Abstract
This study aimed to investigate the association between cognitive impairment and polypharmacy in patients with atrial fibrillation prone to cognitive decline, and to elucidate if the Dementia Assessment Sheet for Community-based Integrated Care System 21-Items (DASC-21) severity classification indicates drug adjustment. This retrospective cohort study used the DASC-21 and Diagnosis Procedure Combination data at a specialised geriatric hospital with patients hospitalised between April 2019 and March 2022. The association between cognitive severity evaluated using the DASC-21 and polypharmacy was investigated using a multivariate logistic regression model. Data of 1191 inpatients (44.3% aged ≥85 years, 49.0% male) were analysed. Compared with severe cognitive impairment, mild (odds ratio [OR]: 3.33, 95% confidence interval [CI]: 1.29–8.57) and moderate (OR: 2.46, 95% CI: 1.06–5.72) impairments were associated with concurrent use of ≥6 medications. Antithrombotics were related to polypharmacy. The ORs did not change with 6, 8, or 10 medications (2.11 [95% CI: 1.51–2.95, p < 0.001], 2.42 [95% CI: 1.79–3.27, p < 0.001], and 2.01 [95% CI: 1.46–2.77, p < 0.001], respectively). DASC-21 severity was associated with polypharmacy in patients with atrial fibrillation, with a trend toward decreased polypharmacy from moderate to severe. The DASC-21 may serve as an indicator for drug adjustment in clinical practice.
1. Introduction
Atrial fibrillation is a disease whose severity increases with age, and its global prevalence increases yearly. A 10-year analysis of the Framingham Heart Study revealed high rates of the incidence, prevalence, and risk factors of atrial fibrillation, such as post-onset stroke and mortality [1]. The prevalence of atrial fibrillation increases approximately four-fold in men and women. An age-adjusted incidence rate also shows a similar trend [1]. Since atrial fibrillation is a high risk factor for cerebral infarction, antithrombotic medications are the mainstay of medication therapy to prevent cerebral infarction.
Older patients often have multimorbidity, difficulties in diet and exercise therapy, and poor medication adherence; therefore, polypharmacy is common among them. Polypharmacy is associated with increased rates of adverse events, including drug interactions, adverse drug events (ADEs), falls due to dizziness, hospitalisation, prolonged hospital stays, readmission immediately after discharge, and mortality [2,3,4]. In a meta-analysis, the pooled prevalence of adverse drug reactions in older inpatients was 22%, and polypharmacy and potentially inappropriate medication use were predictors of ADEs during hospitalisation [5]. In line with this, various methods for improving polypharmacy have been assessed, including the development of qualitative assessment tools such as the Screening Tool of Older Persons’ Prescriptions (STOPP) criteria [6] and Beers criteria [7], as well as proposals to understand the reasons for taking medications and assessing medication risks and benefits [8]. Reducing or modifying inappropriate prescriptions has been validated in randomised trials [9,10,11] and observational studies [12,13], thereby recognising the effectiveness of pharmacist-led interventions.
Cognitive decline has also been reported to be closely associated with medication adherence [14,15,16,17] and may be an essential indicator for clinicians to decide whether or not to continue medication therapy. Regarding medication adjustment, which includes deprescribing as a process to improve medication adherence in the older patients, the suitability of the Comprehensive Geriatric Assessment (CGA) [18,19], a method that represents problems in areas such as cognitive function, activities of daily living (ADLs), psychology, nutrition, medications, and social status of patients, has been reported [20,21,22,23,24]. The Dementia Assessment Sheet for Community-based Integrated Care System 21-Items (DASC-21) is an effective tool for CGA. It can be used to evaluate behavioural changes related to cognitive impairment and impairment in daily living. It is characterised by its full range of instrumental activities of daily living (IADLs; six items), making it easy to detect impairment in daily living among individuals with mild dementia [25]. In this regard, the DASC-21 may be more likely to identify executive functions, including self-management of medications, than other cognitive function screenings, and is considered reliable, especially when completed by family members and caregivers who know the patient well [25]. The higher the DASC-21 score, the more severe the cognitive impairment and the likelier it is the time to consider medication adjustments for older patients with advanced functional disability. Patients with atrial fibrillation are prone to multimorbidity and polypharmacy due to rate control. Medication adjustments are often required to avoid adverse events because polypharmacy in atrial fibrillation is associated with increased breeding and all-cause mortality [26]. However, it is unclear how cognitive function affects polypharmacy in older patients with atrial fibrillation.
