Frailty and Survivability of Polish Caucasian Nonagenarians and Centenarians
Abstract
1. Introduction
2. Material and Methods
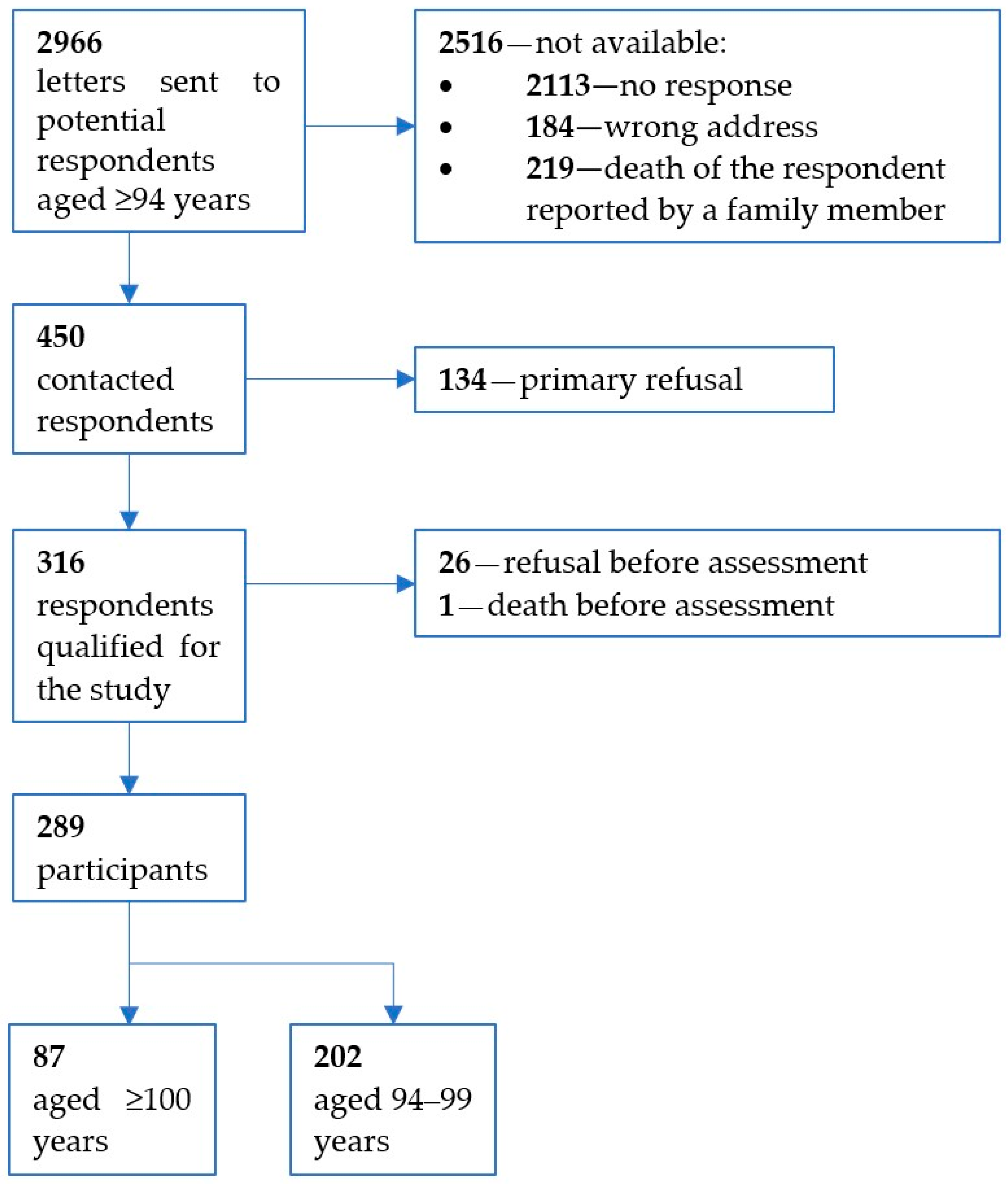
2.1. Study Sample and Procedures
2.2. Assessment of Frailty
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Cohort
3.2. Frailty and Handgrip Strength Measurement
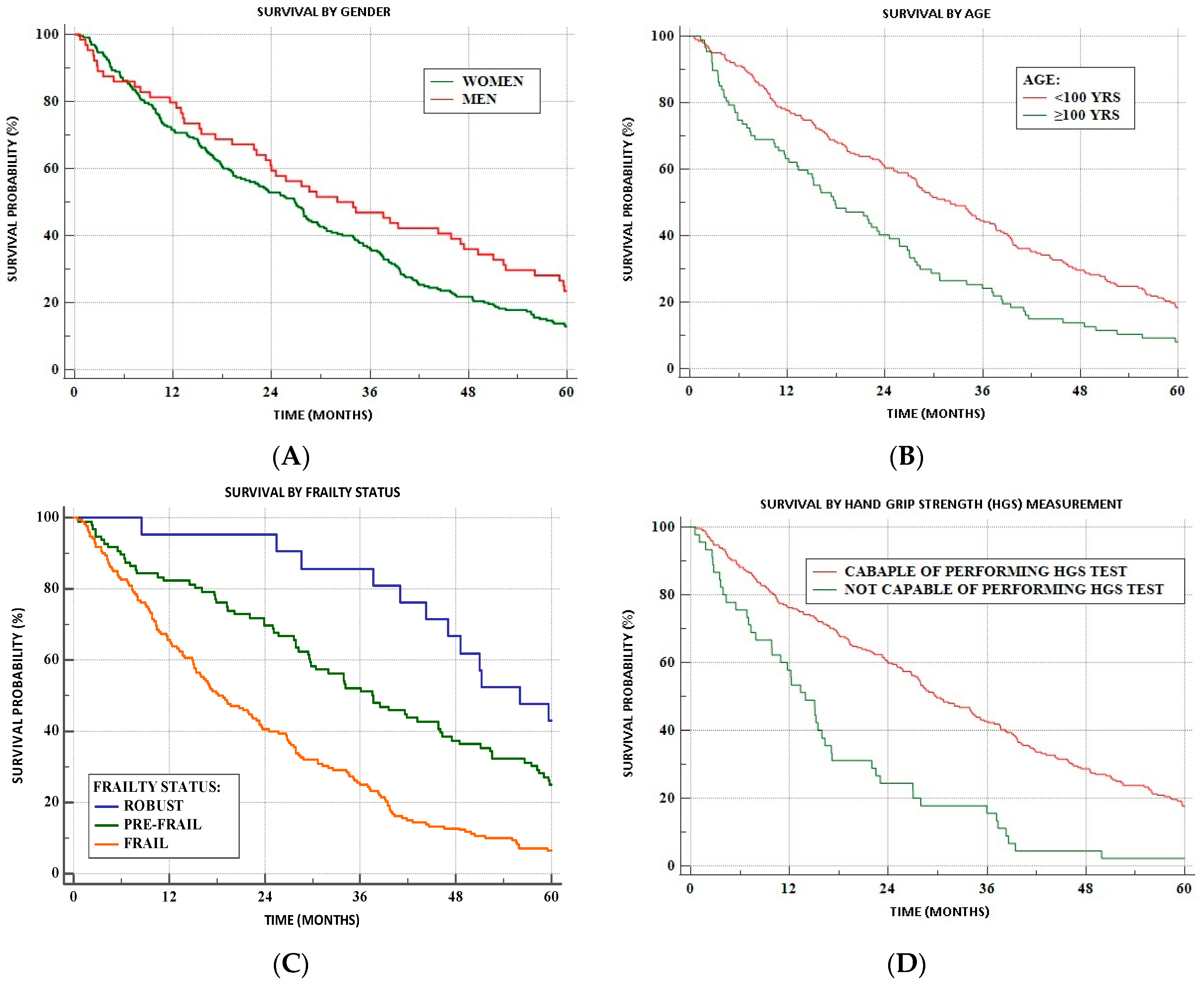
3.3. Survivability
4. Discussion
4.1. Prevalence of Frailty Using Frailty Phenotype
4.2. Prevalence of Frailty Criteria
4.3. Other Frailty Scales
4.4. Correlates of Frailty
4.5. Frailty and Mortality
4.6. Handgrip Strength
4.7. Strengths and Limitations of the Study
4.8. Future Perspectives for Frailty Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Takeda, C.; Angioni, D.; Setphan, E.; Macaron, T.; De Souto Barreto, P.; Sourdet, S.; Sierra, F.; Vellas, B. Age-related frailty: A clinical model for geroscience? J. Nutr. Health Aging 2020, 24, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Gregson, E.; Theou, O.; Rockwood, K.; Howlett, S.E. The association between frailty, the metabolic syndrome, and mortality over the lifespan. GeroScience 2017, 39, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.H.; Peel, N.M.; Samanta, M.; Theou, O.; Howlett, S.E.; Hubbard, R.E. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 2017, 89, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Howlett, S.E. Sex differences in frailty: Comparisons between humans and preclinical models. Mech. Ageing Dev. 2021, 198, 111546. [Google Scholar] [CrossRef]
- Pilotto, A.; Custodero, C.; Maggi, S.; Polidori, M.C.; Veronese, N.; Ferruci, L. A multidimensional approach to frailty in older people. Ageing Res. Rev. 2020, 60, 101047. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Csete, M.E. Basic science of frailty—Biological mechanisms of age-related sarcopenia. Anesth. Analg. 2021, 132, 293–304. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kochlik, B.; Franz, K.; Henning, T.; Weber, D.; Wernitz, A.; Herpich, C.; Jannasch, F.; Aykaç, V.; Müller-Werdan, U.; Schulze, M.B.; et al. Frailty is characterized by biomarker patterns reflecting inflammation or muscle catabolism in multi-morbid patients. J. Cachexia Sarcopenia Muscle 2023, 14, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; Abellan Van Kan, G.; Ousset, P.-J.; Gillette-Guyonet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J. Nutr. Health Aging 2013, 17, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Facal, D.; Burgo, C.; Spuch, C.; Gaspar, P.; Campos-Magdaleno, M. Cognitive frailty: An update. Front. Psychol. 2021, 12, 813398. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Arai, H.; Sakurai, T. An update on cognitive frailty: Its definition, impact, associated factors and underlying mechanisms, and interventions. Geriatr. Gerontol. Int. 2022, 22, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.-L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 21, 53–61. [Google Scholar] [CrossRef]
- Checa-Lopez, M.; Oviedo-Briones, M.; Pardo-Gomez, A.; Gonzalez-Turin, J.; Guevera-Guevara, T.; Carnicero, J.A.; Alamo-Ascencio, S.; Landi, F.; Cesari, M.; Grodzicki, T.; et al. FRAILTOOLS study protocol: A comprehensive validation of frailty assessment tools to screen and diagnose frailty in different clinical and social settings and to provide instruments for integrated care in older adults. BMC Geriatr. 2019, 19, 86. [Google Scholar] [CrossRef]
