Simple Summary
Cytology is a minimally invasive and cost-effective diagnostic method used in veterinary medicine to examine cells from tissues, fluids, and other body structures. This study analysed over 3000 cases from cats across Portugal to evaluate the effectiveness of cytology in diagnosing various conditions. The highest diagnostic success rates were observed in fluids and gland tissues, while samples from skin lesions and lymph nodes were more challenging. Tumours were more frequent in older cats, while younger cats often had inflammation. Some tumours, such as epithelial cancers, appeared more in females, whereas other types, like mesenchymal cancers, were more common in males. Poor-quality or incomplete samples often resulted in inconclusive findings, highlighting the need for proper collection and preparation. This research demonstrates the vital role of cytology in detecting diseases early, guiding treatment decisions, and improving the overall care of feline patients. With meticulous sample handling and integration with other diagnostic methods, cytology remains an invaluable tool in veterinary medicine.
Abstract
This study evaluated diagnostic trends and the overall utility of cytology in feline patients through the analysis of a large, multicentric dataset from Portugal. A retrospective review of 3068 cytological cases from 130 veterinary practices was conducted, with samples categorised by anatomical location and lesion type. Diagnostic outcomes were statistically assessed, revealing an overall success rate of 66.20%. The highest diagnostic yields occurred in fluid samples (83.48%), glandular tissues (76.67%), and mucous membranes (75.81%), followed by organ-based samples (67.79%), miscellaneous tissues (66.98%), cutaneous/subcutaneous nodules (62.16%), and lymph nodes (57.93%). Neoplastic lesions showed age-associated prevalence, being more common in older cats, with epithelial and melanocytic lesions more frequent in females and round cell/mesenchymal lesions predominating in males. Non-diagnostic samples (33.80%) primarily resulted from insufficient cellularity or suboptimal quality, though no significant correlation existed between diagnostic success and clinical setting. This study underscores that cytology remains a fundamental diagnostic tool in feline medicine, particularly when combined with proper sampling techniques and complementary diagnostic methods, and reinforces its value in clinical decision-making, thereby supporting its broader utilisation in routine veterinary practice.
Keywords:
anatomic location; cancer; clinical pathology; cytology; diagnosis; cat; epidemiology; neoplasms 1. Introduction
Cytology is an essential diagnostic tool in veterinary medicine, widely recognised for its ease of execution, minimally invasive nature, and cost-effectiveness [1,2,3,4]. It provides a rapid and accessible means of assessing the cellular composition of lesions [4,5], thereby facilitating the diagnosis and classification of a broad spectrum of pathological conditions. Owing to its versatility, cytology is routinely employed in the evaluation of cutaneous and subcutaneous masses, cystic structures, ulcerative lesions, and fistulous tracts. Furthermore, it plays a pivotal role in the examination of organ samples and body fluids, including effusions, cerebrospinal fluid (CSF), urine, and synovial fluid [2,3,4,6,7,8,9,10,11].
A variety of techniques are available for collecting cytological samples, including fine-needle aspiration (FNA), fine-needle non-aspiration, impression smears, swabs, and scrapings [12,13,14]. The selection of technique largely depends on the lesion’s characteristics, anatomical location, and the practitioner’s preference [13]. Despite its numerous advantages, cytology has inherent limitations [15,16], primarily due to the absence of tissue architecture, which is critical for differentiating between benign and malignant conditions [17]. Despite these limitations, cytology often provides sufficient information for a definitive diagnosis. In instances where a specific conclusion is elusive, the insights gained by cytology can direct further diagnostic efforts to achieve a conclusive diagnosis [7,18,19,20].
The diagnostic accuracy of cytology is influenced by multiple factors, including sample quality, cellular preservation, and preparation techniques. Inadequate sample selection, suboptimal collection methods, and improper slide preparation can lead to non-diagnostic or inconclusive results [2,18,21]. Therefore, meticulous attention to sample acquisition and preparation is essential to maximise the clinical utility of cytological evaluation. Numerous studies in companion animals have highlighted the diagnostic value of cytology [1,5,6,7,11,15,19,20,22,23,24,25]. In feline medicine, cytology is extensively used to diagnose neoplastic and inflammatory conditions affecting cutaneous and subcutaneous lesions [7,11,26], internal organs such as the spleen [27] and urinary tract/kidney [28], bone [25], skeletal muscle [29], oral lesions [1,24], the lymphatic system [5,15], and the mammary gland [30].
This study conducts a comprehensive retrospective analysis of cytological diagnoses in cats over a seven-year period, encompassing 3068 cases from veterinary practices throughout Portugal. To the authors’ best knowledge, this represents the first large-scale investigation into the epidemiological relevance of cytology for feline diagnostics in the country. By examining diagnostic trends and outcomes, this study highlights the fundamental role of cytology in veterinary medicine, reinforcing its significance as a critical tool for early disease detection and informed clinical decision-making.
2. Materials and Methods
2.1. Data Collection, Sampling, and Diagnostic Procedures
Cytology samples were submitted to INNO Veterinary Laboratories (Braga, Portugal) over a seven-year period. The samples were submitted by a total of 130 veterinary practices located across all districts of mainland Portugal and from the Insular Autonomous Regions.
The vast majority of cytological samples received were submitted unstained. These were processed in-house and stained using a Romanowsky-type stain (Hemacolor®, Merck KGaA, Darmstadt, Germany) with an automated RAL Stainer (RAL Diagnostics, Martillac, France), ensuring consistent and reproducible staining quality. The staining protocol followed the manufacturer’s specifications. Pre-stained slides were submitted only occasionally (<5%), but due to the retrospective nature of the study, the exact proportion and details of externally applied staining methods could not be determined.