Therefore, this study aimed to investigate the association between cognitive impairment and polypharmacy in patients with atrial fibrillation to elucidate an indicator for medication adjustment by professionals in health care.
2. Methods
2.1. Study Design and Data Sources
This retrospective, cross-sectional study used the DASC-21 with the Diagnosis Procedure Combination (DPC) data [27] at the Tokyo Metropolitan Institute for Geriatrics and Gerontology, an acute-care hospital for older patients. Since its establishment, this hospital has been conducting interdisciplinary research on ageing as a core institute in Japan. It functions as a knowledge bank and a source for capable researchers in gerontology. In our study, the data of patients hospitalised and discharged between April 2019 and March 2022 were used. DPC data have been collected in Japan since 2003 as a component of the case-mix system implemented in acute-care hospitals [28]. The DASC-21 questionnaire reflects cognitive and life functions [25] and is frequently used in geriatric hospitals.
2.2. Study Population
Data on hospitalised patients diagnosed with atrial fibrillation following the International Classification of Diseases, 10th Revision (ICD-10) codes I480, I481, I482, and I489 were extracted from the DPC database and disease names at discharge. We extracted data on patients with atrial fibrillation comorbidities that could not be identified in the DPC data to extract more patients with atrial fibrillation. Furthermore, patient responses to the DASC-21 were identified. Patients without regular oral medications, missing co-payment and ADL information, and who died during hospitalisation were excluded.
2.3. Ethics Consideration
The ethics committee of the Tokyo Metropolitan Geriatric Hospital and Research Institute approved this study (approval no. R22-021). The opt-out consent model was adopted because the authors received all data after anonymisation. All analyses followed the tenets of the Declaration of Helsinki.
2.4. Variables
The outcome measurement was polypharmacy, defined as the concurrent use of ≥6 medications (the definition of polypharmacy in other countries is five or more medications, but this study was conducted only in one hospital in Japan, so the Japanese definition of six or more medications was used.). The thresholds were changed to ≥8 or ≥10 to assess the level of polypharmacy. Since prescriptions for primary medical care are determined the day before discharge, the number of medications (oral, patch, and inhalation medications) used a day before discharge was considered as the number of medications used regularly at home. DASC-21 responses provided by the patient’s family or caregiver were obtained before admission at the hospital [25]. If family members or caregivers were unavailable, the responses of the patient or a care manager were considered. A previous study reported that the Cronbach’s alpha coefficient values for the DASC-21 responses provided by patients’ family members, other responders, and trained nurses were 0.934, 0.950, and 0.808, respectively, and that the tool is sufficiently reliable and valid for assessing cognitive and life function impairment, detecting dementia, and assessing dementia severity [25]. The results obtained using the DASC-21 correlate significantly with those obtained using the Mini-Mental State Examination (MMSE), Frontal Assessment Battery, and Clinical Dementia Rating (CDR) total and box scores [25]. The DASC-21 data were classified into four categories based on the DASC-21 Assessment Manual: (1) normal (no cognitive impairment), (2) mild cognitive impairment, (3) moderate cognitive impairment, and (4) severe cognitive impairment [29]. Patients’ characteristics, such as sex, age, height, weight, patient co-payment information, ADLs at admission and discharge, length of hospital stay (LOS), diagnosis codes, emergency hospitalisation, discharge destination (household, transfer), hospitalisation pathway (household, transfer), and medications were extracted from the DPC data. Age was classified as ≤74 years, 75–84 years (the late stage of ageing in Japan), and ≥85 years. LOS was divided into interquartile ranges, and we used the fourth quartile, where hospitalisation is prolonged, as the variable. Furthermore, data on antithrombotic medications (direct oral anticoagulants and warfarin), benzodiazepines (BZs), proton pump inhibitors (PPIs), 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), and medications identified using the Screening Tool for Older Person’s Appropriate Prescriptions for Japanese (STOPP-J) [30] (Supplementary File S1), were extracted from the DPC data. BZs were excluded from the original STOPP-J. The Charlson Comorbidity Index (CCI) was calculated following the coding algorithms by Quan et al. and used as a measure of the chronic illness burden [31].