- Mediouni, M.; Schlatterer, D. Frailty as an outcome predictor after ankle fractures: Where are we now? Geriatr. Ortop. Surg. Rehabil. 2018, 9, 1–3. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip strength: An indispensable biomarker for older adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef]
- Robine, J.M.; Cheung, S.L.; Saito, Y.; Jeune, B.; Parker, M.G.; Herrmann, F.R. Centenarians today: New insights on selection from the 5-COOP study. Curr. Gerontol. Geriatr. Res. 2010, 2010, 120354. [Google Scholar] [CrossRef]
- Herr, M.; Jeune, B.; Fors, S.; Andersen-Ranberg, K.; Ankri, J.; Arai, Y.; Cubaynes, S.; Santos-Eggiman, B.; Zekry, D.; Parker, M.; et al. Frailty and Associated Factors among Centenarians in the 5-COOP Countries. Gerontology 2018, 64, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Downs, T.D.; Cash, H.R.; Grotz, R.C. Progress in Development of the Index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, A.E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Population Projection 2014–2050; Statistical Analyses and Studies; Central Statistical Office (Poland): Warsaw, Poland, 2014.
- Rohrmann, S. Epidemiology of frailty in older people. In Frailty and Cardiovascular Diseases: Research into an Elderly Population; Veronese, N., Ed.; Springer: Cham, Switzerland, 2020; pp. 21–27. [Google Scholar] [CrossRef]
- Lewis, E.G.; Coles, S.; Howorth, K.; Kissima, J.; Gray, W.; Urasa, S.; Walker, R.; Dotchin, C. The prevalence and characteristics of frailty by frailty index in rural Tanzania. BMC Geriatr. 2018, 18, 283. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Lv, X.; Du, J.; Kong, G.; Zhang, L. Age- and Gender-Specific Prevalence of Frailty and Its Outcomes in the Longevous Population: The Chinese Longitudinal Healthy Longevity Study. Front. Med. 2021, 8, 719806. [Google Scholar] [CrossRef]
- Alqahtani, B.A.; Alenazi, A.M.; Alshehri, M.M.; Osailan, A.M.; Alsubaie, S.F.; Alqahtani, M.A. Prevalence of frailty and associated factors among Saudi community-dwelling older adults: A cross-sectional study. BMC Geriatr. 2021, 21, 185. [Google Scholar] [CrossRef]
- Cobden, J.; de Noronha, M.; Kingsley, M. Prevalence of frailty and mobility disability in older people living in retirement villages. Australas. J. Ageing 2022, 41, 222–228. [Google Scholar] [CrossRef]
- Menendez-Gonzalez, L.; Izaguirre-Riesgo, A.; Tranche-Iparraguirre, S.; Montero-Rodriguez, A.; Orts-Cortes, M.I. Prevalencia y factores asociados de fragilidad en adultos mayores de 70 anos en la comunidad. Aten. Primaria 2021, 53, 1021128. [Google Scholar] [CrossRef]
- Gagesch, M.; Chocano-Bedoya, P.O.; Abderhalden, L.A.; Freystaetter, G.; Sadlon, A.; Kanis, J.A.; Kressig, R.W.; Guyonnet, S.; DaSilva, J.A.P.; Felsenberg, D.; et al. Prevalence of physical frailty: Results from the DO-HEALTH Study. J. Frailty Ageing 2022, 11, 18–25. [Google Scholar] [CrossRef]
