Fall-Related Adverse Events of Anti-Epileptic Drugs Used for Neuropathic Pain in Older Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Collection and Quality Evaluation
2.4. Statistical Analysis
3. Results
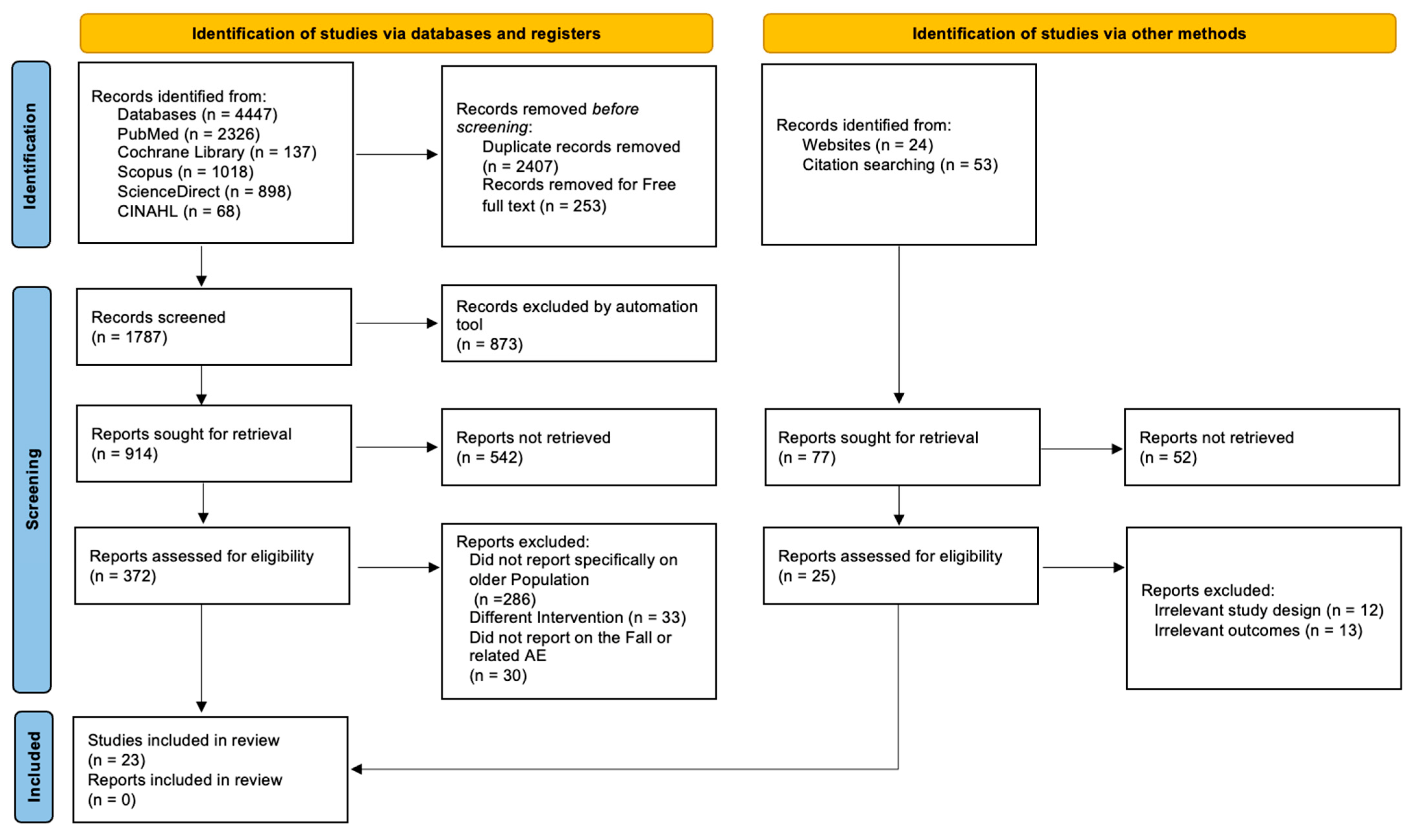
3.1. Study Selection
3.2. Study Characteristics