2.5. Statistical Analysis
To summarise patient characteristics, continuous variables are expressed as the mean and standard deviation or the median and interquartile range (IQR), depending on the variable distribution. Categorical variables are expressed as proportions. Differences between groups were compared using the Mann–Whitney U test or chi-squared test. The association between the severity of cognitive impairment, as assessed using the DASC-21, and polypharmacy was investigated using a multivariate logistic regression model adjusted for the following covariates: sex, age, body mass index (BMI), LOS, hospitalisation pathway, emergency admission, discharge destination, CCI, connective tissue disease/rheumatic disease, peptic ulcer disease, diabetes without complications, diabetes with complications, renal disease, antithrombotics, BZs, medications identified using STOPP-J, and statins. Adjusted odds ratios and 95% confidence intervals (CIs) were calculated. Multiple comparisons of the number of medications per 100 patients according to the four categories based on the DASC-21 were performed using Steel’s test and referenced severe classification. Statistical significance was set at a two-tailed p-value of ≤0.05. All analyses were conducted using JMP Pro 16.2.0 (SAS Institute Inc., Cary, NC, USA).
3. Results
Figure 1 shows the flowchart of patient selection. A total of 1933 inpatients participated in this study. However, after excluding 598 patients without the DASC-21 responses, 45 with unknown or no medications, 11 with missing co-payment information, 2 with unknown ADL assessment results at admission, 3 with unknown ADL assessment results at discharge, and 84 who died during hospitalisation, only 1191 patients were included in the final analysis. Figure 2 shows the relationship between the four DASC-21 severity categories and the number of medications.
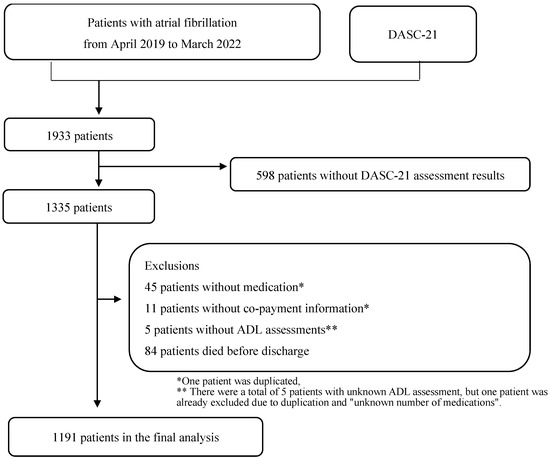
Figure 1.
Flow diagram of patient selection.
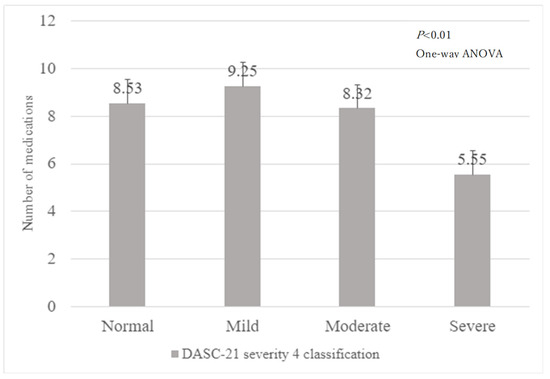
Figure 2.
Number of medications by DASC-21 severity 4 classification.
DASC-21: Dementia Assessment Sheet for Community-based Integrated Care System 21-Items and ADL: activity of daily living.
Patient characteristics are summarised in Table 1. Of the 1191 patients, 15.6%, 40.1%, and 44.3% were aged ≤74 years, 75–84 years, and ≥85 years, respectively. Furthermore, 16.5%, 60.1%, and 23.3% had a BMI of <18.5 kg/m2, 18.5–25 kg/m2, and ≥25 kg/m2, respectively. The CCI was 0, 1 or 2, and 3 for 10.1%, 57.6%, and 32.3% of the patients, respectively. Medications identified using STOPP-J were used by 14.0% of the patients. The overall mean number of medications was 8.0 (IQR: 6–11), with 4.0 (IQR: 3–5) patients taking ≤5 medications, and 9.0 (IQR: 8–12) taking ≥6 medications. The mean DASC-21 score of all patients was 28.0 (IQR: 23–45).

Table 1.
Characteristics of the study population.
