomycetidae dominated by corticioid forms. Mycologia 2010, 102, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.T.; Li, Y.C.; Wu, G.; Feng, B.; Zhao, K.; Gelardi, M.; Kost, G.W.; Yang, Z.L. The genus Imleria (Boletaceae) in East Asia. Phytotaxa 2014, 191, 81–98. [Google Scholar] [CrossRef]
- Chuankid, B.; Vadthanarat, S.; Thongbai, B.; Stadler, M.; Lumyong, S.; Hyde, K.D.; Raspe, O. Retiboletus (Boletaceae) in northern Thailand: One novel species and two first records. Mycoscience 2021, 62, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Khmelnitsky, O.; Davoodian, N.; Singh, P.; Raspé, O.; Lee, S.M.; Fechner, N.; Bonito, G.; Lebel, T. Ionosporus: A new genus for Boletus longipes (Boletaceae), with a new species, I. australis, from Australia. Mycol. Prog. 2019, 18, 439–451. [Google Scholar] [CrossRef]
- Halling, R.E.; Fechner, N.A.; Holmes, G.; Davoodian, N. Kgaria (Boletaceae, Boletoideae) gen. nov. in Australia: Neither a Tylopilus nor a Porphyrellus. Fungal Syst. Evol. 2023, 12, 31–45. [Google Scholar] [CrossRef]
- Orihara, T.; Smith, M.; Shimomura, N.; Iwase, K.; Maekawa, N. Diversity and systematics of the sequestrate genus Octaviania in Japan: Two new subgenera and eleven new species. Persoonia 2012, 28, 85–112. [Google Scholar] [CrossRef]
- Jargeat, P.; Chaumeton, J.P.; Navaud, O.; Vizzini, A.; Gryta, H. The Paxillus involutus (Boletales, Paxillaceae) complex in Europe: Genetic diversity and morphological description of the new species Paxillus cuprinus, typification of P. involutus s.s., and synthesis of species boundaries. Fungal Biol. 2014, 118, 12–31. [Google Scholar] [CrossRef]
- Binder, M.; Bresinsky, A. Derivation of a polymorphic lineage of Gasteromycetes from boletoid ancestors. Mycologia 2002, 94, 85–98. [Google Scholar] [CrossRef]
- Xie, H.J.; Zhang, C.X.; He, M.X.; Liang, Z.Q.; Deng, X.H.; Zeng, N.K. Buchwaldoboletus xylophilus and Phlebopus portentosus, two non-ectomycorrhizal boletes from tropical China. Phytotaxa 2021, 520, 137–154. [Google Scholar] [CrossRef]
- Binder, M.; Hibbett, D.S. Molecular systematics and biological diversification of Boletales. Mycologia 2006, 98, 971–981. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, F.; Zeng, N.K.; Cui, Y.Y.; Yang, Z.L. A new genus Pseudoaustroboletus (Boletaceae, Boletales) from Asia as inferred from molecular and morphological data. Mycol. Prog. 2014, 13, 1207–1216. [Google Scholar] [CrossRef]
- Li, Y.C.; Yang, Z.L. The Boletes of China: Tylopilus s.l.; Science Press: Beijing, China, 2021. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Raghoonundon, B.; Lumyong, S.; Raspé, O. Rostrupomyces, a new genus to accommodate Xerocomussisongkhramensis, and a new Hemileccinum species (Xerocomoideae, Boletaceae) from Thailand. MycoKeys 2024, 103, 129–165. [Google Scholar] [CrossRef] [PubMed]
- Raspe, O.; Vadthanarat, S.; De Kesel, A.; Degreef, J.; Hyde, K.D.; Lumyong, S. Pulveroboletus fragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycol. Prog. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Raspé, O.; Lumyong, S. Rubinosporus auriporus gen. et sp. nov. (Boletaceae: Xerocomoideae) from tropical forests of Thailand, producing unusual dark ruby spore deposits. J. Fungi 2022, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wu, G.; Yang, Z.L. A new genus, Rubroboletus, to accommodate Boletus sinicus and its allies. Phytotaxa 2014, 188, 61–77. [Google Scholar] [CrossRef]
- Liang, Z.Q.; An, D.Y.; Jiang, S.; Su, M.S.; Zeng, N.K. Butyriboletus hainanensis (Boletaceae, Boletales), a new species from tropical China. Phytotaxa 2016, 267, 256–262. [Google Scholar] [CrossRef]
- Desjardin, D.E.; Binder, M.; Roekring, S.; Flegel, T. Spongiforma, a new genus of gasteroid boletes from Thailand. Fungal Divers. 2009, 37, 1–8. [Google Scholar]
- Orihara, T.; Lebel, T.; Ge, Z.W.; Smith, M.; Maekawa, N. Evolutionary history of the sequestrate genus Rossbeevera (Boletaceae) reveals a new genus Turmalinea and highlights the utility of ITS minisatellite–like insertions for molecular identification. Persoonia 2016, 37, 173–198. [Google Scholar] [CrossRef]