- Duarte, N.; Teixeira, L.; Ribeiro, O.; Paul, C. Frailty phenotype criteria in centenarians: Findings from the Oporto Centenarians Study. Eur. Geriatr. Med. 2014, 5, 371–376. [Google Scholar]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.L.; Walston, J.D.; Kasper, J.D. Frailty in older adults: A nationally representative profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Kawas, C.H.; Gibbs, L.; Corrada, M.M. Prevalence of frailty and factors associated with frailty in individuals aged 90 and older: The 90+ Study. J. Am. Geriatr. Soc. 2016, 64, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Marzetti, R.; Picca, A.; Calvani, R.; Cesari, M.; Uchida, M.C. Prevalence of prefrailty and frailty in South America: A systematic review of observational studies. J. Frailty Aging 2020, 9, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Geronikola, N.; Zalonis, I.; Ntanasi, E.; Charisis, S.; Kosmidis, M.H.; Anastasiou, C.A.; Dardiotis, E.; Hadjigeorgiou, G.; Megalou, M.; Velonakis, G.; et al. Sex differences in frailty incidence in Greek community-dwelling older people: The HELIAD Study. J. Frailty Aging 2022, 11, 250–255. [Google Scholar] [CrossRef]
- Sousa, V.D.; Rojjanasrirat, W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: A clear and user-friendly guideline. J. Eval. Clin. Pract. 2011, 17, 268–274. [Google Scholar] [CrossRef]
- De Oliveira, D.C.; de Oliveira Maximo, R.; Ramirez, P.C.; de Souza, A.F.; Luiz, M.M.; Delinocente, M.L.B.; Chagas, M.H.N.; Steptoe, A.; de Oliveira, C.; da Silva Alexandre, T. Is slowness a better discriminator of disability than frailty in older adults? J. Cachexia Sarcopenia Muscle 2021, 12, 2069–2078. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Kujawska-Danecka, H.; Jagiello, K.; Hajduk, A.; Skalska, A.; Mossakowska, M.; Zdrojewski, T.; Grodzicki, T.; Gasowski, J. The national burden of frailty and disproportionate distribution of its components—The predominance of slow gate speed: A 2018-19 face-to-face epidemiologic assessment representative of population of older Poles. Aging Clin. Exp. Res. 2023, 12, 1–9. [Google Scholar] [CrossRef]
- Van der Elst, M.C.J.; Schoenmakers, B.; Op het Veld, L.P.M.; De Roeck, E.E.; Van der Vorst, A.; Schols, J.M.G.A.; De Lepeleire, J.; Kempen, G.I.J.M.; D-SCOPE Consortium. Validation of replacement questions for slowness and weakness to assess the Fried Phenotype: A cross-sectional study. Eur. Geriatr. Med. 2020, 11, 793–801. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fan, W.; Zhu, B.; Ma, C.; Tan, X.; Gu, Y. Effects of age, period and cohort on the prevalence of frailty in Chinese older adults from 2022 to 2014. Front. Public Health 2022, 10, 935163. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.M.; Olivieri-Mui, B.; McCarthy, E.P.; Kim, D.H. Changes in a frailty index and association with mortality. J. Am. Geriatr. Soc. 2021, 69, 1057–1062. [Google Scholar] [CrossRef]
- Danilovich, M.K.; Diaz, L.; Corcos, D.M.; Ciolino, J.D. Relationship between SHARE-FI Frailty Scores and Physical Performance Measures in Older Adult Medicaid Recipients. Geriatrics 2018, 3, 51. [Google Scholar] [CrossRef]