Table 2 shows the association between cognitive impairment severity as determined based on the DASC-21 and polypharmacy. Compared with severe impairment, mild and moderate cognitive impairments were associated with using ≥6 medications (OR: 3.33, 95% CI: 1.29–8.57; OR: 2.46, 95% CI: 1.06–5.72, respectively). However, DASC-21 cognitive severity, normally referenced as severe, was not associated with the use of ≥6 polypharmacy medications (OR: 2.04, 95% CI: 0.84–4.94), but was associated with the use of ≥8 (OR: 3.13, 95% CI: 1.23–8.01) and ≥10 medications (OR: 3.76, 95% CI: 1.04–13.5). The patients who used ≥10 medications were older (OR: 1.55, 95% CI: 1.01–2.40), had a longer LOS (OR: 1.69, 95% CI: 1.21–2.37), and had many comorbidities (OR: 1.63, 95% CI: 0.95–2.81). Antithrombotic medication use was associated with polypharmacy, and the ORs did not change significantly regardless of whether the patients were taking >6, 8, or 10 medications (2.11 [95% CI: 1.51–2.95, p < 0.001], 2.42 [95% CI: 1.79–3.27, p < 0.001], and 2.01 [95% CI 1.46–2.77, p < 0.001], respectively).

Table 2.
Association between cognitive impairment severity evaluated using the DASC-21 and polypharmacy.
Table 3 shows a multiple comparison analysis for the mean number of medications on each severity referenced as severe classification. Regarding the numbers of medication for antithrombotics (Normal: p = 0.501, Mild: p = 0.855, and Moderate: p = 0.855) and STOPP-J (Normal: p = 0.577, Mild: p = 0.491, and Moderate: p = 0.356), there was no decrease in the number of medications compared with severe classification. The number of medications in antithrombotics was stable among normal, mild, and moderate DASC-21 severity cognitive impairment compared with severe (76.7 ± 42.3, 73.4 ± 44.3, 73.2 ± 44.3, and 69.6 ± 46.7, respectively). A similar trend was observed for STOPP-J (8.9 ± 31.6, 19.6 ± 41.4, 21.7 ± 43.9, and 12.1 ± 33.1, respectively).

Table 3.
Multiple comparison analysis of the number of medications per the severity of cognitive impairment based on the DASC-21.
DASC-21: Dementia Assessment Sheet for Community-based Integrated Care System 21-Items; SD: standard deviation; and STOPP-J: Screening Tool for Older Person’s Appropriate Prescriptions for Japanese.
4. Discussion
This study assessed the association between cognitive impairment and polypharmacy in patients with atrial fibrillation to determine the indicators of medication adjustment by healthcare professionals. We found that severe cognitive impairment was negatively associated with concurrently using ≥6 medications, compared with mild or moderate cognitive impairment. A similar trend was noted in patients who used ≥8 and ≥10 medications. The results showed that the OR for each polypharmacy medication decreased from mild to severe cognitive impairment, and the total number of medications decreased with worsening cognitive function severity. Moderate DASC-21 severity is associated with impairments in remote memory, location orientation, social judgement, or physical ADLs [29]. The presence of moderate dementia and polypharmacy may prompt the goals of care discussion regarding the relative benefits of individual medications. This period is likely to be characterised by moderate to severe executive dysfunction and difficulty maintaining medication adherence, and may be the starting point for medication adjustment. Furthermore, severely impaired patients have impairments in remote memory, location awareness, social judgement, and physical ADLs, which may require considering treatment with end-of-life care in mind. Current or anticipated side effects, inappropriate polypharmacy, progression of frailty and cognitive and physical dysfunction, and shorter life expectancy as triggers for prescription discontinuation or drug adjustment have been referred to in various references [32,33,34], supporting the present study.