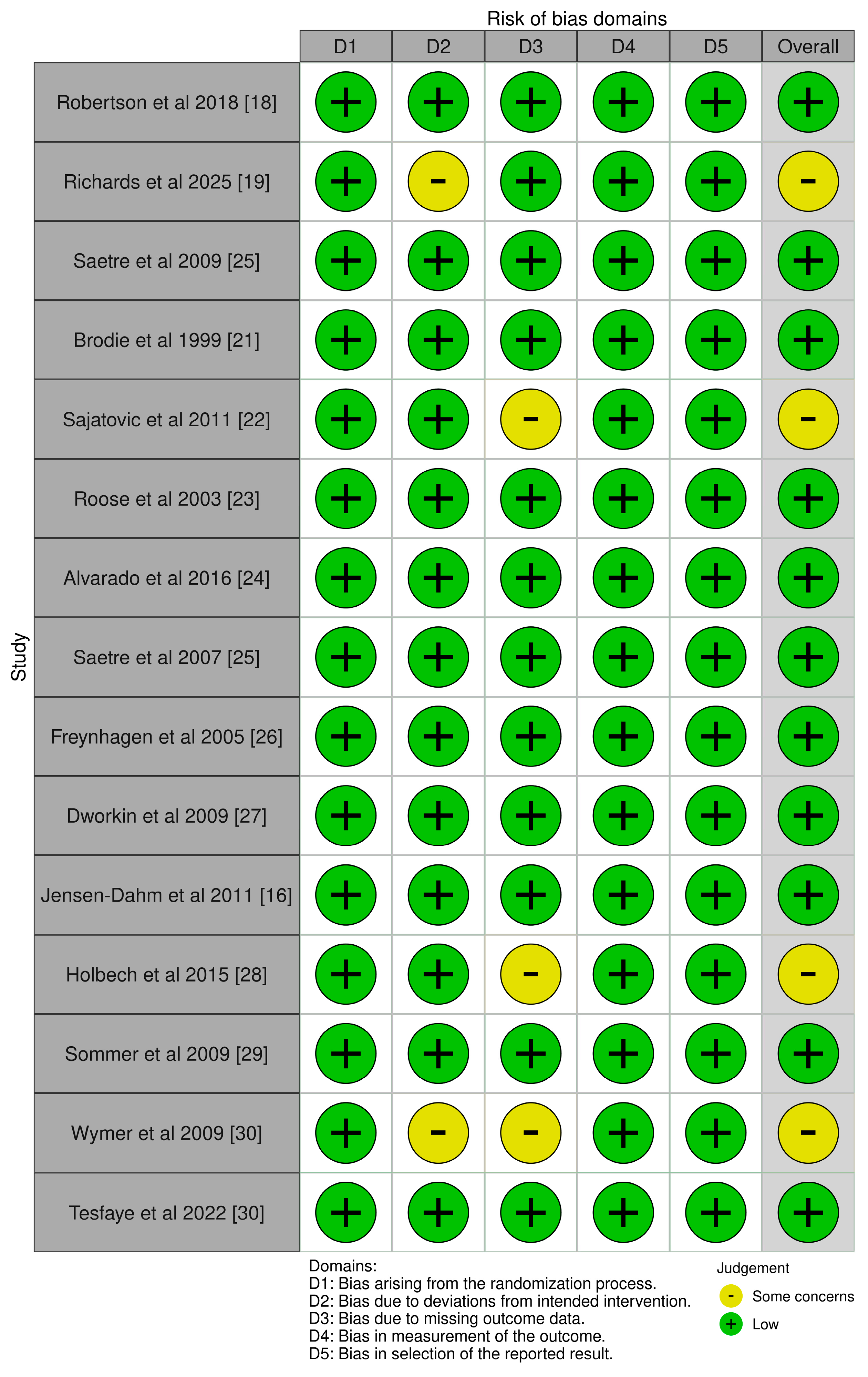
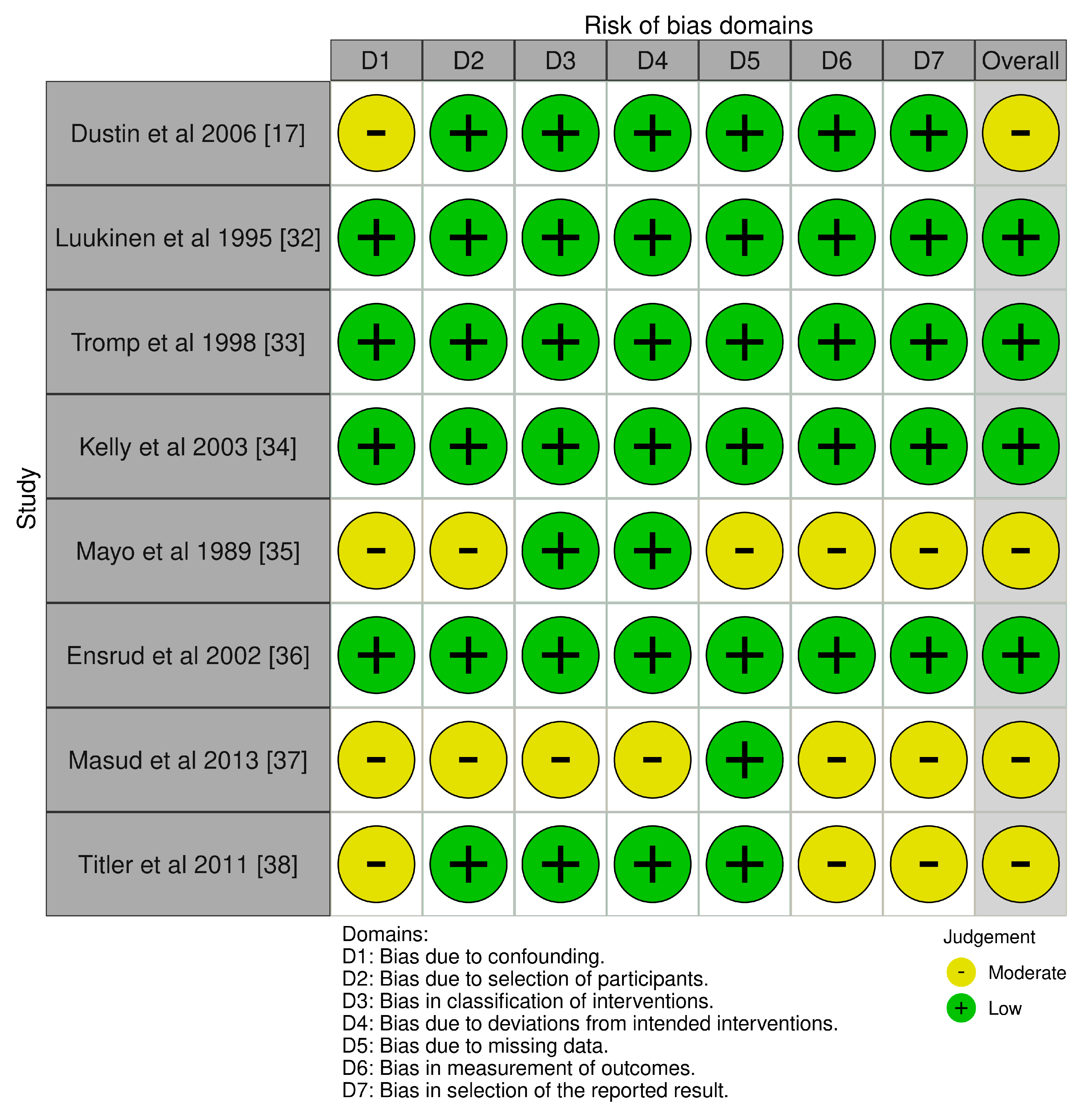
3.3. Risk of Bias Assessment
3.4. GRADE Assessment
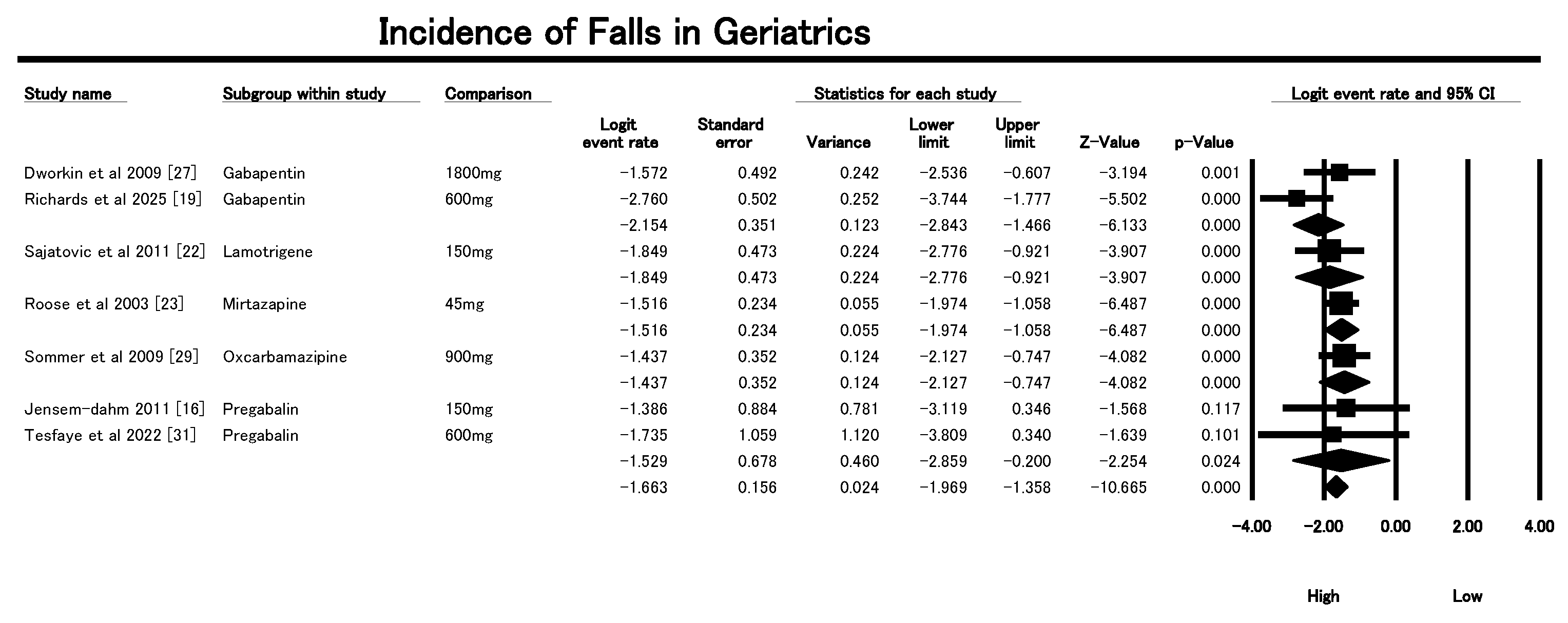
3.5. Incidence on Falls
3.6. AE Relating to Falls
3.6.1. Incidence of Dizziness
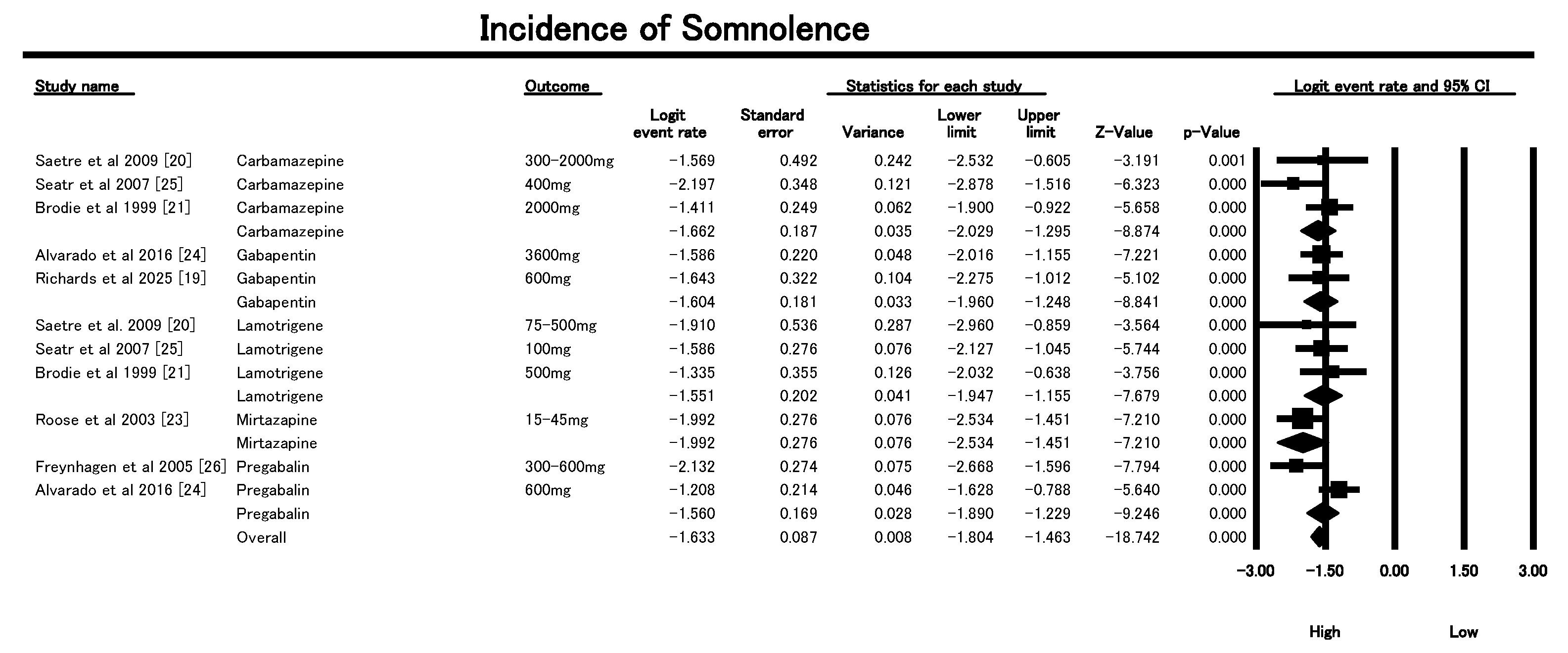