- Salaffi, F.; Di Carlo, M.; Carotti, M.; Farah, S.; Giovagnoni, A. Frailty prevalence according to the Survey of Health, Ageing and Retirement in Europe-Frailty Instrument (SHARE-FI) definition, and its variables associated, in patients with symptomatic knee osteoarthritis: Findings from a cross-sectional study. Aging Clin. Exp. Res. 2021, 33, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Briones, M.; Rodriguez-Laso, A.; Carnicero, J.A.; Gryglewska, B.; Sinclair, A.J.; Landi, F.; Vellas, B.; Rodriguez Artalejo, F.; Checa-Lopez, M.; Rodriguez-Manas, L. The ability of eight frailty instruments to identify adverse outcomes across different settings: The FRAILTOOLS project. J. Cachexia Sarcopenia Muscle 2022, 13, 1487–1501. [Google Scholar] [CrossRef]
- Ziller, C.; Braun, T.; Thiel, C. Frailty phenotype prevalence in community-dwelling older adults according to physical frailty assessment method. Clin. Interv. Aging 2020, 15, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Tolley, A.P.L.; Ramsey, K.A.; Rojer, A.G.M.; Reijnierse, E.M.; Maier, A.B. Objectively measured physical activity is associated with frailty in community-dwelling older adults: A systematic review. J. Clin. Epidemiol. 2021, 137, 218–230. [Google Scholar] [CrossRef]
- Veronese, N.; Custodero, C.; Cella, A.; Demurtas, J.; Zora, S.; Maggi, S.; Barbagallo, M.; Sabba, C.; Ferrucci, L.; Pilotto, A. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and metaanalysis. Ageing Res. Rev. 2021, 72, 101498. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatments—Facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Arosio, B.; Geraci, A.; Ferri, E.; Mari, D.; Cesari, M. Biological Frailty Index in centenarians. Aging Clin. Exp. Res. 2022, 34, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Agredano, R.S.; Fraile, V.M.; Estrada-Masllorens, J.M.; Guix-Comellas, E.M.; Masclans, J.G.; Poyato, M.L. Comprehensive Geriatric Assessment of the nonagenarian population. Procedia Soc. Behav. Sci. 2017, 237, 1371–1375. [Google Scholar] [CrossRef]
- Ribeiro, O.; Duarte, N.; Teixeira, L.; Paul, C. Frailty and depression in centenarians. Int. Psychogeriatr. 2018, 30, 115–124. [Google Scholar]
- Hwang, A.C.; Chen, L.Y.; Tang, T.C.; Peng, M.N.; Lin, M.H.; Chou, Y.J.; Hsiao, F.-Y.; Chen, L.K. Transitions in frailty and 4-year mortality risk in Taiwan Longitudinal Study on Aging. JAMDA 2023, 24, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lohman, M.C.; Sonnega, A.J.; Resciniti, N.V.; Leggett, A.N. Frailty phenotype and cause-specific mortality in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1935–1942. [Google Scholar] [CrossRef]
- Perez-Zepeda, M.U.; Cesari, M.; Garcia-Pena, C. Predictive value of frailty indices for adverse outcomes in older adults. Rev. Investig. Clin. 2016, 68, 92–98. [Google Scholar] [PubMed]
- Strandberg, T.E.; Lindstrom, L.; Jyvakorpi, S.; Urtamo, A.; Pitkala, K.H.; Kivimaki, M. Phenotypic frailty and multimorbidity are independent 18-year mortality risk indicators in older men. Eur. Geriatr. Med. 2021, 12, 953–961. [Google Scholar] [CrossRef]
- Dupre, M.E.; Gu, D.; Warner, D.F.; Yi, Z. Frailty and type of death among older adults in China: Prospective cohort study. BMJ 2009, 338, b1175. [Google Scholar] [CrossRef]
- Gu, D.; Dupre, M.E.; Sautter, J.; Zhu, H.; Liu, Y.; Yi, Z. Frailty and mortality among Chinese at advanced ages. J. Gerontol. B Psychol. Sci. Soc. Sci. 2009, 64B, 279–289. [Google Scholar]