After staining, all slides were mounted using Entellan™ (Sigma-Aldrich, St. Louis, MO, USA) to preserve the preparations. Microscopic evaluation was performed using a Nikon Eclipse 600 microscope (Nikon Corporation, Tokyo, Japan) equipped with CFI Plan Achromat objectives: 10× (Ref. MRP70100), 20× (Ref. MRL00202), 40× (Ref. MRP70400), 50× (Ref. MRL01502), and 100× oil immersion (Ref. MRL01903). No digital scanning methods were employed in this study; all evaluations were conducted using direct light microscopy. Cytological interpretation was performed by two experienced clinical pathologists, each with over 30 years of continuous practice in diagnostic cytopathology. Although not board-certified diplomates, both professionals have extensive training and a well-established record in the field, ensuring a high level of diagnostic consistency and reliability throughout the study. The same two evaluators remained consistent throughout the seven-year study period (2011–2016). All samples were initially evaluated independently; in cases of uncertainty or inconclusiveness, the pathologists discussed the findings collaboratively to reach a consensus diagnosis. In cases of diagnostic uncertainty, the remaining co-authors, also experienced in cytological interpretation, were involved in the review process to reach a broad consensus. Additionally, in selected cases, samples were submitted to external specialised veterinary laboratories for second-opinion evaluation, thereby supporting the accuracy and consistency of the final diagnosis. Fluid samples were submitted either in plain tubes or in tubes with EDTA. These were transported under refrigeration, with sample stability ensured through controlled temperature conditions maintained by the laboratory’s dedicated logistics team. In cases of low cellularity, centrifugation was performed upon arrival to concentrate cellular components prior to smear preparation. Due to the retrospective design of the study, data on the specific sample collection techniques were not available (e.g., fine-needle aspiration, non-aspiration, and touch imprint). However, all samples were collected in accordance with the sampling guidelines and best practice recommendations provided by the laboratory to all participating clinics.
Accompanying each sample was a laboratory request form that provided essential clinical information, including breed, sex, age, clinical suspicions or signs; the anatomical location of the sample; and the analyses requested. The age of the animals was categorised into seven groups following a previous methodology [31].
Samples were categorised by anatomical location as follows: subcutaneous and cutaneous locations; lymph nodes; glandular tissues (including mammary glands, endocrine glands, and pancreas); fluids (comprising joint fluid, urine, and transtracheal or bronchoalveolar washes); miscellaneous (including intra-abdominal and intrathoracic masses, mediastinum, mesentery, bone, ear, testicle, and penis); mucous membranes (covering vaginal, oral, ocular, nasal, and anal locations); and organs (including the lungs, kidneys, liver, and spleen). Samples requiring additional diagnostic modalities, such as bone marrow myelograms, thoracic, abdominal, and pericardial effusions, or cerebrospinal fluid, were excluded from the study.
Furthermore, samples were classified into diagnostic categories—neoplastic (comprising epithelial, spindle cells, round cells, and melanocytic lesions) [28,32]; inflammatory (subdivided into infectious, suppurative, pyogranulomatous/granulomatous, eosinophilic, lymphocytic, plasmocytic, and those of unknown origin) [12,28,32,33]; non-neoplastic/non-inflammatory (including cystic, degenerative and haemorrhagic lesions, hyperplasia, corticosteroid-induced hepatopathy, and extramedullary haematopoiesis, among others) [34,35]; and the “other” category, which included normal samples and vaginal cytology for oestrous cycle staging. The designation “infectious” was applied when there was cytological evidence of infectious organisms, regardless of the predominant inflammatory cell type. Non-diagnostic categories were designated as indicative (providing suggestive information towards a potential diagnosis, e.g., when it is not possible to determine the subtype of neoplasia) and inconclusive (characterised by low cellularity, haemodilution, poor sample quality, or destroyed cells) [36,37].
The classifications were further simplified into two overarching categories: neoplastic and non-neoplastic, with the latter encompassing inflammatory and non-neoplastic/non-inflammatory processes and other sample types.
This study is based exclusively on cytological diagnoses. Although histopathological confirmation may have been performed in some individual cases as part of routine clinical follow-up, such data were not systematically collected or analysed, and histology was not used to validate or override the cytological findings presented. The primary objective of this study was not to assess the diagnostic accuracy of cytology against histopathology, but rather to provide a large-scale epidemiological overview of cytological utilisation and diagnostic outcomes in feline patients.
2.2. Statistical Analysis
Statistical evaluations were conducted using JMP®, version 14.3 (SAS Institute, Cary, NC, USA, 1989–2023), DATAtab® (DATAtab e.U., Graz, Austria, 2024), and MedCalc® Statistical Software version 20.006 (MedCalc Software Ltd., Ostend, Belgium). Proportional differences were examined using the Chi-Square test (χ2), utilising Fisher’s exact test when expected frequencies fell below five. In analysing breed predisposition, univariable logistic regression was utilised to determine the odds of neoplastic versus non-neoplastic conditions, considering potential biases in breed representation. A significance threshold of p ≤ 0.05 was considered.
3. Results
A total of 3068 animal samples were received for analysis. Of these, 1597 samples (52.05%; 95% CI: 50.3–53.8%) were from females and 1471 (48.0%; 95% CI: 46.2–49.7%) were from males.
Information on animal breed was absent in 590 (19.24%) of the request forms. From the available data (n = 2478), 13 different breeds were identified. The breakdown included 2018 Domestic Shorthair (DSH) (65.80%), 247 Persian (8.05%), 141 Siamese (4.60%), 30 Norwegian Forest (0.98%), 10 Sphynx (0.33%), 9 Main Coon (0.29%), 6 Turkish Angora (0.20%), 6 Burmese (0.20%), 4 British Shorthair (0.13%), 2 Scottish Fold (0.07%), 2 Somali (0.07%), 1 Bobtail (0.03%), and 1 Chartreux (0.03%).