3.6.2. Incidence of Somnolence
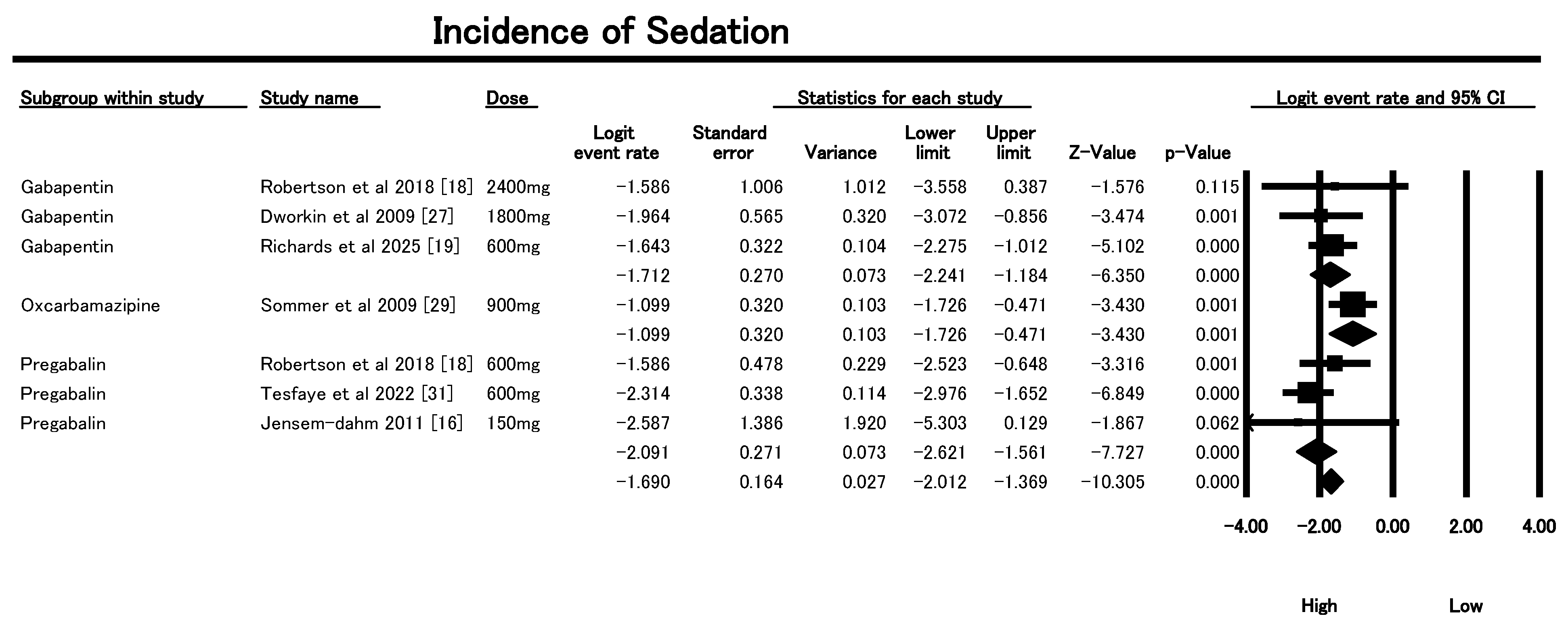
3.6.3. Incidence of Sedation
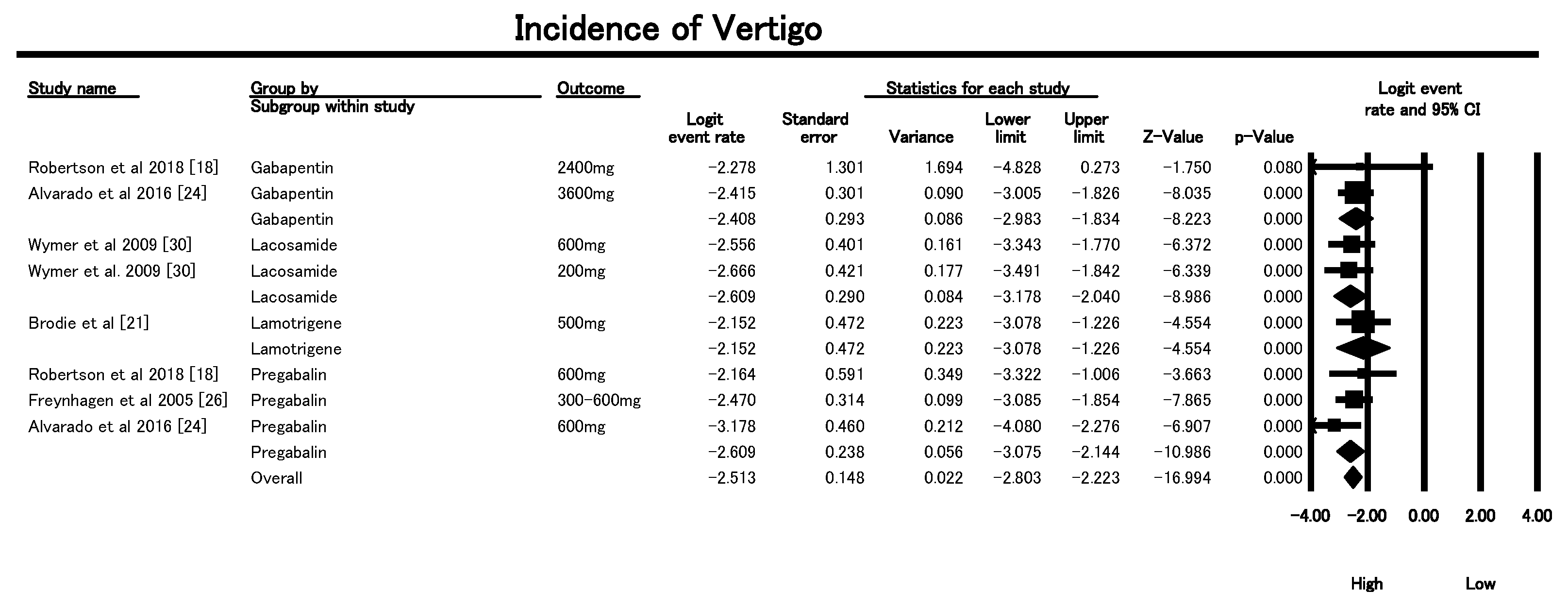
3.6.4. Vertigo Incidence
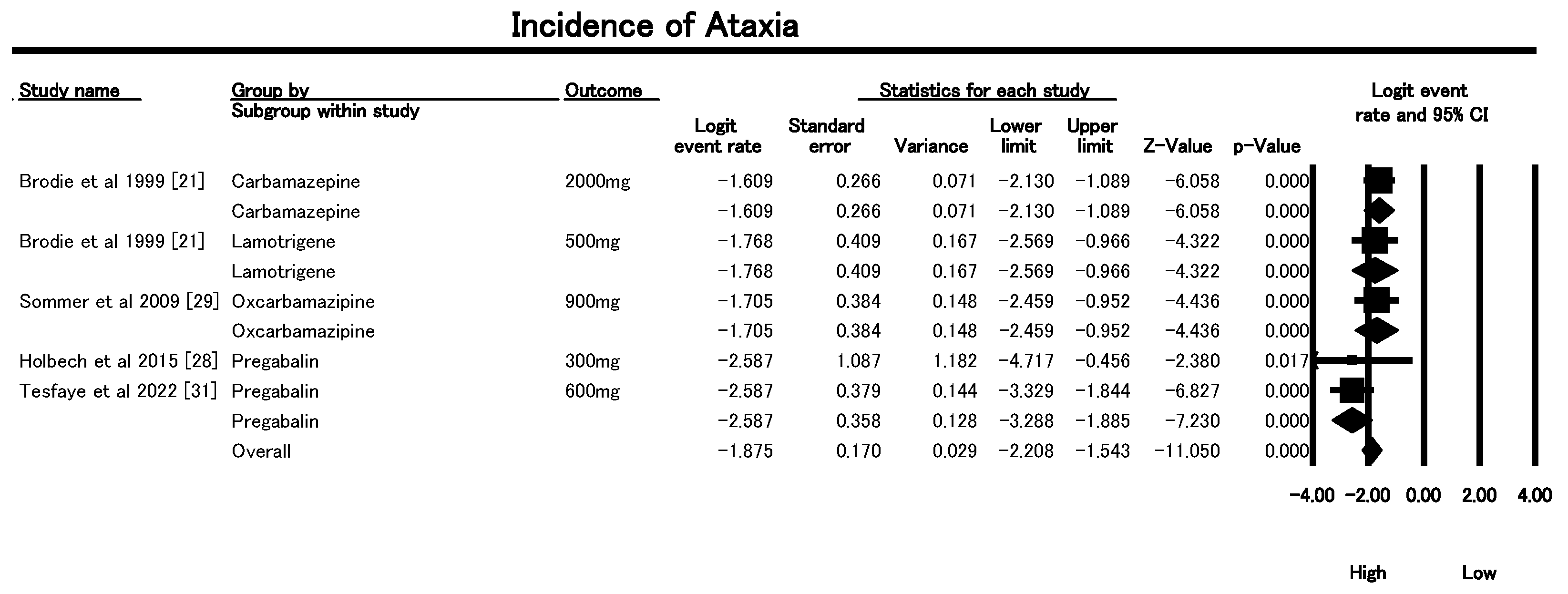
3.6.5. Incidence of Ataxia
3.7. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Schug, S.A.; Stannard, K.J. Treatment of Neuropathic Pain. J. Korean Med. Assoc. 2011, 64, 484–490. [Google Scholar] [CrossRef]
- Haasum, Y.; Johnell, K. Use of antiepileptic drugs and risk of falls in old age: A systematic review. Epilepsy Res. 2017, 138, 98–104. [Google Scholar] [CrossRef]
- Ambrose, A.F.; Paul, G.; Hausdorff, J.M. Risk factors for falls among older adults: A review of the literature. Maturitas 2013, 75, 51–61. [Google Scholar] [CrossRef]
- Falls [Online]. Available online: https://www.who.int/news-room/fact-sheets/detail/falls (accessed on 2 October 2025).