In the DASC-21 severity classifications, BZ, PPI, and statin use was significantly lower in severe disease than in normal and mild disease, however, this trend was not observed for STOPP-J and antithrombotic drugs. The use of antithrombotic medications to prevent cerebral infarction can have serious outcomes if discontinued. Previous studies have also demonstrated that these medications cannot be easily discontinued, even in the presence of cognitive decline or deterioration in ADLs [35], which is in line with our findings. In addition, STOPP-J contains many antipsychotics, which may increase the likelihood of prescribing due to delirium or altered consciousness associated with the progression of frailty. Reportedly, the prescribing of antipsychotics increases toward the end of life [36], which is also in line with our findings. Furthermore, it has been reported that statins should not be discontinued, even in patients with advanced severe dementia, because of the prognostic value of coronary artery disease and other conditions [37]. On the other hand, in a report of attempted statin discontinuation in patients with poor prognosis for life, discontinuation was safe [38]. In the present study, the number of medications was reduced in patients with severe disease on the DASC-21. This may be attributed to the fact that the DASC-21 reflects cognitive as well as life functions, thus identifying patients with more severe disease than other scales, resulting in a reduction of statin use as well. It is also reasonable to assume that the use of BZ was discontinued because of the high risk of delirium and falls, and concerns regarding adverse events and cognitive dysfunction associated with worsening ADLs [39]. In older patients with atrial fibrillation, the DASC-21 severity classification may be used as a discussion tool to initiate medication adjustments, including medication reductions, especially for moderate to severe cases. To our knowledge, this is the first study to report on the clinical assessment of cognitive impairment to determine the association between cognitive impairment severity and the number of medications in older patients with atrial fibrillation. There has been no consensus on the various factors that lead to initiating medication adjustments. While studies using the CGA and other methods have been reported [23], no evaluation method solely focuses on medication adjustment, and much is left to the healthcare professional’s judgement. It is also important to consider medication adjustments when changes in physical function occur. In a retrospective study conducted in the UK among hypertensive patients aged >80 years, there was a higher rate of non-adherence to antihypertensive medication in the 5 years before death among non-survivors than among survivors [40]. Patients with severe cognitive impairment, judged using the DASC-21, were frail, and based on the present results, medication adjustment at that point appeared to be a common clinical response. However, the number of medications administered reportedly did not decrease in patients with heart failure, even if they showed functional impairment and other factors, such as the underlying disease [41]. As previously mentioned, moderate severity of DASC-21 may be associated with impairments in remote memory, place orientation, social judgment, or physical ADLs, [29]; however, its association with a decreased number of medications remains unknown. As the severity of DASC-21 increases, instrumental, which are executive functions, and physical ADLs are more likely to decline, and inadequate social support, such as medication assistance, could be a risk for rehospitalisation [42,43]. Therefore, from the perspective of healthcare professionals, a decline in physical and executive functions may be the starting point for medication reconciliation. It is important to use CGAs, such as the DASC-21, to adjust medications while considering the disease significance and prioritisation of medications from a geriatric viewpoint.
Antithrombotic medication use was associated with polypharmacy, even in cases in which polypharmacy continued. In addition, there was no decrease in antithrombotic medication use over the cognitive severity classification. This suggests that antithrombotic medications may be preferentially continued despite worse cognitive status using the DASC-21, reflecting goals of care considerations. Frail patients were reported as less likely to be prescribed direct oral anticoagulants than cognitively intact patients [44]; however, antithrombotic medications were continued in terminally ill patients with severe dementia despite them showing a variety of coexisting functional impairments [35]. Non-adherence to antithrombotic medication was associated with cognitive decline (as judged using the MMSE), and it was not linked to ADL or IADL impairment [45]. Therefore, it is important to use antithrombotic medications safely in frail patients. There is no doubt that eliminating polypharmacy, including the adjustment of inappropriate medications, is vital to reduce the risk of adverse events and interactions in frail patients with atrial fibrillation. In this context, when a patient is unable to take their medication, advance care planning (ACP) should be agreed upon with the patient and family.
This study has several limitations. Due to data construction, the number of medications in the inpatient setting was used in the analysis. Essentially, the number of medications for outpatients with stable disease is desirable for analysis. Since the Japanese healthcare system is still in the process of functional differentiation of hospital beds compared to other countries, patients are often treated in the hospital until their diseases stabilise, and the number of medications at discharge approximates the number of medications in the outpatient setting. Therefore, to minimise the impact of the number of medications, the number of medications on the day before discharge was extracted. We used DPC data recorded during insurance claims for this analysis, however, an important limitation is that insurance claims data may not capture all diseases present in patients. To address these, we used the CCI to account for comorbidities and further extracted and adjusted the number of contributing diseases to eliminate as many influences as possible. The breakdown of responders to the DASC-21 questionnaire in this study was 5 from unknown, 321 from the patients themselves, 21 from facility staff, and 844 (70.9%) from family members. The DASC-21 assessment showed that when family members responded, Cronbach’s alpha was higher, and the association with various dementia assessments (MMSE and CDR) was stronger [25]. The high response rate of family members in this study suggests that the DASC-21 questionnaire is more reliable than that intended in the original paper. However, it is unclear whether the family members who responded under the special circumstances of hospitalisation accurately understood the patient’s cognitive and life functions. Since this study was conducted at a single institution, the results of this study cannot be applied to other countries. Therefore, it is important to consider each country’s healthcare systems and demographics, and future studies examining this issue in multiple populations are warranted.