- Evans, C.J.; Ho, Y.; Daveson, B.A.; Hall, S.; Higginson, I.J.; Gao, W. Place and Cause of Death in Centenarians: A Population-Based Observational Study in England, 2001 to 2010. PLoS Med. 2014, 11, e1001653. [Google Scholar] [CrossRef]
- Gu, D.; Feng, Q. Frailty still matters to health and survival in centenarians: The case of China. Geriatrics 2015, 15, 159. [Google Scholar] [CrossRef]
- Mossakowska, M.; Broczek, K.; Wieczorowska-Tobis, K.; Klich-Raczka, A.; Jonas, M.; Pawlik-Pachucka, E.; Safranow, K.; Kuznicki, J.; Puzianowska-Kuznicka, M. Cognitive performance and functional status are the major factors predicting survival of centenarians in Poland. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1269–1275. [Google Scholar] [CrossRef]
- Choe, Y.R.; Jeong, J.R.; Kim, Y.P. Grip strength mediates the relationship between muscle mass and frailty. J. Cachexia Sarcopenia Muscle 2020, 11, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Granic, A.; Davies, K.; Kirkwood, T.B.L.; Jagger, C.; Sayer, A.A. Prevalence and incidence of sarcopenia in the very old: Findings from the Newcastle 85+ Study. J. Cachexia Sarcopenia Muscle 2017, 8, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Suetta, C.; Haddock, B.; Alcazar, J.; Noerst, T.; Hansen, O.M.; Ludvig, H.; Kamper, R.S.; Schnohr, P.; Presscot, E.; Andersen, L.L.; et al. The Copenhagen Sarcopenia Study: Lean mass, strength, power, and physical function in a Danish cohort aged 20-93 years. J. Cachexia Sarcopenia Muscle 2019, 10, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Syddall, H.E.; Westbury, L.D.; Dodds, R.; Dennison, E.M.; Cooper, C.; Sayer, A.A. Mortality in the Hertfordshire Ageing Study: Association with level and loss of hand grip strength in later life. Age Ageing 2017, 46, 407–412. [Google Scholar] [CrossRef]
- Stessman, J.; Rottenberg, J.; Fisher, M.; Hammerman-Rozenberg, A.; Jacobs, J.M. Hand grip strength in old and very old adults: Mood, cognition, function and mortality. J. Am. Geriatr. Soc. 2017, 65, 526–532. [Google Scholar] [CrossRef]
- Lee, S.H.; Gong, H.S. Measurement and interpretation of handgrip strength for research on sarcopenia and osteoporosis. J. Bone Metab. 2020, 27, 85–96. [Google Scholar] [CrossRef]
- Woo, J. Challenges of population ageing: Putting frailty as a cornerstone of health and social care systems. Eur. Geriatr. Med. 2018, 9, 273–276. [Google Scholar] [CrossRef]
- Avgerinou, C.; Kotsani, M.; Gavana, M.; Andreou, M.; Papageorgiou, D.I.; Roca, V.; Symintiridou, D.; Manolaki, C.; Soulis, G.; Smyrnakis, E. Perceptions, attitudes, and training needs of primary healthcare professionals in identifying and managing frailty: A qualitative study. Eur. Geriatr. Med. 2021, 12, 321–332. [Google Scholar] [CrossRef]
| Symptom | Criterion | Score |
|---|---|---|
| Weight loss | Self-reported weight loss of 5 kg during the past year and/or self-reported weight loss of 3 kg during the past 3 months | 0—without weight loss 1—weight loss |
| Low level of physical activity | Self-reported outdoor activity and/or difficulty walking up a flight of stairs and/or walking a distance of 500 m without rest | 0—no difficulty/some difficulty 1—a lot of difficulties or unable to perform at least one walking task |