Age data were available for 2625 animals (85.56%), while 443 request forms (14.44%) did not specify the animals’ age. The age distribution ranged from ≤1 year (6 months) to 23 years, with a median age of 8 years (interquartile range: 4–12 years). A total of 13.2% (95% CI: 12.0–14.6; n = 347) were classified as kittens, 6.2% (95% CI: 5.4–7.2; n = 163) as young, 10.0% (95% CI: 8.9–11.2; n = 263) as young adults, 16.0% (95% CI: 14.6–17.4; n = 419) as adults, 19.1% (95% CI: 17.7–20.7; n = 502) as mature adults, 30.0% (95% CI: 27.8–31.3; n = 775) as seniors, and 5.9% (95% CI: 5.1–6.9; n = 156) as geriatrics.
3.1. Diagnostic Yield
In this study, of the 3068 cytological samples analysed, 2031 (66.20%) yielded a definitive diagnosis, while 1037 (33.80%) were non-diagnostic. Within the diagnostic outcomes, 714 samples (23.27% of the total) were identified as neoplastic, 691 samples (22.52%) showed inflammatory changes, 439 samples (14.31%) were categorised as non-neoplastic and non-inflammatory, and 187 samples (6.1%) fell into the “other” category. On the non-diagnostic side, 564 samples (18.38%) provided indicative findings that suggested a possible diagnosis, and 473 samples (15.42%) were inconclusive, yielding neither specific nor suggestive results.
Based on the anatomical origin, the majority of the samples (n = 1094) were obtained from cutaneous and subcutaneous nodules. Of these, 62.16% contained sufficient cytological features to support a definitive interpretation. A total of 444 samples were collected from internal organs, with 67.79% meeting the criteria for cytological assessment. Lymph node aspirates comprised 274 samples, of which 57.93% were considered cytologically interpretable. Glandular tissues were sampled in 138 cases, yielding a high proportion of interpretable results (76.67%). Mucosal tissues contributed 257 samples, with 75.81% deemed adequate for diagnostic evaluation. Among the 142 samples classified as miscellaneous, 66.98% were suitable for interpretation. Finally, fluid samples (n = 96) showed the highest proportion of interpretable material, at 83.48%. The distribution of sample adequacy by anatomical site is illustrated in Figure 1.
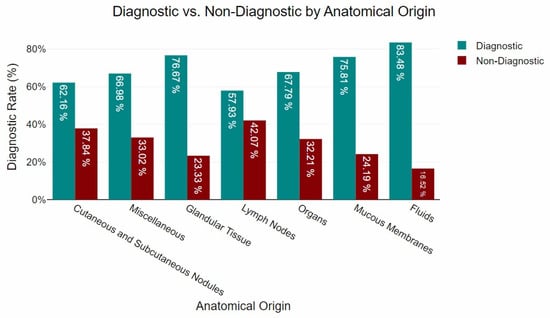
Figure 1.
Diagnostic yield by anatomical origin.
3.2. Neoplastic Versus Non-Neoplastic Diagnoses
The incidence of neoplastic processes was first analysed in relation to epidemiological factors (sex, breed, and age), followed by an assessment based on anatomical location.
3.2.1. Distribution of Neoplastic and Non-Neoplastic Lesions by Sex, Breed, and Age
A total of 2031 samples were evaluated, with 1083 from females and 948 from males. Among the female samples, 392 (54.90%) were diagnosed as neoplastic, whereas 691 (52.47%) were non-neoplastic. In the male group, 322 samples (45.10%) were neoplastic, and 626 (47.53%) were non-neoplastic. Overall, neoplastic lesions accounted for 714 cases (35.16%) of the total sample population, while non-neoplastic cases represented the majority, with 1317 cases (64.84%). The Chi-Square test of independence between sexes did not indicate a significant association between male and female neoplastic lesions (p = 0.294), indicating a consistent frequency of neoplastic occurrences among the sexes evaluated.
Regarding the breed, no significant association was found between the breed and the occurrence of neoplastic conditions (Chi-Square test, p = 0.303), indicating a uniform distribution of neoplastic occurrences across the various breeds evaluated.
In contrast to sex and breed, age showed a significant association with the incidence of neoplasia (Chi-Square test, p < 0.001). In animals aged ≤ 1 year, 10.3% of lesions were neoplastic. This proportion increased to 21.4% in the >1 to ≤2 years group and 24.3% in the >2 to ≤4 years group. These younger animals served as the baseline for comparison, with the ≤1 year group set as the reference (odds ratio (OR) = 1).
The likelihood of neoplastic diagnosis increased progressively with age:
- >1 to ≤2 years: OR = 0.97 (95% CI: 0.60–1.55);
- >2 to ≤4 years: OR = 1.14 (95% CI: 0.76–1.70);
- >4 to ≤7 years: OR = 1.39 (95% CI: 1.00–1.93);
- >7 to ≤10 years: OR = 2.68 (95% CI: 1.98–3.62);
- >10 to ≤15 years: OR = 3.73 (95% CI: 2.85–4.88);
- >15 years: OR = 4.90 (95% CI: 3.14–7.66).
Table 1 displays the occurrence of non-neoplastic versus neoplastic lesions according to age group.

Table 1.
Occurrence of types of non-neoplastic/neoplastic lesions by age group.