- Hartikainen, S.; Lönnroos, E.; Louhivuori, K. Medication as a risk factor for falls: Critical systematic review. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 1172–1181. [Google Scholar] [CrossRef]
- Florisson, S.; Aagesen, E.K.; Bertelsen, A.S.; Nielsen, L.P.; Rosholm, J.U. Are older adults insufficiently included in clinical trials?—An umbrella review. Basic. Clin. Pharmacol. Toxicol. 2021, 128, 213–223. [Google Scholar] [CrossRef]
- Seppala, L.J.; van de Glind, E.M.; Daams, J.G.; Ploegmakers, K.J.; de Vries, M.; Wermelink, A.M.; van der Velde, N.; Blain, H.; Bousquet, J.; Bucht, G.; et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-analysis: III. Others. J. Am. Med. Dir. Assoc. 2018, 19, 372.e1–372.e8. [Google Scholar] [CrossRef]
- Zia, A.; Kamaruzzaman, S.B.; Tan, M.P. The consumption of two or more fall risk-increasing drugs rather than polypharmacy is associated with falls. Geriatr. Gerontol. Int. 2017, 17, 463–470. [Google Scholar] [CrossRef]
- Dassieu, L.; Paul-Savoie, E.; Develay, É.; Guilhon, A.C.V.; Lacasse, A.; Guénette, L.; Perreault, K.; Beaudry, H.; Dupuis, L.; The Quebec Consortium on Adverse Effects of Pain Medications. Swallowing the pill of adverse effects: A qualitative study of patients’ and pharmacists’ experiences and decision-making regarding the adverse effects of chronic pain medications. Health Expect. 2021, 25, 394. [Google Scholar] [CrossRef] [PubMed]
- Hilmer, S.N.; Gnjidic, D. The effects of polypharmacy in older adults. Clin. Pharmacol. Ther. 2009, 85, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Zeber, J.E.; Copeland, L.A.; Pugh, M.J.V. Variation in antiepileptic drug adherence among older patients with new-onset epilepsy. Ann. Pharmacother. 2010, 44, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, I4919. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ Br. Med. J. 2008, 336, 924. [Google Scholar] [CrossRef]
- Jensen-Dahm, C.; Rowbotham, M.C.; Reda, H.; Petersen, K.L. Effect of a single dose of pregabalin on herpes zoster pain. Trials 2011, 12, 55. [Google Scholar] [CrossRef]
- French, D.D.; Campbell, R.; Spehar, A.; Cunningham, F.; Bulat, T.; Luther, S.L. Drugs and falls in community-dwelling older people: A national veterans study. Clin. Ther. 2006, 28, 619–630. [Google Scholar] [CrossRef]
- Robertson, K.; Marshman, L.A.G.; Plummer, D.; Downs, E. Effect of Gabapentin vs Pregabalin on Pain Intensity in Adults With Chronic Sciatica: A Randomized Clinical Trial. JAMA Neurol. 2018, 76, 28. [Google Scholar] [CrossRef]
- Richards, K.C.; Fry, L.M.; Lozano, A.J.; Ji, W.; Morrison, J.D.; Britt, K.C.; Bliwise, D.L.; Gooneratne, N.S.; Hanlon, A.L. Treatment of Restless Legs Syndrome Improves Agitation and Sleep in Persons with Dementia: A Randomized Trial. J. Am. Med. Dir. Assoc. 2025, 26, 105485. [Google Scholar] [CrossRef]
- Saetre, E.; Abdelnoor, M.; Amlie, J.P.; Tossebro, M.; Perucca, E.; Taubøll, E.; Anfinsen, O.G.; Isojärvi, J.; Gjerstad, L. Cardiac function and antiepileptic drug treatment in the elderly: A comparison between lamotrigine and sustained-release carbamazepine. Epilepsia 2009, 50, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J.; Overstall, P.W.; Giorgi, L. Multicentre, double-blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. Epilepsy Res. 1999, 37, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sajatovic, M.; Gildengers, A.; Al Jurdi, R.K.; Gyulai, L.; Cassidy, K.A.; Greenberg, R.L.; Bruce, M.L.; Mulsant, B.H.; Have, T.T.; Young, R.C. Multisite, open-label, prospective trial of lamotrigine for geriatric bipolar depression: A preliminary report. Bipolar Disord. 2011, 13, 294–302. [Google Scholar] [CrossRef]
- Roose, S.P.; Nelson, J.C.; Salzman, C.; Hollander, S.B.; Rodrigues, H. Open-label study of mirtazapine orally disintegrating tablets in depressed patients in the nursing home. Curr. Med. Res. Opin. 2003, 19, 737–746. [Google Scholar] [CrossRef]
- Alvarado, A.M.; Navarro, S.A. Clinical trial assessing the efficacy of gabapentin plus B complex (B1/B12) versus pregabalin for treating painful diabetic neuropathy. J. Diabetes Res. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Saetre, E.; Perucca, E.; Isojärvi, J.; Gjerstad, L. An international multicenter randomized double-blind controlled trial of lamotrigine and sustained-release carbamazepine in the treatment of newly diagnosed epilepsy in the elderly. Epilepsia 2007, 48, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Freynhagen, R.; Strojek, K.; Griesing, T.; Whalen, E.; Balkenohl, M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005, 115, 254–263. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Barbano, R.L.; Tyring, S.K.; Betts, R.F.; McDermott, M.P.; Pennella-Vaughan, J.; Bennett, G.J.; Berber, E.; Gnann, J.W.; Irvine, C.; et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain 2009, 142, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Holbech, J.V.; Bach, F.W.; Finnerup, N.B.; Brøsen, K.; Jensen, T.S.; Sindrup, S.H. Imipramine and pregabalin combination for painful polyneuropathy: A randomized controlled trial. Pain 2015, 156, 958–966. [Google Scholar] [CrossRef]
- Sommer, O.H.; Aga, O.; Cvancarova, M.; Olsen, I.C.; Selbaek, G.; Engedal, K. Effect of oxcarbazepine in the treatment of agitation and aggression in severe dementia. Dement. Geriatr. Cogn. Disord. 2009, 27, 155–163. [Google Scholar] [CrossRef]