| Slow walking speed | Self-reported walking speed | 0—fast or normal walking speed 1—slow, very slow, or unable to walk |
| Weakness | Self-reported difficulty carrying a 5 kg bag | 0—no difficulty/some difficulty 1—a lot of difficulties, or unable to perform the task |
| Fatigue | Self-reported fatigue (when moving or resting) | 0—never, rarely, sometimes 1—often, most of the time, or always |
| All (n = 289) | Women (n = 225) | Men (n = 64) | 94–99 Years (n = 202) | ≥100 Years (n = 87) | |
|---|---|---|---|---|---|
| Age [yrs ± SD] | 98.3 ± 2.6 | 98.4 ± 2.5 | 98.2 ± 2.8 | 96.9 ± 1.5 | 101.5 ± 1.5 *** |
| Living at home [n (%)] | 239 (82.7) | 180 (80.0) | 59 (92.2) | 169 (83.7) | 70 (80.5) |
| Living in an institution [n (%)] | 50 (17.3) | 45 (20.0) | 5 (7.8) * | 33 (16.3) | 17 (19.5) |
| Education level | |||||
| <8 yrs [n (%)] | 88 (37.9) | 77 (42.5) | 11 (21.6) | 67 (39.9) | 21 (32.8) |
| 8–13 yrs [n (%)] | 92 (39.7) | 71 (39.2) | 21 (41.2) | 64 (38.1) | 28 (43.8) |
| >13 yrs [n (%)] | 52 (22.4) | 33 (18.2) | 19 (37.3) ** | 37 (22.0) | 15 (23.4) |
| Missing data [n] | 57 | 44 | 13 | 34 | 23 |
| ADL score | 3.8 ± 1.8 | 3.6 ± 1.8 | 4.4 ± 1.8 ** | 4.1 ± 1.8 | 3.1 ± 1.8 *** |
| Dependent [n (%)] | 67 (23.2) | 55 (24.4) | 12 (18.8) | 36 (17.8) | 31 (35.6) |
| Partially dependent [n (%)] | 102 (35.3) | 90 (40.0) | 12 (18.8) | 65 (32.2) | 37 (42.5) |
| Independent [n (%)] | 120 (41.5) | 80 (35.6) | 40 (62.5) *** | 101 (50.0) | 19 (21.8) *** |
| IADL score | 13.6 ± 4.9 | 13.1 ± 4.6 | 15.2 ± 5.5 ** | 14.4 ± 5.0 | 11.6 ± 4.2 *** |
| Dependent [n (%)] | 235 (81.3) | 190 (84.4) | 45 (70.3) | 156 (77.2) | 79 (90.8) |
| Partially dependent [n (%)] | 44 (15.2) | 31 (13.8) | 13 (20.3) | 37 (18.3) | 7 (8.0) |
| Independent [n (%)] | 10 (3.5) | 4 (1.8) | 6 (9.4) ** | 9 (4.5) | 1 (1.1) * |
| MMSE score | 22 (15–26) | 21 (14–25) | 24 (20–26) ** | 23 (18–26) | 17 (11–23) *** |
| No dementia [n (%)] | 119 (42.0) | 84 (38.4) | 35 (54.7) | 100 (49.8) | 19 (23.2) |
| Mild/moderate dementia [n (%)] | 126 (44.5) | 99 (45.2) | 27 (42.2) | 82 (40.8) | 44 (53.7) |
| Severe dementia [n (%)] | 38 (13.4) | 36 (16.4) | 2 (3.1) ** | 19 (9.5) | 19 (23.2) *** |
| Missing data [n] | 6 | 6 | 0 | 1 | 5 |
| Vision impairment | |||||
| No impairment [n (%)] | 119 (41.5) | 82 (36.8) | 37 (57.8) | 97 (48.5) | 22 (25.3) |
| Mild impairment [n (%)] | 50 (17.4) | 42 (18.8) | 8 (12.5) | 34 (17.0) | 16 (18.4) |
| Moderate impairment [n (%)] | 73 (25.4) | 61 (27.4) | 12 (18.8) | 49 (24.5) | 24 (27.6) |
| Severe impairment [n (%)] | 45 (15.7) | 38 (17.0) | 7 (10.9) * | 20 (10.0) | 25 (28.7) *** |
| Missing data [n] | 2 | 2 | 0 | 2 | 0 |
| Hearing impairment | |||||
| No impairment [n (%)] | 84 (30.2) | 61 (28.5) | 23 (35.9) | 65 (32.8) | 19 (23.8) |
| Moderate impairment [n (%)] | 134 (48.2) | 107 (50.0) | 27 (42.2) | 92 (46.5) | 42 (52.5) |
| Severe impairment [n (%)] | 60 (21.6) | 46 (21.5) | 14 (21.9) | 41 (20.7) | 19 (23.8) |
| Missing data [n] | 11 | 11 | 0 | 4 | 7 |
| CVD [n (%)] | 185 (64.9) | 144 (64.9) | 41 (65.1) | 130 (65.3) | 55 (64.0) |
| Missing data [n] | 4 | 3 | 1 | 3 | 1 |