5. Conclusions
We found that severe cognitive impairment, as judged using the DASC-21, was associated with decreased polypharmacy in patients with atrial fibrillation. On the other hand, antithrombotic medications continued to be used in these patients regardless of DASC-21 severity. This suggests that there may be some risk/benefit-related ACP, depending on the class of medications. We believe that the DASC-21-based evaluation of cognition among patients with atrial fibrillation may allow for medication adjustment in clinical practice, and that healthcare professionals can maximise patient benefits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/geriatrics9010015/s1, File S1: Generic name and ATC code for each drug type.
Author Contributions
Conceptualisation, Y.S., K.K., J.I., R.I., A.A. and S.I.; Data curation, Y.S.; Formal analysis, Y.S. and S.I.; Funding acquisition, S.I.; Methodology, Y.S., K.K., J.I., R.I., A.A. and S.I.; Project administration, Y.S. and S.I.; Software, Y.S. and S.I.; Supervision, J.I., R.I., A.A. and S.I.; Writing—original draft, Y.S.; Writing—review and editing, K.K., J.I., R.I., A.A. and S.I. All authors will be informed about each step of manuscript processing, including submission, revision, and revision reminder, via emails from our system or assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Tokyo Metropolitan Institute for Geriatrics and Gerontology (Approval no. R22-021 and date of Approval 18 August 2022).
Informed Consent Statement
This study used opt-out consent because all data were anonymized before being received by the authors.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available to protect the participants’ privacy.
Conflicts of Interest
The authors, except for A. Araki, have no conflicts of interest to declare. A. Araki has received honoraria from Dainippon Sumitomo Pharma Co, Ono Pharmaceuticals, and Novo Nordisk Pharma K.K.
References
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Rollason, V.; Vogt, N. Reduction of polypharmacy in the elderly: A systematic review of the role of the pharmacist. Drugs Aging 2003, 20, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Seymour, D.G.; Primrose, W.R. A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing 2004, 33, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Frazier, S.C. Health outcomes and polypharmacy in elderly individuals: An integrated literature review. J. Gerontol. Nurs. 2005, 31, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Yadesa, T.M.; Kitutu, F.E.; Deyno, S.; Ogwang, P.W.; Tamukong, R.; Alele, P.E. Prevalence, characteristics and predicting risk factors of adverse drug reactions among hospitalized older adults: A systematic review and meta-analysis. SAGE Open Med. 2021, 9, 20503121211039100. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef]
- 2019 American Geriatrics Society Beers Criteria® Update Expert Panel; Fick, D.M.; Semla, T.P.; Steinman, M.; Beizer, J.; Brandt, N.; Dombrowski, R.; DuBeau, C.E.; Pezzullo, L.; Epplin, J.J.; et al. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Scott, I.A.; Hilmer, S.N.; Reeve, E.; Potter, K.; Le Couteur, D.; Rigby, D.; Gnjidic, D.; Del Mar, C.B.; Roughhead, E.E.; Page, A.; et al. Reducing inappropriate polypharmacy: The process of deprescribing. JAMA Intern. Med. 2015, 175, 827–834. [Google Scholar] [CrossRef]
- Frankenthal, D.; Lerman, Y.; Kalendaryev, E.; Lerman, Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: A randomized clinical trial. J. Am. Geriatr. Soc. 2014, 62, 1658–1665. [Google Scholar] [CrossRef]
- Ravn-Nielsen, L.V.; Duckert, M.-L.; Lund, M.L.; Henriksen, J.P.; Nielsen, M.L.; Eriksen, C.S.; Buck, T.C.; Pottergard, A.; Hansen, M.R.; Hallas, J. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: A randomized clinical trial. JAMA Intern. Med. 2018, 178, 375–382. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, M.; Izquierdo, M.; Beobide-Telleria, I.; Ferro-Uriguen, A.; Alonso-Renedo, J.; Casas-Herrero, Á.; Martínez-Velilla, N. Medicine optimization strategy in an acute geriatric unit: The pharmacist in the geriatric team. Geriatr. Gerontol. Int. 2019, 19, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Hatano, M.; Mizuno, T.; Arakawa, Y.; Inagaki, R.; Kato, A.; Matsuzaki, H.; Mizokami, F.; Koseki, T.; Yamada, S. Efficacy of a pharmacist team clinical medication review in older adults: A prospective and retrospective observational study. Biol. Pharm. Bull. 2022, 45, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Stuijt, C.C.M.; Franssen, E.J.F.; Egberts, A.C.G.; Hudson, S.A. Appropriateness of prescribing among elderly patients in a Dutch residential home: Observational study of outcomes after a pharmacist-led medication review. Drugs Aging 2008, 25, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.