- Wymer, J.P.; Simpson, J.; Sen, D.; Bongardt, S. Efficacy and safety of lacosamide in diabetic neuropathic pain: An 18-week double-blind placebo-controlled trial of fixed-dose regimens. Clin. J. Pain 2009, 25, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Sloan, G.; Petrie, J.; White, D.; Bradburn, M.; Julious, S.; Rajbhandari, S.; Sharma, S.; Rayman, G.; Gouni, R.; et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): A multicentre, double-blind, randomised crossover trial. Lancet 2022, 400, 680–690. [Google Scholar] [CrossRef]
- Luukinen, H.; Koski, K.; Laippala, P.; Kivelä, S.L. Predictors for recurrent falls among the home-dwelling elderly. Scand. J. Prim. Health Care 1995, 13, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Tromp, A.M.; Smit, J.H.; Deeg, D.J.H.; Bouter, L.M.; Lips, P. Predictors for falls and fractures in the longitudinal aging study Amsterdam. J. Bone Miner. Res. 1998, 13, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.D.; Pickett, W.; Yiannakoulias, N.; Rowe, B.H.; Schopflocher, D.P.; Svenson, L.; Voaklander, D.C. Medication use and falls in community-dwelling older persons. Age Ageing 2003, 32, 503–509. [Google Scholar] [CrossRef]
- Mayo, N.E.; Korner-Bitensky, N.; Becker, R.; Georges, P. Predicting falls among patients in a rehabilitation hospital. Am. J. Phys. Med. Rehabil. 1989, 68, 139–146. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Blackwell, T.L.; Mangione, C.M.; Bowman, P.J.; Whooley, M.A.; Bauer, D.C.; Schwartz, A.V.; Hanlon, J.T.; Nevitt, M.C. Central nervous system-active medications and risk for falls in older women. J. Am. Geriatr. Soc. 2002, 50, 1629–1637. [Google Scholar] [CrossRef]
- Masud, T.; Frost, M.; Ryg, J.; Matzen, L.; Ibsen, M.; Abrahamsen, B.; Brixen, K. Central nervous system medications and falls risk in men aged 60-75 years: The study on male osteoporosis and aging (SOMA). Age Ageing 2013, 42, 121–124. [Google Scholar] [CrossRef]
- Titler, M.G.; Shever, L.L.; Kanak, M.F.; Picone, D.M.; Qin, R. Factors associated with falls during hospitalization in an older adult population. Res. Theory Nurs. Pract. 2011, 25, 127–148. [Google Scholar] [CrossRef]
- Maximos, M.; Chang, F.; Patel, T. Risk of falls associated with antiepileptic drug use in ambulatory elderly populations: A systematic review. Can. Pharm. J. CPJ 2017, 150, 101. [Google Scholar] [CrossRef]
- Ishida, J.H.; McCulloch, C.E.; Steinman, M.A.; Grimes, B.A.; Johansen, K.L. Gabapentin and Pregabalin Use and Association with Adverse Outcomes among Hemodialysis Patients. J. Am. Soc. Nephrol. 2018, 29, 1970–1978. [Google Scholar] [CrossRef]
- Randolph, A.; Lin, Y.; Volpi, E.; Kuo, Y. Impact of Anticonvulsant Use on Incidence of Falls in Older Patients with Diabetic Peripheral Neuropathy. Innov. Aging 2018, 2, 151. [Google Scholar] [CrossRef]
- Woolcott, J.C.; Richardson, K.J.; Wiens, M.O.; Patel, B.; Marin, J.; Khan, K.M.; Marra, C.A. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch. Intern. Med. 2009, 169, 1952–1960. [Google Scholar] [CrossRef]
- de Vries, M.; Seppala, L.J.; Daams, J.G.; van de Glind, E.M.; Masud, T.; van der Velde, N.; Blain, H.; Bousquet, J.; Bucht, G.; Caballero-Mora, M.A.; et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: I. Cardiovascular Drugs. J. Am. Med. Dir. Assoc. 2018, 19, 371.e1–371.e9. [Google Scholar] [CrossRef] [PubMed]
- Zaccara, G.; Gangemi, P.; Perucca, P.; Specchio, L. The adverse event profile of pregabalin: A systematic review and meta-analysis of randomized controlled trials. Epilepsia 2011, 52, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Bauquier, S.H. Benzodiazepines. In Pharmacology in Veterinary Anesthesia and Analgesia; Wiley: Hoboken, NJ, USA, 2024; pp. 60–74. [Google Scholar] [CrossRef]
- Rozing, M.P.; Wium-Andersen, M.K.; Wium-Andersen, I.K.; Jørgensen, T.S.H.; Jørgensen, M.B.; Osler, M. Use of hypnotic-sedative medication and risk of falls and fractures in adults: A self-controlled case series study. Acta Psychiatr. Scand. 2023, 148, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Masse, M.; Henry, H.; Cuvelier, E.; Pinçon, C.; Pavy, M.; Beeuwsaert, A.; Barthélémy, C.; Cuny, D.; Gautier, S.; Kambia, N.; et al. Sleep Medication in Older Adults: Identifying the Need for Support by a Community Pharmacist. Healthcare 2022, 10, 147. [Google Scholar] [CrossRef]

| Study | Country | Sample Size | Mean Age | Diagnosis | Interventions | Key Findings |
|---|---|---|---|---|---|---|
| Robertson et al., 2018 [18] | Australia | 18 | 57 ± 16.5 | Chronic Sciatica | Gabapentin vs. Pregabalin | Gabapentin superior in pain reduction and fewer adverse events compared to pregabalin. |
| Richards et al., 2025 [19] | USA | 147 | 83.4 ± 9.1 | RLS with dementia | Gabapentin enacarbil vs. Placebo | Reduced nighttime agitation; trend toward more falls in the gabapentin group (p = 0.066). |
| Saetre et al., 2009 [20] | Norway | 108 | ≥65 | Newly diagnosed epilepsy | Carbamazepine vs. Lamotrigine | No significant ECG changes; both drugs tolerated well in older Adults. |
| Brodie et al., 1999 [21] | UK/Europe | 150 | 77 | Newly diagnosed epilepsy | Lamotrigine vs. Carbamazepine | Lamotrigine had fewer adverse events and better continuation than carbamazepine. |
| Sajatovic et al., 2011 [22] | USA | 57 | 66.5 ± 6.7 | Bipolar depression | Lamotrigine (augmentation) | Improvement in depression and function; some adverse events including unsteady gait. |
| Roose et al., 2003 [23] | USA | 119 | ≥70 | Depression | Mirtazapine (15–45 mg/day) | Effective in depression; common AEs were falls (18%) and somnolence (12%). |
| Alvarado et al., 2016 [24] | Mexico | 270 | >50 | Painful diabetic neuropathy | Gabapentin + B1/B12 vs. Pregabalin | Comparable efficacy; less vertigo with gabapentin/B1/B12 (p = 0.014); lower gabapentin doses needed for pain relief. |