| Hypertension [n (%)] | 193 (66.8) | 154 (68.4) | 39 (60.9) | 140 (69.3) | 53 (60.9) |
| Past stroke [n (%)] | 33 (12.1) | 26 (12.2) | 7 (11.7) | 18 (9.5) | 15 (18.1) * |
| Missing data [n] | 16 | 12 | 4 | 12 | 4 |
| Diabetes [n (%)] | 40 (13.9) | 30 (13.5) | 10 (15.6) | 34 (17.0) | 6 (6.9) * |
| Missing data [n] | 2 | 2 | 0 | 2 | 0 |
| Arthritis [n (%)] | 77 (26.6) | 69 (30.7) | 8 (12.5) ** | 52 (25.7) | 25 (28.7) |
| Osteoporosis [n (%)] | 54 (19.9) | 45 (21.2) | 9 (15.0) | 44 (23.0) | 10 (12.3) * |
| Missing data [n] | 17 | 13 | 4 | 11 | 6 |
| COPD [n (%)] | 7 (2.4) | 5 (2.2) | 2 (3.1) | 5 (2.5) | 2 (2.3) |
| Missing data [n] | 1 | 1 | 0 | 1 | 0 |
| All (n = 289) | Women (n = 225) | Men (n = 64) | 94–99 yrs (n = 202) | ≥100 yrs (n = 87) | |
|---|---|---|---|---|---|
| Frailty status | |||||
| Robust [n (%)] | 21 (7.3) | 10 (4.4) | 11 (17.2) | 19 (9.4) | 2 (2.3) |
| Prefrail [n (%)] | 96 (33.2) | 66 (29.3) | 30 (46.9) | 71 (35.2) | 25 (28.7) |
| Frail [n (%)] | 172 (59.5) | 149 (66.3) | 23 (35.9) | 112 (55.4) | 60 (69.0) |
| Frailty criteria | |||||
| Weight loss [n (%)] | 17 (5.9) | 15 (6.7) | 2 (3.1) | 13 (6.4) | 4 (4.6) |
| Low physical activity [n (%)] | 200 (69.2) | 172 (76.4) | 28 (43.8) *** | 133 (65.8) | 67 (77.0) |
| Slow walking speed [n (%)] | 204 (70.6) | 162 (72.0) | 42 (65.6) | 137 (67.8) | 67 (77.0) |
| Fatigue [n (%)] | 68 (23.5) | 62 (27.6) | 6 (9.4) ** | 43 (21.3) | 25 (28.7) |
| Weakness [n (%)] | 246 (85.4) | 203 (90.6) | 43 (67.2) *** | 166 (82.2) | 80 (93.0) * |
| Handgrip strength measurement | |||||
| Performed [n (%)] | 244 (84.4) | 186 (82.7) | 58 (90.6) | 182 (90.1) | 62 (71.3) *** |
| Not performed [n (%)] | 45 (15.6) | 39 (17.3) | 6 (9.4) | 20 (9.9) | 25 (28.7) *** |
| Robust (n = 21) | Prefrail (n = 96) | Frail (n = 172) | Comparison of Subgroups | |
|---|---|---|---|---|
| Age [yrs ± SD] | 96.3 ± 1.7 | 98.0 ± 2.1 ** | 98.7 ± 2.8 *** | p < 0.001 |
| Min–max | 94–100 | 94–102 | 94–106 | |
| Female [n (%)] | 10 (47.6) | 66 (68.8) | 149 (86.6) | p < 0.001 |
| Male [n (%)] | 11 (52.4) | 30 (31.3) | 23 (13.4) *** | |
| ADL | 5.8 ± 0.4 | 4.8 ± 1.3 *** | 3.0 ± 1.8 *** | p < 0.001 |
| Dependent [n (%)] | 0 | 4 (4.2) | 63 (36.6) | p < 0.001 |
| Partially dependent [n (%)] | 0 | 31 (32.3) | 71 (41.3) | |
| Independent [n (%)] | 21 (100) | 61 (63.5) ** | 38 (22.1) *** | |
| IADL | 19.5 ± 3.9 | 16.2 ± 5.1 ** | 11.4 ± 3.4 *** | p < 0.001 |
| Dependent [n (%)] | 9 (42.9) | 62 (64.6) | 164 (95.3) | p < 0.001 |
| Partially dependent [n (%)] | 8 (38.1) | 28 (29.2) | 8 (4.7) | |
| Independent [n (%)] | 4 (19.0) | 6 (6.2) | 0 *** | |
| MMSE [score (Q1–Q3)] | 23 (21–26) | 24 (20–27) | 20 (14–25) ** | p < 0.001 |
| No dementia [n (%)] | 10 (47.6) | 53 (55.2) | 56 (33.7) | p < 0.001 |
| Mild or moderate dementia [n (%)] | 11 (52.4) | 39 (40.6) | 76 (45.8) | |
| Severe dementia [n (%)] | 0 | 4 (4.2) | 34 (20.5) * | |
| Missing data [n] | 0 | 0 | 6 | |
| Vision impairment | ||||
| No impairment [n (%)] | 17 (81.0) | 51 (53.7) | 51 (29.8) *** | p < 0.001 |
| Mild impairment [n (%)] | 1 (4.7) | 20 (21.0) | 29 (17.0) | |
| Moderate impairment [n (%)] | 3 (14.3) | 19 (20.0) | 51 (29.8) | |
| Severe impairment [n (%)] | 0 | 5 (5.3) | 40 (23.4) *** | |