- Li, Y.C.; Ortiz-Santana, B.; Zeng, N.K.; Feng, B.; Yang, Z.L. Molecular phylogeny and taxonomy of the genus Veloporphyrellus. Mycol. 2014, 106, 291–306. [Google Scholar] [CrossRef]
- Li, Y.C.; Feng, B.; Yang, Z.L. Zangia, a new genus of Boletaceae supported by molecular and morphological evidence. Fungal Divers. 2011, 49, 125–143. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. Bayesian Analysis of Molecular Evolution Using MrBayes; Springer: New York, NY, USA, 2005; pp. 183–226. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest 2.3. Program Distributed by the Author; Evolutionary Biology Center, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Singer, R. Das System der Agaricales. II. Ann. Mycol. 1942, 40, 1–132. [Google Scholar]
- Watling, R. A Manual and Source Book of the Boletes and Their Allies; Fungiflora: Oslo, Norway, 2008. [Google Scholar]
- Bandala, V.M.; Montoya, L.; Jarvio, D. Two interesting records of boletes found in coffee plantations in eastern Mexico. Persoonia-Mol. Phylogeny Evol. Fungi 2004, 18, 365–380. [Google Scholar]
- Šutara, J. Xerocomus s. l. in the light of the present state of knowledge. Czech Mycol. 2008, 60, 29–62. [Google Scholar] [CrossRef]
- Li, M.X.; Wu, G.; Yang, Z.L. Four new species of Hemileccinum (Xerocomoideae, Boletaceae) from southwestern China. J. Fungi 2021, 7, 823. [Google Scholar] [CrossRef]
- Bresinsky, A.; Besl, H. Schlüssel zur Gattungsbestimmung der Blätter-, Leisten-und Röhrenpilze mit Literaturhinweisen zur Artbestimmung. Regensbg. Mykol. Schriften 2003, 11, 5–236. [Google Scholar]
- Xue, R.; Wu, L.L.; Jiang, S.; Hao, Y.J.; Chai, H.; Liang, Z.Q.; Zeng, N.K.; Su, M.S. Two new species of the genus Leccinellum (Boletaceae, Boletales) from the south of China. Phytotaxa 2019, 175, 133–140. [Google Scholar] [CrossRef]
- Binder, M.; Besl, H. 28S rDNA sequence data and chemotaxonomical analyses on the generic concept of Leccinum (Boletales). In Micologia; Associazone Micologica Bresadola: Trento, Italy, 2000; pp. 71–82. [Google Scholar]
- Bakker, H.C.; Noordeloos, M.E. A revision of European species of Leccinum Gray and notes on extralimital species. Persoonia 2005, 18, 511–574. [Google Scholar]
- Halling, R.E.; Mueller, G.M. Leccinum (Boletaceae) in Costa Rica. Mycologia 2003, 95, 488–499. [Google Scholar] [CrossRef]
- Meng, X.; Wang, G.S.; Wu, G.; Wang, P.M.; Yang, Z.L.; Li, Y.C. The genus Leccinum (Boletaceae, Boletales) from China based on morphological and molecular data. J. Fungi 2021, 7, 732. [Google Scholar] [CrossRef]
- Halling, R.E.; Nuhn, M.; Fechner, N.A.; Osmundson, T.W.; Soytong, K.; Arora, D.; Hibbett, D.S.; Binder, M. Sutorius: A new genus for Boletus eximius. Mycologia 2012, 104, 951–961. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Halling, R.E.; Amalfi, M.; Lumyong, S.; Raspé, O. An unexpectedly high number of new Sutorius (Boletaceae) species from northern and northeastern Thailand. Front. Microbiol. 2021, 12, 643505. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Geng, C.A.; Li, T.Z.; Huang, X.Y.; Ma, Y.B.; Zhang, X.M.; Wu, G.; Yang, Z.L.; Chen, J.J. Spiroseoflosterol, a Rearranged Ergostane-Steroid from the Fruiting Bodies of Butyriboletus roseoflavus. J. Nat. Prod. 2020, 83, 1706–1710. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Di, Y.T.; Zhang, Y.; Hu, X.J. Four new compounds from Neoboletus magnificus. Nat. Prod. Res. 2020, 34, 1152–1157. [Google Scholar] [CrossRef]
- Bahram, M.; Netherway, T. Fungi as mediators linking organisms and ecosystems. FEMS Microbiol. Rev. 2022, 46, fuab058. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Miyauchi, S.; Morin, E.; Kuo, A.; Drula, E.; Varga, T.; Kohler, A.; Feng, B.; Cao, Y.; Lipzen, A.; et al. Evolutionary innovations through gain and loss of genes in the ectomycorrhizal Boletales. New Phytol. 2022, 233, 1383–1400. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).