3.2.2. Distribution of Neoplastic and Non-Neoplastic Lesions by Anatomical Location
In the analysis, cutaneous and subcutaneous nodules constituted the primary category, encompassing 680 samples, of which 48.09% (n = 327) were characterised as neoplastic and 51.91% (n = 353) as non-neoplastic. The second largest category, consisting of 142 miscellaneous samples, exhibited a neoplastic incidence of 46.48% (n = 66) versus 53.52% (n = 77) of non-neoplastic. Glandular tissue, contributing 138 samples, displayed a neoplastic lesion rate of 45.65% (n = 63) against 54.35% (n = 75) non-neoplastic. Lymph nodes, with 274 samples assessed, showed a lower neoplastic proportion at 33.94% (n = 93) versus non-neoplastic (n = 181; 66.6%). Organ samples, which totalled 444, had a neoplastic lesion proportion of 29.28% and a non-neoplastic proportion of 70.72% (n = 314). Mucous membranes, accounting for 257 samples, primarily consisted of non-neoplastic lesions, with a significantly lower rate of 13.23% and 86.77% (n = 223) non-neoplastic. Fluid samples, the smallest group in this study with 96 specimens, demonstrated the lowest incidence of neoplastic conditions, at just 1.04% and 98.96% (n = 95) non-neoplastic.
Figure 2 presents the distribution of neoplastic and non-neoplastic lesions by category in this study.
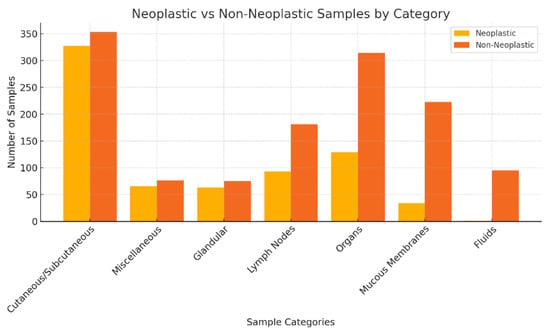
Figure 2.
Neoplastic vs. non-neoplastic samples by category.
3.2.3. Neoplastic Diagnosis
In an attempt to determine whether the incidence of different cell types was associated with sex and age within the group of neoplastic diagnoses, Table 2 presents a descriptive summary of the occurrences of various types of neoplastic lesions, categorised according to their nature: round cell, mesenchymal, epithelial, and melanocytic tumours.

Table 2.
Occurrence of different types of neoplastic lesions categorised by sex and age.
In terms of sex, females showed a higher proportion of epithelial tumours (38.01%), whereas mesenchymal lesions were more frequently observed in males (42.86%). Round cell tumours had a similar distribution between sexes (31.12% in females and 34.16% in males), and melanocytic tumours were rare in both groups, accounting for less than 1% of cases. Regarding age, among the 621 cases evaluated, round cell lesions were the most frequent overall (35.99%), followed by mesenchymal lesions (32.49%), epithelial lesions (31.09%), and melanocytic lesions (0.42%). Round cell tumours predominated in animals aged ≤1 year and >1 to ≤2 years, representing over 80% of the neoplasms in these age groups. Conversely, epithelial and mesenchymal lesions became more prevalent in older animals, particularly in the age groups >10 to ≤15 years and >15 years, where epithelial tumours accounted for up to 39.66% of the diagnoses (Table 2).
4. Discussion
This study presents a comprehensive retrospective analysis of cytology as a diagnostic tool in feline veterinary medicine over a seven-year period. Analysis of 3068 samples from 130 veterinary practices across Portugal yielded a diagnostic success rate of 66.20%, emphasising the critical relevance of cytology in routine practice. Our findings align with the existing literature on the application of cytology in veterinary medicine, particularly its use in diagnosing neoplastic and non-neoplastic conditions. While cytology is widely recognised for its rapid turnaround and accessibility, several limitations remain, primarily linked to the absence of tissue architecture, sample quality, and diagnostic sensitivity when compared to histopathology [5,28,33,34].
Cytology remains a fundamental tool for the preliminary assessment and classification of lesions, particularly through FNA, which offers valuable insights into cellular composition [23]. However, a significant proportion of samples were classified as non-diagnostic, mirroring results observed in canine studies [38,39]. Contributing factors included insufficient cellularity, haemodilution, and suboptimal slide preparation technique [18,21]. Despite these limitations, cytology has demonstrated high diagnostic concordance with histopathology in specific lesion types, particularly in the identification of round cell neoplasms [5,40,41]. Nonetheless, epithelial and mesenchymal neoplasms exhibited a higher rate of inconclusive cytological diagnoses, reinforcing the recommendation for histopathological confirmation in such cases [42]. It is important to note that, unlike other studies, cytological diagnoses in this research were not confirmed by histopathology, as the primary objective was not to assess the accuracy of cytological examinations [2,15]. Therefore, the identified factors should not be interpreted as determinants of increased diagnostic reliability, but rather as variables that enhance the likelihood of a cytopathologist formulating a clinically meaningful interpretation [2].
The present study revealed significant variations in diagnostic yield across anatomical groupings, reinforcing the influence of sample origin on cytological outcomes. Fluids exhibited the highest diagnostic success rate (83.48%), followed by glandular tissue (76.67%) and mucous membranes (75.81%). These findings align with previous research highlighting the superior cytological yield of fluid-based samples, which generally provide higher cellularity and well-preserved morphology, facilitating interpretation [43,44,45]. Similarly, the high yield observed in mucous membranes and glandular tissue may be attributed to the relative homogeneity of their cellular composition, which enhances diagnostic accuracy [24,25,46,47]. Organs demonstrated a notable diagnostic success rate (67.79%), consistent with previous reports, despite the inherent challenges in obtaining representative samples from these tissues [20,25,48]. Conversely, lower rates were observed in miscellaneous (66.98%), cutaneous and subcutaneous nodules (62.16%), and lymph nodes (57.93%). These findings corroborate previous studies [7,11,49], suggesting that cutaneous and subcutaneous samples, despite being more accessible, are prone to artefacts such as haemodilution, poor cellular preservation, and inflammatory contamination, which can reduce diagnostic accuracy. The diagnostic performance of FNA cytology in lymph node evaluation underscores the challenges associated with differentiating reactive hyperplasia from neoplastic infiltration, as previously documented [5,15,50]. While cytology remains a valuable tool for preliminary assessment, histopathological confirmation is often required for definitive classification, particularly in cases with ambiguous cytological features [24].