| Missing data [n] | 0 | 1 | 1 | |
| Hearing impairment | ||||
| No impairment [n (%)] | 6 (28.6) | 42 (44.7) | 36 (22.1) *** | p < 0.001 |
| Moderate impairment [n (%)] | 13 (61.9) | 40 (42.5) | 81 (49.7) | |
| Severe impairment [n (%)] | 2 (9.5) | 12 (12.8) | 46 (28.2) ** | |
| Missing data [n] | 0 | 2 | 9 | |
| Frailty criteria | ||||
| Weight loss [n (%)] | 0 | 0 | 17 (9.9) | p = 0.07 |
| Low level of physical activity [n (%)] | 0 | 31 (32.3) | 169 (98.3) | p < 0.001 |
| Slow walking speed [n (%)] | 0 | 45 (46.9) | 159 (92.4) | p < 0.001 |
| Fatigue [n (%)] | 0 | 0 | 68 (39.5) | p < 0.001 |
| Weakness [n (%)] | 0 | 78 (81.3) | 168 (98.2) | p < 0.001 |
| Handgrip strength measurement | ||||
| Performed [n (%)] | 21 (100) | 94 (97.9) | 129 (75.0) | p < 0.001 |
| Not performed [n (%)] | 0 | 2 (2.1) | 43 (26.0) | |
| Variable | Univariable Model HR (95% CI) | Multivariable Model 1 HR (95% CI) | Multivariable Model 2 HR (95% CI) | Multivariable Model 3 HR (95% CI) | Multivariable Model 4 HR (95% CI) | Multivariable Model 5 HR (95% CI) | Multivariable Model 6 HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Age by year | 0.889 (0.847–0.932) *** | 0.902 (0.861–0.946) *** | Not included | 0.915 (0.869–0.963) *** | Not included | 0.919 (0.874–0.966) *** | Not included |
| Age ≥ 100 yrs | 0.617 (0.476–0.799) *** | Not included | 0.647 (0.499–0.839) ** | Not included | 0.752 (0.567–0.997) * | Not included | 0.740 (0.558–0.982) * |
| Men | 1.393 (1.038–1.869) * | ns | ns | 1.356 (1.009–1.821) * | 1.368 (1.019–1.837) * | ns | ns |
| Prefrail | 0.609 (0.366–1.014) ^ | ns | ns | Not included | Not included | ns | ns |
| Frail | 0.328 (0.200–0.539) *** | 0.474 (0.368–0.611) *** | 0.466 (0.362–0.599) *** | Not included | Not included | 0.503 (0.388–0.653) *** | 0.498 (0.384–0.645) *** |
| Individuals not capable of performing HGS measurement | 0.338 (0.237–0.481) *** | Not included | Not included | 0.507 (0.357–0.721) *** | 0.472 (0.329–0.676) *** | 0.631 (0.442–0.902) * | 0.599 (0.415–0.865) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skubiszewska, A.; Broczek, K.; Maruniak-Chudek, I.; Oledzka, G.; Jonas, M.I.; Puzianowska-Kuznicka, M.; Mossakowska, M. Frailty and Survivability of Polish Caucasian Nonagenarians and Centenarians. Geriatrics 2024, 9, 14. https://doi.org/10.3390/geriatrics9010014
Skubiszewska A, Broczek K, Maruniak-Chudek I, Oledzka G, Jonas MI, Puzianowska-Kuznicka M, Mossakowska M. Frailty and Survivability of Polish Caucasian Nonagenarians and Centenarians. Geriatrics. 2024; 9(1):14. https://doi.org/10.3390/geriatrics9010014
Chicago/Turabian StyleSkubiszewska, Agnieszka, Katarzyna Broczek, Iwona Maruniak-Chudek, Gabriela Oledzka, Marta Izabela Jonas, Monika Puzianowska-Kuznicka, and Malgorzata Mossakowska. 2024. "Frailty and Survivability of Polish Caucasian Nonagenarians and Centenarians" Geriatrics 9, no. 1: 14. https://doi.org/10.3390/geriatrics9010014
APA StyleSkubiszewska, A., Broczek, K., Maruniak-Chudek, I., Oledzka, G., Jonas, M. I., Puzianowska-Kuznicka, M., & Mossakowska, M. (2024). Frailty and Survivability of Polish Caucasian Nonagenarians and Centenarians. Geriatrics, 9(1), 14. https://doi.org/10.3390/geriatrics9010014