| Saetre et al., 2007 [25] | Norway/Multicenter | 185 | ≥65 | Newly diagnosed epilepsy | Lamotrigine (25–500 mg/day), Carbamazepine SR (100–2000 mg/day) | LTG and CBZ had similar effectiveness; CBZ had higher seizure freedom, LTG better tolerability. |
| Freynhagen et al., 2005 [26] | Multicenter | 338 | 62.7 ± 10.6 | DPN or PHN (neuropathic pain) | Pregabalin (flexible: 150–600 mg/day or fixed: 300–600 mg/day) | Both regimens reduced pain and sleep interference; dizziness and somnolence common AEs. |
| Dworkin et al., 2009 [27] | USA | 87 | ≥50 | Acute pain in herpes zoster | Gabapentin (1200–1800 mg/day), CR-oxycodone, placebo | Oxycodone reduced pain more than placebo; gabapentin had modest effect; common AEs included constipation. |
| Jensen-Dahm et al., 2011 [16] | USA | 8 | 65 | Acute herpes zoster pain | Pregabalin (150 mg single dose) | 33% pain reduction vs. 14% placebo; well tolerated; effects on allodynia not significant. |
| Holbech et al. (2015) [28] | Multicenter (EU) | 73 | 20–85 years | Painful polyneuropathy | Imipramine 75 mg/day vs. Pregabalin 300 mg/day vs. Combination vs. Placebo | Combination significantly more effective than either monotherapy but had more adverse events. |
| Sommer et al. (2009) [29] | Germany | 103 | 83 | Agitation/aggression in dementia | Oxcarbazepine vs. Placebo | No significant difference in agitation/aggression; slight trend favoring oxcarbazepine. |
| Wymer et al. (2009) [30] | International | NR | 58 | Painful diabetic neuropathy | Lacosamide 200 mg/d, 400 mg/d, 600 mg/d vs. Placebo | Lacosamide 400 mg/d showed optimal efficacy/tolerability; 600 mg/d had high AE dropout rate. |
| Tesfaye et al. (2022) [31] | UK (13 sites) | 130 | Mean: 60 s | Diabetic peripheral neuropathic pain (DPNP) | A-P, P-A, D-P pathways combining amitriptyline, duloxetine, pregabalin | All had similar efficacy; combination better than monotherapy if pain relief suboptimal. |
| Dustin et al., 2006 [17] | USA | 41,102 (20,551 cases, 20,551 controls) | >65 | Fall-related outpatient visits vs. nonspecific chest pain | Analysis of CNS, CVS, and MSS drugs prescribed | CNS drugs (e.g., antidepressants, anticonvulsants, antipsychotics) more common in fall patients. CVS drugs more common in controls. Highlighted medication-related fall risk. |
| Luukinen et al., 1995 [32] | Finland | 1016 | ≥70 | Recurrent falls in home-dwelling older Adults | Community-based observation and fall history | Prior falls, peripheral neuropathy, psychotropics, and slow gait identified as predictors of recurrent falls. |
| Tromp et al., 1998 [33] | Netherlands | 1469 | >60 (Born before 1931) | Falls, recurrent falls, fractures | Population-based cohort analysis | Impaired mobility, analgesics, and antiepileptics predicted recurrent falls. Fractures predicted by inactivity, female gender, and prior fractures. |
| Kelly et al., 2003 [34] | Netherlands | 1469 | >60 | Recurrent falls and fractures | Baseline interview with follow-up over 38 months | Similarly to Tromp et al.: identified analgesics and inactivity as strong predictors. Recurrent falls occurred in 15% of the cohort. |
| Mayo et al., 1989 [35] | Canada | 402 (201 cases, 201 controls) | >60 | Falls in rehabilitation hospital | Retrospective case–control from admission records | Stroke, incontinence, anticonvulsants, and topical eye meds predicted falls. Risk model validated in second cohort year. |
| Ensrud et al., 2002 [36] | USA | 8127 women | ≥65 years | Community-dwelling older women | CNS-active drugs: benzodiazepines, antidepressants, anticonvulsants, narcotics | Use of benzodiazepines (OR: 1.51), antidepressants (OR: 1.54), and anticonvulsants (OR: 2.56) significantly increased risk of frequent falls. Narcotics not associated. |
| Masud et al., 2013 [37] | Denmark | 4696 men | Median: 66.3 years | Men aged 60–75 years, general population | CNS drugs: opiates, antidepressants, anxiolytics, SSRIs, TCAs | Opiates (OR: 2.4), antidepressants (OR: 2.8), SSRIs (OR: 3.1), TCAs (OR: 2.2), and antiepileptics (OR: 2.8) significantly associated with falls. |
| Titler et al., 2011 [38] | USA | 10,187 hospitalizations | ≥60 years | Hospitalized older adults | Medical/pharmacy/nursing treatments; CNS meds; fall prevention interventions | Antidepressants, antipsychotics, benzodiazepines, restraints, and neurologic monitoring linked to falls. RN skill mix, ulcer care, and pain management inversely related. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamadevan, A.; Vijayan, V.; Marwein, F.; Yoosuf, N. Fall-Related Adverse Events of Anti-Epileptic Drugs Used for Neuropathic Pain in Older Adults: A Systematic Review and Meta-Analysis. Geriatrics 2025, 10, 130. https://doi.org/10.3390/geriatrics10050130
Vamadevan A, Vijayan V, Marwein F, Yoosuf N. Fall-Related Adverse Events of Anti-Epileptic Drugs Used for Neuropathic Pain in Older Adults: A Systematic Review and Meta-Analysis. Geriatrics. 2025; 10(5):130. https://doi.org/10.3390/geriatrics10050130
Chicago/Turabian StyleVamadevan, Arun, Vijesh Vijayan, Fellisha Marwein, and Nishad Yoosuf. 2025. "Fall-Related Adverse Events of Anti-Epileptic Drugs Used for Neuropathic Pain in Older Adults: A Systematic Review and Meta-Analysis" Geriatrics 10, no. 5: 130. https://doi.org/10.3390/geriatrics10050130
APA StyleVamadevan, A., Vijayan, V., Marwein, F., & Yoosuf, N. (2025). Fall-Related Adverse Events of Anti-Epileptic Drugs Used for Neuropathic Pain in Older Adults: A Systematic Review and Meta-Analysis. Geriatrics, 10(5), 130. https://doi.org/10.3390/geriatrics10050130