Non-diagnostic samples constituted 33.80% of the total cases, with the highest proportions observed in lymph nodes, cutaneous and subcutaneous nodules, and miscellaneous. This finding highlights the impact of sampling limitations, including insufficient cellularity, excessive blood contamination, and poor slide preparation techniques. Given the considerable proportion of non-diagnostic cases, optimising sample collection and preparation protocols is crucial to enhancing cytological diagnostic utility [18].
No statistically significant association was found between the feline breed and the likelihood of neoplastic lesions, suggesting a relatively uniform distribution of neoplasia across breeds. This observation is consistent with existing epidemiological data indicating that while certain breeds may carry genetic predispositions, age and environmental exposures are more influential determinants in tumour development [51].
Similarly, no significant overall association was observed between sex and the occurrence of neoplastic lesions, suggesting that neoplasia affects male and female cats at comparable rates. However, a closer examination of specific tumour types revealed notable trends, with epithelial and melanocytic neoplasms more frequently diagnosed in females, while round cell and mesenchymal neoplasms were more prevalent in males. These findings align with prior research indicating that hormonal influences and genetic factors may contribute to differences in tumour distribution between sexes [51,52].
Certain tumour types are known to have sex-associated prevalence patterns in cats, with some neoplasms, such as mammary gland tumours, being significantly more common in females [30,47,52,53,54].
Some neoplasms, namely those of mesenchymal origin, have been reported to occur more frequently in male cats, although the underlying mechanisms remain incompletely understood [47,54]. Similarly, male cats were more frequently diagnosed with round cell neoplasms, like lymphoma, mast cell tumours, and plasma cell tumours. This aligns with prior research suggesting that male cats may have a higher risk of developing lymphoma, particularly in association with feline leukaemia virus (FeLV) infection, influenced by differences in immune function and retroviral susceptibility [47,52]. Beyond neoplasia, cytological diagnoses of inflammatory and non-neoplastic conditions did not demonstrate breed predisposition. However, previous research suggests that certain inflammatory conditions, such as eosinophilic granulomas, may be more common in breeds such as Siamese and other oriental breeds due to their unique immune responses [55,56]. The absence of a general sex predisposition to neoplasia in this study is consistent with previous large-scale epidemiological studies [47,52], which identify sex as a minor determinant of cancer risk, apart from hormonally driven tumours such as mammary carcinoma. However, the observed variations in tumour subtypes between sexes underscore the relevance of considering sex as a potential influencing factor during cytological evaluation.
The present study reinforces the well-documented association between advancing age and an increased prevalence of neoplastic conditions in felines. The cumulative effects of environmental carcinogens, chronic inflammation, and genetic mutations contribute to the heightened neoplastic risk observed in senior and geriatric patients [52,53]. Nonetheless, some studies challenge this association, suggesting that certain tumour types do not follow a clear age-related pattern and may even be more prevalent in younger individuals [51]. Although some tumours, such as lymphomas, may present in younger adult cats, particularly in those with retroviral infections like FeLV, widespread testing and vaccination led to a decline in this type of lymphoma, which are now more frequently diagnosed in older, FeLV-negative cats [57]. In contrast, inflammatory and non-neoplastic lesions were more common in younger cats, particularly those involving infectious processes or immune reactivity, like eosinophilic granulomas and pyogranulomatous inflammations.
Given the marked increase in neoplastic diagnoses in ageing cats, these findings underscore the importance of proactive diagnostic strategies, particularly in senior and geriatric patients. Regular cytological evaluation of suspicious lesions, coupled with imaging and histopathological confirmation where needed, can facilitate early detection and improve clinical outcomes [18,28,58,59]. In younger cats, clinicians should consider inflammatory and reactive processes as primary differential diagnoses before suspecting neoplasia. The highest odds ratio for neoplastic lesions was observed in geriatric cats (>15 years), who were nearly five times more likely to develop neoplasia compared to the reference group. This stark increase in cancer risk further supports the necessity for rigorous neoplastic surveillance in older felines [47,52,53,60].
5. Conclusions
The study underscores cytology as a minimally invasive, quick, and cost-effective technique for identifying neoplastic, inflammatory, and non-neoplastic lesions, especially in fluid-based, glandular, and mucous membrane samples with high diagnostic yields. However, the high rate of non-diagnostic samples (33.80%) calls for improved sampling protocols, operator training, and standardised cytological methods to achieve better diagnostic results.
Diagnostic performance varied with anatomical location, age, and sex of the subjects. Older cats showed a significantly higher rate of neoplastic conditions, with geriatric patients having a nearly fivefold increase in neoplastic diagnoses compared to younger counterparts, suggesting the need for age-specific screening. Although no link was found between breed and neoplasia, sex-based differences were noted; females had higher rates of epithelial and melanocytic neoplasms, whereas mesenchymal and round cell neoplasms were more common in males, indicating that genetic, hormonal, and environmental factors may influence neoplastic development in felines.
Author Contributions
Conceptualization, P.B.-S., R.M., J.P. and F.Q.; methodology, P.B.-S., A.M. and F.Q.; software, P.B.-S., R.L., A.S. and Â.M.; validation, P.B.-S., A.S., R.M., J.P. and F.Q.; formal analysis, P.B.-S., R.L. and Â.M.; investigation, P.B.-S.; resources, P.B.-S., A.S. and L.D.; data curation, P.B.-S., R.L. and Â.M.; writing—original draft preparation, P.B.-S., R.L. and F.Q.; writing—review and editing, P.B.-S., R.L., L.D., A.M., A.S., Â.M., R.M., J.P. and F.Q.; visualisation, P.B.-S. and R.L.; supervision, R.M., J.P. and F.Q.; project administration, R.M., J.P. and F.Q.; funding acquisition, Â.M., J.P. and F.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by project UIDB/00772/2020 (http://doi:10.54499/UIDB/00772/2020), funded by the Portuguese Foundation for Science and Technology (FCT).