-J.; Lee, K.; Kim, B.-H.; Son, Y.-J. Cognitive impairment is independently associated with non-adherence to antithrombotic therapy in older patients with atrial fibrillation. Int. J. Environ. Res. Public Health 2019, 16, 2698. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.L.; Boustani, M.A.; Skopelja, E.N.; Gao, S.; Unverzagt, F.W.; Murray, M.D. Medication adherence in older adults with cognitive impairment: A systematic evidence-based review. Am. J. Geriatr. Pharmacother. 2012, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Shin, D.W.; Chang, S.-A.; Lee, J.E.; Jeong, S.-M.; Kim, S.H.; Yun, J.M.; Son, K. Association between cognitive impairment and poor antihypertensive medication adherence in elderly hypertensive patients without dementia. Sci. Rep. 2018, 8, 11688. [Google Scholar] [CrossRef]
- Salas, M.; In’t Veld, B.A.; van der Linden, P.D.; Hofman, A.; Breteleer, M.; Stricker, B.H. Impaired cognitive function and compliance with antihypertensive drugs in elderly: The Rotterdam Study. Clin. Pharmacol. Ther. 2001, 70, 561–566. [Google Scholar] [CrossRef]
- Stuck, A.E.; Siu, A.L.; Wieland, G.D.; Adams, J.; Rubenstein, L.Z. Comprehensive geriatric assessment: A meta-analysis of controlled trials. Lancet 1993, 342, 1032–1036. [Google Scholar] [CrossRef]
- Devons, C.A.J. Comprehensive geriatric assessment: Making the most of the aging years. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 19–24. [Google Scholar] [CrossRef]
- Unutmaz, G.D.; Soysal, P.; Tuven, B.; Isik, A.T. Costs of medication in older patients: Before and after comprehensive geriatric assessment. Clin. Interv. Aging 2018, 13, 607–613. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Sarti, S.; Manzato, E. Polypharmacy in the elderly: Can comprehensive geriatric assessment reduce inappropriate medication use? Drugs Aging 2011, 28, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, T.; Yamana, H.; Tamiya, H.; Matsui, H.; Fushimi, K.; Akishita, M.; Yasunaga, H.; Ogawa, S. Association between comprehensive geriatric assessment and polypharmacy at discharge in patients with ischaemic stroke: A nationwide, retrospective, cohort study. eClinicalMedicine 2022, 50, 101528. [Google Scholar] [CrossRef] [PubMed]
- Lea, S.C.; Watts, K.L.; Davis, N.A.; Panayiotou, B.; Bankart, M.J.; Arora, A.; Chanmbers, R. The potential clinical benefits of medicines optimisation through comprehensive geriatric assessment, carried out by secondary care geriatricians, in a general practice care setting in North Staffordshire, UK: A feasibility study. BMJ Open 2017, 7, e015278. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Woolford, S.J.; Patel, H.P. Multi-morbidity and polypharmacy in older people: Challenges and opportunities for clinical practice. Geriatrics 2020, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Awata, S.; Sugiyama, M.; Ito, K.; Ura, C.; Miyamae, F.; Sakuma, N.; Niikawa, H.; Okamuram, T.; Inagaki, H.; Ijuin, M. Development of the dementia assessment sheet for community-based integrated care system. Geriatr. Gerontol. Int. 2016, 16 (Suppl. S1), 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.; Nyfort-Hansen, K.; Rowett, D.; Wong, C.X.; Middeldorp, M.E.; Mahajan, R.; Lau, D.H.; Sanders, P.; Hendriks, J.M. Polypharmacy and health outcomes in atrial fibrillation: A systematic review and meta-analysis. Open Heart 2020, 7, 001257. [Google Scholar] [CrossRef]
- Yamana, H.; Moriwaki, M.; Horiguchi, H.; Kodan, M.; Fushimi, K.; Yasunaga, H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J. Epidemiol. 2017, 27, 476–482. [Google Scholar] [CrossRef]
- Hayashida, K.; Murakami, G.; Matsuda, S.; Fushimi, K. History and profile of diagnosis procedure combination (DPC): Development of a real data collection system for acute inpatient care in Japan. J. Epidemiol. 2021, 31, 1–11. [Google Scholar] [CrossRef]
- dasc.jp. What Is DASC-21; DASC-21 Manual. Available online: https://dasc.jp/en/about (accessed on 18 January 2023).