Institutional Review Board Statement
All procedures complied with the Portuguese legislation for the protection of animals used for scientific purposes (i.e., Decree–Law no. 113/2013, of 7 August 2013), which transposes European legislation (i.e., Directive 2010/63/EU of the European Parliament and of the Council, of 22 September 2010). The study project was approved by the Institutional Review Board of INNO Veterinary Laboratories (protocol code INNO.007 and INNO.0026 approved on 29 September 2021), which ensures that the analysed samples of veterinary medical centres can be used anonymously in studies and scientific research works related to this project.
Informed Consent Statement
Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers, tissues, and samples) for all procedures undertaken (prospective or retrospective studies). No animals or people were identifiable within this publication, and therefore additional informed consent for publication was not required.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors extend sincere gratitude to the INNO Veterinary Laboratories (Braga, Portugal) for the generous provision of results that facilitated the research conducted in this study.
Conflicts of Interest
Authors P.B.-S., L.D., A.M. and A.S. are employed by the company INNO Veterinary Laboratories (Braga, Portugal). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bonfanti, U.; Bertazzolo, W.; Gracis, M.; Roccabianca, P.; Romanelli, G.; Palermo, G.; Zini, E. Diagnostic value of cytological analysis of tumours and tumour-like lesions of the oral cavity in dogs and cats: A prospective study on 114 cases. Vet. J. 2015, 205, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sapierzyński, R.; Czopowicz, M.; Ostrzeszewicz, M. Factors affecting the diagnostic utility of canine and feline cytological samples. J. Small Anim. Pract. 2017, 58, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.C.; Seelig, D.M.; Overmann, J. All lesions great and small, part 2. Diagnostic cytology in veterinary medicine. Diagn. Cytopathol. 2014, 42, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.C.; Seelig, D.M.; Overmann, J. All lesions great and small, part 1: Diagnostic cytology in veterinary medicine. Diagn. Cytopathol. 2014, 42, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.K.; Kass, P.H.; Christopher, M.M. Cytologic-histologic concordance in the diagnosis of neoplasia in canine and feline lymph nodes: A retrospective study of 367 cases. Vet. Comp. Oncol. 2017, 15, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Ayele, L.; Mohammed, C.; Yimer, L. Review on diagnostic cytology: Techniques and applications in veterinary medicine. J. Vet. Sci. Technol. 2017, 8, 2. [Google Scholar] [CrossRef]
- MacNeill, A. Cytology of canine and feline cutaneous and subcutaneous lesions and lymph nodes. Top. Companion Anim. Med. 2011, 26, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, C.; Fish, E.; Miller, A.; Snyder, L.; Labadie, J.; Avery, P. Evaluation of Region of Interest Digital Cytology Compared to Light Microscopy for Veterinary Medicine. Vet. Pathol. 2019, 56, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Coleto, A.F.; De Almeida Moreira, T.; Gundim, L.; De Almeida Silva, S.; Castro, M.; Bandarra, M.; Ronchi, A.M. Perfil de exames citológicos, sensibilidade e especificidade da punção por agulha fina em amostras cutâneas e subcutâneas em cães. Braz. J. Vet. Med. 2016, 38, 311–315. [Google Scholar]
- Allen, B.; Evans, S. Diagnostic accuracy of cytology for the detection of bacterial infection in fluid samples from veterinary patients. Vet. Clin. Pathol. 2022, 51, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Ghisleni, G.; Roccabianca, P.; Ceruti, R.; Stefanello, D.; Bertazzolo, W.; Bonfanti, U.; Caniatti, M. Correlation between fine-needle aspiration cytology and histopathology in the evaluation of cutaneous and subcutaneous masses from dogs and cats. Vet. Clin. Pathol. 2006, 35, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.J. Cutaneous and subcutaneous lesions. In Diagnostic Cytology and Hematology of the Dog and Cat, 5th ed.; Valenciano, A.C., Cowell, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 74–101. [Google Scholar]
- Fisher, K.J.; Meyer, D.J. Acquisition and Management of Cytologic Specimens. In Canine and Feline Cytopathology: A Color Atlas and Interpretation Guide, 4th ed.; Raskin, R.E., Meyer, D.J., Boes, K.M., Eds.; Elsevier: St. Louis, MO, USA, 2023; pp. 1–14. [Google Scholar]
- Meinkoth, J.H.; Cowell, R.L.; Tyler, R.D.; Morton, R.J. Sample Collection and Preparation. In Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–17. [Google Scholar]
- Amores-Fuster, I.; Cripps, P.; Graham, P.; Marrington, A.M.; Blackwood, L. The diagnostic utility of lymph node cytology samples in dogs and cats. J. Small Anim. Pract. 2015, 56, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Rodolfo, F.A.; Colodel, M.M.; Rocha, N.S. Cytological examination in veterinary medicine: Retrospective study of 11,468 cases (1994–2008). Pesq. Vet. Bras. 2012, 32, 1169–1173. [Google Scholar]
- Masserdotti, C. Architectural patterns in cytology: Correlation with histology. Vet. Clin. Pathol. 2006, 35, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.; Taeymans, O.; Monti, P. A comparison of cytological quality between fine-needle aspiration and non-aspiration techniques for obtaining ultrasound-guided samples from canine and feline lymph nodes. Vet. Rec. 2021, 188, e25. [Google Scholar] [CrossRef]
- Dolka, I.; Czopowicz, M.; Gruk-Jurka, A.; Wojtkowska, A.; Sapierzyński, R.; Jurka, P. Diagnostic efficacy of smear cytology and Robinson’s cytological grading of canine mammary tumors with respect to histopathology, cytomorphometry, metastases and overall survival. PLoS ONE 2018, 13, e0191595. [Google Scholar] [CrossRef] [PubMed]
- Liffman, R.; Courtman, N. Fine needle aspiration of abdominal organs: A review of current recommendations for achieving a diagnostic sample. J. Small Anim. Pract. 2017, 58, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Fleming, K.L.; Howells, E.J.; Villiers, E.J.; Maddox, T.W. A randomised controlled comparison of aspiration and non-aspiration fine-needle techniques for obtaining ultrasound-guided cytological samples from canine livers. Vet. J. 2019, 252, 105372. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, D.A.; Avery, A.C.; Henson, M.S.; Overmann, J.A.; Rendahl, A.K.; Walz, J.Z.; Seelig, D.M. Cytology and the cell block method in diagnostic characterization of canine lymphadenopathy and in the immunophenotyping of nodal lymphoma. Vet. Comp. Oncol. 2019, 17, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Rist, P.; Clancey, N.; Gilroy, C.; Stryhn, H.; Amsellem, P. Fine-needle aspiration of cutaneous, subcutaneous, and intracavitary masses in dogs and cats using 22- vs 25-gauge needles. Vet. Clin. Pathol. 2019, 48, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Brilhante-Simões, P.; Delgado, L.; Martins, Â.; Silva, A.; Monteiro, L.; Marcos, R.; Prada, J. Association Between Cytological and Histopathological Diagnoses of Neoplastic and Non-Neoplastic Lesions in Oral Cavity from Dogs and Cats: An Observational Retrospective Study of 103 Cases. Vet. Sci. 2025, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Wypij, J.M. Getting to the point: Indications for fine-needle aspiration of internal organs and bone. Top. Companion Anim. Med. 2011, 26, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Lee, J.-J.; Liao, A.T. Comparison of cytological and histopathological validation on fine needle aspiration of superficial masses. Taiwan Vet. J. 2014, 40, 191–198. [Google Scholar] [CrossRef]
- Ballegeer, E.A.; Forrest, L.J.; Dickinson, R.M.; Schutten, M.M.; Delaney, F.A.; Young, K.M. Correlation of ultrasonographic appearance of lesions and cytologic and histologic diagnoses in splenic aspirates from dogs and cats: 32 cases (2002–2005). J. Am. Vet. Med. Assoc. 2007, 230, 690–696. [Google Scholar] [CrossRef] [PubMed]
- McAloney, C.A.; Sharkey, L.C.; Feeney, D.A.; Seelig, D.M. Diagnostic utility of renal fine-needle aspirate cytology and ultrasound in the cat. J. Feline Med. Surg. 2018, 20, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.B.; Rossato, C.K.; Silva, S.L.; Almeida, S.S.N.; Ribeiro, L.S. Fine needle aspiration cytology in feline skeletal muscle as a diagnostic tool for extramedullary plasmacytoma. Arq. Bras. Med. Vet. Zootec. 2017, 69, 587–592. [Google Scholar] [CrossRef]
- Gregório, H.; Pires, I.; Seixas, F.; Queiroga, F. Mammary invasive micropapillary carcinoma in a male cat: Immunohistochemical description and clinical follow-up. Acta Vet. Hung. 2012, 60, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Villamil, J.A.; Henry, C.J.; Bryan, J.N.; Ellersieck, M.; Schultz, L.; Tyler, J.W.; Hahn, A.W. Identification of the most common cutaneous neoplasms in dogs and evaluation of breed and age distributions for selected neoplasms. J. Am. Vet. Med. Assoc. 2011, 239, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Raskin, R. General Categories of Cytologic Interpretation. In Canine and Feline Cytopathology: A Color Atlas and Interpretation Guide, 4th ed.; Raskin, R.E., Meyer, D.J., Boes, K.M., Eds.; Elsevier: St. Louis, MO, USA, 2023; pp. 15–34. [Google Scholar]
- Christopher, M.M.; Ku, C.K. Likelihood of Neoplasia for Diagnoses Modified by Probability Terms in Canine and Feline Lymph Node Cytology: How Probable Is Probable? Front. Vet. Sci. 2018, 5, 246. [Google Scholar] [CrossRef] [PubMed]
- Isaza, D.; Robinson, N.; Pizzirani, S.; Pumphrey, S. Evaluation of cytology and histopathology for the diagnosis of feline orbital neoplasia: 81 cases (2004–2019) and review of the literature. Vet. Ophthalmol. 2020, 23, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Smith, K.C.; Dobromylskyj, M.J. Retrospective study of more than 9000 feline cutaneous tumours in the UK: 2006–2013. J. Feline Med. Surg. 2018, 20, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Skeldon, N.; Dewhurst, E. The perceived and actual diagnostic utility of veterinary cytological samples. J. Small Anim. Pract. 2009, 50, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Rishniw, M.; Freeman, K.P. Veterinary clinicians prefer template-style reports with personal confidence estimates for cytologic sample evaluations. J. Am. Vet. Med. Assoc. 2024, 262, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Tecilla, M.; Gambini, M.; Forlani, A.; Caniatti, M.; Ghisleni, G.; Roccabianca, P. Evaluation of cytological diagnostic accuracy for canine splenic neoplasms: An investigation in 78 cases using STARD guidelines. PLoS ONE 2019, 14, e0224945. [Google Scholar] [CrossRef] [PubMed]