- Kojima, T.; Mizukami, K.; Tomita, N.; Arai, H.; Ohrui, T.; Eto, M.; Takeya, Y.; Isaka, Y.; Rakugi, H.; Sudo, N.; et al. Screening Tool for Older Persons’ Appropriate Prescriptions for Japanese: Report of the Japan Geriatrics Society Working Group on “Guidelines for medical treatment and its safety in the elderly”. Geriatr. Gerontol. Int. 2016, 16, 983–1001. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-H.; Saunders, L.D.; Beck, C.A.; Feasby, T.; Ghali, W. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Fried, T.R.; Mecca, M.C. Medication Appropriateness in Vulnerable Older Adults: Healthy Skepticism of Appropriate Polypharmacy. J. Am. Geriatr. Soc. 2019, 67, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Vordenberg, S.E.; Malani, P.N.; Kullgren, J.T. Polypharmacy and deprescribing. JAMA 2023, 330, 672. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, A.; Steinman, M.A.; Goyal, P.; Zullo, A.R.; Anderson, T.S.; Birtcher, K.K.; Goodlin, S.J.; Maurer, M.S.; Alexander, K.P.; Rich, M.W.; et al. Deprescribing in Older Adults With Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 73, 2584–2595. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, G.M.; Fried, T.R.; Gilstrap, L.G.; O’Leary, J.R.; Austin, A.M.; Skinner, J.S.; Cohen, A.B. Anticoagulant use for atrial fibrillation among persons with advanced dementia at the end of life. JAMA Intern. Med. 2021, 181, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Paque, K.; De Schreye, R.; Elseviers, M.; Stichele, R.V.; Pardon, K.; Dilles, T.; Christiaens, T.; Deliens, L.; Cohen, J. Discontinuation of medications at the end of life: A population study in Belgium, based on linked administrative databases. Br J. Clin. Pharmacol. 2019, 85, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Tjia, J.; Cutrona, S.L.; Peterson, D.; Reed, G.; Andrade, S.E.; Mitchell, S.L. Statin discontinuation in nursing home residents with advanced dementia. J. Am. Geriatr. Soc. 2014, 62, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Kutner, J.S.; Blatchford, P.J.; Taylor, D.H., Jr.; Ritchie, C.S.; Bull, J.H.; Fairclough, D.L.; Hanson, L.C.; LeBlanc, T.W.; Samsa, G.P.; Wolf, S.; et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 691–700. [Google Scholar] [CrossRef]
- 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef]
- Ravindrarajah, R.; Hazra, N.C.; Hamada, S.; Charlton, J.; Jackson, S.H.D.; Dregan, A.; Gulliford, M.C. Systolic blood pressure trajectory, frailty, and all-cause mortality >80 years of age: Cohort study using electronic health records. Circulation 2017, 135, 2357–2368. [Google Scholar] [CrossRef]
- Goyal, P.; Bryan, J.; Kneifati-Hayek, J.; Sterling, M.R.; Banerjee, S.; Maurer, M.S.; Lachs, M.S.; Safford, M.M. Association between functional impairment and medication burden in adults with heart failure. J. Am. Geriatr. Soc. 2019, 67, 284–291. [Google Scholar] [CrossRef]
- Kuzuya, M.; Hirakawa, Y.; Suzuki, Y.; Iwata, M.; Enoki, H.; Hasegawa, J.; Iguchi, A. Association between unmet needs for medication support and all-cause hospitalization in community-dwelling disabled elderly people. J. Am. Geriatr. Soc. 2008, 56, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Birge, S.J. Cognitive dysfunction, medication management, and the risk of readmission in hospital inpatients. J. Am. Geriatr. Soc. 2016, 64, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Romiti, G.F.; Vitolo, M.; Harrison, S.L.; Lane, D.A.; Fauchier, L.; Marin, F.; Näbauer, M.; Potpara, T.S.; Dan, G.-A.; et al. Epidemiology and impact of frailty in patients with atrial fibrillation in Europe. Age Ageing 2022, 51, afac192. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barba, B.; Navarrete-Reyes, A.P.; Avila-Funes, J.A. Are geriatric syndromes associated with reluctance to initiate oral anticoagulation therapy in elderly adults with nonvalvular atrial fibrillation? J. Am. Geriatr. Soc. 2013, 61, 2236–2237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).