- Sabattini, S.; Renzi, A.; Buracco, P.; Defourny, S.; Garnier-Moiroux, M.; Capitani, O.; Bettini, G. Comparative Assessment of the Accuracy of Cytological and Histologic Biopsies in the Diagnosis of Canine Bone Lesions. J. Vet. Intern. Med. 2017, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Auch, C.L.; Michael, A. Cutaneous plasmacytoma with Mott cell differentiation in a dog. J. Vet. Diagn. Investig. 2024, 36, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.C.; Myers, A.N. Cytology of Skin Neoplasms. Vet. Clin. North. Am. Small Anim. Pract. 2017, 47, 85–110. [Google Scholar] [CrossRef] [PubMed]
- Ipek, V.; Cangul, I.T.; Akkoc, A. Comparative Evaluation of the Cytological, Histopathological and Immunohistochemical Findings of Canine Cutaneous and Subcutaneous Masses. Acta Vet. 2021, 71, 61–84. [Google Scholar] [CrossRef]
- Fernandes, N.; Guerra, J.; Réssio, R.; Wasques, D.; Etlinger-Colonelli, D.; Lorente, S.; Nogueira, E.; Dagli, M. Liquid-based cytology and cell block immunocytochemistry in veterinary medicine: Comparison with standard cytology for the evaluation of canine lymphoid samples. Vet. Comp. Oncol. 2016, 14 (Suppl. S1), 107–116. [Google Scholar] [CrossRef] [PubMed]
- Prickett, J.R.; Zimmerman, J.J. The development of oral fluid-based diagnostics and applications in veterinary medicine. Anim. Health Res. Rev. 2010, 11, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; She, D.L.; Xiong, H.; Yang, L.; Fu, S.J. Diagnostic Value of Liquid-Based Cytology in Urothelial Carcinoma Diagnosis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0134940. [Google Scholar] [CrossRef] [PubMed]
- Eördögh, R.; Schwendenwein, I.; Tichy, A.; Nell, B. Impression cytology: A novel sampling technique for conjunctival cytology of the feline eye. Vet. Ophthalmol. 2015, 18, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Grüntzig, K.; Hässig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Guscetti, F.; Folkers, G.; Otto, V.; et al. Swiss Feline Cancer Registry: A Retrospective Study of the Occurrence of Tumours in Cats in Switzerland from 1965 to 2008. J. Comp. Pathol. 2015, 153, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Oblak, M.L.; Lu, H.Y.; Ram, A.S.; McKenna, C. Comparative aspects of targeted sentinel lymph node mapping in veterinary and human medicine: Opportunities for future research. Front. Med. 2024, 11, 1342456. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.B.E.; Engelmann, A.; Kommers, G.; Flores, M.M.; Fighera, R.A.; Rodrigues, B.R.; Lamego, É.C.; Da Silva, C.B.; Bueno, A.; De Andrade, C.M. Fine needle aspiration cytology: High accuracy in diagnosing cutaneous and subcutaneous neoplasms in dogs. Comp. Clin. Pathol. 2022, 32, 155–164. [Google Scholar] [CrossRef]
- Beer, P.; Pozzi, A.; Rohrer Bley, C.; Bacon, N.; Pfammatter, N.S.; Venzin, C. The role of sentinel lymph node mapping in small animal veterinary medicine: A comparison with current approaches in human medicine. Vet. Comp. Oncol. 2018, 16, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Ristevski, T.; Kurilj, A.; Maurić, M.; Zagradišnik, L.; Hohšteter, M.; Šoštarić-Zuckermann, I. Prevalence of pathological lesions diagnosed by cytology in cats, with association of diagnosis to age, breed and gender. Vet. Arhiv. 2021, 91, 169–177. [Google Scholar] [CrossRef]
- Graf, R.; Grüntzig, K.; Boo, G.; Hässig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Guscetti, F.; Folkers, G.; et al. Swiss Feline Cancer Registry 1965–2008: The Influence of Sex, Breed and Age on Tumour Types and Tumour Locations. J. Comp. Pathol. 2016, 154, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Severin, K.; Vlahović, D.; Križanac, S.; Mofardin, S.; Buhin, I.M.; Zagradišnik, L.M.; Šoštarić-Zuckermann, I.-C.; Kurilj, A.G.; Artuković, B.; et al. Cancer morbidity in Croatian cats: Retrospective study on spontaneously arising tumors (2009–2019). Top. Companion Anim. Med. 2024, 58, 100841. [Google Scholar] [CrossRef] [PubMed]
- Morris, J. Mammary Tumours in the Cat: Size matters, so early intervention saves lives. J. Feline Med. Surg. 2013, 15, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Omelchenko, H.; Avramenko, N.; Kulynych, S.; Petrenko, M.; Volosovets, V.; Volosovets, N.; Woźniakowski, G. Some aspects of the diagnosis and treatment of eosinophilic granuloma in cats. J. Vet. Res. 2023, 67, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Falcão, F.; Faísca, P.; Viegas, I.; de Oliveira, J.T.; Requicha, J.F. Feline oral cavity lesions diagnosed by histopathology: A 6-year retrospective study in Portugal. J. Feline Med. Surg. 2020, 22, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Rolph, K.E.; Cavanaugh, R.P. Infectious Causes of Neoplasia in the Domestic Cat. Vet. Sci. 2022, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.; Liffman, R.; Woodward, A.; Beck, C.; Courtman, N.; Dandrieux, J. Assessment of the clinical usefulness of ultrasound-guided cytological specimens obtained from gastrointestinal lesions in dogs and cats. J. Small Anim. Pract. 2021, 62, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, M.; Tamburro, R.; Felici, A.; Del Signore, F.; Dettori, A.; Di Tommaso, M.; Ghiraldelli, A.; Terragni, R.; Simeoni, F.; Falerno, I.; et al. Clinical Value of CT-Guided Fine Needle Aspiration and Tissue-Core Biopsy of Thoracic Masses in the Dog and Cat. Animals 2021, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.; Dobromylskyj, M.; Wood, G.A.; van der Weyden, L. Feline Oncogenomics: What Do We Know about the Genetics of Cancer in Domestic Cats? Vet. Sci. 2022, 9, 547. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).